Abstract
Chronic hepatitis C virus (HCV) infection is associated with cirrhosis, autoimmunity and lymphoproliferative disorders. We have previously reported a differential regulation of T and B lymphocytes by HCV core protein in vitro. In this report, we employed a translational approach to characterize the activation status of peripheral B cells from individuals with chronic HCV infection and to explore potential mechanisms for B-cell dysregulation in the setting of HCV infection. In contrast to the T-cell suppression observed in HCV-infected individuals, B cells exhibit a non-specific polyclonal activation phenotype, characterized by significantly higher levels of (1) the early activation marker, CD69, (2) the costimulatory molecule, CD86, and (3) the CCR5 chemokine receptor, CD195, when compared with B cells from healthy donors in response to phytohaemagglutinin (PHA) stimulation. Importantly, tumour necrosis factor- and Apo-L-related leucocyte-expressed ligand-1 (TALL-1), also known as B-lymphocyte stimulator (BLYS), was found to be up-regulated on the surface of B cells from HCV patients in response to PHA as well as HCV core antigen stimulation. This up-regulation of TALL-1 was associated with vigorous memory B-cell responses to viral antigenic stimulation. Additionally, suppressor of cytokine signalling-1 (SOCS-1), a negative feedback immunoregulator that is inhibited in B lymphocytes by HCV core in vitro, was also inhibited in B cells from HCV patients when compared with healthy donors. These findings suggest that TALL-1 over-expression and SOCS-1 suppression are associated with aberrant B-cell activation, providing a plausible basis for the B-cell clonal expansion underlying the lymphoproliferative disorders and autoimmune phenomena observed during chronic HCV infection.
Keywords: autoimmunity, B cells, hepatitis C, suppressor of cytokine signalling-1 (SOCS-1), tumour necrosis factor- and Apo-L-related leucocyte-expressed ligand-1 (TALL-1)/BAFF/BLYS
Introduction
Hepatitis C virus (HCV) infects over 170 million people worldwide and exhibits a remarkable propensity toward chronic viral persistence. It remains the major factor in the development of chronic hepatitis leading to cirrhosis and hepatocellular carcinoma. Chronic HCV infection is also associated with B-cell lymphoproliferative disorders,1,2 including most notably mixed cryoglobulinaemia (MC) and B-cell non-Hodgkin’s lymphoma (NHL). While extensive studies explore the mechanisms of HCV-specific CD4+ CD8+ T-cell dysfunction, less well-understood are the mechanisms for the B-cell dysregulation that occurs during chronic HCV infection.3 This dysregulation is manifest in several ways, including aberrant B-cell proliferation, a decreased threshold for B-cell activation, and overproduction of antibodies that are ineffective in controlling viral infection.3 Interestingly, while T-cell functions in chronically HCV-infected individuals are characterized by an anergic/exhausted phenotype, limited studies suggest that B-cell functions are characterized by an activation phenotype with polyclonal expansion and production of ‘nonsense’ immunoglobulins that may be associated with autoimmune disorders. We have previously shown that the core antigen of HCV can enhance B-cell activation and proliferation in vitro while suppressing T-cell functions, and that this differential regulation is associated with modulation of suppressor of cytokine signalling-1 (SOCS-1) proteins, negative immunomodulators that can inhibit signal transducer and activator of transcription (STAT) members.4 Translational studies on the regulation of SOCS-1 and B-cell signalling in HCV-infected patients are, however, lacking.
An understanding of the mechanisms underlying B-cell activation and proliferation has been advanced by the identification of tumour necrosis factor- and Apo-L-related leucocyte-expressed ligand-1 (TALL-1), also called B-cell activating factor (BAFF) or B-lymphocyte stimulator (BLYS).5,6 TALL-1 is a 285-amino-acid protein encoded by a gene on chromosome 13q32-34 and expressed by myeloid cells, including activated B cells and monocytes/macrophages/dendritic cells.5–7 Compelling evidence suggests that TALL-1 plays a pivotal role in B-cell haematological and autoimmune diseases in humans, including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, Sjögren’s syndrome, NHL and myeloma; limited data suggest a role in viral infections such as human immunodeficiency virus (HIV) and HCV as well.4,8–15 Interestingly, transgenic mice over-expressing TALL-1 develop B-cell hyperactivation/proliferation in both the peripheral blood as well as marginal zones of lymph nodes, with production of high titres of immunoglobulins, rheumatoid factor, anti-DNA antibodies and cryoglobulins.16–19 An increased serum level of TALL-1 detected by enzyme-linked immunosorbent assay has been recently reported in the setting of MC and HCV infection.4,14,15 TALL-1 expression levels on B cells in the setting of HCV infection remain unknown.
We have previously identified a differential regulation of B-cell and T-cell functions by HCV core antigen in vitro.20 In the translational studies described in this report, we have examined the activation status of B cells from HCV-infected patients and explored the underlying mechanisms. We found that, in contrast to the well-documented T-cell exhaustion that occurs in HCV-infected individuals, B lymphocytes from these individuals exhibit a polyclonal activation phenotype and TALL-1 plays an important role in B-cell over-activation in response to both viral antigen and non-specific immune signalling. Additionally, we observed SOCS-1 suppression in vivo in B cells from chronically HCV-infected individuals, supporting the concept of a differential regulation of lymphocyte function in the setting of chronic HCV infection.
Materials and methods
Subjects
An institutional review-board-approved protocol at East Tennessee State University (Johnson City, TN) has contributed to a database for the storage of blood samples from HCV-infected individuals. Blood from healthy donors serves as a normal control. Thirty-eight chronically HCV-infected patients, two spontaneously resolved HCV patients, one pegylated interferon + ribavirin-treated HCV patient with a sustained virological response, and 10 healthy donors are included in this study. For certain experiments, such as reverse transcription–polymerase chain reaction (RT-PCR), isolation of enough cells required the use of several patient samples at once and limited our ability to use the results for additional studies. We included those individuals with chronic HCV infection confirmed by measurable HCV RNA and not on therapy, and we did not include those patients with diagnosed autoimmune disease.
Cell isolation and culture
Human peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood of healthy donors by Ficoll density centrifugation with lympholyte-H (Cedarlane Labs, Burlington, NC). In certain experiments, B and T lymphocytes were further purified from isolated PBMC by incubation with a magnetic beads-conjugated anti-CD3 or fluorescein isothiocyanate (FITC)-conjugated anti-CD20 antibody, followed by positive selection (Miltenyi Biotec, Auburn, CA) per the manufacturer’s instructions. Purified cells were washed twice and cultured with RPMI-1640 (Life Technologies, Gaithersburg, MD), containing 10% (v/v) fetal bovine serum (Life Technologies), penicillin–streptomycin (100 μg/ml for each drug; Life Technologies), l-glutamine (2 mm) and 2-mercaptoethanol (5·5 × 10−5 m; Life Technologies) at 37° with 5% CO2 in a humidified atmosphere.
Flow cytometry
To determine TALL-1 expression on B lymphocytes, 1 × 106 purified PBMC were stimulated with either 5 μg/ml phytohaemagglutinin (PHA; Sigma, Saint Louis, MI), or 1 μg/ml HCV core or β-gal protein (ViroGen, Watertown, MA), for 24 hr. The treated cells were then washed three times in fluorescence-activated cell sorting (FACS) medium (RPMI-1640 supplemented with 10% fetal bovine serum and 1% NaN3) at 200 g for 5 min at 4°, resuspended in 100 μl of FACS medium containing 20 μl of phycoerythrin (PE)-conjugated anti-TALL-1 conjugate (BD Pharmingen, San Diego, CA), and double-stained with 20 μl FITC-anti-CD20 conjugate (BD Pharmingen), or triple-stained with 20 μl of allophycocyanin-conjugated anti-CD27 (eBioscience, San Diego, CA) by incubating at 4° in the dark for 1 hr. The cells were then washed three times in phosphate-buffered saline before flow cytometric analysis (Becton Dickinson, San Jose, CA). The primary isotype controls were used to determine the level of background staining; 20 000 events were collected after gating on lymphocyte populations.
To determine the expressions of CD69, CD86, CD195 and immunoglobulin G (IgG) on the surface of B cells, 1 × 106 isolated PBMC were activated with PHA for 24 hr. The PE-anti-CD69, -CD86, -CD154, -CD195 and FITC-anti-CD20 double staining and flow cytometry analysis were carried out as above.
RT-PCR
Between 2 × 106 and 3 × 106 B lymphocytes purified by magnetic beads (Miltenyi Biotec) from six HCV patients or three healthy donors were freeze–thawed once, and total RNA was isolated from these cells using an RNA isolation kit (Qiagen Sci., Valencia, CA). A total of 1 μg RNA was treated with DNAse to digest genomic DNA and 1 μl RNA was then reverse-transcribed using murine leukaemia virus (MuLV) reverse transcriptase under conditions of 10 min at room temperature, 20 min at 42°, 5 min at 99° and 5 min at 4°. One microlitre of 1 : 10 series diluted complementary DNA (cDNA: 1 : 1, 1 : 10, 1 : 100) generated in the RT reaction was added to the PCR. The PCR was carried out using the following primer pairs: SOCS-1 sense 5′-ATG GTA GCA CAC AAC CAG GTG-3′; antisense 5′-TCA AAT CTG GAA GGG GAA GGA-3′; hB2M sense 5′-ATG CCT GCC GTG TGA ACC AT-3′; antisense 5′-CAT CCA ATC CAA ATG CGG CAT CT-3′ for 35 cycles of 95° for 45 seconds, 58° for 45 seconds, 72° for 45 seconds, followed by a single 10-min extension at 72°. To control for genomic DNA contamination, equal amounts of cDNA from each sample were PCR amplified without RT. The resulting PCR products were separated on a 2% BioGel (Bio 101; Molnlycke Health Care US, Carlsbad, CA) and viewed using a multi-imager.
Immunoblotting. B lymphocytes were purified from human PBMC using magnetic beads (Miltenyi Biotec) in accordance with the manufacturer’s instruction. A total of 2 × 106 purified cells were activated with PHA (5 μg/ml; Sigma) at 37° in a 5% CO2 atmosphere for 24 hr. Cell lysates were prepared for 30 min at 4° with a lysis buffer (Life Technologies). Cell lysates were sonicated three times for 1 min each time. Cellular debris was pelleted by centrifugation at 16 000 g and supernatants were collected and frozen at −80°.
A total of 80 μg protein was denatured with sample loading buffer at 100° for 5 min and resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, followed by semi-dry transfer (Amersham Pharmacia Biotech, Pittsburg, PA) to a Hybond-P membrane (Amersham Biosciences, Arlington Heights, IL). After blocking in Blotto-Tween-20 (10 mm Tris–HCl, 0·9% NaCl, 0·1% Tween-20, 5% non-fat dry milk) at room temperature for 1 hr, the membrane was probed with monoclonal antibody (Millipore, Billerica, MA) to SOCS-1 (1 : 500). After several 5-min washes with Tris-buffered saline–Tween and Tris-buffered saline, the membrane was incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (1 : 10 000) and subsequently developed by enhanced chemiluminescence (ECL-plus; Amersham Biosciences) on X-OMAT-LS X-ray film (Kodak, Rochester, NY). The membrane was stripped and re-probed with goat polyclonal antibody to β-actin (1 : 500; Santa Cruz Technologies, Santa Cruz, CA) followed by polyclonal anti-goat IgG secondary antibody (1 : 10 000) before development as described above.
Statistical analysis
Data were shown as mean ± SD and the level of significance was determined using an anova program/Stata/SE 8·0 software (StataCorp LP, College Station, TX). P-values < 0·05 were considered significant and < 0·01 were considered very significant.
Results
B cells exhibit an activation phenotype during chronic HCV infection
As an initial approach to explore the underlying mechanisms for these HCV-associated B-cell lymphoproliferative disorders, we first compared the activation status of B cells from chronic HCV patients and healthy subjects, including the cell surface expression levels of CD69 – an early lymphocyte activation marker; CD86 – a B7-2 costimulatory molecule; CD195 – a chemokine receptor (CCR5); and their ability to produce IgG.
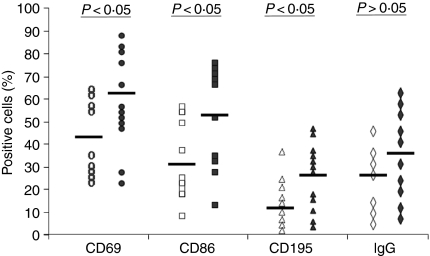
To this end, PBMC isolated from both HCV patients and healthy donors were stimulated with PHA for 24 hr and the expressions of B-cell activation markers were measured by flow cytometry analysis following PE-anti-CD69, PE-anti-CD86, PE-anti-CD195, or PE-anti-IgG plus FITC-anti-CD20 double-staining. As shown in Fig. 1, B cells from chronically HCV-infected patients had relatively higher expression levels of CD69 (62·31 ± 21·99%, n = 12), CD86 (54·24 ± 32·65%, n = 12), CD195 (29·30 ± 15·92%, n = 12), compared with those from healthy donors (43·66 ± 17·80%, n = 9, P = 0·045; 32·65 ± 18·81%n = 9, P = 0·039; 13·41 ± 7·28%, n = 9, P = 0·022, respectively), suggesting that B lymphocytes in HCV infection exhibit an aberrant activation status. Expression levels of IgG, although increased in the HCV population, did not reach statistical significance (38·54 ± 19·79%, n = 10) when compared with healthy donors (29·91 ± 17·51%, n = 7, P > 0·05).
Figure 1.
B cells from chronically hepatitis C virus (HCV)-infected individuals exhibit an activation phenotype compared with those from healthy donors. Peripheral blood mononuclear cells (PBMC) were isolated from 12 chronically HCV-infected patients (filled symbols) and nine healthy donors (open symbols). The cells were stimulated with phytohaemagglutinin for 24 hr, and the expressions of CD69, CD86, CD195 and immunoglobulin G (IgG) on CD20+ B cells were examined by flow cytometry as described in the Materials and methods. The percentage of lymphocyte activation marker-positive cells in the gated B-cell population, and the statistical analysis (P-values) between chronic HCV-infected individuals versus healthy donors, are shown.
TALL-1 is up-regulated on B cells from patients with HCV infection
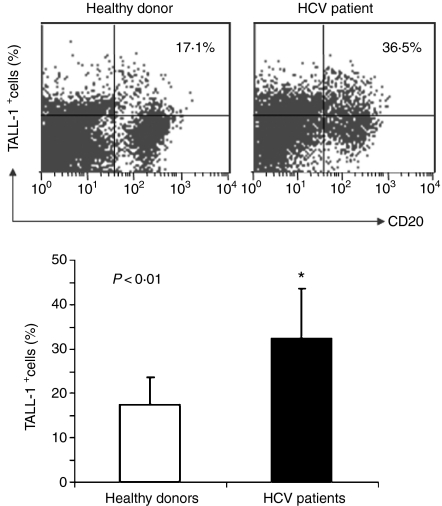
We next detected the expression levels of TALL-1 on the surface of B cells from HCV-infected individuals and healthy donors by flow cytometry. As shown in Fig. 2, B cells from HCV patients exhibit relatively higher levels of TALL-1 expression in response to PHA stimulation than healthy control subjects (32·23 ± 11·39%n = 12 versus 17·42 ± 6·29%, n = 9; P < 0·01). The B cells employed in these experiments were also employed in the studies of B-cell activation markers, and TALL-1 up-regulation correlated with the up-regulation of B-cell markers including CD69 (Pearson correlation R = 0·588, P = 0·027) as well as CD195 (R = 0·763, P = 0·002). Interestingly, we also examined TALL-1 expression on B cells from two spontaneously resolved, HCV-infected individuals and one successfully treated HCV-infected individual and found these levels to be comparable to those in healthy subjects, with the individual recently successfully treated still exhibiting slightly elevated levels of TALL-1 expression (data not shown).
Figure 2.
Tumour necrosis factor- and Apo-L-related leucocyte-expressed ligand-1 (TALL-1) up-regulation on B cells from hepatitis C virus (HCV)-infected patients versus healthy donors in response to mitogenic stimulation. Peripheral blood mononuclear cells (PBMC) isolated from both HCV patients (n = 12) and healthy donors (n = 9) were stimulated with phytohaemagglutinin for 24 hr, and TALL-1 expression on the surface of B lymphocytes was measured by flow cytometric analysis following phycoerythrin-conjugated anti-TALL-1 and fluorescein isothiocyanate-conjugated anti-CD20 double staining. A representative dot plot with percentage of TALL-1-positive cells in the B cells from an HCV patient versus healthy donor is shown above; and the mean ± SD percentages of TALL-1 expression on B lymphocytes from 12 HCV patients versus nine healthy donors are shown below; *P < 0·05.
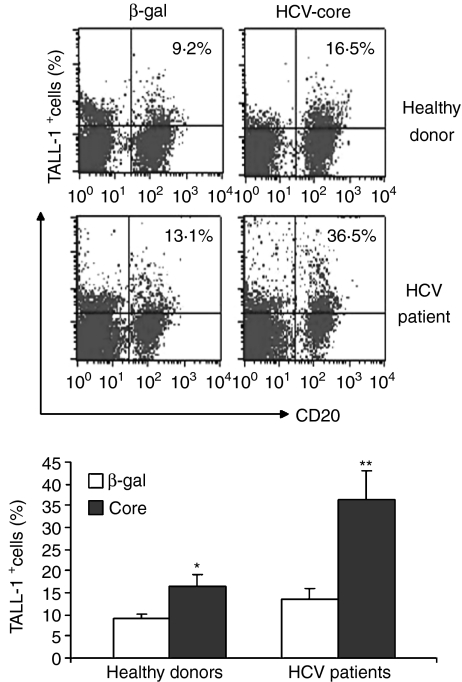
In addition to responses to non-specific stimulation, we also determined whether TALL-1 expression on B cells might be activated in an HCV antigen-driven, specific manner. We therefore examined TALL-1 expression in B cells from HCV-infected individuals and healthy donors exposed to HCV core or a control protein, β-gal. As shown in Fig. 3, HCV core protein can drive TALL-1 expression on B cells derived from healthy donors from 9·2% to 16·5%; whereas core up-regulated TALL-1 expression from 13·1% to 36·5% on B cells derived from chronically infected individuals. In addition, a comparison of TALL-1 expression levels on B cells exposed to only β-gal in the two groups revealed that HCV-infected individuals exhibit a modestly higher level of TALL-1 expression on B cells relative to those of healthy donors at baseline (13·1% versus 9·2%, P > 0·05); whereas B cells treated with HCV core exhibit a much higher level of TALL-1 in HCV patients compared with healthy donors (36·5% versus 16·5%, P < 0·05). This very significant increase in HCV core-mediated TALL-1 expression on B cells from HCV patients suggests the possibility of a vigorous memory response of B lymphocytes in HCV-infected individuals to viral antigen stimulation.
Figure 3.
Hepatitis C virus (HCV) core protein up-regulates tumour necrosis factor- and Apo-L-related leucocyte-expressed ligand-1 (TALL-1) expression on B lymphocytes. Peripheral blood mononuclear cells (PBMC) isolated from both HCV patients and healthy donors were stimulated with either HCV core or control protein, β-gal, for 24 hr; and TALL-1 expression on the surface of B lymphocytes was measured by flow cytometry following phycoerythrin-conjugated anti-TALL-1 and fluorescein isothiocyanate- conjugated anti-CD20 double staining. A representative dot plot with percentage of TALL-1-positive cells on the B cells from an HCV patient versus a healthy donor is shown above; and the mean ± SD percentage of TALL-1 expression on B lymphocytes from three HCV patients versus three healthy donors are shown below; *P < 0·05; **P < 0·01.
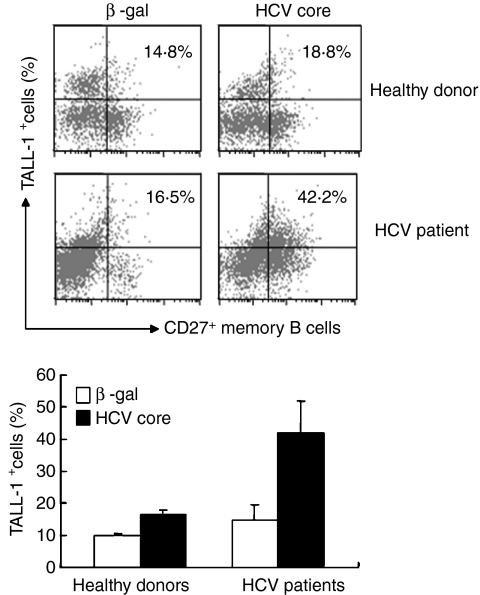
It has been well recognized that chronic HCV infection is associated with polyclonal B-cell proliferation in both the periphery and lymph nodes/organs, characterized by a low threshold memory B-cell (CD20+ CD27+) expansion upon antigenic stimulation. Whether TALL-1, a known factor for B-cell survival, is associated with viral antigen-driven memory B-cell responses during HCV infection is unknown. We therefore further examined TALL-1 expression on CD20+ CD27+ memory B lymphocytes from HCV-infected patients compared with those from healthy donors in response to HCV core antigen by triple staining. As shown in Fig. 4, PBMC gated on lymphocytes were further gated on CD20+ B lymphocytes based on isotype control, and then TALL-1 expression on CD27+ memory B cells was assayed. TALL-1 expression on memory B cells from healthy donors was up-regulated by HCV core protein (from 10·1% to 16·8%); whereas its expression on memory B cells from HCV patients was increased by a very significant margin (from 15·1% to 41·7%) upon exposure to HCV core protein. This is equivalent to what was observed upon core antigen stimulation of the entire B-cell population (as shown in Fig. 3), suggesting that the viral antigen-mediated increases in TALL-1 expression on B lymphocytes from HCV-infected individuals is primarily a function of a vigorous memory B-cell recall response to viral antigenic stimulation.
Figure 4.
Hepatitis C virus (HCV) core protein up-regulates tumour necrosis factor- and Apo-L-related leucocyte-expressed ligand-1 (TALL-1) expression on memory B lymphocytes. Peripheral blood mononuclear cells (PBMC) isolated from HCV-infected patients and healthy donors were stimulated with either HCV core or control protein, β-gal, for 24 hr; and TALL-1 expression on the surface of memory B lymphocytes was measured by flow cytometry following phycoerythrin-conjugated anti-TALL-1, allophycocyanin-conjugated anti-CD27 and fluorescein isothiocyanate-conjugated anti-CD20 triple staining. PBMC were first gated on lymphocyte populations, and CD20+ B lymphocytes were further gated based on FITC isotype control; TALL-1 expression on the CD20+ CD27+ cells was then analysed. Above, a representative dot plot with percentage of TALL-1-positive cells on the memory B cells from an HCV patient versus a healthy donor is shown; below, the mean ± SD percentage of TALL-1 expression on memory B lymphocytes from three HCV patients versus three healthy donors is shown; **P < 0·01.
SOCS-1 and programmed death ligand-1 expression levels are altered in B cells from individuals with HCV infection
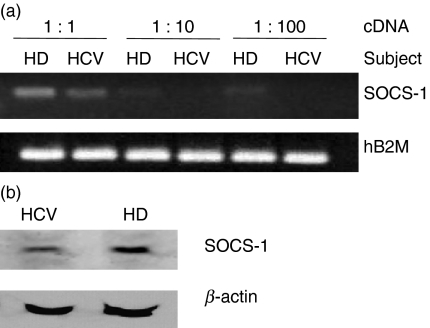
Because we have previously found down-regulation of SOCS-1 and over-activation of B cells exposed to HCV core in vitro, we next, investigated whether SOCS-1 suppression is associated with up-regulation of TALL-1 in B cells during HCV infection. We measured SOCS-1 messenger RNA (mRNA) in purified B cells from HCV-infected patients and healthy donors by semi-quantitative RT-PCR. As shown in Fig. 5(a), B cells isolated from a pool of chronically HCV-infected individuals exhibit significantly less SOCS-1 expression compared with those from healthy subjects. These data were reproducible in two independent experiments using B cells isolated from six HCV patients and three healthy donors to achieve sufficient RNA. In addition, SOCS-1 protein expression was similarly suppressed in the setting of HCV infection when assayed by immunoblotting (Fig. 5b). These data confirm our in vitro studies suggesting that HCV alters SOCS-1 mRNA and protein expression, and provide a novel model for the observed B-cell hyperproliferation observed in vitro in response to HCV core antigen and observed in vivo in chronically infected subjects.
Figure 5.
Suppressor of cytokine signalling-1 (SOCS-1) is suppressed in B lymphocytes from hepatitis C virus (HCV) patients versus healthy donors. (a) SOCS-1 RNA expression is suppressed in B cells from individuals with HCV infection. B cells were purified from six chronically HCV-infected patients and three healthy donors using magnetic beads, and total RNAs were isolated for semi-quantitative reverse transcription–polymerase chain reaction (RT-PCR) amplifying the SOCS-1 gene, as described in the Materials and methods. The hB2M housekeeping gene was amplified from the same amount of complementary DNA (cDNA) as a control. The amplified gene products from 1 : 1, 1 : 10, and 1 : 100 diluted cDNA used for PCR are shown. HD, healthy donors; HCV, HCV patients. The results were reproducible in two independent experiments using RNAs isolated from six chronically HCV-infected patients and three healthy donors. (b) SOCS-1 protein expression is suppressed in B cells during HCV infection. Magnetic antibody cell sorting-purified B cells were isolated from either a healthy donor (HD) or an HCV-infected individual (HCV) and subjected to immunoblotting. β-Actin serves as a control. Immunoblot results were reproducible in at least two independent experiments.
We and others have previously shown that, like SOCS-1, the programmed death-1 (PD-1)/PD ligand-1 (PDL-1) pathway is involved in T-cell exhaustion during HCV infection; a role in B-cell dysfunction, however, is unknown.21,22 To determine whether this negative regulatory pathway participates in dysregulating B-cell activation, we examined the expression levels of PD-1 and PDL-1 on the surface of B cells isolated from both HCV-infected patients and healthy subjects. The PD-1 expression levels on CD20+ B cells in both groups of subjects were low and not significantly different (11·60 ± 2·49% versus 9·31 ± 2·44%, P > 0·05), whereas PDL-1 expression levels were significantly higher on the surface of B cells from HCV patients compared with those from healthy subjects (42·80 ± 24·00% versus 20·60 ± 10·32%, P < 0·05). While it appears that B-cell PD-1/PDL-1 signalling may not be contributing to the observed B-cell abnormalities, it is certainly possible that the higher level of PDL-1 expression on B cells contributes to the suppression of T cells because these have been shown to express significantly higher levels of PD-1 in the setting of chronic HCV infection.
Discussion
In addition to chronic hepatic disease, HCV infection is strikingly associated with multiple extrahepatic manifestations that are related to B-cell proliferative disorders.3 Anti-HCV antibodies, monoclonal IgM with anti-IgG rheumatoid factor activity, and autoimmune anti-nuclear and anti-cardiolipin antibodies are observed in liver tissue, bone marrow and peripheral blood in about half of HCV-infected individuals.23–25 Although some data suggest that TALL-1 secretion or the anti-apoptotic Bcl-2 protein may be involved in non-specific B-cell proliferation to antigen-driven stimulation, the mechanisms by which B-cell activation occurs during HCV infection remain poorly defined. In this translational study, we have found abnormal B-cell activation during HCV infection that is associated with dysregulation of key proteins that control cellular proliferation, with over-expression of TALL-1 and suppression of SOCS-1. This involved regulation of lymphocyte activation markers, boosting of memory lymphocyte responses, and alterations in adapter pathways is fundamental to lymphocyte functions.
Mixed cryoglobulinaemia is the major extrahepatic manifestation of HCV infection and is found in 30–50% of HCV patients, who also have a high prevalence of B-NHL;26–28 over 90% of individuals with MC are found to have HCV infection.29,30 Investigators have suggested that persistent HCV antigen may stimulate crucial cell signalling pathways, including Toll-like receptor pathways, leading to T-dependent B-cell expansion and predisposing the patient to autoimmune and lymphoproliferative disorders such as MC.31,32 In addition, B-cell expansion followed by the induction of autoimmunity may initiate the production of pro-inflammatory cytokines such as tumour necrosis factor and contribute to liver inflammatory necrosis and fibrosis. Consequently, successful treatment of HCV infection with interferon/ribavirin, or elimination of B cells with novel anti-CD20 chimeric antibodies such as rituximab, may lead to the resolution of MC, regression of IgM overproduction, and clearance of B-cell clones.33–36
The mechanisms underlying B-cell activation during chronic HCV infection are not completely known. An antigen-driven process might be involved, and our data clearly support a role for viral antigen and innate immune stimulation in abnormal B-cell activation. Intriguingly, HCV core is secreted from infected cells and circulates in the bloodstream of infected individuals at levels consistent with those used in our experiments.37,38 In addition, the amount of free core protein or core protein expressed on the surface of infected cells is greater in the micro-environment of the liver, where virus replication occurs quite vigorously in early infection. Hence, HCV core protein appears to be present in the setting of clinical infection and could play a role in antigen-mediated B-cell expansion. We have previously demonstrated that HCV core binds gC1qR, a receptor that is expressed on CD20+ B cells. This interaction induces increased cell surface expression of IgM, IgG and key B-cell activation markers including CD69, CD40L, B7-2 and CCR5. In the current study, we have extended these findings in vivo and found similar B-cell activation profiles in chronically HCV-infected individuals.
Importantly, we found that TALL-1 was up-regulated on B lymphocytes by viral antigen and non-specific immune stimulation during HCV infection, and that the up-regulation of TALL-1 on B cells from HCV-infected patients was primarily comprised of a vigorous memory B-cell response to viral antigenic stimulation. The biological meaning of this over-expression deserves careful interpretation and additional investigation. It may represent a pathogenic epiphenomenon of activated B cells that secrete TALL-1, or an intrinsic factor that stimulates aberrant B-cell activation, favouring both autoimmunity and lymphoproliferation. Alternatively, an endogenous interferon response to viral antigen may increase TALL-1 expression by myeloid cells,6 initiating the cell stimulation cascade. Nevertheless, over-expression of TALL-1 in chronic HCV infection may attenuate the sensitivity of B cells to spontaneous apoptosis, and in doing so may favour their survival. Indeed, the TALL-1 pathway has been recently demonstrated to alter B-cell homeostasis in the course of Epstein–Barr virus and HIV infections,39,40 suggesting that a similar mechanism might be employed in different chronic viral infections. In light of this, TALL-1 would represent a link between infection, lymphoproliferation and autoimmunity. It is also feasible that the loss of effective T-cell responses observed in HCV infection may contribute to poor control of viral replication and in turn drive antigen-mediated B-cell expansion through up-regulation of TALL-1. We have shown that HCV core can alter multiple T-cell functions via gC1qR.41–44
It is interesting to note that our limited data suggest that TALL-1 levels on B cells from spontaneously resolved HCV patients are comparable to those of healthy subjects. It has been reported that treatment of HCV-infected patients with interferon may transiently increase the expression of TALL-1, with normalization when antiviral therapy is suspended or when HCV viraemia is controlled.4 While interferon therapy can be effective in HCV-related MC,33–35 a proportion of MC patients may have persistent active disease despite clearance of viral RNA; MC sequelae such as neuropathy, nephritis and skin ulcers may even worsen on therapy, and the onset of MC may occur after interferon therapy is initiated.45–47 The possibility that TALL-1 up-regulation by interferon-based therapy might in part contribute to these effects in predisposed individuals should be considered in this scenario and further studies are warranted.
A relationship between abnormal B-cell activation and TALL-1 over-expression appears evident, and as such the cellular and molecular basis of such TALL-1 up-regulation becomes crucial. The SOCS proteins are considered immunomodulators and represent a powerful mechanism for regulating Janus kinase (JAK)/STAT pathways and ultimately cytokine production and cell proliferation.48 The role of this negative immunomodulator in B cells during HCV infection, however, has not been well-studied. Our results suggest that B-cell activation in HCV infection is associated with suppression of SOCS-1 expression. This finding represents the first evidence of an association between over-expression of TALL-1 and suppression of SOCS-1 during abnormal B-cell activation during HCV infection. A potential model would include chronic antigen exposure to circulating HCV core inducing dysregulation of T-cell responses and both promoting an anergic state via up-regulation of SOCS proteins and inducing down-regulation of SOCS-1 expression and up-regulation of TALL-1 in B cells.20 This might contribute to the B-cell clonal expansion that is currently thought to be the impetus behind the development of MC and NHL (Fig. 6). Other potential viral antigens, including E1/E2 interacting with CD81, could potentially also be involved in this process but data in this area are as yet limited.
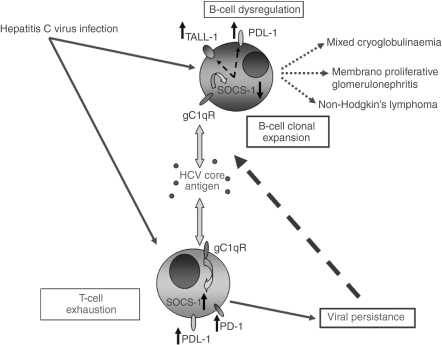
Figure 6.
Model for B-cell over-activation in the setting of chronic hepatitis C virus (HCV) infection. The HCV viral gene products, including most notably HCV core antigen signalling via gC1qR, lead to down-regulation of suppressor of cytokine signalling-1 (SOCS-1) expression and up-regulation of TALL-1 expression in B cells, favouring the cellular proliferation/clonal expansion that is the driving force underlying HCV-related extrahepatic diseases. T-cell exhaustion mediated by programmed death-1/programmed death ligand-1 leads to poor viral clearance and persistent antigenaemia.
Interestingly, several investigators have found either inactivation or suppression of SOCS-1 in the setting of uncontrolled growth and malignancies, including notably multiple myeloma but also breast, pancreatic and ovarian cancers.49–51 How this regulatory molecule might be involved in the development of MC and NHL in chronically HCV-infected patients is unknown but is currently under investigation. It is feasible that abnormal B-cell activation and over-expression of TALL-1 are linked through the SOCS-1 intracellular signalling pathway. This hypothesis reinforces the critical role for additional research to elucidate the intrinsic mechanisms for TALL-1 up-regulation and SOCS-1 suppression during chronic HCV infection. It also creates exciting possibilities for decoying TALL-1 or boosting B-cell SOCS-1 signalling in future therapeutic algorithms for HCV-associated autoimmune disease.
Acknowledgments
We thank our colleagues for constructive criticism and comments. This work was supported in part by ETSU major research grants to Z.Q.Y. (RDC #07-002M) and to J.P.M. (RDC #06-002M) and by an NIH NIAID grant to J.P.M./Z.Q.Y. (R15AI072750).
Disclosures
No authors have conflicting financial interests to declare.
References
- 1.Sansonno D, Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect Dis. 2005;5:227–36. doi: 10.1016/S1473-3099(05)70053-0. [DOI] [PubMed] [Google Scholar]
- 2.Racanelli V, Sansonno D, Piccoli C, D’Amore FP, Tucci FA, Dammacco F. Molecular characterization of B cell clonal expansions in the liver of chronically hepatitis C virus-infected patients. J Immunol. 2001;167:21–9. doi: 10.4049/jimmunol.167.1.21. [DOI] [PubMed] [Google Scholar]
- 3.King E, Trabue C, Yin D, Yao ZQ, Moorman JP, Hepatitis C. The complications of immune dysfunction. Expert Rev Clin Immunol. 2007;3:145–57. doi: 10.1586/1744666X.3.2.145. [DOI] [PubMed] [Google Scholar]
- 4.Fabris M, Quartuccio S, Sacco S, et al. B-lymphocyte stimulator (BLyS) up-regulation in mixed cryoglobulinemia syndrome and hepatitis C virus infection. Rheumatology. 2007;46:37–43. doi: 10.1093/rheumatology/kel174. [DOI] [PubMed] [Google Scholar]
- 5.Moore PA, Belvedere O, Orr A, et al. BLys: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 6.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardelli B, Belvedere O, Roschke V, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–9. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Mariette X, Roux S, Zhang J, et al. The level of BLys (BAFF) correlates with the titer of autoantibodies in human Sjögren’s syndrome. Ann Rheum Dis. 2003;62:168–71. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 2006;54:192–201. doi: 10.1002/art.21526. [DOI] [PubMed] [Google Scholar]
- 12.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100:2973–9. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 13.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–41. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 14.Toubi E, Gordon S, Kessel A, Rosner I, Rozenbaum M, Shoenfeld Y, Zuckerman E. Elevated serum B-lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27:134–9. doi: 10.1016/j.jaut.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Sene D, Limal P, Ghillani-Dalbin P, Saadoun D, Piette JC, Cacoub P. Hepatitis C virus-associated B-cell proliferation – the role of serum B lymphocyte stimulator (BLyS/BAFF) Rheumatology. 2007;46:65–9. doi: 10.1093/rheumatology/kel177. [DOI] [PubMed] [Google Scholar]
- 16.Khare SD, Sarosi I, Xia XZ, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA. 2000;97:3370–5. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes SLI, Leon BF, Rozas VMF, Gonzalez JP, Naves PR. BAFF: a regulatory cytokine of B lymphocytes involved in autoimmunity and lymphoid cancer. Rev Med Chil. 2006;134:1175–84. doi: 10.4067/s0034-98872006000900014. [DOI] [PubMed] [Google Scholar]
- 20.Yao ZQ, Prayther D, Trabue C, Dong ZP, Moorman J. Differential regulation of SOCS-1 signaling in B and T lymphocytes by HCV core protein. Immunology. 2008;125:197–207. doi: 10.1111/j.1365-2567.2008.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao ZQ, King E, Prayther D, Yin D, Moorman J. T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral Immunol. 2007;20:276–87. doi: 10.1089/vim.2006.0096. [DOI] [PubMed] [Google Scholar]
- 22.Golden-Mason L, Klarquist J, Wabed AS, Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol. 2008;180:3637–41. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 23.Vallat L, Benhamou Y, Gutierrez M, et al. Clonal B cell populations in the blood and liver of patients with chronic hepatitis C virus infection. Arthritis Rheum. 2004;50:3668–78. doi: 10.1002/art.20594. [DOI] [PubMed] [Google Scholar]
- 24.Clifford BD, Donahue D, Smith L, et al. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology. 1995;21:613–9. [PubMed] [Google Scholar]
- 25.Zachou K, Liaskos C, Christodoulou DK, et al. Anti-cardiolipin antibodies in patients with chronic viral hepatitis are independent of beta2-glycoprotein I cofactor or features of antiphospholipid syndrome. Eur J Clin Invest. 2003;33:161–8. doi: 10.1046/j.1365-2362.2003.01110.x. [DOI] [PubMed] [Google Scholar]
- 26.Germanidis G, haioun C, Dhumeaux D, Reyes F, Pawlotsky JM. Hepatitis C virus infection, mixed cryoglobulinemia, and B-cell non-Hodgkin’s lymphoma. Hepatology (Baltimore, MD) 1999;30:822–3. doi: 10.1002/hep.510300323. [DOI] [PubMed] [Google Scholar]
- 27.Kashyap A, Nademanee A, Molina A. Hepatitis C and B-cell lymphoma. Ann Intern Med. 1998;128:695. doi: 10.7326/0003-4819-128-8-199804150-00022. [DOI] [PubMed] [Google Scholar]
- 28.Ohsawa M, Shingu N, Miwa H, et al. Risk of non-Hodgkin’s lymphoma in patients with hepatitis C virus infection. Int J Cancer. 1999;80:237–9. doi: 10.1002/(sici)1097-0215(19990118)80:2<237::aid-ijc12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Agnello V. Hepatitis C virus infection and type II cryoglobulinemia: an immunological perspective. Hepatology (Baltimore, MD) 1997;26:1375–9. doi: 10.1053/jhep.1997.v26.ajhep0261375. [DOI] [PubMed] [Google Scholar]
- 30.Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606–10. doi: 10.1002/art.1780341221. [DOI] [PubMed] [Google Scholar]
- 31.Franzin F, Efremov DG, Pozzato G, Tulissi P, Batista F, Burrone OR. Clonal B-cell expansions in peripheral blood of HCV-infected patients. Br J Haematol. 1995;90:548–52. doi: 10.1111/j.1365-2141.1995.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 32.Vallat L, Benhamou Y, Gutierrez M, et al. Clonal B cell populations in the blood and liver of patients with chronic hepatitis C virus infection. Arthritis Rheum. 2004;50:3668–78. doi: 10.1002/art.20594. [DOI] [PubMed] [Google Scholar]
- 33.D’Amico E, Chincoli C, Cacciatore P, et al. Effects of combined antiviral therapy on asymptomatic mixed cryoglobulinemia in naive patients with chronic hepatitis C virus infection: a preliminary study. Dig Dis Sci. 2005;50:2344–7. doi: 10.1007/s10620-005-3059-x. [DOI] [PubMed] [Google Scholar]
- 34.Mazzaro C, Zorat F, Caizzi M, et al. Treatment with peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study. J Hepatol. 2005;42:632–8. doi: 10.1016/j.jhep.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 35.Mazzaro C, Zorat F, Comar C, et al. Interferon plus ribavirin in patients with hepatitis C virus positive mixed cryoglobulinemia resistant to interferon. J Rheumatol. 2003;30:1775–81. [PubMed] [Google Scholar]
- 36.Zaja F, De Vita S, Russo D, Michelutti A, Fanin R, Ferraccioli G, Baccarani M. Rituximab for the treatment of type II mixed cryoglobulinemia. Arthritis Rheum. 2002;46:2252–4. doi: 10.1002/art.10345. [DOI] [PubMed] [Google Scholar]
- 37.Maillard P, Krawczynski K, Nitkiewicz J, et al. Nonenveloped nucleocapsids of hepatitis C virus in the serum of infected patients. J Virol. 2001;75:8240–50. doi: 10.1128/JVI.75.17.8240-8250.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masalova OV, Atanadze SN, Samokhvalov EI, et al. Detection of hepatitis C virus core protein circulating within different virus particle populations. J Med Virol. 1998;55:1–6. doi: 10.1002/(sici)1096-9071(199805)55:1<1::aid-jmv1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003;171:5215–24. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, Lederman MM. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS. 2003;17:1983–5. doi: 10.1097/00002030-200309050-00018. [DOI] [PubMed] [Google Scholar]
- 41.Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 2000;106:1239–49. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao ZQ, Nguyen DT, Hiotellis AI, Hahn YS. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J Immunol. 2001;167:5264–72. doi: 10.4049/jimmunol.167.9.5264. [DOI] [PubMed] [Google Scholar]
- 43.Yao ZQ, Eisen-Vandervelde A, Ray S, Hahn YS. HCV core/gC1qR interaction arrests T cell cycle progression through stabilization of the cell cycle inhibitor p27Kip1. Virology. 2003;314:271–82. doi: 10.1016/s0042-6822(03)00419-7. [DOI] [PubMed] [Google Scholar]
- 44.Yao ZQ, Eisen-Vandervelde A, Waggoner SN, Cale EM, Hahn YS. Direct binding of hepatitis C virus core to gC1qR on CD4+ and CD8+ T cells leads to impaired activation of Lck and Akt. J Virol. 2004;78:6409–19. doi: 10.1128/JVI.78.12.6409-6419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine JW, Gota C, Fessler B, Valabrese L, Cooper SM. Persistent cryoglobulinemic vasculitis following successful treatment of hepatitis C virus. J Rheumatol. 2005;32:1164–7. [PubMed] [Google Scholar]
- 46.La Civita L, Zignego AL, Lombardini F, et al. Exacerbation of peripheral neuropathy during alpha-interferon therapy in a patient with mixed cryoglobulinemia and hepatitis B virus infection. J Rheumatol. 1996;23:1641–3. [PubMed] [Google Scholar]
- 47.Beuthien W, Mellinghoff HU, Kempis J. Vasculitic complications of interferon-alpha treatment for chronic hepatitis C virus infection: case report and review of the literature. Clin Rheumatol. 2005;24:507–15. doi: 10.1007/s10067-005-1093-x. [DOI] [PubMed] [Google Scholar]
- 48.Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev. 2002;2:410–6. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto M, Nishimoto N, Davydova J, Kishimoto T, Curiel DT. Suppressor of cytokine signaling-1 expression by infectivity-enhanced adenoviral vector inhibits IL-6-dependent proliferation of multiple myeloma cells. Cancer Gene Ther. 2006;13:194–202. doi: 10.1038/sj.cgt.7700873. [DOI] [PubMed] [Google Scholar]
- 50.Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, Campbell IG, Visvader JE. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene. 2004;23:7726–33. doi: 10.1038/sj.onc.1207787. [DOI] [PubMed] [Google Scholar]
- 51.Komazaki T, Nagai H, Emi M, et al. Hypermethylation-associated inactivation of the SOCS-1 gene, a JAK/STAT inhibitor, in human pancreatic cancers. Jpn J Clin Oncol. 2004;34:191–4. doi: 10.1093/jjco/hyh035. [DOI] [PubMed] [Google Scholar]