Abstract
AIMS
To investigate the pharmacokinetics and safety of PD 0200390 in healthy subjects and subjects with renal impairment (RI) and to examine the relationship between oral and renal PD 0200390 clearance and estimated creatinine clearance (CLcr).
METHODS
In this open-label study, 26 subjects were categorized into four groups based on renal function: no RI (CLcr >80 ml min−1; n= 6); mild RI (CLcr 51 to ≤80 ml min−1; n= 6); moderate RI (CLcr >30 to 50 ml min−1; n= 6); and severe RI (CLcr ≤30 ml min−1; n= 8). Subjects received a single, oral dose of PD 0200390 25 mg. Noncompartmental pharmacokinetic parameters were determined from plasma and urine concentration–time data.
RESULTS
PD 0200390 was rapidly absorbed; mean time to maximum plasma concentration was 1.66–3.24 h. Mean half-life in subjects with normal renal function was 5.36 h, and increased with worsening RI. Oral (CL/F) and renal (CLR) clearance rates decreased with deteriorating renal function, whereas area under the concentration–time curve (AUC0–∞) values increased by 56, 117 and 436% in subjects with mild, moderate and severe RI, respectively, indicating increased PD 0200390 exposure. Regression analysis demonstrated that CL/F and CLR correlated with CLcr (r= 0.953 and 0.961, respectively). PD 0200390 was well tolerated in subjects with mild, moderate or no RI. The most common adverse events were somnolence, dizziness and headache; these occurred with greatest intensity in the severe RI group.
CONCLUSIONS
PD 0200390 pharmacokinetic parameters (CL/F, CLR and AUC0–∞) vary predictably with decreases in renal function; therefore dose adjustment may be required in individuals with RI.
Keywords: insomnia, PD 0200390, pharmacokinetics, renal impairment, safety
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
PD 0200390 is a ligand for the alpha-2-delta protein, an auxiliary subunit of voltage-gated calcium channels, which is the first in a new class being investigated for the treatment of insomnia.
Preclinical studies showed that PD 0200390 increases slow-wave sleep in rats; in humans, data in healthy volunteers showed that PD 0200390 is safe and well tolerated, and that renal excretion of unchanged drug is the primary route of elimination of PD 0200390.
This study investigated the effect of renal impairment on the single-dose pharmacokinetics and tolerability of PD 0200390, to determine whether dose adjustments may be required in individuals with renal dysfunction.
WHAT THIS STUDY ADDS
PD 0200390 was well tolerated in subjects with mild, moderate or no renal impairment, whereas the group of patients with severe renal impairment experienced an increased frequency of treatment-associated adverse events.
The degree of renal impairment had a predictable effect on the clearance of PD 0200390; correlation between key pharmacokinetic parameters (renal and oral clearance, and drug exposure) and changes in renal function were confirmed by regression analysis.
Dose adjustment may be required when PD 0200390 is administered to patients with impaired renal function, to compensate for increased exposure.
Introduction
Insomnia, which is characterized by difficulty falling asleep, difficulty staying asleep or nonrestorative sleep, can cause significant distress to those affected, impairing both social and occupational daytime functioning [1]. The disorder is relatively common, with over one-third of individuals surveyed in the UK reporting insomnia symptoms [2, 3]. Compared with good sleepers, those with insomnia have lower productivity, increased utilization of medical care and increased medication usage [4, 5], all of which pose a significant financial burden on society. In recent years several pharmacological agents have been used to treat insomnia, including the benzodiazepines, newer allosteric modulators of the GABAA receptor, antihistamines, antidepressants and antipsychotics [1].
PD 0200390 [(3S,4S)-(1-aminomethyl-3,4-dimethyl-cyclopentyl)-acetic acid] is a ligand for the alpha-2-delta (α2δ) protein, an auxiliary subunit of the voltage-gated calcium channel (VGCC), and is the first in a new class being investigated for the treatment of insomnia. Ligand binding to the VGCC α2δ subunit is thought to exert effects consistent with promoting restorative sleep by modulating neurotransmitter release in the central nervous system via inhibition of calcium influx at high rates of neuronal firing [6]. Preclinical studies have shown that PD 0200390 increases slow-wave sleep in rats, with no effect on rapid eye movement sleep [7]. Single- and multiple-dose studies of the safety and pharmacokinetics of PD 0200390 in a total of 84 healthy human subjects indicated that PD 0200390 is safe and well tolerated at single doses up to 150 mg and multiple doses up to 100 mg twice daily and 150 mg once daily for 14 days [8, 9]. A dose-proportional pharmacokinetic profile with low intersubject variability was observed in these subjects. The half-life of 5–6 h and time to reach maximum plasma concentration (Cmax) of 1.3 h, associated with 50% of maximal pharmacodynamic response (EC50) occurring at plasma concentrations of 1.1–1.6 µg ml−1 for pharmacodynamic measures of sleepiness (visual analogue scale for sleepiness and Stanford Sleepiness Scale), were consistent with potential efficacy in sleep onset and maintenance, and minimal risk of next-day effects with appropriate dosing [8].
Further data obtained from healthy volunteer studies demonstrated that PD 0200390 was rapidly absorbed, and urinary recovery of unchanged drug was high (85–97%, on average) [8, 9], indicating that the drug has high oral bioavailability and undergoes minimal metabolism. The high level of urinary recovery also indicates that renal excretion is the primary route of elimination of PD 0200390. It is, therefore, important to determine the safety of PD 0200390 in individuals with impaired renal function. In the current study, we investigated the single-dose pharmacokinetics and safety profile of PD 0200390 in healthy subjects and subjects with varying degrees of renal impairment (RI). In addition, we aimed to determine the relationship between PD 0200390 clearance (both oral and renal) and estimated creatinine clearance (CLcr). These findings will help to determine if dose restriction is required in this patient population.
Methods
Subjects
Subjects aged 18–80 years and weighing 45–110 kg were eligible for inclusion. Women were of nonreproductive potential [prior hysterectomy, tubal ligation, or postmenopausal (1 year without menses)], with a negative pregnancy test prior to study entry. Subjects were required to be in good health relative to the degree of RI; subjects with no RI were required to have haematology, clinical chemistry and urinalysis values within normal limits. Subjects were also required to have normal electrocardiograms at screening (QTc intervals <430 ms for men or <450 ms for women). Subjects with any history or clinical evidence of significant respiratory, cardiovascular, gastrointestinal, hepatic, haematological, neurological or psychiatric disease were excluded from the study. Subjects were also excluded if they had a significant urine concentration of a drug that could interfere with the study, or if they had used medication that investigators judged could interfere with the parameters measured in this study within 14 days prior to the study.
Study design
This was a Phase I, open-label, parallel-group, single-dose study. The study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice, the Declaration of Helsinki, and in compliance with United States Food and Drug Administration regulations for informed consent and protection of subject rights. The Arkansas Institutional Review Board (Little Rock, AR, USA) approved the study protocol, and all participants gave written, informed consent prior to enrolment.
At screening, CLcr was determined based on serum creatinine measurements taken at least 72 h apart. CLcr was estimated using the Cockcroft–Gault equation [10], and subjects for whom the lower of the two estimated values was within 20% of the higher value were admitted to the study. Subjects were assigned to one of four groups based on the first estimated CLcr values: Group 1, normal renal function (CLcr >80 ml min−1 and normal haematology, clinical chemistry, and urinalysis); Group 2, mild RI (CLcr 51 to ≤80 ml min−1); Group 3, moderate RI (CLcr >30 to 50 ml min−1); and Group 4, severe RI (CLcr ≤30 ml min−1). Groups were matched as closely as possible for gender, age and weight. Subjects received a single 25-mg dose of PD 0200390, taken orally in a capsule with approximately 230 ml water, administered within 2 weeks of the first screening assessment. The median time from the first screening assessment of CLcr to dosing was 5 days. The 25-mg dose was chosen for this study following demonstration of its safety in healthy volunteer tolerance studies. In a single-dose study the maximum tolerated single dose was 150 mg [8]; the 25-mg dose, at one-sixth of the maximum tolerated dose in healthy subjects, was selected to allow for increased exposure that was expected in subjects with impaired renal function.
Plasma and urine sampling and assay
Venous blood samples were collected predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 9, 12, 24, 36 and 48 h post dose. Additional plasma collections were made at 72 h for Group 3 (moderate RI) and at 72, 96, 120, 144 and 168 h for Group 4 (severe RI). Urine specimens were collected predose and all urine passed during 0–4, 4–8, 8–12, 12–24 and 24–48 h post dose was collected. Urine was also collected over 48–72 h for Groups 3 and 4, and over 72–96, 96–120, 120–144 and 144–168 h for Group 4 only.
Blood samples were diluted in potassium ethylenediamine tetraaceticacid and centrifuged to separate plasma. Plasma and urine samples were stored at −20°C until assayed, when concentrations of PD 0200390 were measured using validated liquid chromatography/tandem mass spectrometry methods (PPD Development, Richmond, VA, USA). Data acquisition used Mass Chrom Version 1.2 software. The analytical range of the study assay was 5000–10 000 ng ml−1 for plasma, and 100 ng ml−1 to 200 µg ml−1 for urine. Quality control samples showed that the precision (% coefficient of variation) of the study assay was ≤6.94% for plasma and ≤3.97% for urine, and accuracy (% relative error) was –8.15% to –1.13% for plasma and –1.75% to 1.70% for urine. The specificity of the assay was confirmed using blank standards and internal reference standards ([2H2,13C2]PD 0200390), run with each batch of samples. No interfering peaks that significantly impacted quantification were observed at the retention time of PD 0200390 or the internal standard in plasma samples. Carryover contamination was observed in some cases during urine sample assays, but further tests confirmed that carryover did not influence sample results.
Statistical analysis of pharmacokinetic parameters
Pharmacokinetic parameters determined included Cmax, time to Cmax (tmax), area under the concentration–time curve from zero to infinity (AUC0–∞), terminal elimination half-life (t1/2), percent of dose excreted in urine, oral clearance (CL/F), renal clearance (CLR) and apparent volume of distribution (Vd/F). Pharmacokinetic parameter values were calculated for each subject using noncompartmental analysis of concentration–time and urinary excretion data. Actual sampling times were used for all pharmacokinetic evaluations. Calculations were made using WinNonlin Pro Version 2.1.
The relationship between key PD 0200390 parameters (including CL/F, CLR and AUC) and CLcr was examined by linear regression analysis to assess the potential relationship between renal function and PD 0200390 pharmacokinetic parameters. For the linear regression analysis, CLcr was treated as a continuous variable rather than categorized as representing normal renal function or mild, moderate or severe RI.
Safety and tolerability assessments
Safety was evaluated by adverse event (AE) monitoring, clinical observation, physical examination, vital signs, clinical laboratory parameters and ECGs. All AEs reported by subjects following administration of PD 0200390 were recorded and evaluated by the investigator for intensity and relationship to treatment. AEs that began during the study were defined as treatment-emergent signs and symptoms (TESS). Descriptive statistics were used to summarize AEs by degree of RI.
Results
Subject demographics
Twenty-six subjects (15 male, 11 female) entered and completed the study. Six subjects had normal renal function, six had mild RI, six had moderate RI and eight had severe RI. Table 1 shows the demographics of the subjects in each of the four renal function groups. Mean age, overall, was 55.3 years (range 25–76 years) and mean weight was 79.3 kg (range 56.4–104.5 kg). Mean age and weight were slightly higher in the severe RI group; however, these factors were controlled for in the calculation of CLcr by use of the Cockcroft–Gault equation. Three male subjects who had screening QTc intervals of 439, 457 and 467 ms were enrolled as eligibility exceptions. The investigator did not consider these QTc findings to warrant exclusion from the study.
Table 1.
Subject demographics
| Degree of renal function | ||||
|---|---|---|---|---|
| Normal RF (n= 6) | Mild RI (n= 6) | Moderate RI (n= 6) | Severe RI (n= 8) | |
| Age (years; mean [SD]) | 48.2 [15.1] | 56.7 [12.2] | 52.5 [19.5] | 59.8 [8.9] |
| Gender (n[%]) | ||||
| Male | 3 [50] | 4 [67] | 4 [67] | 4 [50] |
| Female | 3 [50] | 2 [33] | 2 [33] | 4 [50] |
| Weight (kg; mean [SD]) | 75.9 [10.4] | 76.6 [15.1] | 78.6 [14.4] | 84.4 [14.6] |
| BMI (kg m−2; mean [SD]) | 26.4 [3.6] | 27.3 [4.2] | 25.5 [2.9] | 30.3 [7.4] |
| Serum creatinine (mg dl−1) | 0.85 [0.19] | 1.30 [0.46] | 2.13 [0.60] | 4.59 [2.70] |
| CLcr (ml min−1; mean [SD]) | 106.4 [21.4] | 66.9 [11.2] | 42.7 [6.3] | 22.3 [7.3] |
BMI, body mass index; CLcr, creatinine clearance; RF, renal function; RI, renal impairment.
Pharmacokinetics of PD 0200390
In general, PD 0200390 was absorbed rapidly, with mean tmax ranging from 1.66 to 3.24 h (Table 2, Figure 1). Cmax values were generally consistent across the groups; mean values ranged from 0.45 to 0.56 µg ml−1. The mean half-life in subjects with normal renal function was 5.36 h, and increased with increasing degree of RI. CL/F and CLR values decreased with deteriorating renal function, while AUC0–∞ values increased, indicating increased drug exposure in patients with impaired renal function. Mean Vd/F was 48 l, and was similar across groups with different degrees of RI. Mean urinary recovery was 92.0% in subjects with normal renal function and 94.4% in subjects with mild RI, and was lower in subjects with moderate and severe RI, averaging 75.3% and 67.1%, respectively (probably due to insufficient time for complete collection).
Table 2.
Mean PD 0200390 pharmacokinetic parameters following administration of single 25-mg doses in subjects with various degrees of renal function
| Pharmacokinetic parameter Mean (SD, 95% CI) | Degree of renal function Normal RF (n= 6) | Mild RI(n= 6) | Moderate RI(n= 6) | Severe RI(n= 8) |
|---|---|---|---|---|
| CLcr (ml min−1) | 106 | 66.9 | 42.7 | 22.3 |
| (21.2, 89.4–123) | (11.2, 57.9–75.8) | (6.3, 37.7–47.7) | (7.3, 17.3–27.4) | |
| Cmax (µg ml−1) | 0.45 | 0.55 | 0.50 | 0.56 |
| (0.12, 0.36–0.55) | (0.11, 0.46–0.63) | (0.14, 0.39–0.61) | (0.11, 0.48–0.64) | |
| tmax (h) | 1.91 | 2.14 | 1.66 | 3.24 |
| (1.08, 1.04–2.77) | (0.67, 1.60–2.68) | (0.52, 1.24–2.07) | (2.59, 1.44–5.04) | |
| AUC0–∞ (µg h−1 ml−1) | 4.14 | 6.44 | 9.00 | 22.2 |
| (0.88, 3.43–4.84) | (0.93, 5.69–7.18) | (2.54, 6.97–11.0) | (10.5, 14.9–29.4) | |
| t1/2 (h) | 5.36 | 7.46 | 14.0 | 28.6 |
| (0.98, 4.57–6.15) | (1.87, 5.96–8.95) | (7.44, 8.08–20.0) | (15.3, 18.0–39.2) | |
| CL/F (ml min−1) | 105 | 65.9 | 49.6 | 23.3 |
| (23.1, 86.3–123) | (9.53, 58.3–73.5) | (14.1, 38.3–60.9) | (11.4, 15.4–31.2) | |
| CLR (ml min−1) | 95.2 | 62.0 | 37.7 | 17.0 |
| (19.0, 80.0–110) | (12.6, 51.9–72.2) | (15.7, 25.1–50.3) | (11.2, 9.31–24.8) | |
| Vd/F (l) | 47.7 | 42.1 | 54.6 | 47.3 |
| (8.58, 40.8–54.5) | (10.5, 33.8–50.5) | (17.7, 40.4–68.7) | (9.37, 40.8–53.8) | |
| Ae% | 92.0 | 94.4 | 75.3 | 67.0 |
| (12.9, 81.7–102) | (16.0, 81.6–107) | (17.1, 61.6–89.0) | (20.4, 52.9–81.1) |
SD, standard deviation; CI, confidence interval; Ae%, percent of dose excreted unchanged in urine; AUC0–∞, area under plasma concentration–time curve from zero to infinity; CLcr, creatinine clearance; CL/F, oral clearance; CLR, renal clearance; Cmax, maximum observed plasma concentration; RF, renal function; RI, renal impairment; tmax, time to reach Cmax; t1/2, terminal half-life; Vd/F, apparent volume of distribution.
Figure 1.
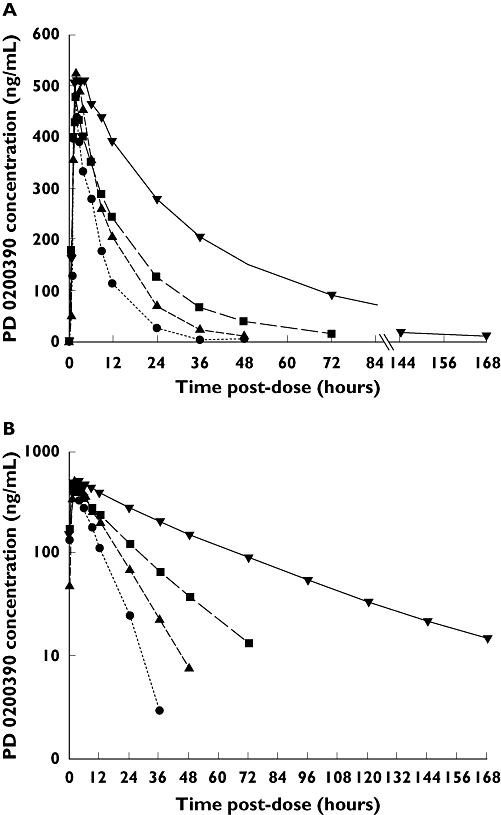
Mean plasma PD 0200390 concentration–time profile following administration of single PD 0200390 doses to subjects with varying degrees of renal function [linear scale (A) and logarithmic scale (B)]. Severe renal impairment (n= 8) ( ); Moderate renal impairment (n= 6) (
); Moderate renal impairment (n= 6) ( ); Mild renal impairment (n= 6) (
); Mild renal impairment (n= 6) ( ); Normal renal function (n= 6) (
); Normal renal function (n= 6) ( )
)
Relationship between PD 0200390 clearance and estimated CLcr
The regression analysis investigating the relationship between key PD 0200390 pharmacokinetic parameters and subject CLcr demonstrated that both oral and renal clearance of PD 0200390 correlated strongly with glomerular filtration rate, as measured according to CLcr, with correlation coefficients of 0.953 and 0.961 (Table 3). The net clearance at or near the net glomerular filtration rate suggests that the renal clearance mechanism is probably predominantly passive. The intercept of the relationship between CL/F and CLcr values was not statistically significantly different from zero, indicating that nonrenal clearance was negligible (Figure 2). In addition, the slope of that relationship was close to one, at 0.937; this suggests complete bioavailability after oral administration (assuming no active secretion takes place). PD 0200390 exposure (as represented by AUC) also correlated with CLcr (r= 0.723), with a negative slope (Table 3).
Table 3.
Summary of regression analysis investigating the relationship between PD 0200390 pharmacokinetic parameters and subject creatinine clearance values
| Parameter | Slope | P-value* | Intercept | P-value** | r† |
|---|---|---|---|---|---|
| CL/F | 0.937 | <0.001 | 4.86 | 0.236 | 0.953 |
| CLR | 0.927 | <0.001 | −2.33 | 0.523 | 0.961 |
| AUC | −0.199 | <0.001 | 22.6 | <0.001 | 0.723 |
CL/F, oral clearance; CLR, renal clearance; AUC, area under the plasma concentration–time curve.
P < 0.05 indicates slope is significantly different from 0;
P < 0.05 indicates intercept is significantly different from 0.
Correlation coefficient.
Figure 2.
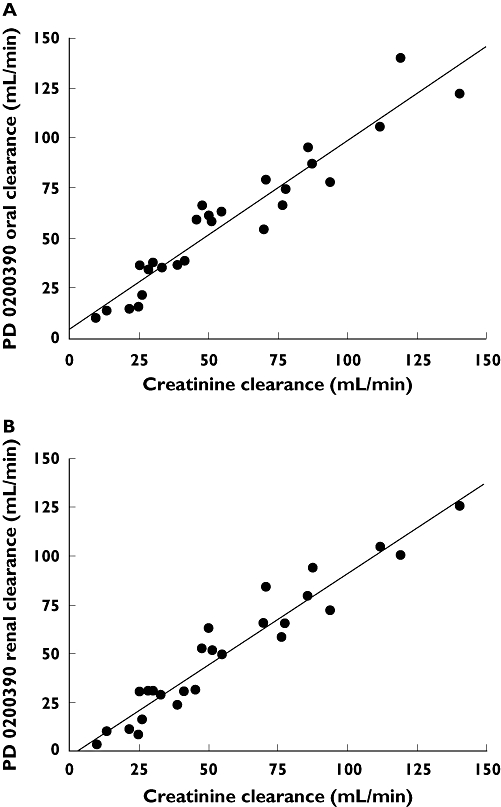
Relationship between individual PD 0200390 pharmacokinetic parameter values and subject creatinine clearance: (A) CL/F and IB) CLR
Safety
A total of 40 TESS AEs were reported by 20 of 26 subjects during the study period. Of these, 38 were considered to be associated with PD 0200390 treatment: 14 were mild, 21 were moderate and three were severe. All three severe AEs were somnolence and occurred in the severe RI group. The most frequently reported treatment-related AEs in subjects receiving PD 0200390 were somnolence (17 subjects) and dizziness (six subjects). One subject in the severe RI group experienced nausea and vomiting. All AEs were of limited duration, and resolved. There were no deaths, serious AEs or withdrawals due to AEs.
There were no clinically significant findings based on clinical laboratory measurements or physical examination. With regard to vital signs, one subject, a 70-year-old woman in the severe RI group, had a possibly clinically significant postdose increase in systolic blood pressure (>180 mmHg, and an increase from baseline ≥40 mmHg). From a predose measurement of 120/56 mmHg, the subject's blood pressure increased to 184/72 mmHg 1 h post dose. This subject's blood pressure returned to 120/47 mmHg 6 h post dose, and was elevated again at closeout (193/74 mmHg on day 8), when no PD 0200390 remained detectable in plasma.
Discussion
PD 0200390 exposure following a single 25-mg dose changed proportionally with the degree of RI. The AUC for plasma concentration increased, whereas mean oral and renal clearance of PD 0200390 decreased, as the degree of renal function declined. Regression analysis demonstrated that CL/F, CLR and AUC correlated strongly with CLcr. Cmax, tmax and Vd/F were similar across all groups, whereas t1/2 was prolonged in subjects with impaired renal function. The findings of this study suggest that, because individuals with impaired renal function have increased exposure to PD 0200390 compared with individuals with normal renal function, it may be necessary to use lower doses in patients with renal impairment.
PD 0200390 pharmacokinetics were investigated in this study in subjects with impaired renal function following the observation in healthy volunteer studies that the major route of PD 0200390 elimination was renal excretion of unchanged drug [8, 9]. Urinary recovery was similarly high in this study in subjects with normal renal function and mild RI, averaging 92 and 94%, respectively. The results suggest that PD 0200390 is highly permeable, nearly completely absorbed, and undergoes little or no metabolism. Urinary recovery was lower in subjects with moderate and severe RI (75 and 67%, respectively). However, plasma concentrations of PD 0200390 were predictable across groups, and were consistent with urinary recovery of PD 0200390, as would be expected when urinary excretion is the major route of elimination. Furthermore, the relationship between CL/F and CLcr, with a slope of almost one and an intercept of approximately zero, indicated that nonrenal clearance of PD 0200390 is absent or negligible. Therefore, it is likely that other factors, such as incomplete urine collection and the difficulty of collecting over longer periods (up to 72 h for the moderate RI group and 168 h for the severe RI group), may have confounded measurement of urinary excretion in these groups. Indeed, most urine samples taken during the last collection interval still had detectable PD 0200390 concentrations. It is also possible that large-volume samples collected over long intervals may have been inadequately mixed prior to sampling for analysis.
Renal excretion, and a requirement for dose adjustment in patients with renal impairment, are typical of the α2δ class. For example pregabalin (Lyrica®; Pfizer Inc.) should be administered at reduced doses in patients with impaired renal function, with the degree of dose reduction determined based on the patient's CLcr [11]. For most agents used to treat insomnia, hepatic metabolism is a more important route of elimination than urinary excretion. This obviates a requirement to adjust dosing for patients with impaired renal function; for example, studies of zolpidem (Ambien®; Sanofi-Aventis) [12] and eszopiclone (Lunesta®; Sepracor Inc.) [13] indicated that dose adjustment is not necessary in individuals with RI. However, these drugs should be used at low doses, with caution, in patients with hepatic impairment [14, 15], and may be associated with a risk of interactions with drugs that inhibit or induce hepatic metabolic enzymes. PD 0200390 has a low risk of drug–drug interactions, owing to its negligible metabolism prior to excretion as unchanged drug. As such, PD 0200390 exposure is unlikely to be impacted by concomitant medications, and any potential requirement for dose adjustment should be determined by the level of renal impairment, which had a highly predictable effect on PD 0200390 exposure in this study. The predictable relationship between renal function and PD 0200390 clearance, as demonstrated in the regression analyses reported in this study, also suggests a low degree of intersubject variability relating to factors other than renal function. This is consistent with observations of low intersubject variability in pharmacokinetic parameters in healthy volunteers [8, 9].
One potential consequence of increased exposure to drugs used to treat insomnia is next-day residual effects. Thus, an important goal of dose adjustment is to avoid effects persisting more than approximately 8 h after dosing. However, such dose adjustments would reduce Cmax, a parameter that did not change proportionally with RI; the consequences of this on sleep parameters are not known. Final dosing recommendations for PD 0200390 in patients with RI and insomnia will depend on understanding the relationship between efficacy, tolerability and drug exposure, as measured by Cmax and AUC, and the therapeutic index of PD 0200390.
A further consideration is to ensure acceptable tolerability. The 25-mg dose of PD 0200390 was well tolerated in subjects with mild and moderate RI, and less so in subjects with severe RI. All AEs experienced by subjects in the severe RI group were moderate or severe in intensity, whereas the AEs reported for subjects in the other three groups were considered mild or moderate. These findings support the pharmacokinetic data indicating a potential need for dose restriction in individuals with more severe renal dysfunction.
Conclusions
Oral administration of a single 25-mg dose of PD 0200390 was well tolerated in subjects with mild or moderate RI, or normal renal function. The findings of this study demonstrate that changes in the key PD 0200390 pharmacokinetic parameters CL/F, CLR and AUC are highly correlated with decreases in renal function. The degree of RI had a predictable effect on the clearance of PD 0200390, whereby a 50% reduction of CLcr was associated with similar reductions in oral and renal clearance of PD 0200390. Owing to the reduced clearance of PD 0200390 and consequent increased exposure and risk of AEs in patients with impaired renal function, it may be necessary to adjust dosing in these individuals. Once a therapeutic dose range for PD 0200390 in insomnia is established, further investigation of the relationship between pharmacokinetic parameters such as AUC, Cmax, t1/2 and efficacy and tolerability in patients with RI will help to determine final dosing recommendations.
Acknowledgments
This research was sponsored by Pfizer Inc. All authors were employees of Pfizer Inc. at the time of conducting the study, except G.G., who was employed by Clinical Study Centers LLC, the organization contracted by Pfizer to perform the research. Editorial support was provided by Samantha Stanbury, a medical writer at GCL, UK, and was funded by Pfizer Inc.
REFERENCES
- 1.Zammit GK. The prevalence, morbidities, and treatments of insomnia. CNS Neurol Disord Drug Targets. 2007;6:3–16. doi: 10.2174/187152707779940754. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Caulet M, Priest RG, Guilleminault C. DSM-IV and ICSD-90 insomnia symptoms and sleep dissatisfaction. Br J Psychiatry. 1997;171:382–8. doi: 10.1192/bjp.171.4.382. [DOI] [PubMed] [Google Scholar]
- 3.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 4.Simon GE, VonKorff M. Prevalence, burden and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 5.Léger D, Guilleminault C, Bader G, Lévy E, Paillard M. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–9. [PubMed] [Google Scholar]
- 6.Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel α2δ ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Donevan S, Li Z, Serpa K, Meltzer L, Taylor C, Thorpe A. PD 0200390, a novel α2δ ligand, enhances slow wave sleep [abstract. Biol Psychiatry. 2008;63(Suppl. 1):s102. Abstract no. 310. [Google Scholar]
- 8.Corrigan B, Werth J, Bramson C, Alvey C, Abel R, Feltner D, Ouellet D. Pharmacokinetics and pharmacodynamics of PD 0200390 following single-dose administration to healthy volunteers. Clin Pharmacol Ther. 2008;83(Suppl 1):S29. Abstract no. PI-69. [Google Scholar]
- 9.Corrigan B, Werth J, Moton A, Alvey C, Feltner D, Ouellet D. Pharmacokinetics of PD 0200390 following multiple dose administration to healthy volunteers. Clin Pharmacol Ther. 2008;83(Suppl 1):S30. Abstract no. PI-71. [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Pfizer Inc. Lyrica® (pregabalin) prescribing information. June 2007.
- 12.Fillastre JP, Geffroy-Josse S, Etienne I, Dhib M, Rosenzweig P, Danjou P, Dubruc C, Bianchetti G. Pharmacokinetics and pharmacodynamics of zolpidem following repeated doses in hemodialyzed uraemic patients. Fundam Clin Pharmacol. 1993;7:1–9. doi: 10.1111/j.1472-8206.1993.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 13.Halas CJ. Eszopiclone. Am J Health Syst Pharm. 2006;63:41–8. doi: 10.2146/ajhp050357. [DOI] [PubMed] [Google Scholar]
- 14.Sanofi-aventis. Ambien® (zolpidem) prescribing information. March 2007.
- 15.Sepracor Inc. Lunesta® (eszopiclone) prescribing information. December 2004.


