Abstract
AIM
To investigate a potential interaction between cranberry juice and diclofenac, a substrate of CYP2C9.
METHODS
The inhibitory effect of cranberry juice on diclofenac metabolism was determined using human liver microsome assay. Subsequently, we performed a clinical trial in healthy human subjects to determine whether the repeated consumption of cranberry juice changed the diclofenac pharmacokinetics.
RESULTS
Cranberry juice significantly suppressed diclofenac metabolism by human liver microsomes. On the other hand, repeated consumption of cranberry juice did not influence the diclofenac pharmacokinetics in human subjects.
CONCLUSIONS
Cranberry juice inhibited diclofenac metabolism by human liver microsomes, but not in human subjects. Based on the present and previous findings, we think that although cranberry juice inhibits CYP2C9 activity in vitro, it does not change the pharmacokinetics of medications metabolized by CYP2C9 in clinical situations.
Keywords: cranberry juice, diclofenac, interaction
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Cranberry juice has a significant inhibitory effect on CYP2C9 activity in vitro, whereas it shows a minimal effect on the pharmacokinetics and pharmacodynamics of warfarin, a CYP2C9 substrate in vivo.
Information regarding the interaction between cranberry juice and other medications metabolized by CYP2C9 is limited.
WHAT THIS STUDY ADDS
Cranberry juice suppressed the metabolism of diclofenac, another CYP2C9 substrate, by human liver microsomes.
Pharmacokinetic parameters of diclofenac were not altered by cranberry juice consumption in human subjects.
Introduction
Potential interactions of foods and beverages with medications are of deep concern in clinical practice. It is well known that some kinds of fruit juice cause the pharmacokinetic alteration of medications. For example, grapefruit juice increases plasma concentrations of simvastatin, lovastatin and buspiron by inhibiting their CYP3A4-mediated metabolism [1–3]. Several recent case reports have shown that the patients on warfarin therapy suffered from a profound hypoprothrombinaemia after the ingestion of cranberry juice (CrJ) [4, 5]. However, since other studies did not reveal a clinically relevant interaction between CrJ and warfarin [6, 7], these reports seem to be only unvalidated descriptions of clinical events.
In a previous in vitro study, CrJ inhibited the hydroxylation of flurbiprofen, a CYP2C9 substrate, to approximately 50% by human liver microsomes [8]. We also found that CrJ significantly inhibited the metabolism of phenytoin, another CYP2C9 substrate, in vitro[9]. Therefore, it is anticipated that CrJ inhibits CYP2C9-mediated drug metabolism in vivo. However, it is reported that a single consumption of CrJ did not change the flurbiprofen pharmacokinetics in human subjects [8]. In addition, there are several reports indicating that CrJ exerts a minimal effect on the pharmacokinetics and pharmacodynamics of warfarin after repeated drinking of the juice [7, 10–12].
However, because information concerning the influence of CrJ on the pharmacokinetics of CYP2C9 substrates is limited, we think it premature to reach to a definite conclusion about the effect of CrJ on the pharmacokinetics of medications that are mainly metabolized by CYP2C9. To address the issue, we examined the effect of CrJ on another CYP2C9 substrate, diclofenac, in human subjects. We determined the inhibitory effect of CrJ on diclofenac metabolism by human liver microsomes and in human subjects.
Materials and methods
Materials
CrJ (containing 27% cranberry; Ocean Spray Cranberry, Inc., Lakeville-Middleboro, MA, USA) was used in this study. Pooled human liver microsome (from 15 donors) was obtained from BD Bioscience (Franklin Lakes, NJ, USA). Diclofenac sodium, ibuprofen and sulfaphenazole (SFZ) were purchased from Sigma-Aldrich (St Louis, MO, USA).
In vitro study
Human liver microsome assay
Metabolism of diclofenac was determined by the reduction of its concentration. Human liver microsome assay was performed according to a previous report [13] with a minor modification. In brief, 180 µl of reaction mixture was preincubated for 10 min at 37 °C by the addition of human liver microsomes [donated from subjects with CYP2C9*1/*1 (n = 19), *1/*2 (n = 6) and *1/*3 (n = 3)), final concentration 0.05 mg ml−1] to the nicotinamide adenine dinucleotide phosphate (NADPH)-regenerating system (1.3 mM NADP, 3.3 mM glucose 6-phosphate, 0.4 U ml−1 glucose-6-phosphate dehydrogenase, 3.3 mM MgCl2) in 50 mM phosphate buffer (pH 7.4) with 10 µl of SFZ solution (final concentration 0.1, 0.3 and 1.0 µM) or CrJ (final juice concentration 0.11–9.0%). After preincubation, 10 µl of diclofenac solution (final concentration 1 µg ml−1) was added and the mixture was incubated for 40 min at 37 °C, because the rate of reduction of diclofenac remained constant for up to 40 min under these conditions. The reaction was stopped by the addition of 900 µl of dichloromethane containing ibuprofen (internal standard, 5 µg ml−1). Sample was shaken for 5 min vigorously and centrifuged at 750 g for 5 min. The aliquot layer was discarded and the organic layer solvent (800 µl) was evaporated to dryness under a stream of nitrogen. The residue was dissolved in 100 µl of mobile phase and 60 µl of solution was injected into the high-performance liquid chromatography (HPLC) system to determine the diclofenac concentration.
Clinical study
Subjects
Eight healthy male (n = 6) and female (n = 2) volunteers with a mean age of 30.5 years (range 23–44 years) were enrolled into this study. Their CYP2C9 genotype status were all *1/*1. All subjects were prohibited from taking any medications or supplement drugs, or any food or beverages containing cranberry during 7 days before each trial. All subjects gave their written informed consent to participate in this study. The protocol was approved by the Ethics Committee of Jichi Medical University (No. 07-25, Tochigi, Japan). The protocol was conducted in accordance with the guidelines on good clinical practice and with ethical standards for human experimentation established by the Declaration of Helsinki.
Study design
The study was an open-label, two-period, crossover design with a wash-out period of >2 weeks. In one period, the subject was administered one tablet of 25 mg diclofenac (Voltaren®, Novartis Pharma K.K., Tokyo, Japan) with 180 ml of water. In another period, the subject ingested 180 ml of CrJ (containing 27% cranberry; Ocean Spray Cranberry, Inc.) twice a day for 5 days. On day 6, the subject was administered one tablet of 25 mg diclofenac with 180 ml of CrJ. On the day of drug administration in each period, all subjects were instructed to take the same breakfast as usual, to prevent gastrointestinal adverse effects by diclofenac. Diclofenac was given to each subject 2 h after the breakfast.
For the determination of plasma diclofenac concentration, blood samples were collected in the tube with ethylenediamine tetraaceticacid at predose and 0.5, 1, 2, 3, 4, 6 and 9 h after the dosing. Plasma was stored at −80 °C until analysis.
Solid extraction
Plasma sample (300 µl) spiked with 10 µl of internal standard solution (ibuprofen, 1 mg ml−1 in methanol) was mixed with 700 µl of 0.1 N HCl solution. This aqueous was loaded onto the extraction cartridge (Oasis HLB solid phase extraction cartridge; Waters, Milford, MA, USA), which was preactivated with 1 ml of methanol and 1 ml of water. A wash step was performed with 1 ml of 5% methanol. The cartridge was removed to another collection tube and the sample was eluted using 1 ml of methanol. The elution was evaporated to dryness under a stream of nitrogen. The residue was reconstituted in 100 µl of mobile phase and 50 µl was injected into the HPLC system.
Analysis of samples
Diclofenac concentrations were determined by a validated HPLC method with ultraviolet (UV) detection. The HPLC system consisted of a liquid pomp (880-PU; JASCO Co., Tokyo, Japan), an autosampler (851-AS; JASCO), an UV detector (875-UV; JASCO) and a recorder (SIC Chromatocorder12; System Instruments Co., Ltd, Tokyo, Japan). The column was a Mightysil RP-18 (4.6 × 150 mm, 5 µm; KANTO Chemical Co., Inc., Tokyo, Japan) fitted with a RP-18 guard column (4.6 × 5 mm, 5 µm; KANTO Chemical). The mobile phase contained 40% of 25 mM KH2PO4 (pH 3.6 by H3PO4) and 60% of acetonitrile, its flow rate was 0.8 ml min−1, and UV detection was employed at 280 nm. The calibration curves (using peak height ratios) were linear over the range 50–2000 ng ml−1 (r2 = 0.999). The coefficients of variation, determined from 50 and 2000 ng ml−1 of diclofenac, were 2.52 and 0.33% (intraday), and 4.22 and 3.10% (interday), respectively.
Pharmacokinetic analysis
The pharmacokinetic values of maximum plasma concentration (Cmax) and time to maximum concentration (tmax) were directly obtained in each subject. The area under the concentration curve (AUC0–∞) was calculated using WinNonlin® software (Pharsight Co., Mountain View, CA, USA). The population pharmacokinetic parameters were calculated with NONMEM program version VI level 1.0 (Icon Inc., North Wales, PA, USA) following the two-compartment model (PREDPP library, subroutines ADVAN4 and TRANS4), using the first-order conditional estimation with the η–ε interaction method.
Protein-binding ratio of diclofenac
The protein-binding ratio of diclofenac was measured by an ultrafiltration method. In brief, the aqueous of human liver microsomes (final concentration, 0.05 mg ml−1) in 50 mM phosphate buffer or drug-free human plasma was incubated for 10 min at 37 °C with each concentration of diclofenac. After incubation, the sample was applied to Microcon® column (YM-10; Millipore, Billerica, MA, USA) and centrifuged to separate the protein-binding fraction. We collected the residual aqueous on the membrane, and diclofenac concentrations in residual as well as total sample were measured. The extraction and determination of total and protein-binding diclofenac concentration were performed as described above. The protein-binding ratio of diclofenac was calculated as follows:
 |
(1) |
Analysis of phytochemical contents of cranberry juice
CrJ was extracted by the conditioned Strata® SPE Column (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) and eluted by methanol of one-third of the initial respective volume of juice. The phytochemical contents of CrJ were analysed for phenolic compounds (anthocyanins, flavonols, hydroxycinnamic acid and hydroxybenzoic acid) by the validated HPLC method with a modification [14]. Phenolic compounds were qualified using cyaniding-3-glucoside for anthocyanins (520 nm), rutin for flavonols (365 nm), chlorogenic acid for hydroxycinnamic acid (330 nm) and gallic acid for hydroxybenzoic acid (280 nm).
Statistical analysis
In the experiments with human liver microsome, groups were compared by anova, and the difference between the two groups was determined using Bonferroni–Dunn test. Concentration–inhibition relationships were fitted to a four-parameter logistic equation using a nonlinear curve-fitting program (Igor Pro 6.03; WaveMetrics, Lake Oswego, OR, USA), from which the IC50 values were derived [15]. During an iterative curve fitting, no restriction was set to all values of coefficients. The intra- and interassay coefficients of variation were better than 5%. The pharmacokinetics parameters were analysed by paired t-test or χ2 test (for NONMEM analysis). In all analyses, P < 0.05 was considered to be significant.
Results
Effect of CrJ on the diclofenac metabolism by human liver microsomes
To examine whether the extract of CrJ inhibited diclofenac metabolism, we performed the experiment using human liver microsome with NADPH re-generation system. In this assay, we measured the parent drug (diclofenac), and calculated the reduction of diclofenac concentration. SFZ significantly inhibited diclofenac metabolism at >0.3 µM, and the IC50 value was calculated to be 0.4 µM (Figure 1a). CrJ also inhibited diclofenac metabolism concentration-dependently, and the IC50 value was calculated to be 1.44% (Figure 1b).
Figure 1.
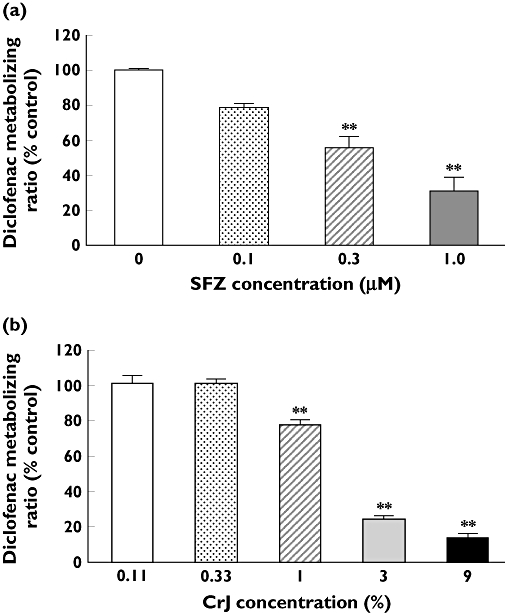
Effects of sulfaphenazole (SFZ) (a) and cranberry juice CrJ (b) on diclofenac metabolism by human liver microsomes The reaction velocity expressed as a percent of control (0 µM inhibitor or 0% of CrJ) value. The IC50 value was estimated to be 0.4 µM (a) and 1.44% (b). **P < 0.01 compared with control. Mean ± SE. n = 3–4
Effect of CrJ ingestion on the diclofenac pharmacokinetics in human subjects
To clarify the influence of repeated ingestion of CrJ on the diclofenac pharmacokinetics, we performed the clinical study in healthy human subjects. Five-day ingestion of CrJ did not markedly influence plasma diclofenac concentration (Figure 2). No significant differences were observed in the tmax, Cmax or AUC0–∞ between the two trials (Table 1a). Estimated population pharmacokinetic parameters in the final model are shown in Table 1b. By hypotheses testing, the ingestion of CrJ did not influence the following parameters: CL/F (apparent clearance), Ktr (transit rate constant), Vc/F (apparent central volume of distribution) or F (bioavailability) (Table 1c).
Figure 2.
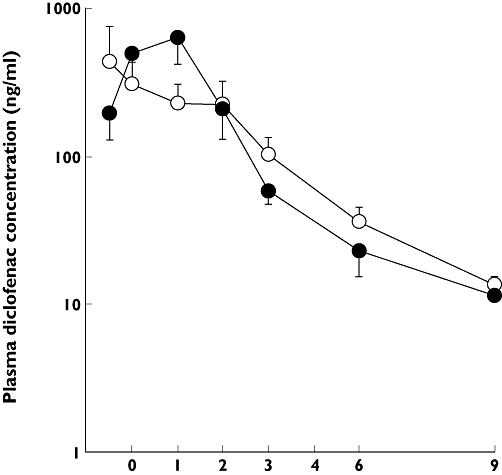
Plasma diclofenac concentration after oral administration of a single dose of 25 mg diclofenac. diclofenac alone (○); diclofenac with cranberry juice (CrJ) (•), mean ± SE. n = 8
Table 1a.
Comparison of pharmacokinetic parameters of diclofenac after cranberry juice (CrJ) consumption for 5 days
| No treatment | After CrJ consumption | Mean difference (95% CI) | |
|---|---|---|---|
| tmax (h) | 1.63 ± 0.31 | 1.75 ± 0.18 | −0.13 (−0.86, 0.61) |
| Cmax (ng ml−1) | 825.53 ± 256.33 | 788.55 ± 227.34 | 36.98 (−725.97, 799.93) |
| AUC0–∞ (ng h−1 ml−1) | 1157.95 ± 198.75 | 1482.03 ± 314.51 | −324.125 (−976.03, 327.78) |
Mean ± SE (n = 8).
Table 1b.
Estimated population pharmacokinetic parameters in final model
| Parameter | Parameter value | RSE (%) | 95% CI |
|---|---|---|---|
| Fixed effects | |||
| Ktr (1 h−1) | 4.70 | 31.1 | (1.84, 7.56) |
| CL/F (l h−1) | 24.6 | 22.0 | (14.0, 35.2) |
| Vc/F (l) | 10.3 | 45.1 | (1.20, 19.4) |
| Q/F (l h−1) | 7.51 | 38.9 | (1.78, 13.2) |
| Vp/F (l) | 14.5 | 35.4 | (4.44, 24.6) |
| Random effects | |||
| ω2 (IIV F; %) | 47.2 | 55.2 | (−3.87, 98.3) |
| ω2 (IIV Ktr; %) | 65.4 | 54.7 | (−4.72, 136) |
| ω2 (IOV F; %) | 46.4 | 87.0 | (−32.7, 126) |
| ω2 (IOV Ktr; %) | 67.8 | 63.2 | (−16.2, 152) |
| Residual variability | |||
| σ2 | 48.4 | 21.3 | (28.2, 68.6) |
CL/F, apparent clearance; IIV, interindividual variability; IOV, intra-occasional variability; Ktr, transit rate constant; Q/F, apparent intercompartmental clearance; RSE, relative standard error; Vc/F, apparent central volume of distribution; Vp/F, apparent peripheral volume of distribution.
Table 1c.
Effect of the ingestion of cranberry juice on pharmacokinetic parameters
| Model equation | θ value (95% CI) | LLD | P-value |
|---|---|---|---|
| CL/F = CL/Fpop × θ | 1.06 (0.802, 1.32) | −0.194 | 0.660 |
| Ktr = Ktrpop × θ | 0.806 (0.292, 1.32) | −0.706 | 0.401 |
| Vc/F = Vc/Fpop × θ | 1.42 (0.184, 2.66) | −0.425 | 0.514 |
| F = 1 × θ | 1.07 (0.632, 1.51) | −0.111 | 0.739 |
θ, population estimate for the fractional increase in each parameters; CL/F, apparent clearance; Ktr, transit rate constant; F, bioavailability assumed 1.0 for subjects without juice intake; LLD, −2 log likelihood difference from the value of final model; Vc/F, apparent central volume of distribution. ‘P’pop represents the population mean value of parameter ‘P’.
Protein-binding ratio of diclofenac
The protein-binding ratio of diclofenac in plasma was approximately 90% or more (Table 2). On the other hand, the ratio of diclofenac in human liver microsome was markedly small in each diclofenac concentration used in this study (0.1–1.0 µg ml−1).
Table 2.
The protein-binding ratio of diclofenac
| Diclofenac concentration (µg ml−1) | The protein-binding ratio (%) | ||
|---|---|---|---|
| (0.10) | (0.33) | (1.00) | |
| Drug-free plasma | 90.9 ± 5.0 | 93.1 ± 3.5 | 86.9 ± 6.5 |
| Human liver microsome | 9.5 ± 1.1 | 13.1 ± 2.6 | 14.8 ± 0.4 |
Mean ± SE (n = 3).
Analysis of phytochemical contents of cranberry juice
The contents of anthocyanins, flavonols, hydroxycinnamic acid and hydroxybenzoic acid are shown in Table 3. The values of these contents, excluding hydroxybenzoic acid, were similar to those in a previous study, in which the same brand of CrJ was used [10].
Table 3.
The phytochemical contents of cranberry juice
| Anthocyanins | Flavonols | HCA | HBA | |
|---|---|---|---|---|
| Content (mg per 100 g of weight) | 1.91 ± 0.06 | 4.88 ± 0.29 | 2.05 ± 0.07 | 10.16 ± 1.52 |
Mean ± SE (n = 3). HBA, hydroxybenzoic acid; HCA, hydroxycinnamic acid.
Discussion
In this study, we examined the inhibitory effect of CrJ on diclofenac metabolism. In human liver microsome experiment, SFZ, an inhibitor of CYP2C9 activity, significantly suppressed diclofenac metabolism, and its IC50 value was estimated to be 0.3–1.0 µM. The IC50 value of SFZ on the CYP2C9 inhibition was reported to be in the range 0.1–1.0 µM [16–18], and therefore we think that the present assay has good validity. In this study, CrJ also concentration-dependently inhibited diclofenac metabolism, and the IC50 value was estimated to be 1.44%. Previously, we found that phenytoin metabolism was suppressed by CrJ in human liver microsome assay [9]. These observations support the hypothesis that CrJ inhibits the metabolism of CYP2C9 substrates.
The IC50 value of CrJ to diclofenac metabolism in this study was approximately twofold lower than that in the previous study, in which the influence of CrJ on flurbiprofen hydroxylation was examined [8]. However, the parameter can not be directly compared between the two studies, because the IC50 value depends on the kinds of substrate used and substrate concentration even if the same enzyme is inhibited. In addition, we measured the parent drug concentration, but not its hydroxyl metabolites. Therefore, apparent differences might reside in the differences in protocols.
Next, we examined the influence of repeated CrJ consumption on the diclofenac pharmacokinetics in human subjects. Pharmacokinetic parameters did not significantly differ between the two trials. CrJ increased the CL/F value of diclofenac in some subjects and decreased it in others, and consequently CrJ did not significantly influence the CL/F value evaluated by population pharmacokinetic analysis. Therefore, CrJ is considered to have minimal influence on the diclofenac pharmacokinetics in human subjects, which is different from the observation in human liver microsome assay. Thus, the inhibitory effect of CrJ on diclofenac metabolism was detected in vitro, but not in vivo.
Diclofenac is extensively converted to several hydroxylated metabolites. The major metabolite is 4′-hydroxy (OH) diclofenac, and minor metabolites are 3′OH- and 5′OH- diclofenac [19]. The 4′-hydroxylation and 3′-hydroxylation of diclofenac are mediated mainly by CYP2C9, whereas 5′-hydroxylation is by other CYP2C enzyme, i.e. CYP2C8, 2C18 and 2C19 in vitro[20]. In addition, some diclofenac is excreted as acyl glucuronide of the parent drug itself [21]. Thus, diclofenac metabolism largely, but not completely depends on CYP2C9. Therefore, it remains possible that CrJ inhibited other metabolic pathways rather than CYP2C9-mediated hydroxylation in the present human liver microsome assay. However, since the inhibitory effect of CrJ on diclofenac metabolism was not detected in human subjects, it is probable that the influence of CrJ on the pharmacokinetics of diclofenac and other CYP2C9-metabolized medications is negligible in the clinical situation.
The influence of CrJ on diclofenac metabolism was different in vitro from in vivo. To evaluate the mechanism of such a discrepancy, we determined the protein-binding ratio of diclofenac in vitro and in vivo. This study showed that the major part of diclofenac existed as unbound form in vitro and protein-bound form in vivo. These findings lead us to speculate that the concentration of unbound-form diclofenac exposed to CYP enzymes was higher in vitro than in vivo, and consequently the inhibitory effect of CrJ on the metabolism of diclofenac seems greater in the in vitro study. We have already reported that CrJ inhibited phenytoin metabolism by human liver microsomes, but not in cultured hepatic cells [9]. These data indicate that the penetration of CrJ-containing inhibitory substance(s) into cells was small, which may be another mechanism for the difference of CrJ effect in vitro from in vivo. Based on the present and previous findings, we think that the inhibitory effect of CrJ on CYP2C9 activity is negligible in vivo, if any.
Several kinds of fruit juice have the capacity to influence drug disposition, but many of these interactions are not clinically important [22]. For example, pomegranate juice inhibited the disposition of midazolam, a CYP3A4 substrate, but did not alter the oral clearance of midazolam in human subjects [23]. Farkas and Greenblatt have provided the potential explanation for this phenomenon that the concentration of the inhibitors is not be high enough in the juice, and inhibitor(s) might be transported out of the target cells [22]. These data indicate that the in vitro model is poorly predictive for the evaluation of a drug–food interaction in vivo.
In summary, the present study has shown that CrJ inhibited diclofenac metabolism by human liver microsomes, but not in human subjects. Based on the present and previous findings, we think that CrJ does not change the pharmacokinetics of medications metabolized by CYP2C9 in clinical situations.
Competing interests
None declared.
REFERENCES
- 1.Kantola T, Kivistö KT, Neuvonen PJ. Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther. 1998;63:397–402. doi: 10.1016/S0009-9236(98)90034-0. [DOI] [PubMed] [Google Scholar]
- 2.Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice–simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther. 1998;64:477–83. doi: 10.1016/S0009-9236(98)90130-8. [DOI] [PubMed] [Google Scholar]
- 3.Lilja JJ, Kivistö KT, Backman JT, Lamberg TS, Neuvonen PJ. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655–60. doi: 10.1016/S0009-9236(98)90056-X. [DOI] [PubMed] [Google Scholar]
- 4.Suvarna R, Pirmohamed M, Henderson L. Possible interaction between warfarin and cranberry juice. BMJ. 2003;327:1454. doi: 10.1136/bmj.327.7429.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rindone JP, Murphy TW. Warfarin–cranberry juice interaction resulting in profound hypoprothrombinemia and bleeding. Am J Ther. 2005;13:283–4. doi: 10.1097/01.mjt.0000178908.32892.2f. [DOI] [PubMed] [Google Scholar]
- 6.Pham DQ, Pham AQ. Interaction potential between cranberry juice and warfarin. Am J Health Syst Pharm. 2007;64:490–4. doi: 10.2146/ajhp060370. [DOI] [PubMed] [Google Scholar]
- 7.Ansell J, McDonough M, Harmatz JS, Greenblatt DJ. A randomized, double-blind trial of the interaction between cranberry juice and warfarin. J Thromb Thrombolysis. 2008;25:112. doi: 10.1177/0091270009337510. [DOI] [PubMed] [Google Scholar]
- 8.Greenblatt DJ, von Moltke LL, Perloff ES, Luo Y, Harmatz JS, Zinny MA. Interaction of flurbiprofen with cranberry juice, grape juice, tea, and fluconazole: in vitro and clinical studies. Clin Pharmacol Ther. 2006;79:125–33. doi: 10.1016/j.clpt.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Ushijima K, Koshimizu T, Fujimura A. The inhibitory effect of cranberry juice on phenytoin metabolism by human liver microsomes. Jpn J Clin Pharmacol Ther. 2009;40:59–64. doi: 10.1111/j.1365-2125.2009.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lilja JJ, Backman JT, Neuvonen PJ. Effects of daily ingestion of cranberry juice on the pharmacokinetics of warfarin, tizanidine, and midazolam-probes of CYP2C9, CYP1A2, and CYP3A4. Clin Pharmacol Ther. 2007;81:833–9. doi: 10.1038/sj.clpt.6100149. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed AMI, Jiang X, Williams KM, Day RO, Roufogalis BD, Liauw WS, Xu H, McLachlan AJ. Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br J Pharmacol. 2008;154:1691–700. doi: 10.1038/bjp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Seeram NP, Carpenter CL, Thames G, Minutti C, Bowerman S. Cranberry does not affect prothrombin time in male subjects on warfarin. J Am Diet Assoc. 2006;106:2057–61. doi: 10.1016/j.jada.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Egashira K, Ohtani H, Itoh S, Koyabu N, Tsujimoto M, Murakami H, Sawada Y. Inhibitory effects of pomelo on the metabolism of tacrolimus and the activities of CYP3A4 and P-glycoprotein. Drug Metab Dispos. 2004;32:828–33. doi: 10.1124/dmd.32.8.828. [DOI] [PubMed] [Google Scholar]
- 14.Kähkönen MP, Hopia AI, Heinonen M. Berry phenolics and their antioxidant activity. J Agric Food Chem. 2001;49:4076–82. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- 15.Koshimizu T, Ueno S, Tanoue A, Yanagihara N, Stojilkovic SS, Tsujimoto G. Heteromultimerization modulates P2X receptor functions through participating extracellular and C-terminal subdomains. J Biol Chem. 2002;277:46891–9. doi: 10.1074/jbc.M205274200. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin SJ, Bloomer JC, Smith GJ, Ayrton AD, Clarke SE, Chenery RJ. Ketoconazole and sulphaphenazole as the respective selective inhibitors of P4503A and 2C9. Xenobiotica. 1995;25:261–70. doi: 10.3109/00498259509061850. [DOI] [PubMed] [Google Scholar]
- 17.Dierks EA, Stams KR, Lim HK, Cornelius G, Zhang H, Ball SE. A method for the simultaneous evaluation of the activities of seven major human drug-metabolizing cytochrome P450s using an in vitro cocktail of probe substrates and fast gradient liquid chromatography tandem mass spectrometry. Drug Metab Dispos. 2001;29:23–9. [PubMed] [Google Scholar]
- 18.Lin T, Pan K, Mordenti J, Pan L. In vitro assessment of cytochrome P450 inhibition: strategies for increasing LC/MS-based assay throughput using a one-point IC(50) method and multiplexing high-performance liquid chromatography. J Pharm Sci. 2007;96:2485–93. doi: 10.1002/jps.20884. [DOI] [PubMed] [Google Scholar]
- 19.Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 20.Bort R, Macé K, Boobis A, Gómez-Lechón MJ, Pfeifer A, Castell J. Hepatic metabolism of diclofenac: role of human CYP in the minor oxidative pathways. Biochem Pharmacol. 1999;58:787–96. doi: 10.1016/s0006-2952(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 21.Stierlin H, Faigle JW. Biotransformation of diclofenac sodium (Voltaren) in animals and in man. II. Quantitative determination of the unchanged drug and principal phenolic metabolites, in urine and bile. Xenobiotica. 1979;9:611–21. doi: 10.3109/00498257909042328. [DOI] [PubMed] [Google Scholar]
- 22.Farkas D, Greenblatt DJ. Influence of fruit juices on drug disposition: discrepancies between in vitro and clinical studies. Expert Opin Drug Metab Toxicol. 2008;4:381–93. doi: 10.1517/17425255.4.4.381. [DOI] [PubMed] [Google Scholar]
- 23.Farkas D, Oleson LE, Zhao Y, Harmatz JS, Zinny MA, Court MH, Greenblatt DJ. Pomegranate juice does not impair clearance of oral or intravenous midazolam, a probe for cytochrome P450-3A activity: comparison with grapefruit juice. J Clin Pharmacol. 2007;47:286–94. doi: 10.1177/0091270006298359. [DOI] [PubMed] [Google Scholar]


