Abstract
Alemtuzumab is a humanized monoclonal antibody against CD52, an antigen found on the surface of normal and malignant lymphocytes. It is approved for the treatment of B-cell chronic lymphocytic leukaemia and is undergoing Phase III clinical trials for the treatment of multiple sclerosis. The exact mechanism by which alemtuzumab mediates its biological effects in vivo is not clearly defined and mechanism of action studies have been hampered by the lack of cross-reactivity between human and mouse CD52. To address this issue, a transgenic mouse expressing human CD52 (hCD52) was created. Transgenic mice did not display any phenotypic abnormalities and were able to mount normal immune responses. The tissue distribution of hCD52 and the level of expression by various immune cell populations were comparable to those seen in humans. Treatment with alemtuzumab replicated the transient increase in serum cytokines and depletion of peripheral blood lymphocytes observed in humans. Lymphocyte depletion was not as profound in lymphoid organs, providing a possible explanation for the relatively low incidence of infection in alemtuzumab-treated patients. Interestingly, both lymphocyte depletion and cytokine induction by alemtuzumab were largely independent of complement and appeared to be mediated by neutrophils and natural killer cells because removal of these populations with antibodies to Gr-1 or asialo-GM-1, respectively, strongly inhibited the activity of alemtuzumab whereas removal of complement by treatment with cobra venom factor had no impact. The hCD52 transgenic mouse appears to be a useful model and has provided evidence for the previously uncharacterized involvement of neutrophils in the activity of alemtuzumab.
Keywords: alemtuzumab, antibody, Campath, CD52, neutrophil
Introduction
Alemtuzumab (Campath-1H) is a recombinant humanized immunoglobulin G1 (IgG1) monoclonal antibody directed against human CD52 (hCD52), a 12 amino acid, 28 000 molecular weight glycosylated glycosylphophatidylinositol (GPI)-linked cell surface protein.1,2 CD52 is expressed at high levels by both normal and malignant B and T lymphocytes with lower levels found on monocytes, macrophages and eosinophils and little expression on mature natural killer (NK) cells, neutrophils and haematological stem cells.2–7 CD52 is also produced by epithelial cells in the epididymis and duct deferens and is acquired by sperm during passage through the genital tract.2,8 The exact biological function of CD52 remains unclear but some evidence suggests that it may be involved in T-cell migration and costimulation.9–11
Alemtuzumab is currently approved as a first-line treatment against B-cell chronic lymphocytic leukaemia. Treatment with the antibody results in the depletion of CD52+ tumour cells but the mechanism(s) involved are not well-defined. In vitro studies indicate that alemtuzumab is capable of complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC), as well as induction of apoptosis,12–17 but the extent of the role played by these various mechanisms in vivo remains to be established.
Alemtuzumab has also been tested clinically in the context of autoimmune diseases including rheumatoid arthritis, vasculitis and, most notably, multiple sclerosis (MS).18–22 Recently published results from a Phase II clinical trial in previously untreated relapsing–remitting MS patients showed a 74% reduction in the rate of relapse in patients receiving annual courses of alemtuzumab treatment compared with interferon-β1a given three times per week.22 In addition, patients treated with alemtuzumab showed a 71% reduction in the risk for sustained accumulation of disability compared with interferon-β1a-treated patients over a 36-month period.22 Significant lymphocyte depletion was observed in alemtuzumab-treated patients and probably played a role in controlling autoreactivity but the mechanism responsible for sustaining a long-term therapeutic benefit in the face of lymphocyte repopulation remains unclear.
Even though the properties of alemtuzumab have been studied in vitro using human peripheral blood lymphocytes, more detailed in vivo mechanism of action studies have been hampered by the fact that the antibody does not cross-react with murine CD52. Homologues of CD52 have been identified in the mouse and several other species that possess very similar signal peptides and 5′ and 3′ untranslated sequences but the mature peptides are very different among species, which explains the lack of cross-reactivity.2 Therefore, a transgenic hCD52 mouse was created to allow for in-depth characterization of the biological impact and mechanism of lymphocyte depletion by alemtuzumab in vivo. The hCD52 transgenic mouse effectively reproduced the tissue distribution of CD52 observed in humans and responded to treatment with alemtuzumab in a similar manner. Study results provided insight into the in vivo activity of alemtuzumab and the model represents a useful tool to potentially optimize the use of alemtuzumab in oncology and autoimmune disease applications.
Materials and methods
Human CD52 transgenic mice
The hCD52 transgenic mouse was created on a CD1 mouse strain background at Xenogen Biosciences (Cranbury, NJ) by microinjecting mouse embryonic stem cells with a bacmid construct consisting of ∼ 145 kilobases genomic DNA from human chromosome 1 containing the entire hCD52 gene and promoter sequence. The murine CD52 gene remained present. Genetic determination of homozygosity or heterozygosity in hCD52 transgenic mice was performed on tail clips using polymerase chain reaction. Homozygous or heterozygous hCD52 transgenic mice were found to have a normal physical appearance, physiological activities, body weights and life span when compared with the wild-type CD1 background strain. In all studies, 8- to 12-week-old heterozygous hCD52 transgenic mice were used unless otherwise specified. Experimental protocols were approved by Genzyme’s Institutional Animal Care and Use Committee and studies were conducted in Genzyme’s Association for Assessment and Accreditation of Laboratory Animal Care accredited facility.
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples of spleen, inguinal lymph nodes and associated adipose tissue, thymus, pancreas, stomach, testes, ovary and bone/bone marrow from six heterozygous hCD52 transgenic mice (three males and three females) and two CD1 wild-type mice (one male, one female) were cut into 5-μm sections and stained with haematoxylin & eosin. Serial sections were assessed for tissue morphology and expression of hCD52 by staining with a monoclonal rat anti-human CD52 antibody (Campath-1G clone YTH34.5; Serotec, Duesseldorf, Germany) at a dilution of 1 : 8000. A rabbit anti-rat secondary antibody (Vector Laboratories, Burlingame, CA) was then added at a dilution of 1 : 250. Detection of positive cells was performed using a biotin-free horseradish peroxidase and polymer detection kit (Mach-2 HRP Rabbit; Biocare, Concord, CA) followed by a diaminobenzidine chromogen (Dako, Carpenteria, CA). All tissue sections were evaluated qualitatively for staining intensity and distribution by a board-certified veterinary pathologist.
Evaluation of immune responses
To assess the immune status of hCD52 transgenic mice compared with wild-type CD1 mice, groups of three mice were immunized intradermally with 1 × 109 infectious units of a non-replicating E1-deleted adenovirus (Ad) serotype 2 vector lacking a transgene. Three weeks later, serum samples and spleens were collected from individual mice to assess humoral and cellular immune responses to Ad. Titres of antibodies to Ad were measured by enzyme-linked immunsorbent assay (ELISA) as previously described.23 Briefly, twofold serial dilutions of serum were added to wells coated with inactivated Ad2 particles and bound Ad-specific antibodies were detected by the addition of horseradish peroxidase-conjugated goat anti-mouse IgG, -A or -M (Cappel, Durham, NC) followed by SigmaFAST OPD substrate (Sigma, St Louis, MO). The serum titre was defined as the reciprocal of the highest dilution of serum producing a colorimetric signal with an optical density ≤ 0·1. The cellular immune response was measured as previously described by stimulating spleen cells (5 × 105/well of 96-well plate) with inactivated Ad particles (1 μg/ml) for 5 days. 23 The amount of proliferation induced was measured by pulsing with 1 μCi/well [3H]thymidine for the last 18 hr of incubation. Results shown are mean ± SEM of the values obtained with individual mice.
Quantification of CD52 antigen density on the cell surface
The number of human CD52 molecules present on the surface of different cell populations from hCD52 transgenic mice was quantified using Quantum Simply Cellular anti-human IgG beads from Bangs Laboratories Inc. (Fishers, IN) according to the manufacturer’s instructions. Briefly, beads coated with different amounts of anti-human IgG corresponding to a pre-calibrated antibody-binding capacity (ABC) were incubated with saturating concentrations of alemtuzumab/Campath-1H (Genzyme Corporation, Cambridge, MA) labelled with fluorescein isothiocyanate (FITC) to generate an ABC standard curve based on mean fluorescence intensity (MFI). The ABC standard curve was then used to convert the MFI value obtained with FITC-alemtuzumab binding to a given fluorescence-activated cell sorter (FACS)-delineated subpopulation into the number of human CD52 molecules per cell.
Flow cytometry
Cell staining was performed by incubating 4 × 105 to 2 × 106 cells with FITC-conjugated alemtuzumab/Campath-1H and fluorescently labelled antibodies specific for mouse cell surface markers including B220 (clone RA3-6B2), CD19 (clone 1D3), CD3 (clone 145-2C11), CD4 (clone RM4-5), CD8 (clone 53-6.7), F480 (clone BM8), CD11b (clone MI/70), GR-1 (clone RB6-8L5), NK1.1 (clone PK136), CD49b (clone DX5), CD44 (clone 1M7) and CD25 (clone PC61.5) purchased from BD Bioscience (San Jose, CA) or eBioscience (San Diego, CA). Identification of T regulatory cells was performed by intracellular staining for Fox-P3 (clone FJK-16S) as indicated by the manufacturer (eBioscience). Stem cell identification was performed by staining cells isolated from the bone marrow with Mouse Lineage Antibody cocktail (BD Bioscience) simultaneously with Thy-1.1 (clone H1S51), Sca-1 (clone D7) and c-kit (clone 2B9). Staining of peripheral blood cells was performed by staining 50 μl of whole blood from individual mice with the antibodies described above followed by removal of red blood cells using FACS lysis solution (BD Bioscience) as described by the manufacturer. Fluorescence intensities were measured using either a FACS Calibur or LSR-II (BD Bioscience) and analysis was performed using flowjo software (Tree Star Inc., Ashland, OR).
For quantification of absolute numbers of specific cell populations in the peripheral blood, CountBright Absolute Counting Beads (Invitrogen, Carlsbad, CA) were added to blood samples according to the manufacturer’s instructions. For lymphoid organs, the absolute number of cells in a given population was obtained by multiplying the percentage of FACS-positive cells by the total number of cells recovered from the organ.
Alemtuzumab-mediated cell depletion and repopulation
Human CD52 transgenic mice were treated with a single intraperitoneal (i.p.) injection of alemtuzumab at the doses indicated or with phosphate-buffered saline as a vehicle control. In cell depletion experiments, blood and lymphoid organs from individual mice (n = 5) were collected at 72 hr post-treatment for FACS analysis. In cell repopulation studies, serial blood collections were performed on individual mice (n = 8) at 72 hr and at various intervals up to 25 weeks post-treatment. Results are shown as ‘% control’ calculated by taking the absolute number of cells in a given cell population in individual alemtuzumab-treated mice and dividing it by the mean absolute number of the same population in control mice.
Serum cytokine analysis
To evaluate cytokine induction following treatment with alemtuzumab, hCD52 transgenic mice were given a single i.p. administration of antibody at the doses indicated and serum was collected from individual mice at 1, 2, 4 and 24 hr post-dosing (n = 5 to n = 7). Remicade® (Genentech Inc., San Francisco, CA), a human IgG1 monoclonal antibody against human tumour necrosis factor-α (TNF-α), which does not cross-react with mouse TNF-α, was used as a negative control. Serum cytokine concentrations were determined using a BD Cytometric Bead Array (Mouse Inflammation kit; BD Biosciences, San Jose, CA) according to the manufacturer’s protocol.
Mechanism of action studies
Mice were treated to remove selected effector arms of the immune system to study the impact on the cytokine induction and lymphocyte-depleting activity of alemtuzumab. Complement was inactivated by treatment with cobra venom factor (Calbiochem, San Diego, CA) administered i.p. at 1 mg/kg at 72 and 24 hr before the administration of alemtuzumab. Depletion of complement was confirmed to be 85–90% using an ELISA kit to measure C3 according to the manufacturer’s instructions (Immunology Consultants Laboratory Inc., Newberg, OR). Natural killer cells were removed by treatment with anti-asialo-GM1 antibody (Wake Chemicals USA, Inc., Richmond, VA) administered intravenously (i.v.) at 25 mg/kg, 72 and 24 hr before the administration of alemtuzumab. Neutrophils were depleted with anti-Gr-1 antibody (anti-Ly-6G; eBioscience) given i.v. at 7·5 mg/kg, 72 and 24 hr before the injection of alemtuzumab. Depletion of NK cells and neutrophils from the blood was confirmed by FACS staining and was found to be 85–90% and 95%, respectively.
Statistical analysis
graphpad prism V4.03 (GraphPad Software, San Diego, CA) was used for statistical analysis. Comparisons for multiple groups were made using one-way analysis of variance with the Dunnett’s post hoc test. Differences were considered statistically significant if the P-value was < 0·05.
Results
Tissue distribution of hCD52 in transgenic mice
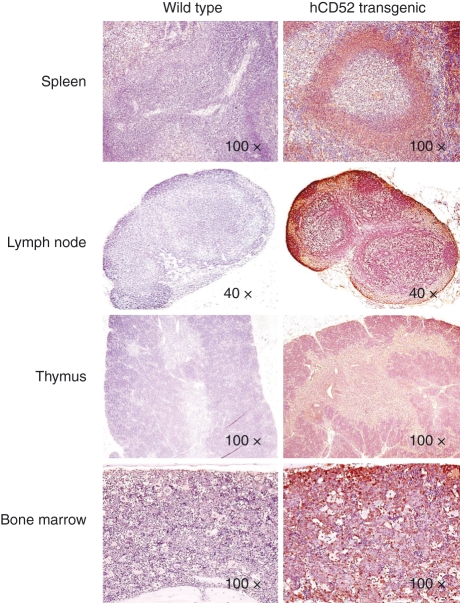
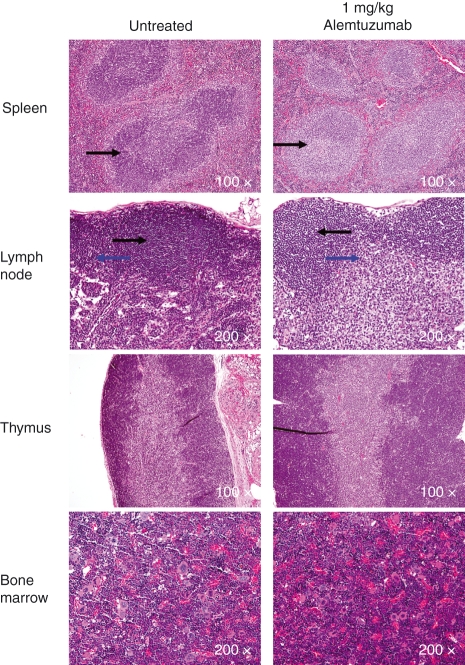
Histological evaluation of tissues from hCD52 transgenic mice showed that the morphology of tissues, including the spleen, inguinal lymph nodes and associated adipose tissue, thymus, bone/bone marrow (Fig. 1) pancreas, stomach, testes and ovary (not shown) was normal and comparable to that observed in CD1 wild-type control mice indicating that expression of the human transgene product did not affect normal tissue architecture. Staining of the tissues for hCD52 expression revealed a tissue distribution similar to that seen in humans with high levels of expression in lymphoid tissues (Fig. 1) and positive scattered mononuclear cells in the stomach, testes and adipose tissue (not shown). As in humans, the proximal epithelium and mature sperm in the epididymis stained positive for hCD52 (not shown). Staining was also observed in some granulosa cells and cumulus oophorous cells in the ovary (not shown), a tissue that does not appear to have been examined in humans. No hCD52 staining was detected in any of the tissues from CD1 wild-type mice (Fig. 1) as expected from the absence of hCD52 in these mice and the lack of cross-reactivity of the detecting antibody for mouse CD52. Staining with a control IgG antibody also failed to generate a signal in either wild-type or hCD52 transgenic mice (not shown).
Figure 1.
Expression of hCD52 in lymphoid tissues. Representative images of immunohistochemical staining for human CD52 in the spleen, lymph nodes, thymus and bone marrow of wild-type CD1 mice (left panels) and hCD52 transgenic mice (right panels) are shown. Human CD52 positivity is indicated by brown staining. As expected, no hCD52 expression was detected in any of the tissues from wild-type mice while hCD52 positivity was prominent in the lymphoid organs of transgenic mice. There was diffuse CD52 staining in the spleen with intensely positive lymphoid cells and macrophages in the white pulp and positive mononuclear cells in the sinusoids (red pulp). The lymph nodes also stained diffusely positive for hCD52 with positive cells in the B-cell and T-cell areas. In the thymus, there was diffuse CD52 staining of lymphocytes in both the cortex and the medulla. In the bone marrow, approximately 50% of cells were positive and the vast majority exhibited mononuclear cell morphology.
Expression of hCD52 in immune cell populations
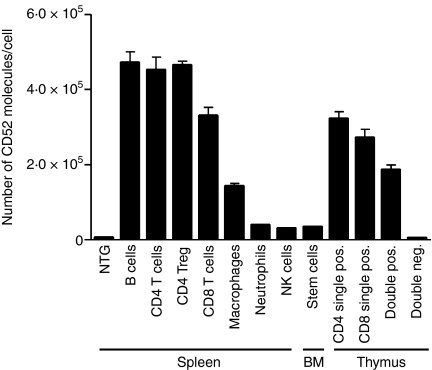
The pattern of expression and density of hCD52 antigen on various immune cell populations was examined by FACS analysis. The pattern of human CD52 expression in transgenic mice was similar to that reported in humans.2–8 The highest levels of expression were seen on B and T lymphocytes with lower levels on macrophages and little or no expression on mature NK cells, neutrophils and bone marrow stem cells (Fig. 2). The actual number of hCD52 molecules per lymphocyte (3·3 × 105 for CD8+ T cells, 4·7 × 105 for CD4+ T cells and B cells) was also comparable to that previously reported for unfractionated human peripheral mononuclear cells (4·2 × 105 to 4·5 × 105)24. CD52 expression in the thymus has not been previously studied in humans but analysis of thymic cells from hCD52 transgenic mice indicated that robust levels of hCD52 were present on CD4/CD8 single-positive as well as double-positive thymocytes with no detectable expression above background on double-negative thymocytes (Fig. 2).
Figure 2.
Level of CD52 expression on immune cell populations. Human CD52 expression was quantified on the indicated cell populations from the spleen, bone marrow (BM) and thymus of hCD52 transgenic mice. Using multi-parameter flow cytometry, hCD52 mean fluorescence intensities were quantified and used to calculate the number of hCD52 molecules/cell as described in the Materials and methods section. The cell populations examined included B220+ B cells, CD4+ T cells, CD4+ CD25+ FoxP3+ T cells (CD4 Treg), CD8+ T cells, CD11b+ CD11c− macrophages, Gr-1+ neutrophils, NK1.1+ CD49b+ mature NK cells, c-kit+ Sca+ CD45− bone marrow stem cells, CD4 and CD8 single-positive thymocytes, double-positive and double-negative thymocytes. Non-transgenic (NTG) B220+ B cells are shown as a representative population to demonstrate the level of background staining for all NTG cell populations. Error bars indicate the SEM of six animals/group.
Immune status of hCD52 transgenic mice
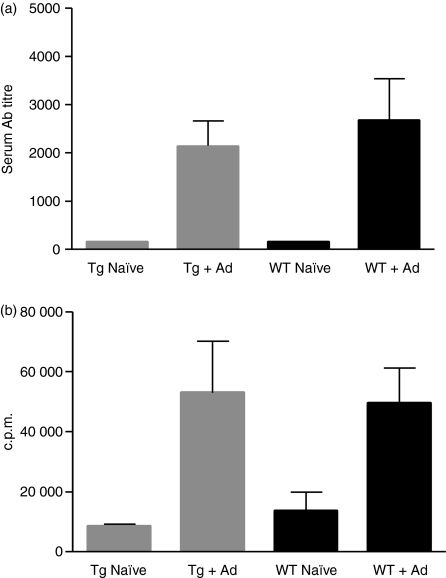
As described above, the architecture of lymphoid organs appeared intact in hCD52 transgenic mice and we next sought to determine whether immune function was also preserved. Wild-type and hCD52 transgenic CD1 mice were immunized with a non-replicating adenovirus (Ad) vector and both humoral and cell-mediated immune responses were measured 3 weeks later. As shown in Fig. 3, transgenic and wild-type mice developed comparable levels of Ad-specific antibodies (Fig. 3a) and splenocytes from the animals displayed equivalent levels of proliferation when stimulated with Ad antigen in vitro (Fig. 3b). These results indicate that immune function is not compromised by the expression of hCD52 in the transgenic mice.
Figure 3.
Immune status of hCD52 transgenic mice. The humoral and cellular immune responses of hCD52 transgenic (Tg) CD-1 mice immunized with adenovirus (Ad) were compared with those of wild type (WT) CD-1 mice; (a) Mean Ad-specific antibody titres ± SEM of serum samples from individual naïve or immunized mice. (b) Mean Ad-induced proliferation ± SEM of spleen cells from individual naïve or immunized mice (n = 3). There were no significant differences between the responses of wild-type and hCD52 transgenic mice (P> 0.05).
Immune cell depletion following treatment with alemtuzumab
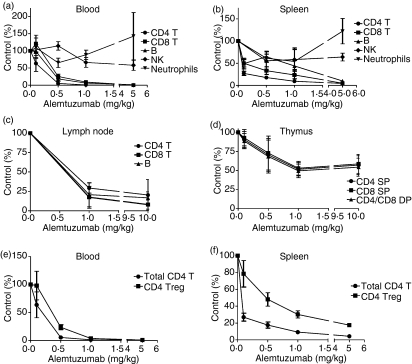
Administration of a single dose of alemtuzumab resulted in the dose-dependent depletion of B and T lymphocytes in the peripheral blood and lymphoid organs (Fig. 4). As observed in humans, treatment with doses of alemtuzumab ≥ 1 mg/kg resulted in a near complete depletion of B and T lymphocytes from the circulation19,21,22,25 (Fig. 4) while mature NK cells and neutrophils which express little CD52 were not as affected.25 There was a trend for increased numbers of neutrophils in the blood and spleen at the highest dose of alemtuzumab (Fig. 4). The reason for this effect is unclear but could involve factors such as recruitment of neutrophils from the bone marrow or demarginalization.
Figure 4.
Immune cell depletion after treatment with alemtuzumab. Absolute numbers of immune cell populations remaining at 72 hr after the administration of various intravenous doses of alemtuzumab were assessed as described in the Materials and methods section. Results shown are the mean ± SEM of individual mice (n = 5) and are expressed as the per cent of cells remaining after treatment relative to the number of cells present in vehicle-treated control mice (% Control). The organs examined included the blood (a), spleen (b), inguinal lymph nodes (c) and thymus (d). The cell populations analysed consisted of CD4+ T cells, CD8+ T cells, single-positive (SP) and double-positive (DP) thymocytes, B220+ B cells, NK1.1+ CD49b+ NK cells and Gr-1+ neutrophils. Analysis of remaining numbers of CD4+ CD25+ FoxP3+ Tregs compared with total CD4+ T cells was also performed for the blood (e) and spleen (f).
Notably, the degree of lymphocyte depletion was not as profound in lymphoid organs, an assessment that has not been possible to conduct in humans. For example, at doses of 0·5–1 mg/kg alemtuzumab, most lymphocytes were depleted from the circulation but significant numbers of B and T cells were still present in the spleen, lymph nodes, bone marrow and thymus as determined by FACS analysis (Fig. 4) and histochemistry (Fig. 5). A higher dose of 5 mg/kg was required to achieve a more complete depletion in the spleen while depletion was still incomplete in the lymph nodes and thymus even at 10 mg/kg. In particular, a maximum depletion of only ∼ 50% of single-positive and double-positive thymocytes was achievable in the thymus even though the level of CD52 expression by these cells is quite robust (Fig. 2). It is possible that penetrance and exposure to alemtuzumab are reduced in lymphoid organs compared to peripheral blood and may, at least in part, account for lower levels of depletion. In addition, effector mechanisms responsible for depletion (e.g. ADCC effector cells) may not be as abundant in some of the organs such as the thymus. Together with the relatively unaffected innate immune system components such as neutrophils, NK cells and macrophages, the preservation of ‘lymphocyte pools’ in lymphoid organs may contribute to the relatively low incidence of infection in alemtuzumab-treated rheumatoid arthritis and MS patients in the face of severe and prolonged lymphopenia.19,20,22,26–28
Figure 5.
Histology of lymphoid organs after treatment with alemtuzumab. Lymphoid tissues collected from hCD52 transgenic mice left untreated or treated intraperitoneally with 1 mg/kg alemtuzumab (72 hr post-dosing) were stained with haematoxylin & eosin for histological examination. There was mild lymphoid depletion in the spleen (periarteriolar lymphoid sheath shown by arrow) and in the paracortex (bottom arrow) and, to a lesser extent, the cortex (top arrow) of inguinal lymph nodes. There were no notable differences in the cellularity of the thymus or bone marrow in treated versus untreated mice. Depletion of bone marrow stem cells was undetectable by fluorescence-activated cell sorting up to a dose of 10 mg/kg (not shown).
Interestingly, a relative sparing of T cells with a regulatory phenotype (CD4+ CD25+ FoxP3+) compared to total CD4+ T cells was observed in both the blood and spleen (Fig. 4) even though both populations express equivalent levels of CD52 on their surface (Fig. 2). A similar enrichment of CD4+ CD25high cells in the depleted CD4+ pool was also observed by Cox et al.21 in MS patients treated with alemtuzumab. The mechanism and significance of this apparent sparing of T cells with a regulatory phenotype remain unclear but could be a factor in the long-term suppression of MS by alemtuzumab.
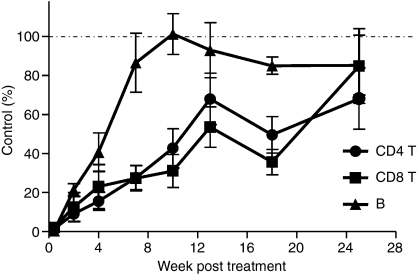
Lymphocyte repopulation after treatment with alemtuzumab
The kinetics of peripheral blood lymphocyte repopulation has been studied in MS patients treated with alemtuzumab.20–22 After near complete depletion from the circulation, B lymphocytes return to pre-treatment levels between 3 and 6 months while T-cell counts rise slowly and remain below normal for several years. A similar repopulation pattern was observed in the hCD52 transgenic mice albeit in a more contracted time frame. Mice were treated with a single 10 mg/kg dose of alemtuzumab resulting in essentially complete depletion of B and T lymphocytes from the circulation. B lymphocytes returned to baseline levels by 7–10 weeks post-treatment while CD4+ and CD8+ T cells recovered more slowly and were still below normal levels at 25 weeks (Fig 6). As shown above (Fig. 5), treatment with alemtuzumab did not significantly affect the bone marrow presumably allowing for the rapid recovery of B lymphocytes. Treatment with the antibody partially depleted thymocytes (Fig. 4), which may account at least in part for the slower recovery of T lymphocytes.
Figure 6.
Pattern of lymphocyte repopulation after treatment with alemtuzumab. Blood samples were collected at various time-points following the intraperitoneal administration of 10 mg/kg alemtuzumab and the absolute numbers of CD4+ T cells, CD8+ T cells and CD19+ B cells were assessed as described in the Materials and methods section. Results shown are the mean ± SEM of individual mice (n = 8) and are expressed as the per cent of cells remaining after treatment relative to the number of cells present in untreated, age-matched control mice (% Control).
Mechanism of lymphocyte depletion
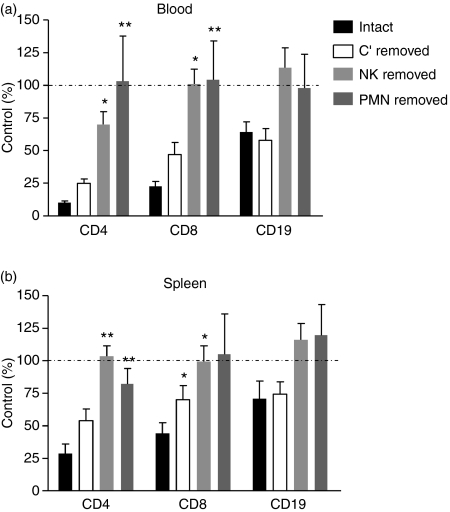
Alemtuzumab has been reported to mediate the lysis of CD52+ cells in vitro via complement-dependent cytotoxicity and ADCC.29 However, the relative contribution of these mechanisms in vivo remains undefined. The hCD52 mice offered a unique opportunity to explore this issue by selectively removing each effector mechanism and assessing the impact on the depleting activity of alemtuzumab. As shown in Fig. 7, removal of complement with cobra venom factor had little or no impact on the depletion of blood or splenic B and T lymphocytes by alemtuzumab. In contrast, removal of NK cells with an anti-asialo GM-1 antibody or neutrophils with an antibody against Gr-1, significantly reduced or ablated the activity of alemtuzumab, suggesting a predominant role for ADCC as opposed to complement-dependent cytotoxicity in lymphocyte depletion. The involvement of neutrophils as effectors in the activity of alemtuzumab is a novel finding.
Figure 7.
Mechanism of lymphocyte depletion by alemtuzumab. Immune effector arms were selectively inactivated to study the impact on the lymphocyte-depleting activity of alemtuzumab. Mice were either left untreated (intact) or were treated with cobra venom factor to remove complement (C′ removed), anti-asialo-GM1 to remove natural killer (NK) cells (NK removed) or anti-Gr-1 to remove neutrophils (PMN removed) before the administration of alemtuzumab (0.1 mg/kg, intraperitoneally). Absolute numbers of CD4+ T cells, CD8+ T cells and CD19+ B cells remaining in the blood (a) and spleen (b) at 72 hr post-alemtuzumab were assessed as described in the Materials and methods. Results shown are the mean ± SEM of individual mice (n = 7) and are expressed as the per cent of cells remaining after treatment relative to the number of cells present in untreated, control mice (% Control). *P < 0.05, **P < 0.01 versus intact mice.
Induction of serum cytokines by alemtuzumab
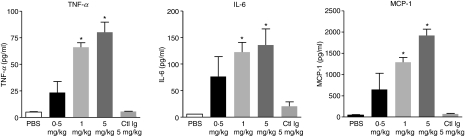
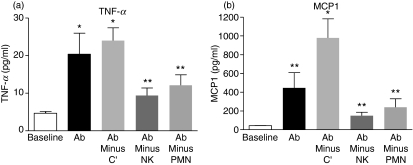
Administration of alemtuzumab in humans results in an infusion reaction associated with the induction of serum cytokines including TNF-α, IL-6 and interferon-γ.19,20,22,28,30 The mechanism of induction and source of the cytokines are not fully characterized. Cytokine induction was studied in the hCD52 transgenic mouse. Serum was collected at different time-points (1, 2, 4, 24 hr) following the i.p. injection of different doses of alemtuzumab. A dose-dependent cytokine peak, including TNF-α, IL-6 and monocyte chemotactic protein-1, was observed at 2 hr post-injection shown in Fig. 8, followed by a return to basal levels by 24 hr (not shown). The mechanism responsible for cytokine release was investigated. As observed for lymphocyte depletion, removal of complement did not significantly affect cytokine induction by alemtuzumab (Fig. 9). Removal of NK cells by treatment with anti-asialo GM-1 or neutrophils by treatment with anti-Gr-1, showed an equivalent and significant dampening of cytokine induction. These results suggest that lymphocyte depletion and cytokine induction are linked processes involving effector cells rather than complement activation. The actual source of the cytokines remains to be determined (e.g. effector NK cells, neutrophils, lysed lymphocytes, indirectly stimulated cells).
Figure 8.
Induction of serum cytokines by alemtuzumab. Mice were injected with various doses of alemtuzumab intraperitoneally (0.5, 1 or 5 mg/kg) or with phosphate-buffered saline (PBS) or Remicade® as an isotype control (Ctl Ig, 5 mg/kg). Remicade® is a human immunoglobulin G1 specific for human tumour necrosis factor-α (TNF-α) and does not cross-react with murine TNF-α. Serum samples were collected at 1, 2, 4, 24 hr post-dosing with alemtuzumab and cytokine levels measured with a multiplex mouse cytokine assay kit. Data are shown for the 2-hr cytokine peak only and represent the mean ± SEM of individual mice (n = 5) for TNF-α, interleukin-6 (IL-6) and monocyte chemoattractant protein 1 (MCP-1). *P < 0.01 versus PBS.
Figure 9.
Mechanism of cytokine induction by alemtuzumab. Immune effector arms were selectively inactivated to study the impact on the cytokine-inducing activity of alemtuzumab. Serum levels of tumour necrosis factor-α (TNF-α) and monocyte chemoattractant protein 1 (MCP-1) at 2 hr post-alemtuzumab (0.1 mg/kg, intraperitoneally) are shown for mice that were either left untreated (Ab) or were treated with cobra venom factor to remove complement (Ab minus C′), anti-asialo-GM1 to remove natural killer (NK) cells (Ab minus NK) or anti-Gr-1 to remove neutrophils (Ab minus PMN) before the administration of alemtuzumab. Results shown are the mean ± SEM of individual mice (n = 5). Background (baseline) levels of serum cytokines in untreated mice are also shown. *P < 0.01, **P > 0.05 versus baseline.
Discussion
A new hCD52 transgenic mouse model was created to allow for investigation of the mechanism of action of alemtuzumab, an anti-hCD52 therapeutic monoclonal antibody that does not cross-react with murine CD52. Histological examination and FACS analysis indicated that the tissue distribution of hCD52 and level of expression by various immune cell populations in the transgenic mouse were comparable to those seen in humans (Figs 1 and 2). The architecture of lymphoid organs, where hCD52 is highly expressed, was found to be normal and transgenic mice developed normal humoral and cell-mediated immune responses to adenovirus comparable to those of wild-type mice (Fig. 3). Treatment of the mice with alemtuzumab resulted in the dose-dependent depletion of B and T lymphocytes from the circulation (Fig. 4). Cells with little expression of CD52 such as mature NK cells and neutrophils were comparably less affected (Fig. 4). At doses comparable to those administered to MS and rheumatoid arthritis patients (≥ 1 mg/kg total), a near total depletion of circulating B and T lymphocytes was observed. This profound loss of peripheral lymphocytes also occurs in patients with autoimmune diseases and is surprisingly not associated with a dramatically higher incidence or severity of infection nor with an unusual spectrum of infection.19,20,22,26–28 It has been hypothesized that preservation of innate immune responses (NK cells, neutrophils) provides a relatively intact first line of defence against pathogens and that residual lymphocytes in lymphoid organs may also still be available to participate in the immune response.19,20,27 Assessment of lymphocyte levels outside the periphery has not been possible in humans but our results in the transgenic mouse suggest that significant pools of lymphocytes are still present in peripheral lymphoid organs (spleen, lymph nodes) even when circulating levels are almost undetectable (Figs 4 and 5).
The pattern of lymphocyte repopulation in transgenic mice treated with alemtuzumab also mirrored that observed in humans. B-lymphocyte recovery was the most rapid with T lymphocytes repopulating more slowly and remaining below normal levels for a prolonged period of time (Fig. 6). In MS patients treated with alemtuzumab, B-cell levels returned to baseline by approximately 3 months while the median recovery time for CD4+ and CD8+ cells was considerably longer at 61 and 30 months, respectively.20 A similar pattern was observed in alemtuzumab-treated rheumatoid arthritis patients.27 Alemtuzumab does not deplete CD52-negative haematological precursors, which presumably accounts for the relatively rapid recovery of B lymphocytes. The mechanism responsible for the prolonged T-cell lymphopenia is unclear. Examination of the thymus has not been possible in humans but, in the transgenic mouse, partial depletion of single-positive and double-positive thymocytes was observed reaching a maximum of approximately 50% at the highest dose of 10 mg/kg of alemtuzumab (Fig. 4). This partial loss of thymocytes may have contributed to the delay in T-cell recovery in spite of the preservation of bone marrow stem cells, but probably does not entirely account for the long duration of T-cell lymphopenia.
Cox et al.21 have shown that in alemtuzumab-treated MS patients, delayed T-cell reconstitution could not be attributed to an impairment in the production of or response to IL-7, a key cytokine in lymphocyte homeostasis, but may involve suboptimal production of IL-15, which maintains the proliferation and survival of CD8+ T cells, and CCL21, which promotes CD4+ T-cell regeneration. It has also been suspected that the decline in thymic function known to occur with age may contribute to the delay in T-cell recovery. This is supported by the reported age dependence of the increase in T-cell receptor rearrangement excision circle, a measure of thymic activity, in MS patients following treatment with alemtuzumab.
The potent T-lymphocyte-depleting activity of alemtuzumab probably plays a role in the efficacy of the antibody in controlling disease in relapsing/remitting MS patients. However, it has been hypothesized that additional factors beyond simple depletion may be involved because the therapeutic benefit persists in the face of lymphocyte repopulation between yearly treatments and long after the last treatment with alemtuzumab. It has been speculated that treatment with alemtuzumab may create a ‘tolerogenic environment’ during lymphocyte repopulation.26 In support of this hypothesis is the observation of a predominance of peripheral blood T cells with a regulatory phenotype during the first 6 months following alemtuzumab treatment in MS patients.21,26 This reported ‘sparing’ of T cells with a regulatory phenotype was also observed in our transgenic mice and was even more prominent in the spleen compared with blood, the only accessible compartment in humans (Fig. 4). The biological significance of these findings remains to be established and the hCD52 transgenic mouse represents a useful tool to explore this issue in future studies.
The mechanism(s) responsible for the depletion of lymphocytes by alemtuzumab in vivo has not been fully characterized. The antibody is capable of complement-dependent cytotoxicity and ADCC in vitro29 but the extent to which each mechanism contributes to lymphocyte depletion in vivo is unknown. To explore this issue, we took advantage of the hCD52 transgenic mouse system to selectively disable individual effector mechanisms and assess the impact on overall lymphocyte depletion. Interestingly, removal of complement had little or no impact on the lymphocyte-depleting activity of alemtuzumab while removal of NK cells or neutrophils essentially ablated its activity, indicating a prominent role for ADCC in lymphocyte depletion (Fig. 7). A role for neutrophils in the ADCC activity of alemtuzumab has not been previously described, possibly because in vitro studies of ADCC typically rely on effector cell preparations consisting of peripheral blood mononuclear cells or purified NK cells, which do not contain neutrophils. Neutrophil-mediated killing, however, has been reported as an effector mechanism in the activity of rituximab31,32 and may represent a more general mechanism of ADCC than previously appreciated. It is perhaps biologically meaningful that NK cells and neutrophils express little CD52 themselves and are therefore relatively unaffected by treatment with alemtuzumab thus remaining available to carry out their depleting activity. A potential involvement of macrophages as effector cells was not characterized but also remains a possibility.
The administration of alemtuzumab to patients causes a first-dose cytokine release syndrome characterized by the production of TNF-α, IL-6 and interferon-γ.19,20,22,28,30 The hCD52 transgenic mouse showed a similar dose-dependent induction of cytokines (Fig. 8) and allowed for an in vivo assessment of the mechanisms involved. Our results indicated that alemtuzumab-induced cytokine release was independent of complement but was potently inhibited by the removal of NK cells or neutrophils. The same mechanism therefore appears to underlie both lymphocyte depletion and cytokine induction, suggesting that the two are closely linked. Our results are also in agreement with early in vitro studies using whole human blood suggesting that cytokine production upon exposure to alemtuzumab did not require complement and appeared to result from the ligation of CD16 on effector NK cells.30 It was postulated that NK cells were the source of the TNF-α produced but our findings suggest that neutrophils could also be a source of cytokines. In addition, the possibility of cytokine release by monocytes, lysed lymphocytes and other ‘bystander’ cells stimulated in the process (e.g. endothelial cells) cannot be excluded.
Overall, the new hCD52 transgenic mouse appears to successfully replicate the pattern of hCD52 expression and response of patients to treatment with alemtuzumab. The manipulations afforded by a mouse system have provided significant insight into the biological activity of alemtuzumab and will be useful in studying the process of lymphocyte reconstitution following treatment-induced lymphopenia. A better understanding of the activity of alemtuzumab in vivo could provide information to optimize its use in autoimmune disease and oncology applications.
Acknowledgments
The authors wish to thank Lisa Woodworth, Michael LaMorte and Carrie Garron for technical help and the Genzyme Department of Comparative Medicine for animal husbandry.
Disclosures
All authors are employees and shareholders of Genzyme Corporation, the manufacturer of Alemtuzumab.
References
- 1.Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1antigen (CDw52) Tissue Antigens. 1990;35:118–27. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 2.Hale G. CD52 (Campath-1) J Biol Regul Homeost Agents. 2001;15:386–91. [PubMed] [Google Scholar]
- 3.Huh Y, Kantarjian H, Pierce SM, et al. Expression of human CD52 in human hematopoietic malignancies. Blood. 1998;92 Abstract 4199. [Google Scholar]
- 4.Elsner J, Hochstetter R, Spiekermann K, Kapp A. Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood. 1996;88:4684–93. [PubMed] [Google Scholar]
- 5.Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood. 1993;82:807–12. [PubMed] [Google Scholar]
- 6.Rodig SJ, Abramson JS, Pinkus GS, Treon SP, Dorfman DM, Dong HY, Shipp MA, Kutok JL. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H) Clin Cancer Res. 2006;12:7174–9. doi: 10.1158/1078-0432.CCR-06-1275. [DOI] [PubMed] [Google Scholar]
- 7.Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res. 1998;22:185–91. doi: 10.1016/s0145-2126(97)00158-6. [DOI] [PubMed] [Google Scholar]
- 8.Domagala A, Kurpisz M. CD52 antigen – a review. Med Sci Monit. 2001;7:325–31. [PubMed] [Google Scholar]
- 9.Rowan WC, Hale G, Tite JP, Brett SJ. Cross-linking of the Campath-1 antigen (CD52) triggers activation of normal human T lymphocytes. Int Immunol. 1995;7:69–77. doi: 10.1093/intimm/7.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Masuyama J-I, Yoshio T, Suzuki K, et al. Characterization of the 4C8 antigen involved in transendothelial migration of CD26hi T cells after tight adhesion to human umbilical vein endothelial cell monolayers. J Exp Med. 1999;189:979–89. doi: 10.1084/jem.189.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, Masuyama J-I, Sohma Y, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin Immunol. 2006;120:247–59. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Zent CS, Secreto CR, LaPlant BR, et al. Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early-intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab. Leuk Res. 2008;32:1849–56. doi: 10.1016/j.leukres.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golay J, Manganini M, Rambaldi A, Introna M. Effect of alemtuzumab on neoplastic B cells. Haematologica. 2004;89:1476–83. [PubMed] [Google Scholar]
- 14.Cruz RI, Hernandez-Ilizaliturri FJ, Olejniczak S, et al. CD52 over-expression affects rituximab-associated complement-mediated cytotoxicity (CMC) but not antibody-dependent cellular cytotoxicity (ADCC): Pre-clinical evidence that targeting CD52 with alemtuzumab may reverse acquired resistance to rituximab in non-Hodgkin’s lymphoma (NHL) Leuk Lymphoma. 2007;48:2424–36. doi: 10.1080/10428190701647879. [DOI] [PubMed] [Google Scholar]
- 15.Rowan W, Tite J, Topley P, Brett SJ. Cross-linking of the CAMPATH-1 antigen (CD52) mediates growth inhibition in human B- and T-lymphoma cell lines, and subsequent emergence of CD52-deficient cells. Immunology. 1998;95:427–36. doi: 10.1046/j.1365-2567.1998.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smolewski P, Szmigielska-Kaplon A, Cebula B, Jamroziak K, Rogalinska M, Kilianska Z, Robak T. Proapoptotic activity of alemtuzumab alone and in combination with rituximab or purine nucleoside analogues in chronic lymphocytic leukemia cells. Leuk Lymphoma. 2005;46:87–100. doi: 10.1080/13693780400007151. [DOI] [PubMed] [Google Scholar]
- 17.Mone AP, Cheney C, Banks AL, et al. Alemtuzumab induces caspase-independent cell death in human chronic lymphocytic leukemia cells through a lipid raft-dependent mechanism. Leukemia. 2006;20:272–9. doi: 10.1038/sj.leu.2404014. [DOI] [PubMed] [Google Scholar]
- 18.Reiff A. A review of Campath in autoimmune disease: biologic therapy in the gray zone between immunosuppression and immunoablation. Hematology. 2005;10:79–93. doi: 10.1080/10245330400026139. [DOI] [PubMed] [Google Scholar]
- 19.Brett S, Baxter G, Cooper H, Johnston JM, Tite J, Rapson N. Repopulation of blood lymphocyte sub-populations in rheumatoid arthritis patients treated with the depleting humanized monoclonal antibody, CAMPATH-1H. Immunology. 1996;88:13–19. doi: 10.1046/j.1365-2567.1996.d01-650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis. Evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 21.Cox AL, Thompson SA, Jones JL, Robertson VH, Hale G, Waldmann H, Compston DAS, Coles AJ. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005;35:3332–42. doi: 10.1002/eji.200535075. [DOI] [PubMed] [Google Scholar]
- 22.Coles AJ, Compston DAS, Selmaj KW, Lake SL, Moran S, Margolin DH, Norris K, Tandon PK. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan JM, Smith AE. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum Gene Ther. 1997;8:1095–104. doi: 10.1089/hum.1997.8.9-1095. [DOI] [PubMed] [Google Scholar]
- 24.Bindon CI, Hale G, Waldmann H. Importance of antigen specificity for complement-mediated lysis by monoclonal antibodies. Eur J Immunol. 1988;18:1507–14. doi: 10.1002/eji.1830181006. [DOI] [PubMed] [Google Scholar]
- 25.Buggins AG, Mufti GJ, Salisbury J, et al. Peripheral blood but not tissue dendritic cells express CD52 and are depleted by treatment with alemtuzumab. Blood. 2002;100:1715–20. [PubMed] [Google Scholar]
- 26.Simpson BS, Coles AJ. Rationale for cytotoxic monoclonal antibodies in MS. Int MS J. 2007;14:48–56. [PubMed] [Google Scholar]
- 27.Isaacs JD, Greer S, Sharma S, et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged and profound therapy-induced lymphopenia. Arthritis Rheum. 2001;44:1998–2008. doi: 10.1002/1529-0131(200109)44:9<1998::AID-ART348>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Coles AJ, Wing M, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1995;354:1691–5. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 29.Lowenstein H, Shah A, Chant A, Khan A. Different mechanisms of Campath-1H-mediated depletion for CD4+ and CD8+ T cells in peripheral blood. Transpl Int. 2006;19:927–36. doi: 10.1111/j.1432-2277.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 30.Wing MG, Moreau T, Greenwood J, et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest. 1996;98:2819–26. doi: 10.1172/JCI119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, Czuczman MS. Neutrophils contribute to the biological antitumor activity of Rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9:5866–73. [PubMed] [Google Scholar]
- 32.Hernandez-Ilizaliturri FJ, Jupudy V, Reising S, Repasky E, Czuczman MS. Concurrent administration of granulocyte colony-stimulating factor or granulocyte-monocyte colony-stimulating factor enhances the biological activity of rituximab in a severe combined immunodeficiency mouse lymphoma model. Leuk Lymphoma. 2005;46:1775–84. doi: 10.1080/17402520500182329. [DOI] [PubMed] [Google Scholar]