Abstract
The design, synthesis and structural analysis of a new foldamer that mimics an extended β-sheet is presented. The non-peptidic mimetic is based on a series of 2,2-disubstituted-indolin-3-one groups linked through their 4,7 positions by an alkyne spacer. An intramolecular hydrogen bond between the carbonyl of one indolinone subunit and the proximal NH of another subunit imposes a conformation that mimics the side chain positioning on a β-strand. X-ray crystallographic studies support the presence of this intramolecular hydrogen bond. The described approach allows extension of the scaffold to longer oligomers that will form the basis of new mimetics for the disruption of therapeutically relevant protein-protein interactions that rely on the contacts of side chain residues on two β-strands.
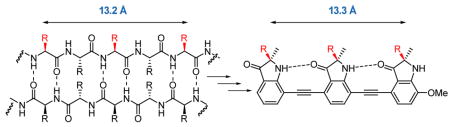
In recent years there has been much interest in the development of synthetic mimics of protein secondary structure. These molecules have the potential to act as effective agents in the disruption of protein-protein interactions involved in disease processes. In particular, substantial progress has been made in the mimicry of α-helix structure and function with several innovative peptidic and non-peptidic scaffolds that reproduce the distance and angular projection of side chains on the helix.1 Surprisingly, less work has been done towards the development of β-strand and β-sheet mimetics. Early examples have been based on conformationally constrained scaffolds that mimic an extended peptide.2, 3 In general, these approaches utilize a rigid template that stabilizes β-sheet structure between the attached peptide strands. The early work of Feigel showed that a biphenyl template can be used to induce β-sheet formation between two attached peptides.4 This subsequently led to the design of templates such as epindolidiones5, dibenzofurans6, metallopeptides7, and oligoureas8 each capable of positioning the peptides such that interstrand hydrogen bonding can occur. There is only one example of a truly non-peptidic β-strand mimetic which is the oligopyrrolinone scaffold from Smith.9
As part of our interest in protein-protein interactions we sought to extend our synthetic approach to α-helix mimics to stretches of protein in a β-strand or β-sheet conformation. One biologically significant example of edge-edge contact between two β-strands is seen with the oncoprotein Ras (or related Rap1A) and one of its key effector proteins, Raf1 kinase (Figure 1) (pdb 1GUA).10 Attenuation of Ras function is a major focus in the development of new cancer therapeutics and reinforces the need for structural mimetics that are able to disrupt this protein-protein interaction. In this paper we present the design, synthesis and structural analysis of a novel and potentially extendable foldamer that both mimics the rigidity and side chain position on β-strands and can be further applied to the inhibition of therapeutically relevant interactions such as Rap1A/Raf1.
Figure 1.
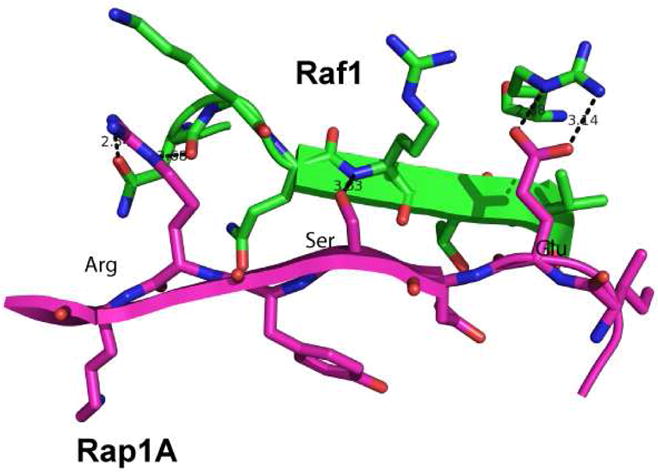
Part of the Rap1A/Raf1 protein-protein interaction interface.10
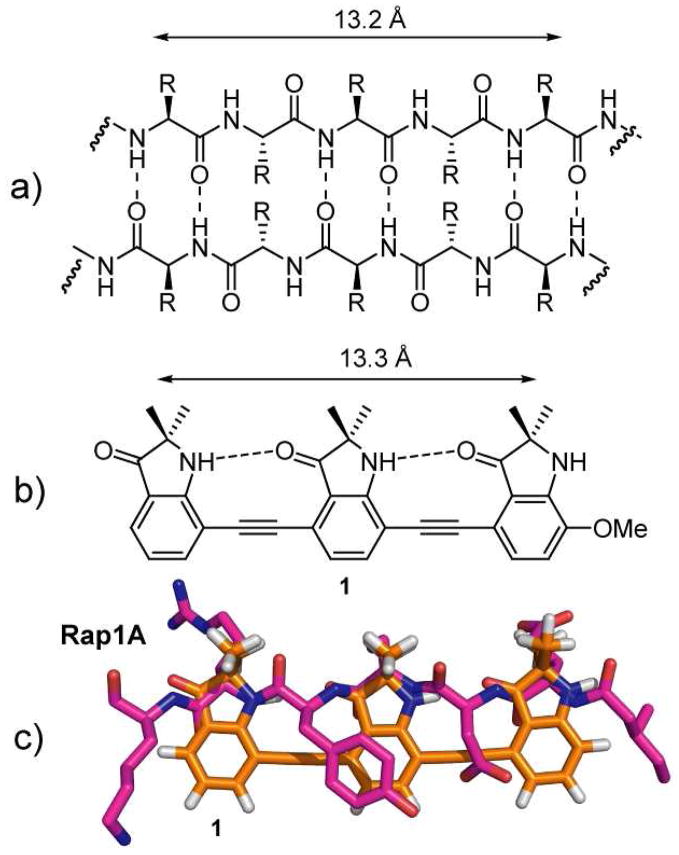
Our design is based on a series of 2,2-disubstituted-indolin-3-one groups linked through their 4,7-positions by an alkyne spacer. The spacing in this molecule allows the formation of an intramolecular hydrogen bond between the carbonyl of one indolinone and the proximal NH of another subunit, enforcing the desired conformation which places all of the substituents on the same side of the molecule. This expectation is supported by energy minimization using the TAFF force field (MOE V2008.10) which predicts the adoption of a planar conformation stabilized by an intramolecular hydrogen bond. Previous work by Kemp11 and Gong12 has shown that 2-amido-2′-carboxamide functionalized diphenylacetylenes form an intramolecular hydrogen bond between adjacent subunits. The i and i + 4 residues of a β-strand found within a β-sheet are 13.2 Å apart3 and the distance between the first and third gem-dimethyl substituents in the energy minimized tris-indolin-3-one corresponds almost exactly to this distance (Figure 2a and b). A superimposition of the tris-indolin-3-one scaffold onto the interacting β-strand of Rap1A shows an almost exact distance alignment of the three methyl substituents with the side chains of the peptide (Figure 2c).
Figure 2.
a) Antiparallel β-sheet. b) Tris-indolin-3-one 1. c) Overlay of the core of 1 with the Rap1A peptide.
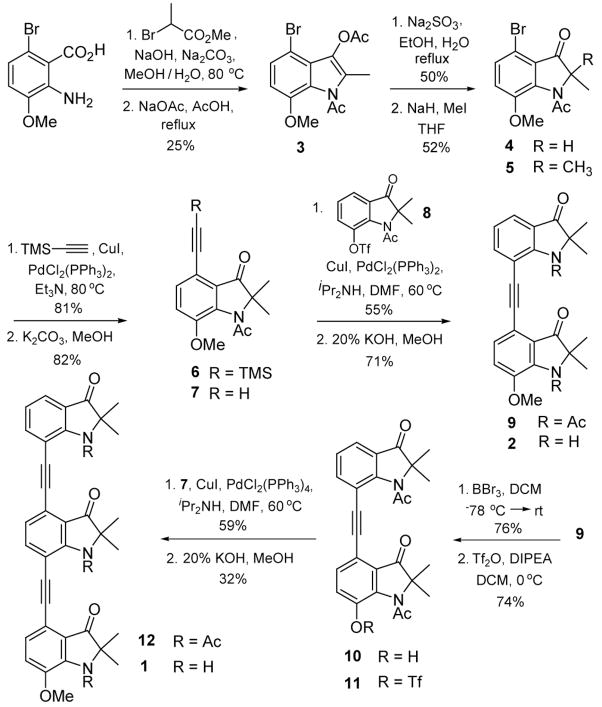
The synthesis of the oligo indolin-3-ones is based on the monomeric unit 7 (Scheme 1). 2-amino-6-bromo-3-methoxy-benzoic acid13 was first alkylated with methyl-2-bromopropionate and then cyclized under Dieckmann conditions to provide 3.14 Selective acetate hydrolysis led to the 2-monalkylated indolinone 4 which was reacted with methyl iodide under basic conditions to give 5.15 Sonogashira coupling followed by deprotection gave the monomeric unit 7 which can be used in the preparation of oligomers of varying lengths. The des-bromo analog of 5 was demethylated and treated with triflic anhydride to afford 8 which was coupled with 7 to give the acetyl protected bis-indolin-3-one 9.16 Deprotection with potassium hydroxide in methanol gave the bis-indolin-3-one 2.17
Scheme 1.
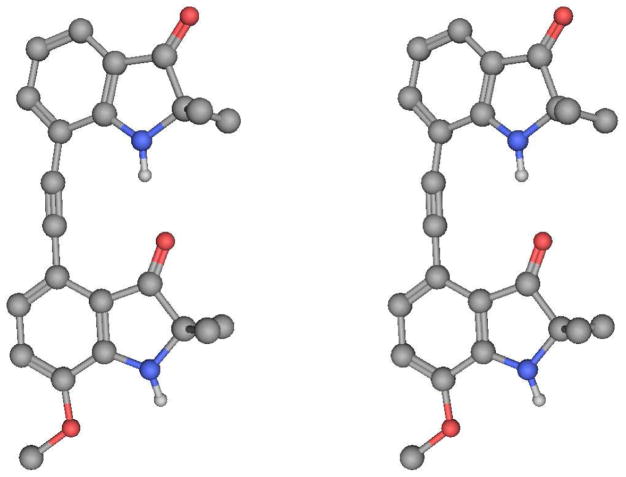
A crystal structure of 2 (Figure 3) confirmed the presence of an intramolecular hydrogen bond between the C=O of one indolinone subunit and the NH of another with H…O and N…O distances of 1.89 and 2.82 Å, respectively, and an NH…O angle of 167.73°. Corresponding values in the second independent molecule for the H…O and N…O distances are 1.88 and 2.86 Å, respectively, and an NH…O angle of 173.19°. In the solid state the hydrogen bond causes a slight bend of the phenylacetylene bond away from linearity (C-C-C, 12°).
Figure 3.
X-ray structure of 2 in stereoview.
The existence of the hydrogen bond in solution was confirmed by variable temperature (VT) NMR studies. At room temperature the bis-indolin-3-one has two NH peaks in the NMR spectrum, one downfield (8.08 ppm in CDCl3 and 8.20 ppm in d6-DMSO) and one upfield (4.84 ppm in CDCl3 and 7.72 ppm in d6-DMSO). The temperature dependence coefficients in d6-DMSO were Δδ/ΔT= −4.9 ppb K−1 for the downfield resonance and Δδ/ΔT= −8.3 ppb K−1 for the upfield resonance. This large difference suggests that the former corresponds to the internal hydrogen bonded proton and the latter to the solvent exposed proton.
The power of this approach is that the scaffold can be readily extended to longer oligomers of indolin-3-one β-strand mimetics as illustrated in Scheme 1. Demethylation of 9 followed by triflation gave triflate 11 which was coupled with 7 to provide the acetyl protected tris-indolin-3-one 12. Deprotection of the three acetates provided the final β-strand mimetic 1.
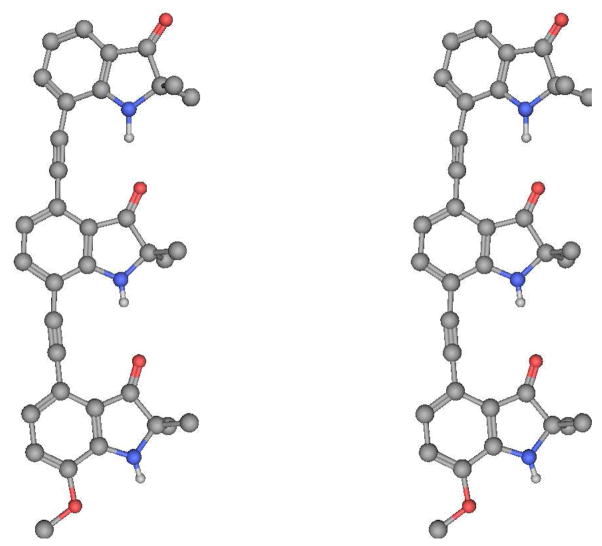
A crystal structure of 1 (Figure 4) supported the presence of two intramolecular hydrogen bonds between two of the three indolinone subunits. The H…O distances were 1.96 and 2.27 Å, the N…O distances were 2.98 and 3.12 Å, and NH…O angles of 167.58° and 170.44°.
Figure 4.
X-ray structure of 1 in stereoview.
We have reported a new foldamer scaffold based on 2,2-disubstituted indolinones in which intramolecular hydrogen bonding promotes a conformation that mimics the residues of a β-strand. The flexibility of the synthetic route to 1 will allow control over both the nature and stereochemistry of the indolinone 2-substituents. Unsymmetrical and accurate strand mimetics of this type are currently under study.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM35208) for financial support of this work, Dr. Chris Incarvito for his assistance with the X-ray crystallographic analysis, and Dr. Nathan T. Ross for helpful discussions.
Footnotes
Supporting Information Available: Experimental procedures, full characterization of all new compounds, and X-ray crystallographic analysis for 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Garner J, Harding MM. Org Biomol Chem. 2007;5(22):3577–3585. doi: 10.1039/b710425a. [DOI] [PubMed] [Google Scholar]
- 2.Glenn MP, Fairlie DP. Mini-Rev Med Chem. 2002;2(5):433–445. doi: 10.2174/1389557023405747. [DOI] [PubMed] [Google Scholar]
- 3.Loughlin WA, Tyndall JDA, Glenn MP, Fairlie DP. Chem Rev. 2004;104(12):6085–6117. doi: 10.1021/cr040648k. [DOI] [PubMed] [Google Scholar]
- 4.Brandmeier V, Sauer WHB, Feigel M. Helv Chim Acta. 1994;77(1):70–85. [Google Scholar]
- 5.Kemp DS, Bowen BR, Muendel CC. J Org Chem. 1990;55(15):4650–4657. [Google Scholar]
- 6.Diaz H, Espina JR, Kelly JW. J Am Chem Soc. 1992;114(21):8316–8318. [Google Scholar]
- 7.Ogawa MY, Gretchikhine AB, Soni SD, Davis SM. Inorg Chem. 1995;34(26):6423. [Google Scholar]
- 8.Nowick JS. Organic & Biomolecular Chemistry. 2006;4(21):3869–3885. doi: 10.1039/b608953b. [DOI] [PubMed] [Google Scholar]
- 9.Smith AB, Guzman MC, Sprengeler PA, Keenan TP, Holcomb RC, Wood JL, Carroll PJ, Hirschmann R. J Am Chem Soc. 1994;116(22):9947–9962. [Google Scholar]
- 10.Nassar N, Horn G, Herrmann C, Block C, Janknecht R, Wittinghofer A. Nat Struct Biol. 1996;3(8):723–729. doi: 10.1038/nsb0896-723. [DOI] [PubMed] [Google Scholar]
- 11.Kemp DS, Li ZQ. Tet Lett. 1995;36(24):4175–4178. [Google Scholar]
- 12.Yang XW, Yuan LH, Yamamoto K, Brown AL, Feng W, Furukawa M, Zeng XC, Gong BJ. Am Chem Soc. 2004;126(10):3148–3162. doi: 10.1021/ja039416d. [DOI] [PubMed] [Google Scholar]
- 13.Prinz W, Kayle A, Levy PR. J Chem Res-S. 1978;(3):116. [Google Scholar]
- 14.Galun A, Kampf A, Markus A. J Heterocycl Chem. 1979;16(4):641–643. [Google Scholar]
- 15.Kawasaki T, Nonaka Y, Kobayashi M, Sakamoto M. J Chem Soc-Perkin Trans. 1991;1(10):2445–2448. [Google Scholar]
- 16.Hiroya K, Suzuki N, Yasuhara A, Egawa Y, Kasano A, Sakamoto T. J Chem Soc-Perkin Trans. 2000;1(24):4339–4346. [Google Scholar]
- 17.Kawada M, Kawano Y, Sugihara H, Takei S, Imada I. Chem Pharm Bull. 1981;29(7):1900–1911. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.