Abstract
Background & Aims
Protein tyrosine kinase 6 (PTK6) is expressed in epithelial linings of the gastrointestinal tract. PTK6 sensitizes the non-transformed Rat1a fibroblast cell line to apoptotic stimuli. The aim of this study was to determine if PTK6 regulates apoptosis in vivo after DNA damage in the small intestine.
Methods
Wild-type and Ptk6 −/− mice were subjected to γ-irradiation; intestinal tissues were collected, protein was isolated, and samples were fixed for immunohistochemical analyses at 0, 6, and 72 hours after the mice were irradiated. Expression of PTK6 was examined in the small intestine before and after irradiation. Apoptosis and proliferation were compared between wild-type and Ptk6 −/− mice. Expression and activation of pro-survival signaling proteins were assessed.
Results
Irradiation induced PTK6 in crypt epithelial cells of the small intestine in wild-type mice. Induction of PTK6 corresponded with DNA-damage induced apoptosis in the wild-type small intestine. Following irradiation, the apoptotic response was impaired in the intestinal crypts of Ptk6 −/− mice. Increased activation of AKT and extracellular signal-regulated kinase (ERK)1/2 and increased inhibitory phosphorylation of the proapoptotic protein BAD were detected in Ptk6 −/− mice after irradiation. In response to the induction of apoptosis, compensatory proliferation increased in the small intestines of wild-type mice, but not in Ptk6 −/− mice at 6 hours after irradiation.
Conclusions
PTK6 is a stress-induced kinase that promotes apoptosis by inhibiting pro-survival signaling. After DNA damage, induction of PTK6 is required for efficient apoptosis and inhibition of AKT and ERK1/2.
Keywords: BRK, Sik, AKT, ERK, BAD
INTRODUCTION
Protein tyrosine kinase 6 (PTK6; also called BRK or Sik) is an intracellular tyrosine kinase that is related to members of the Src-family, and it contains SH3 and SH2 domains as well as a carboxy regulatory tyrosine. PTK6 family kinases include Srms, FRK and Src42A/Dsrc41 that all share a common conserved gene structure. Unlike Src kinases, most PTK6 family members are not myristoylated, resulting in greater flexibility in their intracellular localization (reviewed in 1). PTK6 is expressed in differentiated epithelial cells of the gastrointestinal tract 2–4, skin 5, 6, prostate 7, and oral cavity 8. Although not expressed in the normal mammary gland or ovarian epithelium, PTK6 expression is frequently observed in breast 9 and ovarian tumors 10. Although largely studied in epithelia, expression of PTK6 has also been documented in lymphocytes 11.
A survey of PTK6 expression in adult mouse tissues revealed highest levels of PTK6 expression in the small intestine 12, where PTK6 protein expression is predominantly localized to nondividing differentiated villus epithelial cells 13. The epithelial lining of the small intestine provides a dynamic developmental system that is ideal for studying coordination of mechanisms regulating proliferation, differentiation, and migration. In the small intestine, stem cells anchored in the crypts give rise to proliferating progenitor cells that exit the cell cycle as they migrate and differentiate into four different cell types, including enterocytes, goblet cells, enteroendocrine cells and Paneth cells (reviewed in 14, 15). Proliferation is compartmentalized and confined to crypts. In normal intestine, significant PTK6 expression was not detected in proliferating crypt cells 2, 3, 13.
Disruption of the mouse Ptk6 gene resulted in increased growth and impaired enterocyte differentiation in the small intestine, consistent with a role for PTK6 in promoting differentiation 13. Previously, the serine threonine kinase AKT was identified as a target of PTK6 that was negatively regulated by tyrosine phosphorylation 16. In concordance with these studies, we found increased levels of activated AKT in the intestines of the Ptk6 null mice, indicating that one function of PTK6 in normal nondividing epithelial cells is to suppress AKT to promote growth inhibition as cells differentiate 13. In addition to regulating growth and differentiation, AKT plays a critical role in regulating cell survival (reviewed in 17, 18), suggesting that PTK6 may also have an impact on cell survival in the intestine. In earlier studies, we determined that ectopic expression of PTK6 sensitized nontransformed Rat1a fibroblast cells to apoptotic stimuli such as serum deprivation and UV irradiation 19.
Epithelial cells in the small intestine are sensitive to DNA damage and apoptosis that can be observed within 6 hours after total body irradiation 20, 21. Here we investigated the consequences of PTK6 ablation on apoptosis following irradiation in the mouse small intestine, and have discovered that PTK6 expression is induced in crypt epithelial cells after irradiation, where it negatively regulates survival signaling and contributes to apoptosis after DNA damage.
RESULTS
PTK6 expression is induced in intestinal crypts after γ-irradiation
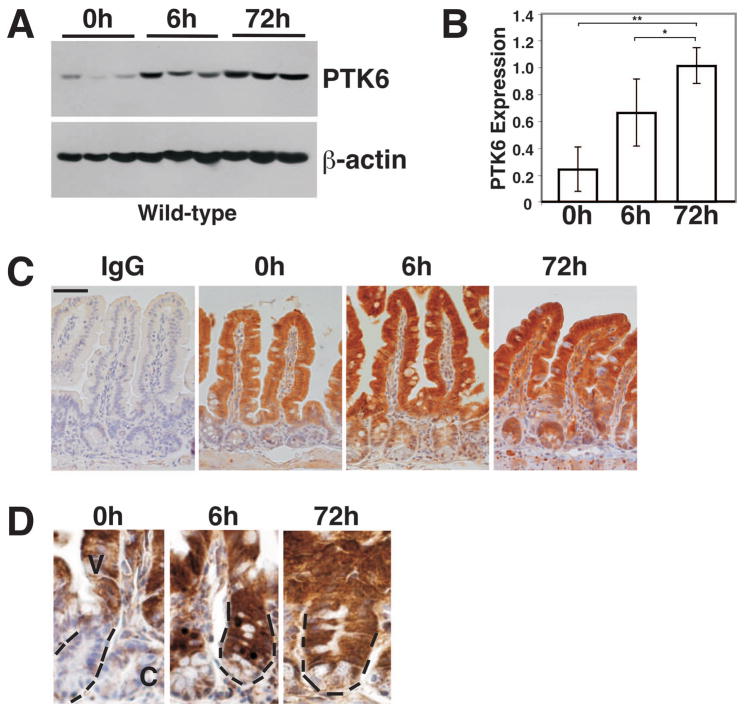
To begin to assess the role of PTK6 following DNA damage, PTK6 protein levels were examined by immunoblotting in the small intestines of wild-type mice following total body γ-irradiation (8 Gy). A significant time-dependent increase in PTK6 protein levels was detected after irradiation (Fig. 1A, B). PTK6 protein expression was localized in untreated and irradiated wild-type mice using immunohistochemistry. Consistent with prior reports 13, PTK6 protein expression was primarily restricted to non-proliferating, terminally differentiated cells of the intestinal villus epithelium in untreated mice (Fig. 1C, 0 hrs). Expression of PTK6 was largely excluded from the proliferative crypt compartment. However, in contrast to untreated animals, PTK6 protein expression was detected not only in the villus epithelium but also in proliferating epithelial cells of the crypt compartment in wild-type mice treated with ionizing radiation (Fig. 1C, D). PTK6 protein was detected throughout the crypt, although expression appeared lower in differentiated granule containing Paneth cells at the very base (Fig. 1D). Positive signals were also detected in some lamina propria cells that may be lymphocytes, as PTK6 has been reported to play a role in lymphocyte activation 11.
Figure 1. PTK6 protein expression is induced by γ-irradiation.
(A) Immunoblotting of untreated (0 h) and γ-irradiated (8 Gy) wild-type mouse lysates (6 h and 72 h following irradiation) from small intestine with antibodies against PTK6 and β-actin as a loading control. Results obtained using lysates from three different animals per timepoint are shown. (B) Quantitation of PTK6 protein expression. PTK6 protein levels were normalized to β-actin expression. The bars represent the mean ± S.D. A significant increase in PTK6 protein expression is detected in irradiated mice (*P-value = 0.028; **P-value = 0.040). (C) PTK6 is induced in the crypt epithelial cells after irradiation. Immunohistochemistry was performed to examine PTK6 expression in untreated and irradiated wild-type animals. Immunostaining for IgG served as a control. While PTK6 protein expression is restricted to the villus epithelium of untreated mice, it is also detected in the crypt of irradiated mice. Size bar represents 50 μm. (D) Higher magnification showing immunolocalization of PTK6 in crypt cells. At 6 and 72 h PTK6 positive cells are evident throughout most of the crypt, with weakest expression in Paneth cells localized at the base. PTK6 immunoreactivity was detected with DAB (brown stain). Dashed lines mark a crypt (C) in each panel at the same magnification.
DNA-damage induced apoptosis is impaired in the PTK6-deficient intestine
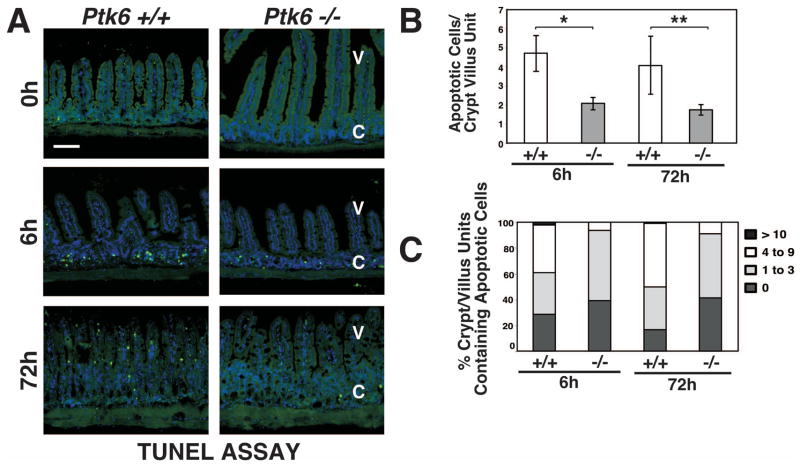
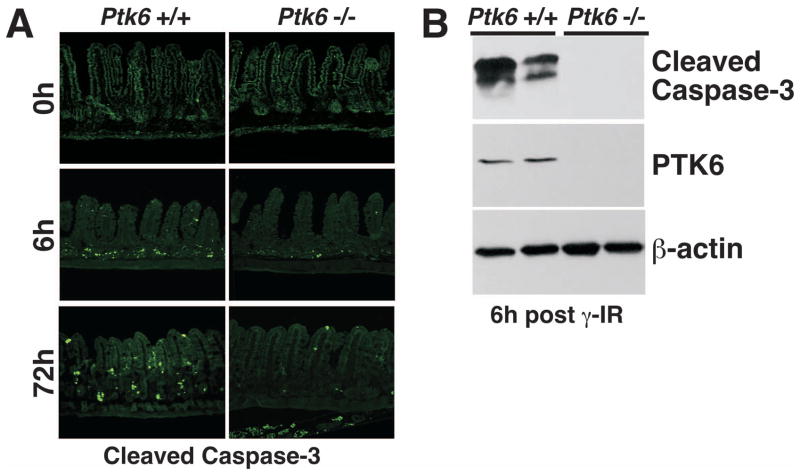
To determine if PTK6 has an impact on DNA-damage induced apoptosis in the small intestine, wild-type and Ptk6 −/− mice were subjected to total body γ-irradiation, and apoptotic cells were identified using the TUNEL assay (Fig. 2) and cleaved-Caspase-3 antibodies (Fig. 3) at 0, 6 and 72 h following treatment. Very little spontaneous apoptosis was detected in the untreated intestines of wild-type and Ptk6 null mice. Consistent with prior reports, exposure to 8 Gy γ-irradiation induced apoptosis of small intestinal crypt epithelial cells 22. However, substantially less apoptosis was detected in Ptk6 −/− mice than Ptk6 +/+ mice, with an approximately 2-fold decrease in number of TUNEL positive cells in Ptk 6−/− mice compared with wild-type controls (Fig. 2B). Wild-type mice exhibited about 5 apoptotic events per crypt/villus unit (Fig. 2B) consistent with the saturation level of apoptotic cells in the small intestinal crypt reported in previous studies 21. Loss of PTK6 resulted in a reduced percentage of crypt-villus units exhibiting significant epithelial apoptosis (4 or more apoptotic cells per unit) (Fig. 2C). Reduced apoptosis in Ptk6 null mice was accompanied by a reduction in Caspase-3 activation that was detected both by immunofluorescence (Fig. 3A) and immunoblotting (Fig. 3B). Thus, induction of PTK6 expression is an important component of the intestinal crypt epithelial cell response to irradiation in vivo, and appears to serve as a determinant of DNA-damage induced apoptosis.
Figure 2. DNA-damage induced apoptosis is compromised in the absence of PTK6.
(A) Radiation–induced apoptosis was examined in the small intestine from untreated and γ-irradiated Ptk6 +/+ and Ptk6 −/− mice at 0, 6 and 72 hours post irradiation using the TUNEL assay. Labeled apoptotic cells were detected with Avidin-FITC (apoptotic cells stain green). The size bar represents 100 μm. Quantification was performed and is presented as a histogram (B) and frequency histogram (C) of apoptotic cells per crypt-villus unit. (B) At least 50 crypt-villus units per section were scored. Values shown are mean ± S.D. from two sections from at least three different mice per group. *P-value = 0.026; **P-value = 0.032. V: villi; C: crypts.
Figure 3. Decreased activation of Caspase-3 correlates with reduced apoptosis in Ptk6 −/− mice.
(A) Activation (cleavage) of Caspase-3 was analyzed in sections of small intestine from untreated and γ-irradiated wild-type (Ptk6 +/+) and Ptk6 −/− mice at 0, 6 and 72 hours post irradiation. The size bar represents 100 μm. (B) Cleaved Caspase-3 levels were determined by immunoblotting. Tissue lysates from small intestine of wild-type (+/+) and knockout (−/−) mice 6 hours after irradiation were subjected to immunoblotting with anti-cleaved Caspase-3 antibodies. PTK6 protein and β-actin expression were examined as controls for genotyping and protein loading, respectively.
Resistance to apoptosis correlates with increased activation of prosurvival signaling in Ptk6 −/− mice
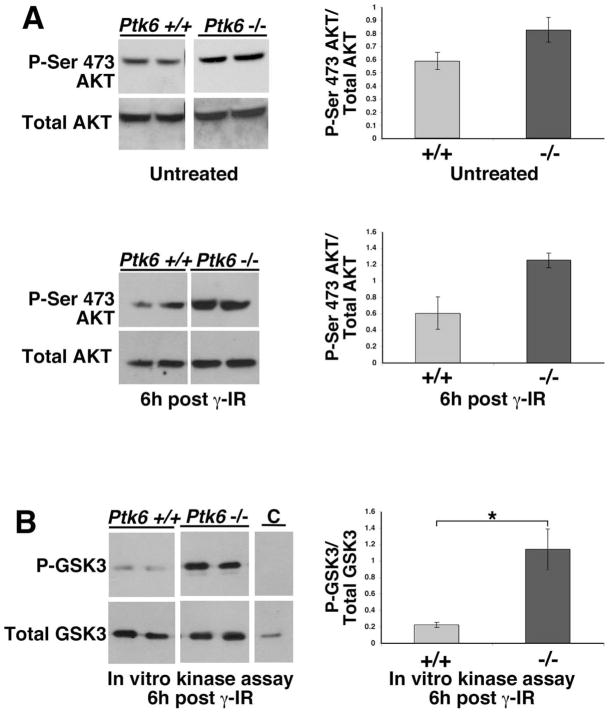
The balance between pro-survival and pro-apoptotic signaling determines the outcome to genotoxic stress. Previously, PTK6 was found to associate with and promote tyrosine phosphorylation of AKT in vitro resulting in inhibition of AKT kinase activity and downstream signaling 16. Enhanced basal AKT activity was detected in PTK6-deficient mice compared with wild-type control mice 13. AKT acts as a survival factor that stimulates progression of the cell cycle and prevents cells from undergoing apoptosis (reviewed in 17). To investigate whether enhanced AKT activation in PTK6-deficient mice might contribute to the apoptotic resistance of these mice following irradiation, lysates from small intestines of age-matched wild-type and Ptk6 −/− mice were analyzed by immunoblotting and AKT in vitro kinase assays (Fig. 4). Phosphorylation of AKT at both Ser 473 and Thr 308 is required for complete activation of AKT kinase. However, phosphorylation of Thr 308 is more transient than Ser 473. Wild-type and PTK6-deficient mice exhibited similar amounts of total AKT protein whereas increased amounts of AKT phosphorylated on Ser 473 were detected in Ptk6 −/− small intestine compared with wild-type controls at 0 and 6 hours (Fig. 4A). Using a two-step in vitro kinase assay to determine AKT activity, AKT precipitated from tissue homogenates of PTK6-deficient mice, but not of wild-type mice, resulted in significant phosphorylation of the GSK-3 substrate after irradiation (Fig. 4B).
Figure 4. AKT activity is increased in PTK6-deficient mice after irradiation.
(A) AKT expression and activation (phosphorylation of Ser 473) was examined by immunoblotting using total tissue lysates from small intestines of Ptk6 +/+ and Ptk6 −/− mice at 0 and 6 hours after whole-body γ-irradiation (γ-IR). (B) AKT activity was determined using in vitro kinase assays. Endogenous AKT was immunoprecipitated from lysates analyzed in (A) with immobilized AKT antibody, and incubated with purified recombinant GSK-3 and ATP in kinase reaction buffer. Reactions were stopped at 0 (control or C) and 30 minutes and subjected to immunoblotting with phospho-GSK-3β and total GSK-3β antibodies. Increased AKT kinase activity was detected in lysates prepared from mice deficient for PTK6 (*P-value = 0.036).
Exposure of cells to ionizing radiation and a variety of other types of toxic stress induces simultaneous compensatory activation of mitogen-activated protein kinase (MAPK) signaling pathways. In addition to the radioprotective and growth-promoting signaling executed by the Phosphoinositide 3-kinase/AKT pathway, the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway has been shown to further stimulate an anti-apoptotic response (reviewed in 23). To delineate the signaling pathways contributing to impaired apoptosis in PTK6-deficient mice, total tissue lysates of irradiated wild-type and PTK6-deficient mice were analyzed for activation of ERK pathway signaling. Mice deficient for PTK6 exhibited increased activation of ERK1/2 at 6 hours post irradiation compared with their wild-type counterparts (Fig. 5A, B).
Figure 5. Increased MAPK signaling is detected in Ptk6−/− mice following irradiation.
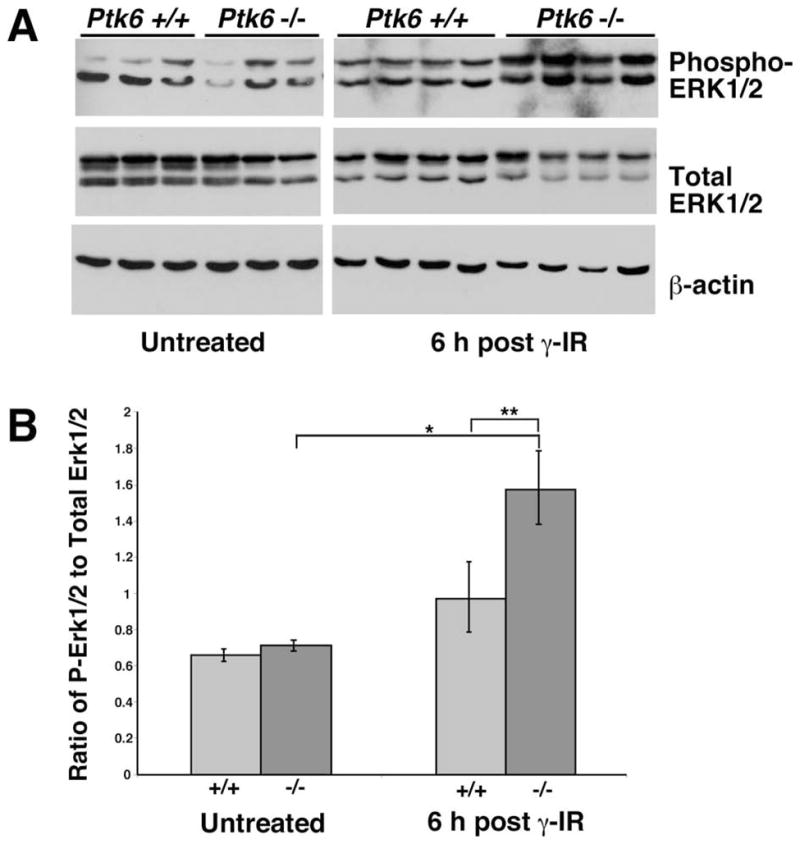
(A) Intestinal protein lysates were harvested from three untreated wild-type (Ptk6 +/+) and three Ptk6 −/− mice, and from four Ptk6 +/+ and four Ptk6 −/− mice at six hours post γ-irradiation (γ-IR). Immunoblotting was performed to examine ERK1/2 expression and phosphorylation. Increased phosphorylation (activation) of ERK1/2 was detected with phospho-specific antibodies. (B) The relative ratios of phospho-ERK1/2 to total ERK are shown. There is a significant increase in phospho-ERK1/2 levels in the Ptk6 null intestine after γ-irradiation and levels of phospho-ERK1/2 are higher in Ptk6 −/− than Ptk6 +/+ mice (*P-value = 0.031; **P-value = 0.016).
BAD is a BH3 domain-only member of the Bcl-2 family that promotes apoptosis by its association with and inhibition of the anti-apoptotic proteins Bcl-2 and Bcl-xL 24. BAD can be inactivated by phosphorylation as a consequence of AKT 25–27 or MAPK 28–31 activation. Since we observed increased activation of these pathways in wild-type and Ptk6 −/− mice, we examined BAD expression and phosphorylation. Although BAD expression remained constant, levels of BAD phosphorylated on Ser 112 that is targeted by ERK1/2 through its activation of the ribosomal S6 kinase (RSK), were higher in PTK6-null small intestinal epithelium following irradiation (Fig. 6A, B). Thus, the increased survival in the Ptk6-null intestine can be attributed in part to ERK1/2 regulated phosphorylation and inactivation of proapoptotic functions of BAD.
Fig. 6. Inhibitory phosphorylation of the pro-apoptotic protein BAD is enhanced in the Ptk6 −/− intestine after irradiation.
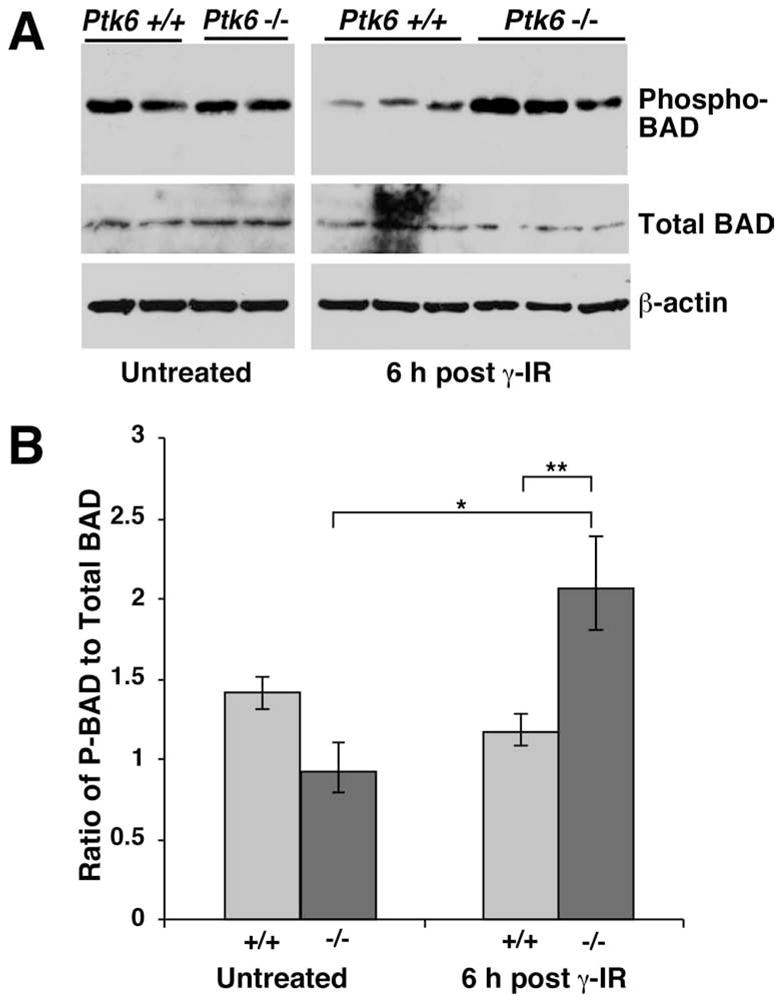
(A) Immunoblotting was performed using lysates from Ptk6 +/+ and Ptk6 −/− intestines at 0 hours and 6 h post γ-irradiation (γ-IR) with BAD phospho-Ser-112 specific antibodies and antibodies against total BAD. Each lane represents lysate from an independent animal. β-actin expression was examined as a control. (B) There was a significant increase in phospho-BAD levels in the Ptk6 −/− intestine at 6 h following γ-irradiation (*), at which time higher levels of phospho-BAD were detected in Ptk6 −/− than Ptk6 +/+ intestines (**) *P-value = 0.034; **P-value = 0.023.
The apoptotic response to irradiation is associated with increased compensatory proliferation in the wild-type small intestinal epithelium
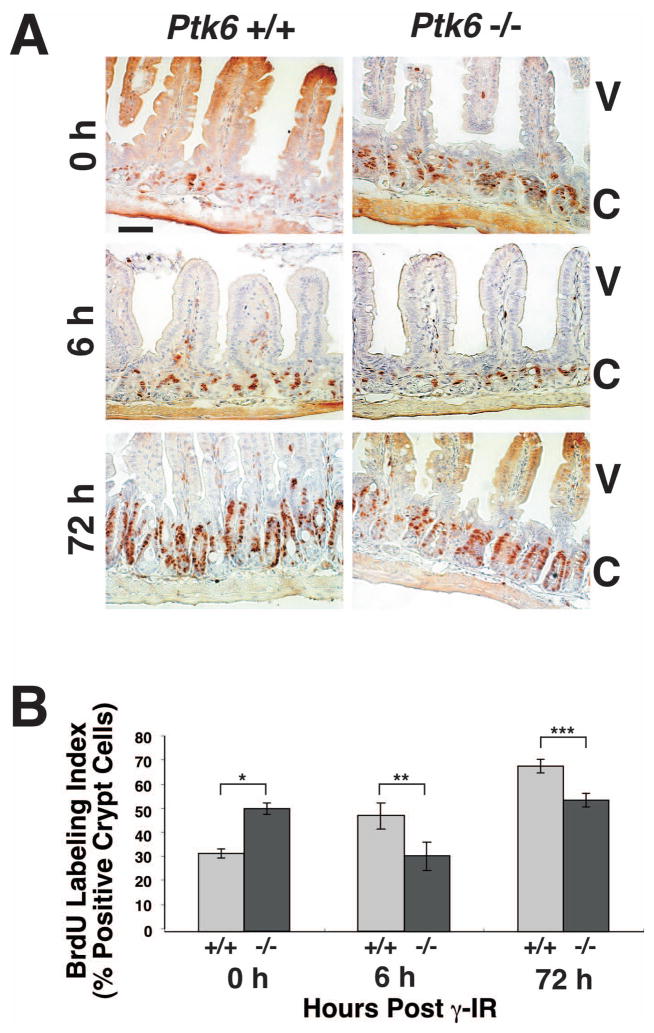
Previous studies of DNA damage induced apoptosis and subsequent crypt survival showed that regenerating crypts are detected 3–4 days after irradiation, and correlated with increased crypt cell proliferation 21. Consistent with these reports, a significant increase in DNA replication as measured by BrdU incorporation can be detected by 3 days after irradiation in wild-type mice (Fig. 7, compare Ptk6 +/+ mice at 0 and 72 hrs). As we previously reported, increased epithelial cell proliferation was detected in intestines of untreated Ptk6 −/− mice when compared with untreated wild-type mice (Fig. 7A, B, 0 hr). However, incorporation of BrdU was significantly lower in Ptk6 −/− mice at 6 hours after exposure to 8 Gy total body γ-irradiation when compared with irradiated wild-type mice (Fig. 7A, B, 6 h). As a consequence of increased apoptosis, the proliferative repair response is enhanced in the small intestines of Ptk6 +/+ mice and higher levels of proliferation were observed at 6 and 72 hours post irradiation.
Figure 7. Increased numbers of intestinal crypt epithelial cells proliferate in wild-type mice than Ptk6 null mice in response to DNA damage.
(A) Proliferation was analyzed by examining BrdU incorporation in sections of small intestine from untreated and γ-irradiated Ptk6 +/+ and Ptk6 −/− mice at 0, 6 and 72 hours post irradiation. S-phase cells were pulse-labeled with BrdU for 1 hour before animals were sacrificed. BrdU incorporation was detected with antibodies against BrdU and DAB (brown). Positive nuclei are detected in the crypts, while some diffuse background staining is seen in the upper villus. V denotes villi and C crypts. Untreated Ptk6 −/− mice exhibit an extended zone of proliferation at 0 h, but proliferation levels are higher in wild-type mice at 6 and 72 h post irradiation. (B) The percent of BrdU positive cells per crypt in wild-type (+/+) and Ptk6 null (−/−) mice at 0, 6, and 72 h post irradiation is shown. Bars, +/− SD. (*P-value = 0.015; **P-value = 0.023; ***P-value = 0.047).
DISCUSSION
Functions of PTK6 are not well understood and often seem paradoxical. A variety of data indicate that this intracellular tyrosine kinase has distinct activities in different cell types. Although PTK6 expression is associated with differentiation in the gastrointestinal tract and skin 3, 5, 6, 13, it is expressed at relatively high levels in a high percentage of human breast tumors but not in the normal mammary gland 9. In the normal prostate PTK6 is localized to nuclei of epithelial cells, but is relocalized to the cytoplasm in prostate cancer cells, suggesting that tissue context as well as its intracellular localization may have an impact on PTK6 signaling 7. A recent report indicated that targeting PTK6 to different cellular compartments has an impact on signaling outcomes 32.
We previously reported that constitutive overexpression of PTK6 in the Rat1a cell line sensitized these cells to apoptotic stimuli, including serum starvation and UV-irradiation 19. However, no significant differences in spontaneous apoptosis were detected in epithelia of untreated wild-type and Ptk6 null mice 13. Under normal conditions, PTK6 expression is primarily confined to differentiating nondividing epithelial cells in the small intestine, where it is a negative regulator of growth that promotes enterocyte differentiation 13.
Here we show that PTK6 is induced in proliferating crypt epithelial cells after DNA damage, and it contributes to apoptosis in response to DNA damage. The tumor suppressor protein p53 contributes to radiation-induced apoptosis by a number of different mechanisms, including transcriptional activation of proapoptotic genes and repression of prosurvival genes (reviewed in 33). Apoptosis in the small intestine following irradiation is regulated by both p53 dependent 34, 35 and independent 20 mechanisms. Although we detected a significant increase in PTK6 protein levels, we did not detect increased levels of PTK6 mRNA expression following irradiation (data not shown), suggesting that regulation of PTK6 occurs primarily at the posttranscriptional level. However, given the important role of p53 in the radiation response, potential cross talk between p53 and PTK6 in regulating DNA damage induced apoptosis in intestinal cells is currently under further investigation.
PTK6 has been shown to associate with and induce phosphorylation of AKT on tyrosine residues leading to inhibition of its activity in cell lines 16. Our data indicate that activated AKT levels are higher in Ptk6 −/− mice subjected to total body irradiation. AKT signaling promotes cell survival through multiple mechanisms (reviewed in 17), and can negatively regulate the DNA damage response. Negative regulation of AKT activity in the intestinal epithelium by PTK6 contributes to the DNA-damage response in the intestine.
Like AKT, activation of the ERK1/2 MAPKs can also promote cell survival after injury (reviewed in 23, 36). The MAPK-activated kinases, RSKs, phosphorylate BAD, a BH3-domain proapoptotic member of the Bcl2 family, on Ser-112 28, 31 disrupting its association with anti-apoptotic proteins Bcl-2 and Bcl-xL, and promoting its sequestration by 14-3-3 protein 37. We detected increased levels of active ERK1/2 in the Ptk6 −/− small intestinal epithelium when compared with wild-type mice after irradiation, indicating that PTK6 signaling negatively regulates ERK1/2 in vivo. Increased ERK1/2 activation corresponded with higher levels of phosphorylation of BAD on Ser 112. Ablation of the pro-apoptotic activity of BAD by ERK1/2 regulated phosphorylation is likely to contribute to the compromised apoptotic response observed in the Ptk6 −/− small intestinal epithelium. We also examined expression and activity of ERK5 that can also promote cell survival and was recently identified as a target of PTK6 in breast cancer cells 38, but saw no differences in ERK5 between wild-type and Ptk6 −/− mice before and after irradiation (data not shown).
Although we detected more proliferating cells in the small intestines of untreated Ptk6 −/− mice, wild-type mice had greater numbers of proliferating cells at six hours post irradiation. The higher number of BrdU positive cells progressing through S-phase of the cell cycle in wild-type mice correlated with increased numbers of cells undergoing apoptosis in response to DNA damage. Other studies have shown that cell death may be counterbalanced by an increase in cell proliferation to promote organismal survival. In the mouse, disruption of MDM2 in the intestine leads to an increase in p53 dependent apoptosis and a corresponding compensatory increase in proliferation 39. Apoptosis can be induced in the intestines of flies by feeding them dextran sulfate sodium and bleomycin, and this leads to an increase in intestinal stem cell proliferation 40. Similarly, the higher level of apoptosis in Ptk6 +/+ mice promotes a more robust regenerative response and increased proliferation after radiation induced injury.
We found that consequences of PTK6 ablation differ under normal growth conditions and following genotoxic stress. Accumulating data suggest that PTK6 activities are cell type specific, and dependent on its intracellular localization and the co-expression of specific substrates and interacting proteins. Here we show that PTK6 may have different functions in the same tissue under different conditions. In a complex tissue such as the small intestine, epithelial cells located in different positions along the crypt-villus axis are subject to different signals emanating from the local microenvironment. PTK6 appears to act as a damage-sensor that participates in the apoptotic response following genotoxic stress.
This is the first study to demonstrate a role for PTK6 in promoting apoptosis after genotoxic stress in vivo. Regulation of apoptosis is important for maintaining intestinal homeostasis in response to a number of factors. Increased epithelial apoptosis has been associated with ulcerative colitis 41. Apoptosis can serve to eliminate damaged and pathogen infected cells from the epithelium 42. While induction of apoptosis plays a pivotal role in determining the response to anti-cancer agents 43, it also plays an important role in chemotherapy and radiation induced mucositis observed in cancer patients undergoing treatment 44, 45. Induction of PTK6 in crypt epithelial cells inhibits prosurvival signaling to promote apoptosis. It will be interesting to determine if disruption of PTK6 signaling has a positive or negative impact on carcinogenesis in the gastrointestinal tract, since impairment of apoptosis plays a central role in the development of cancer and renders tumors refractory to cytotoxic therapies.
MATERIALS AND METHODS
Animals and Tissues
Ptk6-null mice (B6.129SV-Ptk6tm1Aty) have been previously described 13. Sex and age matched male mice at 8–10 weeks of age from heterozygous Ptk6+/− breeding pairs were used. Samples from multiple animals of the same genotype are included in each figure. Whole body irradiation was performed using a 137Caesium γ-source (JL Shepherd Model 6810) at a dose of 8 Gy. At 0, 6 and 72 hours following irradiation, the mice were sacrificed by CO2 anesthesia followed by cervical dislocation. The distal jejunum and entire ileum were removed, flushed with cold PBS and processed for biochemical and histological analysis. The ileum was used for protein and RNA isolation as described 19.
Immunoblotting
Immunoblotting was performed with antibodies specific for cleaved-Caspase-3, phospho-Ser473-AKT, total AKT, phospho-GSK-3 (Ser21/9), phospho-ERK1/2, total-ERK1/2, phosphoserine-112 BAD, BAD and total GSK-3α/β (Cell Signaling Technology, Beverly, MA), and mouse PTK6 (Sik) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Primary antibodies were detected using horseradish peroxidase-conjugated donkey anti-rabbit or sheep anti-mouse secondary antibodies (GE Healthcare, Piscataway, New Jersey). Peroxidase reactions were visualized with SuperSignal West Dura Extended Duration Substrate for chemiluminescence (Pierce Biotechnology, Rockford, IL). Quantitation of band intensity was performed using NIH V1.63 image analysis software. In all figures, multiple lanes represent samples from different mice from the indicated genotypes.
Analysis of Apoptosis
Apoptotic cells were identified by terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assay and activation of Caspase-3 and assessed by immunofluorescence as described 46. TUNEL assays were performed using the In Situ Cell Death Detection Kit according to the manufacturer’s instructions (Roche Diagnostics Corp., Indianapolis, IN). Nuclei were counterstained with 4,6 diamidino-2-phenylindole (DAPI, Sigma-Aldrich, St. Louis, MO). Intestinal sections were incubated with anti-cleaved Caspase-3 polyclonal antibody (Cell Signaling Technology, Beverly, MA) followed by tyramide signal amplification according to the manufacturer’s instructions (TSA Biotin System, PerkinElmer, Boston, MA). Reactions were visualized with FITC-Avidin DCS (Vector Laboratories, Burlingame, CA). The number of cells positive for TUNEL or cleaved Caspase-3 per crypt-villus unit was scored. Positive cells were counted for at least 10 well-oriented crypt-villus units from at least 5 quadrants (a minimum of 50) for each animal and used to determine the average of each animal. Differences between means were compared by Student’s two-tailed t-test. The level of statistical significance was set at P < 0.05.
Immunohistochemistry
Expression of PTK6 was assessed using anti-PTK6 polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a 1:50 dilution followed by use of the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) according to manufacturer’s instructions. Reactions were visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma-Aldrich, St. Louis, MO) and counterstained with hematoxylin. Controls were performed with normal rabbit IgG to assess potential background signals.
Proliferation was detected by the incorporation of BrdU during a one hour pulse before animals were sacrificed. Mice were injected intraperitoneally with 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MO) in PBS at 50 μg/g bodyweight. Tissues were fixed in Carnoy’s (10% glacial acetic acid, 60% ethanol, 30% chloroform) solution. BrdU immunohistochemistry was performed using anti-BrdU monoclonal antibody (BD Biosciences, San Jose, CA) 1:75 with the M.O.M. Immunodetection Kit following manufacturer’s instructions (Vector Laboratories, Burlingame, CA). Reactions were visualized with DAB and nuclei were counterstained with hematoxylin. The percent of BrdU positive cells in well-oriented crypts was quantitated.
In vitro Kinase Assays
Immunoprecipitations were performed using 300 μg of tissue lysate that was incubated with 1 μg anti-AKT polyclonal antibodies (Cell Signaling Technology, Beverly, MA) and 50 μl of 50% Protein A sepharose slurry for 16 hours at 4°C. As controls, lysates were incubated with Sepharose beads and 1 μg of rabbit IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). AKT in vitro kinase assays were performed using a nonradioactive AKT Kinase Assay Kit (Cell Signaling Technology, Beverly, MA) according to the manufacturer’s instructions.
Acknowledgments
This work was supported by National Institutes of Health Grants DK44525 and DK068503 (A. L. T.). A. H. received support from the German Academic Exchange Service (DAAD) and the Schering Foundation (A.H.). J.J.G. is supported by an AGA Foundation Graduate Student Research Fellowship Award and was supported by an NRSA/NIH Institutional T32 training grant, “Training Program in Signal Transduction and Cellular Endocrinology”, T32 DK07739 from the NIDDK.
Footnotes
Financial Disclosures/Conflicts: None
Author Contributions: Tyner: study concept and design; analysis and interpretation of data; manuscript preparation. Haegebarth, Perekatt, Bie, Gierut: acquisition of data; analysis and interpretation of data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13:409–19. doi: 10.3727/096504003108748438. [DOI] [PubMed] [Google Scholar]
- 2.Siyanova EY, Serfas MS, Mazo IA, Tyner AL. Tyrosine kinase gene expression in the mouse small intestine. Oncogene. 1994;9:2053–7. [PubMed] [Google Scholar]
- 3.Vasioukhin V, Serfas MS, Siyanova EY, Polonskaia M, Costigan VJ, Liu B, Thomason A, Tyner AL. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene. 1995;10:349–57. [PubMed] [Google Scholar]
- 4.Llor X, Serfas MS, Bie W, Vasioukhin V, Polonskaia M, Derry J, Abbott CM, Tyner AL. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res. 1999;5:1767–77. [PubMed] [Google Scholar]
- 5.Vasioukhin V, Tyner AL. A role for the epithelial-cell-specific tyrosine kinase Sik during keratinocyte differentiation. Proc Natl Acad Sci U S A. 1997;94:14477–82. doi: 10.1073/pnas.94.26.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TC, Jee SH, Tsai TF, Huang YL, Tsai WL, Chen RH. Role of breast tumour kinase in the in vitro differentiation of HaCaT cells. Br J Dermatol. 2005;153:282–9. doi: 10.1111/j.1365-2133.2005.06604.x. [DOI] [PubMed] [Google Scholar]
- 7.Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene. 2003;22:4212–20. doi: 10.1038/sj.onc.1206465. [DOI] [PubMed] [Google Scholar]
- 8.Petro BJ, Tan RC, Tyner AL, Lingen MW, Watanabe K. Differential expression of the non-receptor tyrosine kinase BRK in oral squamous cell carcinoma and normal oral epithelium. Oral Oncol. 2004;40:1040–7. doi: 10.1016/j.oraloncology.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- 10.Schmandt RE, Bennett M, Clifford S, Thornton A, Jiang F, Broaddus RR, Sun CC, Lu KH, Sood AK, Gershenson DM. The BRK Tyrosine Kinase is Expressed in High-Grade Serous Carcinoma of the Ovary. Cancer Biol Ther. 2006:5. doi: 10.4161/cbt.5.9.2953. [DOI] [PubMed] [Google Scholar]
- 11.Kasprzycka M, Majewski M, Wang ZJ, Ptasznik A, Wysocka M, Zhang Q, Marzec M, Gimotty P, Crompton MR, Wasik MA. Expression and Oncogenic Role of Brk (PTK6/Sik) Protein Tyrosine Kinase in Lymphocytes. Am J Pathol. 2006;168:1631–41. doi: 10.2353/ajpath.2006.050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL. The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem. 2004;279:54398–404. doi: 10.1074/jbc.M409579200. [DOI] [PubMed] [Google Scholar]
- 13.Haegebarth A, Bie W, Yang R, Crawford SE, Vasioukhin V, Fuchs E, Tyner AL. Protein tyrosine kinase 6 negatively regulates growth and promotes enterocyte differentiation in the small intestine. Mol Cell Biol. 2006;26:4949–57. doi: 10.1128/MCB.01901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–21. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Flier LG, Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu Rev Physiol. 2008 doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Ostrander JH, Faivre EJ, Olsen A, Fitzsimmons D, Lange CA. Regulated association of protein kinase B/Akt with breast tumor kinase. J Biol Chem. 2005;280:1982–91. doi: 10.1074/jbc.M412038200. [DOI] [PubMed] [Google Scholar]
- 17.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–96. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 19.Haegebarth A, Nunez R, Tyner AL. The Intracellular Tyrosine Kinase Brk Sensitizes Non-Transformed Cells to Inducers of Apoptosis. Cell Cycle. 2005;4:1239–46. doi: 10.4161/cc.4.9.1965. [DOI] [PubMed] [Google Scholar]
- 20.Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997;14:2759–66. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- 21.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–36. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 22.Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990;58:925–73. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- 23.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 24.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 25.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–9. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 26.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 27.Blume-Jensen P, Janknecht R, Hunter T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol. 1998;8:779–82. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- 28.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 29.Tan Y, Ruan H, Demeter MR, Comb MJ. p90(RSK) blocks bad-mediated cell death via a protein kinase C-dependent pathway. J Biol Chem. 1999;274:34859–67. doi: 10.1074/jbc.274.49.34859. [DOI] [PubMed] [Google Scholar]
- 30.Shimamura A, Ballif BA, Richards SA, Blenis J. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol. 2000;10:127–35. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 31.Fang X, Yu S, Eder A, Mao M, Bast RC, Jr, Boyd D, Mills GB. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–40. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 32.Kim HI, Lee ST. Oncogenic Functions of PTK6 Are Enhanced by Its Targeting to Plasma Membrane But Abolished by Its Targeting to Nucleus. J Biochem. 2009 doi: 10.1093/jb/mvp050. [DOI] [PubMed] [Google Scholar]
- 33.Rozan LM, El-Deiry WS. p53 downstream target genes and tumor suppression: a classical view in evolution. Cell Death Differ. 2007;14:3–9. doi: 10.1038/sj.cdd.4402058. [DOI] [PubMed] [Google Scholar]
- 34.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation induced apapotosis in the gastrointestinal tract of normal and p53 deficient mice. Cancer Res. 1994;5420:614–617. [PubMed] [Google Scholar]
- 35.Clarke AR, Gledhill S, Hoper ML, Bird CC, Wyllie AH. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene. 1994;9:1767–1773. [PubMed] [Google Scholar]
- 36.Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–31. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- 37.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–28. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 38.Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- 39.Valentin-Vega YA, Okano H, Lozano G. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ. 2008;15:1772–81. doi: 10.1038/cdd.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verstege MI, te Velde AA, Hommes DW. Apoptosis as a therapeutic paradigm in inflammatory bowel diseases. Acta Gastroenterol Belg. 2006;69:406–12. [PubMed] [Google Scholar]
- 42.Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815–23. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hickman JA, Potten CS, Merritt AJ, Fisher TC. Apoptosis and cancer chemotherapy. Philos Trans R Soc Lond B Biol Sci. 1994;345:319–25. doi: 10.1098/rstb.1994.0112. [DOI] [PubMed] [Google Scholar]
- 44.Keefe DM. Intestinal mucositis: mechanisms and management. Curr Opin Oncol. 2007;19:323–7. doi: 10.1097/CCO.0b013e3281214412. [DOI] [PubMed] [Google Scholar]
- 45.Bowen JM, Gibson RJ, Cummins AG, Keefe DM. Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer. 2006;14:713–31. doi: 10.1007/s00520-005-0004-7. [DOI] [PubMed] [Google Scholar]
- 46.Poole AJ, Heap D, Carroll RE, Tyner AL. Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon. Oncogene. 2004;23:8128–34. doi: 10.1038/sj.onc.1207994. [DOI] [PubMed] [Google Scholar]