Abstract
Anti-proteinase 3 antibodies are implicated in the pathogenesis of small vessel vasculitis. These are primarily immunoglobulin G (IgG), with different subclasses predominating at different stages of disease. However, little is known of their respective roles in pathogenesis. We have previously shown that patient IgG4 was able to induce superoxide release from human neutrophils. To circumvent difficulties in separating the subclasses and additional differences in polyclonal patient antibodies we have generated monoclonal mouse/human IgG1 and IgG4 anti-proteinase 3 antibodies. Using these antibodies we have compared effects of IgG1 and IgG4 on human neutrophils in terms of superoxide release, cytokine production, degranulation and adhesion. Additionally we have investigated the interaction of the subclasses with Fc receptors expressed by the neutrophil. Chimeric antibodies were generated using human constant regions of each subclass and a variable region taken from a monoclonal antibody directed against proteinase 3. Superoxide release from neutrophils was measured by the reduction of ferricytochrome C, degranulation by the conversion of a synthetic colour substrate, cytokine release by interleukin-8 enzyme-linked immunosorbent assay, and adhesion by a flow-based adhesion assay. Fc receptor binding was assessed using blocking antibodies. The IgG4 anti-proteinase 3 was able to induce a dose-dependent release of superoxide, degranulation and adhesion. The antibody was not able to stimulate the secretion of interleukin-8. Fc receptors were essential for neutrophil stimulation and the constitutive Fc receptors were necessary for different stimulatory pathways. The IgG4 anti-proteinase 3 antibodies are able to stimulate neutrophils to undergo a pro-inflammatory response and may play a role in the pathogenesis of small vessel vasculitis.
Keywords: anti-neutrophil cytoplasmic autoantibody, immunoglobulin, neutrophils, subclass, vasculitis
Introduction
The sensitivity of current systems allows for the detection of low-affinity autoantibodies of the immunoglobulin M (IgM) class to many ‘self’ antigens in normal healthy individuals. However, the provocation of an antigen-specific secondary immune response, with switching to the production of high-affinity IgG antibodies, can result in pathology. In humans four subclasses of IgG are identified that differ in the primary sequence of their heavy chains and each mediates a unique profile of IgG–Fc effector mechanisms. The quantitative distribution of IgG autoantibodies within the IgG subclasses can impact on mechanisms activated and on consequent pathology. Studies of the distribution of anti-neutrophil cytoplasmic autoantibody (ANCA)-mediated small vessel vasculitis (such as Wegener’s granulomatosis) have consistently reported the presence of antibodies of the IgG1 and IgG3 subclasses and it has been suggested that IgG3 titres may correlate with clinical disease activity, possibly because of their potential enhanced capacity to activate neutrophils (reviewed in ref. 1). However, raised IgG1 and IgG4 levels have been observed in patients with ANCA-positive vasculitis and this is not the result of a general increase in titres of IgG4 as a whole.2
To further investigate the contribution of each IgG subclass of anti-PR3 antibody to neutrophil activation, we have purified subclass populations of antibody from the sera of patients with Wegener’s granulomatosis and also generated a panel of monoclonal chimeric antibodies of each IgG subclass sharing identical anti-Proteinase 3 (PR-3) epitope specificity.3 The ability of chimeric IgG1 and IgG3 anti-PR3 antibodies to activate human neutrophils, considered to be integral to their pathogenic potential, has been reported.1,4,5 Both IgG1 and IgG3 PR3-ANCA were shown to elicit similar neutrophil responses for adhesion, superoxide release, degranulation and interleukin-8 (IL-8) production, although quantitatively, the IgG1 subclass induced significantly more degranulation, while the IgG3 subclass generated higher levels of IL-8 production. We suggested that the more potent IL-8 response by IgG3 PR3-ANCA might encourage further neutrophil recruitment and amplify injury.
The ‘matching’ IgG4 PR3-ANCA chimeric antibody has now been produced and characterized. It allows for comparison with PR3-ANCA-positive polyclonal affinity purified human IgG4 antibodies that have previously been shown to activate neutrophils for superoxide production.6 Unexpectedly, stimulatory activity was found to be independent of neutrophil donor or neutrophil expression of FcγRI, the receptor thought to mediate the effects of the IgG4 subclass, suggesting that IgG4 was capable of activating neutrophils via constitutively expressed FcγRIIa, FcγRIIIb or by coligation of other, unidentified, cell surface molecules. If IgG4 PR3-ANCA, in common with IgG1 and IgG3 PR3-ANCA, can ligate FcγRIIa or FcγRIIIb on neutrophils, then other properties such as antibody fucosylation and/or galactosylation and sialylation, Fc receptor glycosylation status or other polymorphisms, antibody allotype, or epitope target might determine the biological outcome. Development of the IgG4 PR3-ANCA chimeric antibody has allowed us to confirm that this IgG subclass can activate neutrophils by engaging with constitutive FcγR. However, in contrast to the IgG1 and IgG3 PR3-ANCA chimeric antibodies, IgG4 PR3-ANCA was unable to stimulate neutrophil IL-8 release, although superoxide release, degranulation and adhesion were supported. The differential effects on functional responses suggest that there are differences in the engagement of signal transduction pathways by the IgG subclasses of ANCA.
Materials and methods
Construction of chimeric IgG4 PR3-ANCA
For all chimeric IgG subclass antibodies to PR3-ANCA, RNA to the variable region of a murine antibody raised against proteinase 3 (4A5; Wieslabs, Lund, Sweden) was isolated and complementary DNA was synthesized. Chimeric antibodies were produced and characterized as previously described, with the following exceptions for the IgG4.1 The variable region of gamma 4 was subcloned into a vector containing the appropriate constant region of the IgG4 chain (AERES Biomedical, Mill Hill, London, UK) and transfected into CHOdhfr− cells. The IgG4 was purified from culture supernatant using Staphylococcal protein A (SpA) Sepharose. The IgG4 sequence was matched to the kabat database (http://www.kabatdatabase.com). For the IgG1 the variable region was subcloned into a vector containing the constant region of human IgG1 chain (AERES Biomedical) and transfected and purified as above.
The IgG4 chimeric antibody was deglycosylated by exposure to PNGase F (72 hr at 37°; 1 unit/mg IgG) (Sigma-Aldrich, Poole, UK) in deglycosylation buffer [40 mm KH2PO4, 10 mm ethylenediamine tetraacetic acid (EDTA) pH7·4]. F(ab′)2 fragments were generated by overnight digestion with 2% pepsin in 0·2 m sodium acetate buffer, pH 4·0.
Chimeric mouse/human IgG4 antibody with specificity for the 4-hydroxyl-3-nitrophenylacetyl (NP) hapten was used as control.7
Verification of IgG production by SDS–PAGE and ELISA
SpA purified IgG4 was subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), under non-reducing and reducing (10 mm dithiothreitol) conditions on a 4–12% Bis–Tris gel. Gels were stained with Coomassie blue.
Cell culture supernatant was assessed for antigen-specific IgG by enzyme-linked immunosorbent assay (ELISA) using PR3-coated plates (Binding Site, Birmingham, UK) and IgG4 PR3-ANCA was detected using goat anti-human IgG4 horseradish peroxidase (HRP; 0·5 mg/ml).
Assessment of specificity of antigen recognition
To confirm the recognition of native antigen, whole cell lysates of neutrophils and recombinant PR3 (160 and 320 ng/ml) were subjected to electrophoresis on a 12% gel and transferred to nitrocellulose membrane.1 Blots were blocked then incubated with purified IgG4 PR3-ANCA 1 μg/ml overnight at 4°. Bands were visualized using sheep anti-human IgG HRP (10 μg/ml).
Indirect immunofluorescence was performed to confirm binding to PR3 expressed in human neutrophils. Ethanol-fixed cytospins of neutrophils (Binding Site) were exposed to purified IgG (1·5 μg/ml) and bound antibody was detected using fluorescein isothiocyanate-conjugated mouse anti-human IgG.
Assessment of functional activation
Neutrophils taken from healthy volunteers with ethical permission and informed consent were used for functional assays. Neutrophils were isolated using a discontinuous Percoll gradient as described previously.8
For all of the following functional assays 1 μmN-formyl-methionyl-leucyl-phenylalanine was used as a positive control for neutrophil stimulation. The ability of chimeric IgG4 PR3-ANCA to elicit superoxide production was measured by the superoxide dismutase inhibitable reduction of ferricytochrome C as described elsewhere.6 Neutrophils at 2 × 106/ml were primed with 2 ng/ml tumour necrosis factor-α and 5 mg/ml cytochalasin B for 15 min at 37°, stimulated with varying concentrations of chimeric IgG4 PR3-ANCA or its F(ab′)2 fragment, 10 μg/ml IgG1 PR3-ANCA, control anti-NP (IgG4) or deglycosylated IgG4 PR3-ANCA and superoxide release was measured over 120 min. The concentration of the IgG4 F(ab′)2 antibodies was adjusted to be in excess of the highest concentration of the whole antibody by 2·5 or 5 times (15 and 30 μg/ml, respectively).
Neutrophil degranulation was assessed by the release of serine proteases, which are contained in the primary granules and are the last granules to be exocytosed, therefore requiring greater stimulus. Neutrophils at 2·5 × 106/ml were primed as above and incubated with varying concentrations of IgG4 PR3-ANCA, 25 μg/ml IgG1 PR3-ANCA or control anti-NP IgG4, for 15 min. Supernatants, containing any released proteases, were removed and enzymatic activity was assessed by incubating them for 22 hr at 30° with 2 mmN-methoxysuccinyl-Ala-Ala-Pro-Val-P-nitroanilide (Sigma-Aldrich). Conversion of this substrate for PR3 and elastase gives rise to a colour change which can be measured at an optical density of 405 nm.
Cytokine production by neutrophils was determined using the release of IL-8. Neutrophils were primed as above and incubated with varying concentrations of chimeric IgG4 PR3-ANCA, 25 μg/ml IgG1 PR3-ANCA or control anti-NP IgG4, for 6 hr. Supernatants were removed and analysed by IL-8 ELISA (BD Biosciences, Oxford, UK).
Blocking studies
Neutrophils were incubated with antibodies to FcγRIIa (IV.3) and/or FcγRIIIb (3G8) (5 μg/ml; Cambridge Bioscience, Cambridge, UK) or isotype control for 5 min at 37°. The cells were subsequently primed and treated as above.
Human umbilical vein endothelial cells (HUVEC)
Umbilical cords were collected with ethical permission and informed consent from Birmingham Women’s Health Care NHS Trust. Endothelial cells were isolated with collagenase type I (Sigma-Aldrich) as previously described and cultured in M199 (Invitrogen, Paisley, UK), with 20% heat inactivated fetal calf serum (Sigma-Aldrich), 1 mg/ml hydrocortisone (Sigma-Aldrich), 10 mg/ml epidermal growth factor (Sigma-Aldrich), 100 U/l penicillin and streptomycin (Sigma-Aldrich) and 2·5 mg/ml amphotericin (Invitrogen).9
Preparation of microslides
Aminopropyltriethoxy-silane-treated microslides were coated with P-selectin (R&D Systems, Abingdon, UK), then blocked with 1% albumin (Sigma-Aldrich). Alternatively, HUVEC were grown to confluence, detached with 0·2% trypsin/EDTA (Sigma-Aldrich) and seeded into microslides. Medium was exchanged through the slides every hour and cells was cultured for 24 hr to allow formation of a monolayer. Confluent HUVEC microslides were treated with 2 IU tumour necrosis factor-α (NIBSC, Potters Bar, UK) for 4 hr before the flow assays.
Flow assay
Experiments were performed essentially as previously described.9 Microslides were perfused with neutrophils at a wall shear stress of 0·1 Pa (1 dyn/cm2), mimicking the microcirculation and ensuring that the initial interaction of neutrophils required selectin expression and not integrin-mediated adhesion. Video recordings were made in four fields in the direction of flow at 2-min intervals for up to 14 min. Recordings were analysed off-line using image pro-plus software (MediaCybernetics, Marlow, UK). Two populations of cells were counted on P-selectin: rolling, which maintained a round shape, and those exhibiting stable adhesion and shape change. On HUVEC, in addition to these populations, neutrophils could also migrate underneath the monolayer.
For experiments involving P-selectin, PR3-ANCA or anti-NP (control) at 60 μg/ml (previously shown to give an optimal response), was added to the flow assay after a 1 min perfusion of neutrophils and 1 min wash out.9 The IgG infusion was continued for 8 min. In HUVEC experiments antibody was added to neutrophils just before perfusion over endothelial cells for 4 min. The bolus of cells was then washed out and antibody was perfused for a further 8 min.
Statistical analysis
One-way analysis of variance (anova) was performed to assess how IgG4 PR3-ANCA-treated samples affected functional responses in comparison to IgG1 PR3-ANCA and controls. Dunnett’s multiple comparison post test was also applied in assays distinguishing Fc-receptor-mediated effects; PR3-ANCA chimeric responses were used as a comparative control. A Wilcoxon signed ranks test was used to test whether the PR3-ANCA chimeric antibodies induced significantly higher stable adhesion in the flow model than controls.
Results
Characterization of IgG4 PR3-ANCA
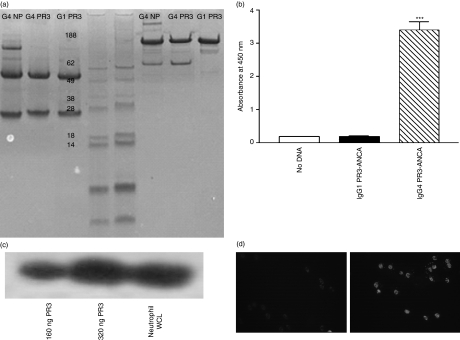
The IgG4 PR3-ANCA was shown to have the characteristics of an IgG and to recognize PR3 by several criteria. First, the antibody was the expected size for an IgG4 on SDS–PAGE (molecular weight 150 000 non-reduced and 50 000 to 25 000 reduced) although smaller bands were also observed under non-reduced conditions which are IgG4 half-molecules (Fig. 1a). The IgG4 PR3-ANCA could be detected in a PR3-specific ELISA using an antibody raised to both human IgG kappa region (data not shown) and human IgG4 (Fig. 1b). In addition, this assay demonstrated recognition of antigen coated onto the surface of the wells. Recognition of PR3 was also confirmed by Western blotting. Bands were seen at 29 000 (the molecular weight of PR3) in lanes containing recombinant PR3 or a lysate of human neutrophils (Fig. 1c). The IgG4 PR3-ANCA was shown to bind to human neutrophils by immunofluorescence (Fig. 1d). The IgG4 anti-NP was negative in the experiments depicted in Fig. 1(b–d).
Figure 1.
Determination of the structural and binding characteristics of recombinant immunoglobulin G4 PR3-anti-neutrophil cytoplasmic autoantibody (IgG4 PR3-ANCA). (a) Confirmation of the molecular weights of the recombinant IgGs by sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Lane 1, IgG4 anti-4-hydroxyl-3-nitrophenylacetyl (NP); lane 2, IgG4 PR3-ANCA; lane 3, IgG1 PR3-ANCA; lanes 4 and 5, molecular weight markers; lane 6, IgG4 anti-NP; lane 7, IgG4 PR3-ANCA; lane 7, IgG1 PR3-ANCA. Lanes 1–3 contain reduced samples and lanes 6–8 contain non-reduced samples. (b) Enzyme-linked immunosorbent assay for the detection of antibodies to PR3. Antibody binding to plates coated with PR3 was detected with a specific anti-IgG4 secondary antibody. Column 1, supernatant from cells which do not contain any plasmids for IgG PR3-ANCA; column 2, supernatant form cells transfected with IgG1 PR3-ANCA; column 3, supernatant from cells transfected with IgG4 PR3-ANCA. ***P = 0·0002 (n = 3). (c) Western blot to determine recognition of antigen by IgG4 PR3-ANCA. Lane 1, recombinant PR3 (160 ng); lane 2, recombinant PR3 (320 ng); lane 3, neutrophil lysate. (d) Indirect immunofluorescence of ethanol-fixed neutrophils using either IgG4 anti-NP (left panel) or IgG4 PR3-ANCA (right panel) as detection antibody.
IgG4 PR3-ANCA can induce a neutrophil respiratory burst or degranulation
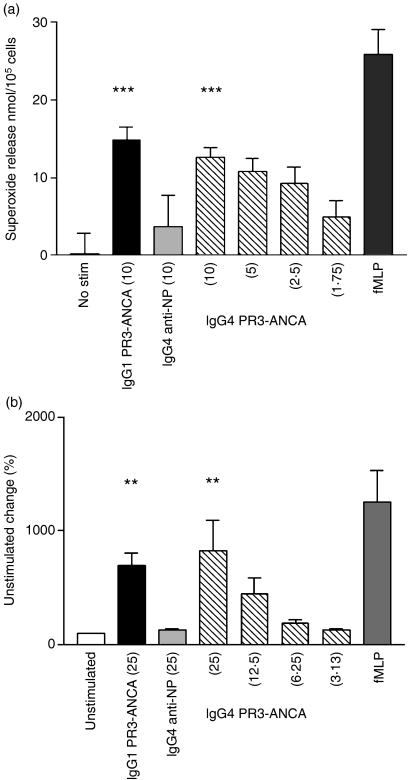
Patient-derived anti-PR3 ANCA, as well as IgG1 and IgG3 PR3-ANCA, are known to induce the release of superoxide from isolated human neutrophils.1Figure 2(a) illustrates that IgG4 can also induce this functional response. The effect of IgG4 PR3-ANCA was statistically significant at the highest concentration, the same magnitude as an equivalent amount of IgG1 PR3-ANCA and showed a dose-dependent decrease in response with decreasing concentrations.
Figure 2.
Determination of the functional characteristics of recombinant immunoglobulin G4 PR3-anti-neutrophil cytoplasmic autoantibody (IgG4 PR3-ANCA). (a) Superoxide production by neutrophils in the presence of recombinant IgG as measured by reduction of ferricytochrome C (n = 6). On the y-axis units of superoxide are expressed as nmol/105 cells. Antibody concentrations were 1·75–10 μg/ml; 1 μmN-formyl-methionyl-leucyl-phenylalanine (FMLP) was used as a positive control. One-way analysis of variance was performed comparing no stimulation, IgG1 PR3-ANCA, IgG4 anti-4-hydroxyl-3-nitrophenylacetyl (NP) and IgG4 PR3-ANCA at the same concentration. ***P < 0·0001. (b) Serine protease release by neutrophils in the presence of the recombinant IgG as measured by conversion of N-methoxysuccinyl-Ala-Ala-Pro-Val-P-nitroanilide (n = 4). Conversion of substrate was measured by changes in absorbance and expressed as a percentage of the unstimulated control. Antibody concentrations were 3·13–25 μg/ml; 1 μm FMLP was used as a positive control. One-way analysis of variance was performed comparing unstimulated, IgG1 PR3-ANCA, IgG4 anti-NP and IgG4 PR3-ANCA at the same concentrations. **P < 0·01.
Neutrophil degranulation has also been shown to be stimulated by patient-derived ANCA IgG, as well as IgG1 and IgG3 PR3-ANCA. We now demonstrate that this is also the case for IgG4 PR3-ANCA (Fig. 2b). Again the effect was statistically significant at the highest concentration, the same magnitude as an equivalent amount of IgG1 PR3-ANCA, and showed a dose-dependent decrease in response with decreasing concentrations. The IgG4 anti-NP did not stimulate any response above unstimulated levels in either of the above assays.
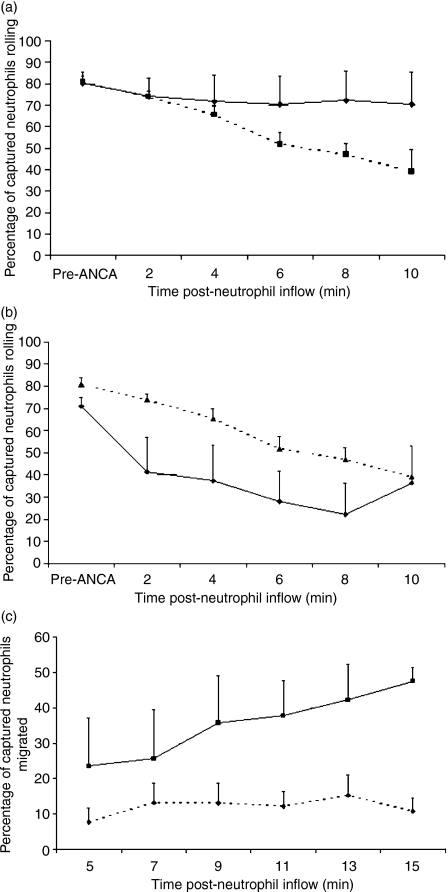
IgG4 PR3-ANCA induces conversion of rolling neutrophils to firm adhesion but is less effective than IgG1 PR3-ANCA
In the flow assay we have previously demonstrated that ANCA IgG can decrease the number of rolling neutrophils on P-selectin-coated slides, with enhanced conversion to stable adhesion.10 Both IgG1 and IgG4 PR3-ANCA can also generate this response, which is statistically significant when compared with the control anti-NP (Fig. 3a). However, fewer neutrophils were seen to be rolling in the presence of the IgG1 than with IgG4 PR3-ANCA (Fig. 3b).
Figure 3.
Determination of the effects of immunoglobulin G4 PR3-anti-neutrophil cytoplasmic autoantibody (IgG4 PR3-ANCA) on neutrophil adhesion under flow. (a) Comparison of neutrophils rolling on a P-selectin surface under flow with time of measurement post-treatment along the x-axis. Diamonds represent IgG4 anti-4-hydroxyl-3-nitrophenylacetyl (NP) and squares represent IgG4 PR3-ANCA. Analysis of variance comparing the two groups gave P < 0·05 (n = 6). (b) Comparison of neutrophils rolling on a P-selectin surface under flow with time of measurement post-treatment along the x-axis. Diamonds represent IgG1 PR3-ANCA and triangles represent IgG4 PR3-ANCA. Analysis of variance comparing the two groups gave P < 0·05 (n = 6). (c) Comparison of neutrophils migrated across human umbilical vein endothelial cells under flow with time of measurement post-treatment along the x-axis. Diamonds represent IgG4 anti-NP and squares IgG4 PR3-ANCA. Analysis of variance comparing the two groups gave P < 0·001 (n = 6).
On HUVEC, IgG4 PR3-ANCA resulted in substantially fewer rolling neutrophils than IgG4 anti-NP. This could be attributed to a significant increase in transmigration of neutrophils through the monolayer (Fig. 3c), although an increase in the number of stable adherent neutrophils was not observed (data not shown).
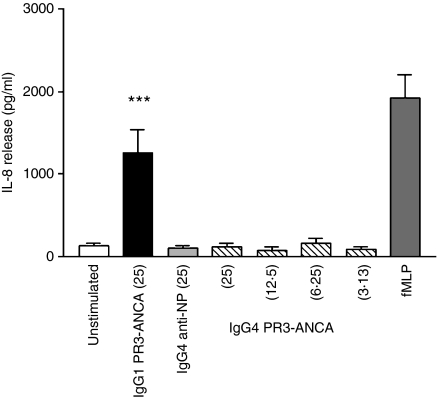
IgG4 PR3-ANCA does not induce IL-8 release
Patient ANCA IgG, IgG1 PR3-ANCA and IgG3 PR3-ANCA induce the release of IL-8.1 In contrast, IgG4 PR3-ANCA was unable to provoke this response even when used at a concentration equivalent to a stimulating dose of IgG1 PR3-ANCA (Fig. 4).
Figure 4.
Interleukin-8 release from neutrophils stimulated with recombinant immunoglobulin Gs (IgG) as measured by enzyme-linked immunosorbent assay. IgG concentrations were 3·13–25 μg/ml; 1 μmN-formyl-methionyl-leucyl-phenylalanine was used as a positive control. One-way analysis of variance was performed comparing unstimulated, immunoglobulin G1 PR3-anti-neutrophil cytoplasmic autoantibody (IgG1 PR3-ANCA), IgG4 anti-4-hydroxyl-3-nitrophenylacetyl (NP) and IgG4 PR3-ANCA at the same concentration. ***P < 0·0005 (n = 4).
Mechanism of engagement of IgG4 PR3-ANCA for functional responses
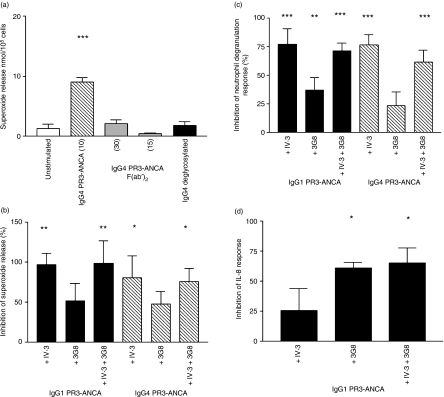
Data from our own studies, as well as from other investigators, have indicated that whole ANCA IgG is necessary to induce a functional response in neutrophils. This is believed to reflect cross-linking of cell-surface PR3 with Fcγ receptors, by concurrent binding of F(ab′)2 and Fc portions of the antibodies, respectively. To ascertain if this was also true for IgG4 PR3-ANCA, F(ab′)2 fragments were made and tested at up to five times the stimulating concentration of whole antibody in a superoxide assay. No increase above basal levels of superoxide was seen (Fig. 5a). Immunoglobulin G4 is not commonly reported to bind to constitutively expressed neutrophil FcγRIIa or FcγRIIIb receptors. Flow cytometric analysis showed that no FcγRI was present on the surface of the freshly isolated neutrophils (data not shown). We therefore investigated if FcγRIIa or FcγRIIIb were indeed involved in the stimulation of the neutrophils by IgG4 PR3-ANCA. For binding to FcγRIIa and FcγRIIIb, glycosylation of IgG is required, which occurs during the normal production of an antibody, and is present on our chimeric antibodies.1 Whole IgG4 PR3-ANCA was deglycosylated and added to neutrophils at a stimulatory concentration. No release of superoxide was observed, suggesting the involvement of Fcγ receptors. To further confirm these observations, blocking antibodies to both the FcγRIIa and FcγRIIIb were used in the assay. Neither blocking antibody alone or in combination stimulated the release of superoxide from neutrophils. Pre-incubation of neutrophils with the blocking antibody to FcγRIIa significantly inhibited the liberation of superoxide both by IgG1 and IgG4 PR3-ANCA (Fig. 5b). Blocking FcγRIIIb had no effect and blocking both together had no additive effect. In the degranulation assay blocking of FcγRIIa again significantly blocked both the IgG1 and IgG4 PR3-ANCA responses but this time antibodies to FcγRIIIb also blocked the IgG1 PR3-ANCA response (Fig. 5c).
Figure 5.
Determination of the role of FcγRs in the activation of neutrophils by immunoglobulin G PR3-anti-neutrophil cytoplasmic autoantibody (IgG PR3-ANCA). (a) Superoxide production from neutrophils in the presence of modified forms of IgG4 PR3-ANCA as measured by reduction of ferricytochrome C (n = 3). On the y-axis units of superoxide are expressed as nmol/105 cells. Antibody concentrations were 10 μg/ml for whole or deglycosylated IgG4 PR3-ANCA and 15 or 30 μg/ml for F(ab′)2 fragments. One-way analysis of variance was performed comparing no stimulation, IgG4 PR3-ANCA, deglycosylated IgG4 PR3-ANCA and IgG4 PR3-ANCA F(ab′)2. ***P < 0·0001. (b) Superoxide production by recombinant IgGs in the presence of blocking antibodies to constitutively expressed FcγRs (n = 5). Data are expressed on the y-axis as percentage inhibition of the unblocked response. Concentration of IgG1 or IgG4 PR3-ANCA was 10 μg/ml and blocking antibodies as described in the Materials and methods were added singly or in combination. FcγRIIa was blocked with IV.3 and FcγRIIIb by 3G8. One-way analysis of variance was performed comparing against stimulated. **P < 0·005 and *P < 0·05. (c) Degranulation by recombinant IgGs in the presence of blocking antibodies to constitutively expressed FcγRs (n = 5). Data are expressed on the y-axis as percentage inhibition of the unblocked response. Concentration of IgG1 or IgG4 PR3-ANCA was 25 μg/ml and blocking antibodies as described in the Materials and methods were added singly or in combination. FcγRIIa was blocked with IV.3 and FcγRIIIb by 3G8. One-way analysis of variance was performed comparing against stimulated. ***P < 0·0001 and **P < 0·001. (d) IL-8 release by IgG1 PR3-ANCA in the presence of blocking antibodies to constitutively expressed FcγRs (n = 4). Data are expressed on the y-axis as percentage inhibition of the unblocked response. IgG1 PR3-ANCA concentration was 25 μg/ml and blocking antibodies as described in the methods were added singly or in combination. FcγRIIa was blocked with IV.3 and FcγRIIIb by 3G8. One-way analysis of variance was performed comparing against stimulated. *P < 0·05.
As the IL-8 response showed a dramatic difference between IgG1 and IgG4 PR3-ANCA, we investigated if the stimulation of cytokine release by IgG1 PR3-ANCA had a different reliance on FcγR to the other functional responses, again using the blocking antibodies. This time inhibiting FcγRIIIb, but not FcγRIIa, induced a significant reduction in release (Fig. 5d). Together the antibodies showed no difference to FcγRIIIb blocking alone.
Discussion
Previous reports have indicated that the subclass distribution of ANCA IgG may be important in disease pathogenesis. Indeed we have found that IgG4 isolated from ANCA-positive patients was unexpectedly able to induce neutrophils to undergo a respiratory burst. Here we show that a monoclonal chimeric IgG4 PR3-ANCA is also able to activate human neutrophils to release superoxide and to degranulate, with a similar efficacy as the structurally similar IgG1 chimeric PR3-ANCA. Interestingly, however, IgG4 PR3-ANCA is unable to induce the release of IL-8 and has a lesser ability to convert neutrophils that are rolling on P-selectin under flow conditions to stable adhesion. Both IgG1 and IgG4 PR3-ANCA required the engagement of Fcγ receptors but there were differences in their patterns of engagement. Specifically, both antibodies induced superoxide release and degranulation primarily via FcγRIIa, while IgG1 PR3-ANCA induction of IL-8 release required FcγRIIIb.
Immunoglobulin G4 has long been thought to be less active than the other subclasses and was often assumed to be a benign blocking antibody; preventing receptor engagement by other more activatory IgG subclasses, although exceptions to this have been recognized.11 It is mainly raised against allergens but also, to a small degree, other protein antigens too. Functionally, IgG4 is unable to bind to the complement component C1q but there is increasing evidence of its involvement in autoimmune diseases, such as autoimmune pancreatitis, nephritis and hepatitis.12,13 Indeed, we ourselves have previously shown that total IgG4 isolated from patients with ANCA-associated vasculitis (AASV) is able to activate human neutrophils to release superoxide in vitro.6 As a result of the lack of complement fixation by this subclass, it is likely that its effects are because of direct interaction of the immunoglobulin with constitutively expressed neutrophil IgG receptors, in association with concurrent cross-linking to PR3 on the neutrophil surface.14 As FcγRI were not up-regulated on the neutrophils used in these studies, which had been isolated from the blood of healthy volunteers, the role of this receptor was not investigated; however, we cannot exclude involvement of this receptor in vivo because it is up-regulated on circulating neutrophils during active ANCA-associated vasculitis.15 It is unlikely that our IgG4 PR3-ANCA chimeric antibody is activating neutrophils through immune complex formation consequent on binding to different antigenic epitopes, with secondary cross-linking of neutrophil FcγRs, as it is a monoclonal product. The antibody blocking studies differ in detail from those previously published using human polyclonal ANCA IgG, where both FcγRIIa and FcγRIIIb appeared to contribute to superoxide release.8 We attribute these apparent differences to the previous use of polyclonal ANCA IgG, which will contain antibodies of multiple IgG subclasses, against multiple epitopes on PR3.
Immunoglobulin G4 is known to be able to interact with FcγRIIa with a Kd of approximately five times that of IgG3 and about three times that of IgG1, but little is known about whether there is an interaction on neutrophils with the more abundant FcγRIIIb.16 We have shown that IgG4 PR3-ANCA does not use this latter receptor for the activation of neutrophils. Furthermore, its inability to bind to this receptor may be the reason for the lack of IL-8 production as IgG1 PR3-ANCA predominantly used this receptor for this function. There is a precedent for the different FcγRs having different functional outputs and these are likely to be because of differential activation of intracellular signalling pathways. FcγRIIa is a more efficient activator of the extracellular signal-regulated kinase mitogen-activated protein kinase (MAPK) than is FcγRIIIb, whereas the reverse is true for p38 MAPK.17 Interestingly, it has been suggested that the source of IL-8 within neutrophils resides within granules distinct from any previously defined and possibly within an endoplasmic reticulum-like structure, but the mechanisms controlling the release of IL-8 from such structures are presently unknown.18
In conclusion, the lack of IL-8 production and the lesser ability to induce firm adhesion of neutrophils by IgG4 PR3-ANCA under pathophysiological conditions may give rise to a difference in vasculitis disease manifestations. It is possible that in patients with increases in IgG4 PR3-ANCA titre there may be less recruitment of neutrophils to inflammatory hotspots and consequently less local tissue damage. In contrast, both IgG1 and IgG3 PR3-ANCA have been shown to induce neutrophil IL-8 release with IgG3 being the most effective in this regard: indeed there is evidence for a greater association between IgG3 PR3-ANCA and clinical disease activity.1 Additionally in Goodpasture’s disease restricted to the lung, a preponderance of IgG4 anti-glomerular basement membrane has been shown,19 so it would be of interest to determine whether high-titre IgG4 PR3-ANCA promotes pulmonary involvement in vasculitis. There may be potential for using subclass distribution of ANCA for tailoring both severity and type of treatment regimens in the future.
Acknowledgments
This work was supported by a studentship from the Arthritis Research Campaign.
Disclosures
The authors declare they have no conflicting financial interests.
References
- 1.Colman R, Hussain A, Goodall M, et al. Chimeric antibodies to proteinase 3 of IgG1 and IgG3 subclasses induce different magnitudes of functional responses in neutrophils. Ann Rheum Dis. 2007;66:676–82. doi: 10.1136/ard.2006.061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouwer E, Cohen Tervaert JW, Horst G, Huitema MG, Van der Giessen M, Limburg PC, Kallenberg CGM. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener’s granulomatosis and clinically related disorders. Clin Exp Immunol. 1991;83:379–86. doi: 10.1111/j.1365-2249.1991.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommarin Y, Rasmussen N, Wieslander J. Characterisation of monoclonal antibodies to proteinase-3 and application in the study of epitopes for classical anti-neutrophil cytoplasm antibodies. Exp Nephrol. 1995;3:249–56. [PubMed] [Google Scholar]
- 4.Morgan MD, Harper L, Williams J, Savage C. Anti-neutrophil cytoplasm-associated glomerulonephritis. J Am Soc Nephrol. 2006;17:1224–34. doi: 10.1681/ASN.2005080882. [DOI] [PubMed] [Google Scholar]
- 5.Kallenberg CG. Antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Curr Opin Rheumatol. 2007;19:17–24. doi: 10.1097/BOR.0b013e3280119842. [DOI] [PubMed] [Google Scholar]
- 6.Holland M, Hewins P, Goodall M, Adu D, Jefferis R, Savage C. Anti-neutrophil cytoplasm antibody IgG subclasses in Wegener’s granulomatosis: a possible pathogenic role for the IgG4 subclass. Clin Exp Immunol. 2004;138:183–92. doi: 10.1111/j.1365-2249.2004.02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruggemann M, Williams GT, Bindon CI, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–61. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Smith A, Dove SK, Martin A, Wakelam MJO, Savage COS. Autoantibodies from patients with systemic vasculitis activate primed neutrophils via Fc gamma receptor dependent pathways. Blood. 2001;98:1448–55. doi: 10.1182/blood.v98.5.1448. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood J, Williams J, Morgan M, Nash G, Savage C. ANCA induces beta2 integrin and CXC chemokine dependent neutrophil–endothelial cell interactions that mimic those of highly cytokine activated endothelium. J Leukoc Biol. 2005;77:33–43. doi: 10.1189/jlb.0104054. [DOI] [PubMed] [Google Scholar]
- 10.Radford DJ, Savage COS, Nash GB. Treatment of rolling neutrophils with anti-neutrophil cytoplasm autoantibodies causes conversion to firm integrin-mediated adhesion. Arthritis Rheum. 2000;43:1337–44. doi: 10.1002/1529-0131(200006)43:6<1337::AID-ANR16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2001;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoneda K, Murata K, Katayama K, et al. Tubulointerstitial nephritis associated with IgG4-related autoimmune disease. Am J Kidney Dis. 2007;50:455–462. doi: 10.1053/j.ajkd.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Umemura T, Zen Y, Hamano H, et al. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut. 2007;56:1471–1472. doi: 10.1136/gut.2007.122283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JM, Ben-Smith A, Hewins P, et al. Activation of the Gi heterotrimeric G protein by ANCA IgG F(ab′)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J Am Soc Nephrol. 2003;14:661–9. doi: 10.1097/01.asn.0000050223.34749.f4. [DOI] [PubMed] [Google Scholar]
- 15.Hewins P, Williams JM, Wakelam MJO, Savage COS. Activation of Syk in neutrophils by anti-neutrophil cytoplasm antibodies occurs via Fcγ receptors and CD18. J Am Soc Nephrol. 2004;15:796–808. doi: 10.1097/01.asn.0000113241.98702.77. [DOI] [PubMed] [Google Scholar]
- 16.Powell MS, Barton PA, Emmanouilidis D, et al. Biohemical analysis and crystallisation of Fc gamma RIIa, the low affinity receptor for IgG. Immunol Lett. 1999;68:17–23. doi: 10.1016/s0165-2478(99)00025-5. [DOI] [PubMed] [Google Scholar]
- 17.Coxon PY, Rane MJ, Powell MS, Klein JB, McLeish KR. Differential mitogen-activated protein kinase stimulation by Fc gamma receptor IIa and Fc gamma receptor IIIb determines the activation phenotype of human neutrophils. J Immunol Methods. 2000;164:6530–7. doi: 10.4049/jimmunol.164.12.6530. [DOI] [PubMed] [Google Scholar]
- 18.Pellme S, Morgelin M, Tapper H, Mellqvist UH, Dahlgren C, Karlson A. Localization of human neutrophil interleukin-8 (CXCL-8) to organelle(s) distinct from the classical granules and secretory vesicles. J Leukoc Biol. 2006;79:564–73. doi: 10.1189/jlb.0505248. [DOI] [PubMed] [Google Scholar]
- 19.Sethi S, Lewin M, Lopez L, Lager D. Linear anti-glomerular basement membrane IgG but no glomerular disease: Goodpasture’s syndrome restricted to the lung. Nephrol Dial Transplant. 2007;22:1233–5. doi: 10.1093/ndt/gfl841. [DOI] [PubMed] [Google Scholar]