Abstract
AIM
Drug–drug interactions (DDIs) may lead to often preventable adverse drug events and health damage. Especially within hospitals, this might be an important factor, as patients are severely ill and multiple medications may be prescribed simultaneously. The objective of this study was to measure the frequency and nature of DDI alerts in a Dutch university hospital.
METHODS
All patients hospitalized in the University Medical Centre Utrecht in 2006 who were prescribed at least one medication were included. The frequency of DDIs was calculated as: (i) the percentage of patients experiencing at least one DDI, and (ii) the percentage of prescriptions generating a DDI alert. Based on the national professional guideline, DDIs were classified into categories of potential clinical outcome, management advice, clinical relevance (A–F) and available evidence (0–4).
RESULTS
Of the 21 277 admissions included, 5909 (27.8%) encountered at least one DDI. Overall, the prescribing physician received a DDI alert in 9.6% of all prescriptions. The most frequently occurring potential clinical consequence of the DDIs was an increased risk of side-effects such as increased bleeding risk (22.0%), hypotension (14.9%), nephrotoxicity (12.6%) and electrolyte disturbances (10.5%). Almost half (48.6%) of the DDIs could be managed by monitoring laboratory values.
CONCLUSIONS
Computerized DDI alerts may be a useful tool to prevent adverse drug events within hospitals, but they may also result in ‘alert fatigue’. The specificity of alerts could significantly improve by the use of more sophisticated clinical decision support systems taking into account, for example, laboratory values.
Keywords: clinical decision support, drug–drug interactions, medication safety
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Drug–drug interactions (DDIs) may lead to often preventable adverse drug events and health damage.
In Dutch community pharmacies approximately 6% of all prescriptions generate a DDI alert.
Hospitalized patients may be especially at risk, as they are more severely ill and multiple medications may be prescribed simultaneously; however, only limited data are available on the frequency and nature of DDIs during hospitalization.
WHAT THIS STUDY ADDS
In a Dutch university hospital 10% of all prescriptions generated a DDI alert; overall 25% of patients encountered at least one potential DDI.
Besides the risk of decreased effectiveness (25% of the DDIs), the most frequently occurring potential clinical consequence of the DDIs was an increased risk of side-effects, such as an increased bleeding risk (22% of DDIs), hypotension (15%) and nephrotoxicity (13%).
Almost half of the DDIs could be managed by monitoring laboratory values.
Introduction
Adverse drug events are increasingly acknowledged as an area of major concern in medical care. The HARM study recently reported that 2.4% of all hospital admissions and 5.6% of all emergency admissions in the Netherlands were related to adverse drug events, of which almost half were considered preventable [1]. Drug–drug interactions (DDI) constitute one of the potential mechanisms leading to often preventable adverse drug events and health damage [2, 3]. Several studies have investigated the frequency and nature of DDIs in community settings [4–7].
Within hospitals, the problem of DDIs may deserve extra attention, as more medications may be prescribed simultaneously, more complex schemes and compounds may be used, and the number of (dose) changes may be larger. Moreover, compared with outpatients, hospitalized patients are more severely ill, and the capacity to counteract and deal with disturbances is often diminished. DDIs may therefore occur more frequently within hospitals than in an outpatient setting and their consequences may be more severe. Krähenbühl et al. estimated that 17% of all adverse drug events in hospitalized patients are caused by DDIs and that approximately 1% of patients will experience an adverse drug event during hospitalization due to a DDI [8]. At discharge about 60% of patients were found to have at least one potentially interacting drug combination [9, 10]. Two studies performed in patients visiting an emergency room found that when medications were added, a potential adverse DDI was introduced in 5–10% of cases [11, 12]. In two internal medicine wards in Finland potentially serious interactions were detected in 1.4% of all prescriptions, as such possibly affecting 6.8% of patients [13]. Overall, data on the occurrence and consequences of DDI alerts within hospitals are scarce. Therefore, the objective of this study was to measure the frequency and nature of DDI alerts in a large university hospital in the Netherlands and to make suggestions for improving the management of DDIs.
Methods
Setting
The University Medical Centre Utrecht (UMCU) is a 1042-bed academic medical centre located in the centre of the Netherlands. It consists of an adults' hospital and a children's hospital. In 2006, 28 888 clinical hospitalizations took place. All medications for hospitalized patients are prescribed using a computerized physician order entry (CPOE) system. For research purposes all prescriptions are routinely exported in the Utrecht Patient Oriented Database (UPOD). UPOD is an infrastructure of relational databases comprising data on patient demographics, hospital discharge diagnoses, medical procedures, medication orders and laboratory tests for all patients treated at the UMCU since 2004, and has been described in detail elsewhere [14].
Study population
All patients hospitalized in the UMCU in 2006 for >24 h and who were prescribed at least one medication were included. If a patient was admitted during the study period to the hospital more than once, the individual hospitalizations contributed to the study multiple times. Prescriptions from the intensive care (IC) units were not included in this study, because in the IC units a different CPOE system is used and the prescription data are not (yet) stored in the UPOD database.
DDIs
In the Netherlands, a working group of the Scientific Institute of Dutch Pharmacists developed and maintains an evidence base and professional guideline for the management of DDIs, described in detail elsewhere [15]. In this professional guideline, the G-standard, DDIs are classified on a six-point potential clinical relevance scale ranging from not very serious to potentially lethal (categories A–F), and on a five-point evidence scale ranging from not proven to very well proven (categories 0–4). This classification in evidence-relevance categories is described in brief in Appendix 1. Based on these categories, 331 DDIs have been classified as ‘relevant’, meaning that they should generate a direct pop-up DDI alert at the moment of prescribing and that assessment of these combinations of drugs is considered necessary. Since the generated DDI alerts had not been logged during the study period, they were reconstructed by combining the G-standard from December 2006 with the prescription data in UPOD.
In addition, the DDIs were classified in ‘potential clinical outcome’ categories and ‘management advice’ categories. The Dutch professional guideline, the G-standard, provides textual information about background, mechanism and advice regarding each DDI. This professional guideline text was converted into ‘potential clinical outcome’ categories and ‘management advice’ categories separately by two hospital pharmacists (J.E.F.Z-v.R. and E.V.U.). Differences in classification were subsequently discussed and settled. One DDI could have multiple outcomes or management advices.
Data analysis
The frequency of DDIs was calculated as: (i) the percentage of patients treated with medication that experienced at least one potential DDI, and (ii) the percentage of all medication orders generating a DDI alert. The frequencies were calculated separately for each clinical specialism in the children's and adults' hospital. The nature of the DDIs was described by listing the 10 most frequent alerts for the children's and the adults' hospital. The percentage of alerts in each clinical outcome and management advice category was calculated.
Finally, the percentages of alerts in each of the G-standard evidence-relevance categories in our hospital were compared with the percentages found in Dutch community pharmacies by Buurma et al.[4].
Results
In total, 21 277 patients who had been prescribed at least one medication were admitted to the hospital in 2006. Of these patients, 5909 (27.8%) encountered at least one DDI; on average these 5909 patients experienced 20 058/5909 = 3.4 DDIs. The percentage of patients experiencing at least one DDI during the hospital admission varied from 3.4% in the paediatric surgery department to 62.0% in the adults' nephrology department (see Table 1).
Table 1.
Frequency of drug–drug interaction alerts
| Admissions | Prescriptions | ||||||
|---|---|---|---|---|---|---|---|
| Number of admissions with ≥1 alerts* | Number of admissions* | % | Average number of alerts per admission with ≥1 alerts | Alerts | Prescriptions | % | |
| Total | 5909 | 21 277 | 27.8 | 3.4 | 20 058 | 208 187 | 9.6 |
| Adult's hospital | 5384 | 15 389 | 32.7 | 3.4 | 18 373 | 167 757 | 11.0 |
| Cardiology and cardiosurgery | 1363 | 2 336 | 58.3 | 3.7 | 5 024 | 29 175 | 17.2 |
| Geriatrics | 172 | 279 | 61.6 | 4.5 | 767 | 5 043 | 15.2 |
| Nephrology | 304 | 490 | 62.0 | 3.4 | 1 026 | 7 150 | 14.3 |
| General internal medicine | 566 | 1 395 | 40.6 | 3.5 | 1 995 | 14 813 | 13.5 |
| Lung diseases | 403 | 798 | 50.5 | 4.0 | 1 610 | 12 275 | 13.1 |
| Oncology and haematology | 334 | 959 | 34.8 | 4.5 | 1 498 | 12 759 | 11.7 |
| Dermatology | 59 | 200 | 29.5 | 5.0 | 297 | 2 920 | 10.2 |
| Neurology and neurosurgery | 698 | 2 193 | 31.8 | 2.8 | 1 958 | 22 415 | 8.7 |
| Surgery | 1497 | 6 668 | 22.5 | 2.7 | 4 024 | 55 446 | 7.3 |
| Psychiatry | 64 | 549 | 11.7 | 2.7 | 174 | 5 761 | 3.0 |
| Children's hospital | 525 | 5 853 | 10.9 | 3.2 | 1 685 | 40 430 | 4.2 |
| Paediatric haematology and nephrology | 180 | 834 | 21.6 | 4.4 | 797 | 9 954 | 8.0 |
| Paediatric neurology | 93 | 679 | 13.7 | 2.8 | 261 | 4 434 | 5.9 |
| Paediatric cardiology | 42 | 292 | 14.4 | 2.1 | 89 | 2 886 | 3.1 |
| Gynaecology and obstetrics | 172 | 2 389 | 7.2 | 2.0 | 338 | 12 697 | 2.7 |
| General paediatrics | 50 | 656 | 7.6 | 2.1 | 107 | 4 696 | 2.3 |
| Paediatric surgery | 37 | 1 093 | 3.4 | 2.5 | 93 | 5 763 | 1.6 |
Numbers do not add up as patients can stay at different departments during one admission.
In 2006, a total of 208 187 prescriptions was registered for clinical patients, of which 20 058 (9.6%) resulted in a DDI alert. The percentage of prescriptions generating an alert varied from 1.6% in the paediatric surgery department to 17.2% in the adults' cardiology department. Although the professional guideline identifies 331 different relevant DDIs, overall only 10 DDIs made up >50% of all DDI alerts, and 50 DDIs made up >90% of all DDI alerts. The 10 most frequently occurring DDIs in the adults' hospital and the children's hospital respectively are shown in Table 2.
Table 2.
Frequency and nature of the 10 most frequently encountered drug–drug interactions
| % of alerts | Cumulative % | Evidence-relevance category | ||
|---|---|---|---|---|
| Adult's hospital | ||||
| 1 | ACE-inhibitors + diuretics | 12.1 | 12.1 | 3D |
| 2 | RAS inhibitors + potassium (saving agents) | 8.5 | 20.7 | 2F |
| 3 | Coumarins + antibiotics (excl. co-trimoxazole/metronidazole/cefamandole) | 7.5 | 28.2 | 3D |
| 4 | NSAIDs (excl. COXIBs) + corticosteroids | 6.0 | 34.2 | 3D |
| 5 | QT-prolongators + QT-prolongators (excl. clarithromycin/erythromycin/voriconazole) | 4.0 | 38.2 | 1E |
| 6 | Bisphosphonates + antacids/iron/calcium | 3.6 | 41.9 | 0A |
| 7 | β-Blockers selective + insulin | 3.6 | 45.4 | 3B |
| 8 | Diuretics + NSAIDs | 3.0 | 48.5 | 3D |
| 9 | β-Blockers + NSAIDs | 2.5 | 51.0 | 3C |
| 10 | β-Blockers + oral antidiabetics | 2.3 | 53.3 | 3B |
| Children's hospital | ||||
| 1 | Ciclosporin + nephrotoxic compounds | 9.3 | 9.3 | 3C |
| 2 | Ciclosporin + CYP3A4-inhibitors | 8.7 | 18.0 | 3D |
| 3 | Ciclosporin + cotrimoxazol/trimethoprim | 8.0 | 25.9 | 3D |
| 4 | NSAIDs (excl. COXIBs) + corticosteroids | 7.8 | 33.8 | 3D |
| 5 | QT-prolongators + QT-prolongators (excl. clarithromycin/erythromycin/voriconazole) | 6.2 | 39.9 | 1E |
| 6 | QT-prolongators + clarithromycin/erythromycin/voriconazole | 4.7 | 44.6 | 3E |
| 7 | Midazolam/alprazolam + enzyme inductors | 4.5 | 49.1 | 3C |
| 8 | Midazolam/alprazolam + CYP3A4-inhibitors | 3.8 | 52.9 | 3B |
| 9 | Diuretics + NSAIDs | 3.6 | 56.5 | 3D |
| 10 | Phenytoin + valproic acid | 3.4 | 59.9 | 3A |
ACE, angiotensin converting enzyme; RAS, renin–angiotensin system; NSAID, nonsteroidal anti-inflammatory drug.
In Table 3 it is shown that an increased risk of side-effects was the most frequently occurring potential clinical consequence of the DDIs in our patient population (81.2% of alerts). This risk encompassed an increased bleeding risk (22.0%), hypotension (14.9%), nephrotoxicity (12.6%) and electrolyte disturbances (10.5%). The DDI could also lead to decreased effectiveness of the medication in 25.2% of cases. This may have severe consequences, for example in the case of antibiotics (ongoing infection) or immunosuppressives (rejection of the transplant). With respect to the management, almost half (48.6%) of all DDI alerts gave the advice to monitor laboratory values. In 36.5% of the occurring DDIs it was advised to avoid the combination, in 35.7% to apply a risk-modifying strategy (such as the addition of a proton pump inhibitor for gastric protection) and in 17.2% to adjust the dose.
Table 3.
Mechanism and advice of drug–drug interactions
| Potential clinical outcome | No. of alerts | %* | No. of alerts | %* | ||
|---|---|---|---|---|---|---|
| Increased risk of side-effects/toxicity | 16 280 | 81.2 | ||||
| Bleeding risk (incl. gastrointestinal ulcer risk) | 4410 | 22.0 | ||||
| Hypotension | 2982 | 14.9 | ||||
| Nephrotoxicity | 2535 | 12.6 | ||||
| Electrolyte disturbances | 2115 | 10.5 | ||||
| Cardiac arrhythmias (incl. QT-prolongation) | 1696 | 8.5 | ||||
| Masking of hypoglycaemia | 1226 | 6.1 | ||||
| Miscellaneous antiepileptics side-effects | 415 | 2.1 | ||||
| Risk of serotonin syndrome | 232 | 1.2 | ||||
| Other | 669 | 3.3 | ||||
| Risk of decreased effectiveness | 5 054 | 25.2 | ||||
| Antihypertensive drugs | 1665 | 8.3 | ||||
| Antibiotics and antimycotics | 722 | 3.6 | ||||
| Biphosphonates | 688 | 3.4 | ||||
| Thyreomimetics | 441 | 2.2 | ||||
| Immunomodifiers | 396 | 2.0 | ||||
| Antiepileptic drugs | 229 | 1.1 | ||||
| Other | 913 | 4.6 | ||||
| Advised management strategy | No. of alerts | %* | No. of alerts | %* | No. of alerts | %* |
| Monitoring | 16 268 | 81.1 | ||||
| Clinical monitoring of toxicity/effectiveness | 3823 | 19.1 | ||||
| Blood pressure monitoring | 1539 | 7.7 | ||||
| ECG monitoring | 1151 | 5.7 | ||||
| Monitoring of laboratory values | 9755 | 48.6 | ||||
| Kidney function (serum creatinine) | 2463 | 12.3 | ||||
| Blood clotting time (International Normalized Ratio) | 2408 | 12.0 | ||||
| Potassium | 2059 | 10.3 | ||||
| Drugs (Therapeutic Drug Monitoring) | 1651 | 8.2 | ||||
| Glucose | 802 | 4.0 | ||||
| Differential blood count | 163 | 0.8 | ||||
| Liver function | 147 | 0.7 | ||||
| Sodium | 62 | 0.3 | ||||
| Avoid combination | 7 330 | 36.5 | ||||
| Risk-modifying strategy | 7 169 | 35.7 | ||||
| Taking medication when sitting or laying down | 2982 | 14.9 | ||||
| Separate moments of oral administration | 2305 | 11.5 | ||||
| Add gastric protection (proton pump inhibitor) | 1789 | 8.9 | ||||
| Other | 88 | 0.4 | ||||
| Adjust dose/titrate dose slowly | 3 429 | 17.1 | ||||
| Other | 889 | 4.4 |
Percentages do not add up to 100% as one alert could encompass multiple outcomes or management advice.
The evidence for the vast majority (77%) of the DDI alerts was of relatively high quality (categories 3–4). The potential clinical relevance was high in 21% (categories E–F), medium in 58% (categories C–D) and low or not classified in 21% of the DDIs (categories A–B). This spreading over the different evidence-relevance categories is presented in Figure 1 and is similar to that found in Dutch community pharmacies by Buurma et al.[4].
Figure 1.
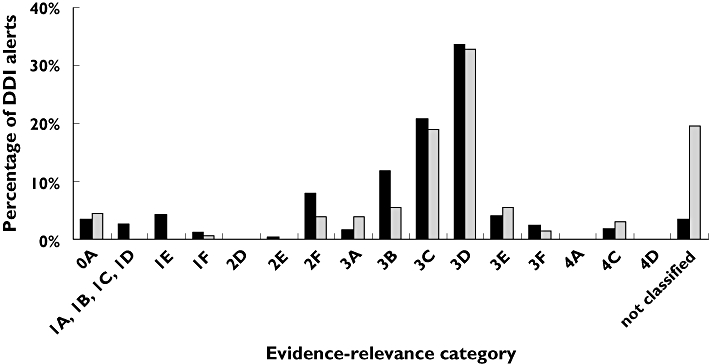
Percentage of drug–drug interaction (DDI) alerts by evidence-relevance category in our study compared with the percentage in Dutch community pharmacies in the study of Buurma et al.[4]. university hospital (this study) ( ); community pharmacies (Buurma et al.) (
); community pharmacies (Buurma et al.) ( )
)
Discussion
We found that of all patients admitted to the hospital and being prescribed medication, exclusive of IC patients, 28% experienced at least one potential DDI. On average this group of patients experienced 3.4 DDIs. Overall, the prescribing physician received a DDI alert in 9.6% of all prescriptions; this percentage largely varied between clinical specialisms. The most frequently occurring potential clinical consequence of the DDIs was an increased risk of side-effects such as increased bleeding risk (22.0%), hypotension (14.9%), nephrotoxicity (12.6%) and electrolyte disturbances (10.5%). Almost half (48.6%) of the DDIs could be managed by monitoring laboratory values.
All admissions in 2006, including re-admissions within the same year, were included in our study as separate cases. Compared with first admissions, the risk for DDIs may be increased during subsequent admissions, as these patients may be more severely ill. To check whether or not this was the case, we also performed the analysis including for each patient only the first admission in 2006. The outcomes of both analyses were similar (29% of first admissions experiencing at least one potential DDI; average number of DDIs 3.2). We consider it therefore justified to combine both first and subsequent admissions in the analysis.
The 10 most frequently encountered DDIs in the adults' hospital did not consist of specific ‘high-tech’ hospital medications, but of medications that are used and initiated on a large scale in the community setting as well. This suggests that home medication caused a substantial proportion of the DDIs. In contrast to the adults' hospital, the DDIs in the children's hospital do seem to be caused by specific ‘hospital medication’, such as, for example, ciclosporin and midazolam. It can be expected that children, in general, will be on less (or no) home medication on (first) admittance to the hospital than adults. Another explanation for our findings in the adults' hospital may be found in the fact that we used the professional guideline, the G-standard. The G-standard is in fact the national standard at this moment and it is used in all hospitals in the Netherlands. However, it has primarily been developed for use within community pharmacies. DDIs concerning specific hospital medications, e.g. anaesthetics and cytostatic agents, were largely missing in the G-standard in 2006. It may be worthwhile considering if our results would be different with the inclusion of the intensive care units and operating rooms and with the use of a specific clinical DDI reference database. However, to our knowledge such a specific clinical DDI database does not yet exist. Dawson and Karalliedde [16] published an overview of DDIs that are important to the clinical anaesthetist. This publication could be a good starting point.
Another kind of interaction that is not included in the professional guideline used but is of high importance in the hospital setting is the chemical or physical incompatibility of two intravenous medications when mixed in the same infusion bag or syringe or when administered simultaneously through the same catheter. These types of interactions were not included in our study. Ideally, to improve patient safety, a CPOE system should also signal this kind of DDI and make proposals for its management.
A limitation may be that the number of separate DDIs may be overestimated, as the same alert may be generated several times, each time the prescription is altered (re-start, dose change). On the other hand, the number of ‘DDI risk moments’ may be underestimated, as a DDI alert was generated only when a DDI interaction started. A warning may also be in place when the DDI stops. For example, in the case of an enzyme induction DDI, the plasma concentration of a drug may decrease and a dose increase may be required. As a consequence, when the interacting medication is stopped, the dose may need to be decreased again. In common practice of our hospital, this alert is not generated, and therefore, and to avoid double-counting, was not included in our study.
Patient safety may be improved by decreasing the frequency of preventable adverse drug events. Computerized alerts may be a useful tool to signal DDIs, but they may also result in ‘alert fatigue’ and the overriding of important signals, especially when the system produces an overload of signals that are of minor clinical relevance [17, 18]. Although 79% of the alerts were of high or medium potential clinical relevance, the actual relevance of some signals may still be low. Currently, the computerized DDI surveillance systems that are routinely used in the Netherlands only take into account the concomitant use of two drugs. Until now they have been unable to check for additional relevant data such as laboratory values, times of administration, absence of gastric protection, age, etc., which could increase the specificity of the signal. For example, the concomitant use of a renin–angiotensin system inhibitor and potassium always results in an alert warning for hyperkalaemia, even when the serum potassium level is low. In this study we did not gather the information to assess the actual relevance of the generated DDI alerts; this may be the topic of our further research.
From Table 3, it can be expected that the specificity of alerts could significantly improve by the use of more sophisticated clinical decision support systems (CDSS) [19–22], taking into account, for example, laboratory values (48.6% of alerts), times of administration (11.5%) and the absence of gastric protection (8.9%). This may be important knowledge for finding strategies to combat ‘alert fatigue’.
Another important finding in this respect is that a relatively small number of combinations (10 different DDIs) is responsible for a large number of alerts (>50%). As these are very well-known DDIs, it may be worthwhile to discuss with the medical board if and when these alerts have indeed to be shown. Turning off some of these top 10 DDI alerts may substantially decrease the ‘alert overload’ and in this way increase attention to dangerous and less known DDIs. In a series of qualitative interviews, Van der Sijs et al.[23] found that indeed the majority of respondents wanted to turn off DDI alerts to reduce alert overload. Since the top 10 alerts varies among clinical specialism and since also knowledge and monitoring practices vary between clinical specialisms within the hospital, it may be wise to suppress DDI alerts differentially between prescribers. This also requires more sophisticated CDSS than we have available at the moment.
In conclusion, 28% of all patients admitted to our hospital are exposed to at least one potential DDI. An increased risk of side-effects (e.g. increased bleeding risk, hypotension, nephrotoxicity, electrolyte disturbances) was the most prevalent potential clinical consequence. Almost half of the DDIs could be managed by monitoring laboratory values. Prescribing physicians receive an automated DDI alert in nearly 10% of all prescriptions. Further research is needed to investigate the clinical relevance of these DDIs and to develop methods to increase the specificity of automated CDSS alerts.
Appendix 1
Classification of drug–drug interactions according to the professional guideline by WINAp [15]
| Category | Description |
|---|---|
| Potential clinical relevance | |
| A | No inconvenience, insignificant effect |
| B | Short-lived inconvenience (<24–48 h) without residual symptoms |
| C | Long-lived inconvenience (48–168 h) without residual symptoms |
| D | Long-lived inconvenience (>168 h) with residual symptoms or handicap |
| E | Potential failure of life-saving therapy, increased risk of pregnancy (without risks concerning mother and/or fetus), cardiac arrhythmia, rhabdomyolysis, malignant hypertension, pseudopheochromocytome, multiorgan failure |
| F | Death, torsade de pointes, ventricular arrhythmia, myocardial infarction, serotonin syndrome, hyperpyrexia (42°C) |
| Quality of evidence | |
| 4 | Controlled, published study with clinically relevant end-points |
| 3 | Controlled, published study with relevant surrogate end-points |
| 2 | Well-documented, published case reports; analysis of case series |
| 1 | Incomplete, published case reports |
| 0 | Animal studies, in vitro studies, data on file |
| - | No evidence |
| Not classified | |
Competing interests
The authors do not have a potential conflict of interest. The Department of Pharmacoepidemiology and Pharmacotherapy employing authors M.J.t.B., W.W.v.S. and A.C.G.E. has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, Novo Nordisk, the private–public-funded Top Institute Pharma (http://www.tipharma.com, includes co-funding from universities, government, and industry), the Dutch Medicines Evaluation Board, and the Dutch Ministry of Health.
The authors are grateful to Hanneke den Breeijen for the data analysis and to their colleagues at the Utrecht Institute for Pharmaceutical Sciences and the UMC Utrecht for their support in establishing and maintaining UPOD.
REFERENCES
- 1.Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–6. doi: 10.1001/archinternmed.2008.3. [DOI] [PubMed] [Google Scholar]
- 2.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug–drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–8. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 3.Bagheri H, Michel F, Lapeyre-Mestre M, Lagier E, Cambus JP, Valdiguie P, Montastruc JL. Detection and incidence of drug-induced liver injuries in hospital: a prospective analysis from laboratory signals. Br J Clin Pharmacol. 2000;50:479–84. doi: 10.1046/j.1365-2125.2000.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buurma H, De Smet PA, Egberts AC. Clinical risk management in Dutch community pharmacies: the case of drug–drug interactions. Drug Saf. 2006;29:723–32. doi: 10.2165/00002018-200629080-00009. [DOI] [PubMed] [Google Scholar]
- 5.Merlo J, Liedholm H, Lindblad U, Bjorck-Linne A, Falt J, Lindberg G, Melander A. Prescriptions with potential drug interactions dispensed at Swedish pharmacies in January 1999: cross sectional study. BMJ. 2001;323:427–8. doi: 10.1136/bmj.323.7310.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buurma H, Schalekamp T, Egberts AC, De Smet PA. Compliance with national guidelines for the management of drug–drug interactions in Dutch community pharmacies. Ann Pharmacother. 2007;41:2024–31. doi: 10.1345/aph.1K240. [DOI] [PubMed] [Google Scholar]
- 7.Peng CC, Glassman PA, Marks IR, Fowler C, Castiglione B, Good CB. Retrospective drug utilization review: incidence of clinically relevant potential drug–drug interactions in a large ambulatory population. J Manag Care Pharm. 2003;9:513–22. doi: 10.18553/jmcp.2003.9.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30:379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 9.Egger SS, Drewe J, Schlienger RG. Potential drug–drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol. 2003;58:773–8. doi: 10.1007/s00228-002-0557-z. [DOI] [PubMed] [Google Scholar]
- 10.Kohler GI, Bode-Boger SM, Busse R, Hoopmann M, Welte T, Boger RH. Drug–drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504–13. doi: 10.5414/cpp38504. [DOI] [PubMed] [Google Scholar]
- 11.Heininger-Rothbucher D, Bischinger S, Ulmer H, Pechlaner C, Speer G, Wiedermann CJ. Incidence and risk of potential adverse drug interactions in the emergency room. Resuscitation. 2001;49:283–8. doi: 10.1016/s0300-9572(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 12.Beers MH, Storrie M, Lee G. Potential adverse drug interactions in the emergency room. An issue in the quality of care. Ann Intern Med. 1990;112:61–4. doi: 10.7326/0003-4819-112-1-61. [DOI] [PubMed] [Google Scholar]
- 13.Gronroos PE, Irjala KM, Huupponen RK, Scheinin H, Forsstrom J, Forsstrom JJ. A medication database – a tool for detecting drug interactions in hospital. Eur J Clin Pharmacol. 1997;53:13–7. doi: 10.1007/s002280050330. [DOI] [PubMed] [Google Scholar]
- 14.ten Berg MJ, Huisman A, van den Bemt PMLA, Schobben AFAM, Egberts ACG, van Sollinge WW. Linking laboratory and medication data: new opportunities for pharmacoepidemiological research. Clin Chem Lab Med. 2007;45:13–9. doi: 10.1515/CCLM.2007.009. [DOI] [PubMed] [Google Scholar]
- 15.Van Roon EN, Flikweert S, Le Comte M, Langendijk PNJ, Kwee-Zuiderwijk WJM, Smits P, Brouwers JRBJ. Clinical relevance of drug–drug interactions: a structured assessment procedure. Drug Saf. 2005;28:1131–9. doi: 10.2165/00002018-200528120-00007. [DOI] [PubMed] [Google Scholar]
- 16.Dawson J, Karalliedde L. Drug interactions and the clinical anaesthetist. Eur J Anaesthesiol. 1998;15:172–89. [PubMed] [Google Scholar]
- 17.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138–47. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuperman GJ, Bobb A, Payne TH, Avery AJ, Gandhi TK, Burns G, Classen DC, Bates DW. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14:29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, Lee R, Mekala A, Song J, Komaroff AL, Bates DW. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839–44. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 20.Schiff GD, Klass D, Peterson J, Shah G, Bates DW. Linking laboratory and pharmacy: opportunities for reducing errors and improving care. Arch Intern Med. 2003;163:893–900. doi: 10.1001/archinte.163.8.893. [DOI] [PubMed] [Google Scholar]
- 21.Raschke RA, Gollihare B, Wunderlich TA, Guidry JR, Leibowitz AI, Peirce JC, Lemelson L, Heisler MA, Susong C. A computer alert system to prevent injury from adverse drug events: development and evaluation in a community teaching hospital. JAMA. 1998;280:1317–20. doi: 10.1001/jama.280.15.1317. [DOI] [PubMed] [Google Scholar]
- 22.Judge J, Field TS, DeFlorio M, Laprino J, Auger J, Rochon P, Bates DW, Gurwitz JH. Prescribers' responses to alerts during medication ordering in the long term care setting. J Am Med Inform Assoc. 2006;13:385–90. doi: 10.1197/jamia.M1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Sijs H, Aarts J, van Gelder T, Berg M, Vulto A. Turning off frequently overridden drug alerts: limited opportunities for doing it safely. J Am Med Inform Assoc. 2008;15:439–48. doi: 10.1197/jamia.M2311. [DOI] [PMC free article] [PubMed] [Google Scholar]


