Abstract
Background
The clinical relevance of exercise-induced pulmonary arterial hypertension (EIPAH) is uncertain, and its existence has never been well-studied by direct measurements of central hemodynamics. Using invasive cardiopulmonary exercise testing, we hypothesized that EIPAH represents a symptomatic stage of PAH, physiologically intermediate between resting pulmonary arterial hypertension and normal.
Methods and Results
406 consecutive clinically indicated cardiopulmonary exercise tests with radial and pulmonary arterial catheters and radionuclide ventriculographic scanning were analyzed. The invasive hemodynamic phenotype of EIPAH (n=78) was compared to resting PAH (n=15), and normals (n=16). Log-log plots of mean pulmonary artery pressure vs. oxygen uptake (VO2) were obtained, and a “join-point” for a least residual sum-of-squares for two straight-line segments (slopes m1, m2) was determined; m2 < m1 = “plateau”, and m2 > m1 = “takeoff” pattern. At maximum exercise, VO2 (55.8±20.3 vs. 66.5±16.3 vs. 91.7±13.7 % predicted) was lowest in resting PAH, intermediate in EIPAH, and highest in normals, whereas mean pulmonary artery pressure (48.4±11.1 vs. 36.6±5.7 vs. 27.4+3.7 mmHg) and pulmonary vascular resistance (294±158 vs. 161±60 vs. 62±20 dynes-sec/cm5, respectively, p<0.05) followed an opposite pattern. An EIPAH plateau (n=32) was associated with lower VO2max (60.6±15.1 vs. 72.0±16.1 %predicted) and maximum cardiac output (78.2±17.1 vs. 87.8±18.3 %predicted), and a higher resting pulmonary vascular resistance (247±101 vs. 199±56 dynes-sec/cm5, p<0.05) than takeoff (n=40). The plateau pattern was most common in resting PAH, while the takeoff pattern was present in nearly all normals.
Conclusions
EIPAH is an early, mild, and clinically relevant phase of the PAH spectrum.
Keywords: Circulation, Physiology, Hemodynamics, Pulmonary hypertension, Exercise
Pulmonary arterial hypertension (PAH) is defined by the NIH registry as a mean pulmonary artery pressure (mPAP) of > 25mmHg at rest or 30mmHg during exercise in the absence of pulmonary venous hypertension1. Exercise-induced PAH (EIPAH), which refers to the patient with normal mPAP at rest but greater than 30mmHg with exercise, is a poorly understood entity. Some believe that exercise-induced PAH is an early2 and more treatable3 phase that precedes resting PAH (RPAH), while others suggest it may be a stable variant4. A recent study of familial PAH relatives demonstrated that exercise-induced PAH can be asymptomatic5 or “preclinical”6 while others have described associated exertional intolerance7-10. Whether exercise-induced PAH inexorably progresses to resting PAH is unknown3, and whether or not to treat10 is controversial.
Most previous descriptions of exercise-induced PAH have been non-invasive, using stress Doppler transthoracic echocardiography7-10. Although an established screening modality for resting PAH11, echocardiography has not been well validated during exercise, when it is technically difficult to accomplish and associated with unique pitfalls. Specifically, the components of pulmonary vascular resistance, which are critical for an accurate diagnosis of pulmonary vasculopathy, cannot be measured directly by stress echocardiography.
There have been surprisingly few direct invasive studies of exercise-induced PAH. Three recent such investigations3, 12, 13 of a total of 29 patients with suspected PAH found the exercise-induced variant in five, but all were limited by the failure to exclude pulmonary venous hypertension.
In the current study, we for the first time fully-characterize exercise-induced PAH in a large group of symptomatic patients with direct measurements of central hemodynamics at rest and during maximum cardiopulmonary exercise testing (CPET). We demonstrate that the pattern and severity of the central hemodynamic response to exercise in exercise-induced PAH is intermediate between that of the normal subject and the patient with resting PAH and provide support for the hypothesis that exercise-induced PAH is a mild yet symptomatic phase of the disease.
Methods
Patients
Four-hundred six complete CPETs performed over a three-year period in the Massachusetts General Hospital Cardiopulmonary Exercise Laboratory, with radial and pulmonary arterial catheters in place and radionuclide ventriculographic scanning, were analyzed. The study was approved by the Partners Human Research Committee. The CPETs were clinically indicated, with the majority ordered for evaluation of dyspnea or fatigue of unclear etiology, or as part of an evaluation for cardiac or pulmonary transplantation.
Cardiopulmonary Exercise Testing
Pulmonary gas exchange and minute ventilation (VE) were measured breath-by-breath using a commercially available metabolic cart (Medical Graphics Corporation CPX/D, St. Paul, MN). The pneumotachograph was calibrated using a 3L syringe at five different flow rates, and the zirconia cell O2 analyzer and single-beam CO2 analyzer were calibrated with room air and a 5% CO2/12% O2 gas. Radial and pulmonary artery catheters (Edwards Scientific, Irvine, CA) were placed using standard techniques, the latter using the internal jugular approach. Systemic and pulmonary artery pressures were measured with HP1290A quartz pressure transducers (Hewlett-Packard Co., Andover, MA). Transducers were interfaced with MT95K2 recorder (Astro-Med Inc., W. Warwick, RI), and mean end-expiratory values were obtained for right atrial (RAP), mPAP, and mean systemic arterial pressure (MAP). Two-ml samples of systemic and pulmonary arterial blood were obtained at rest and during exercise and analyzed at 37 degrees for pO2, pCO2, pH (Model 1620; Instrumentation Laboratories, Lexington, MA), hemoglobin concentration [Hb], and O2 saturation with O2 content calculated from the latter two (Model 482; Instrumentation Laboratories). Right and left ventricular ejection fractions (RVEF, LVEF) and left ventricular end-diastolic volume were measured at rest and near peak exercise by a first-pass cardiac radionuclide scan (Phillips Medical Systems, Valhalla, NY) whose methodology is described elsewhere14.
All patients completed a single bout of incremental cycling (Medical Graphics CPE 2000) exercise to exhaustion. Two minutes of rest were followed by two minutes of unloaded cycling. Work was then continuously increased by 6.25-25 Watts per minute based on history of exertional tolerance. MAP and end-expiratory RAP and mPAP were measured continuously. End-expiratory pulmonary capillary wedge pressure (PCWP) was obtained at rest and during each minute of exercise. Central pressures associated with an end-expiratory pleural pressure swing that is greater than 10mmHg were excluded, or in select cases, incremental exercise was replicated with an esophageal balloon in place, and end-expiratory pleural pressures subtracted. Two ml blood samples were simultaneously drawn from the radial and pulmonary arterial catheters during rest and the last 15 seconds of each minute of exercise. At cessation of exercise, patients were asked which of the following symptoms caused them to stop: shortness of breath, leg fatigue or pain, chest pain, alone or in combination.
Data Analysis
Ventilatory and pulmonary gas exchange data were averaged for the final thirty seconds of the two-minute rest period and over contiguous 30-second intervals during exercise. Predicted values for VO2max utilizing age, gender, and height, were those of Hansen and colleagues15. The ventilatory threshold was determined by the V-slope method16. VE/VCO2 was measured at the ventilatory threshold. Qt was calculated from the Fick Principle Qt = VO2/(Ca-vO2). Predicted maximal Qt was calculated from predicted VO2max and an assumed arterial-venous O2 content difference = ([Hb] × 10)17. PVR was calculated from (mPAP-PCWP)/Qt.
Peak heart rate ≥ 80% of predicted or peak respiratory exchange ratio (RER) ≥ 1.00 were used as indicators of maximum effort. At maximum exercise, PAH was defined as a mPAP ≥ 30mmHg, PCWP < 20mmHg18, and PVR ≥ 80 dynes-sec/cm5 19. PAH was subdivided subsequently into: a) RPAH, indicating a mPAP ≥25 mmHg but a PCWP ≤ 15 mmHg at rest and b) EIPAH, with a resting mPAP < 25mmHg. At maximum exercise, pulmonary venous hypertension (PVH) was defined as PCWP ≥ 20 mmHg. LV systolic dysfunction was defined as PVH with LVEF < 0.55, and LV diastolic dysfunction (LVDD) as PVH with LVEF ≥ 0.55. Peripheral limitation was defined as VO2max < 70% of predicted with Qtmax > 80% of predicted and Ca-vO2 < [Hb]. Normal, including detrained, subjects were defined by a VO2max ≥ 70% predicted and who met none of the abnormal CPET diagnostic criteria above. All others were excluded, including those with a primary pulmonary mechanical limitation (VE/MVV > 0.7 at the ventilatory threshold20).
Statistical programs included Excel and GraphPad Prism. Central tendencies were expressed as mean ± standard deviation and compared by ANOVA with Neuman-Kels finishing test, or unpaired t-tests. Continuous variables were analyzed by linear regression. A log-log plot of mPAP vs.VO2 was obtained for all patients and the VO2 “join-point” for a least residual sum of squares for two straight-line segments determined16, 21. 95% confidence intervals for the slopes of the first (m1) and second (m2) linear regressions were compared; m2 < m1 was classified as a “plateau”, and m2 > m1 as “takeoff” pattern. Identical analyses were performed for mPAP vs. Qt and RAP vs. VO2. A p value < 0.05 was considered significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Patient Demographics
Of the 406 patients, the indication for testing was known in 340. Of these, 255 (75%) were referred for invasive exercise testing for dyspnea of uncertain etiology, 22 (6%) for fatigue, 28 (8%) to differentiate a cardiac from pulmonary limit to exercise, 28 (8%) had known left-ventricular systolic dysfunction and were being considered for cardiac transplantation, and 7 (2%) had other indications. Thus, 305 patients (90%) were referred to clarify the etiology of exertional intolerance.
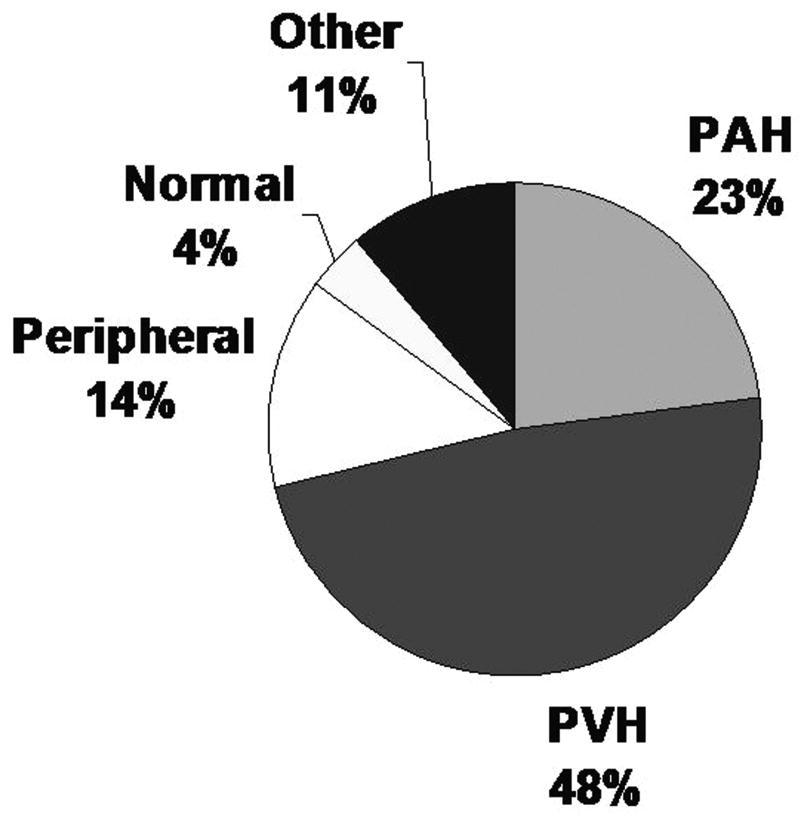
All exercise tests were symptom-limited; no exercise test was stopped by the supervising technician or physician, and there were no complications related to the pulmonary artery catheter or exercise testing. Patients stopped cycling due to either shortness of breath, leg fatigue, or both, with only one patient, in the normal group, additionally experiencing chest pain. CPET diagnoses included 93 cases (23%) of PAH, 196 cases (48%) of PVH or other cardiac limitation, 55 (14%) peripheral limitation, 16 (4%) normal and 46 (11%) other (Fig. 1). Of the 93 PAH cases, 78 had exercise-induced PAH, and 15 had resting PAH. Of the 255 cases referred for dyspnea of uncertain etiology, the two most common CPET diagnoses were PAH (n=86) and left-ventricular diastolic dysfunction (n=69).
Figure 1.
CPET diagnoses. PVH: pulmonary venous hypertension; PAH: pulmonary arterial hypertension
PAH vs. Normal
Thus, 109 subjects constituted the normal and PAH study populations as defined in the Methods. 106 of 109 subjects (except once in the exercise-induced PAH group and two in the normal group) demonstrated adequate maximum effort as defined by peak heart rate ≥ 80% of predicted or peak RER ≥ 1.00.
Exercise-induced PAH and resting PAH groups were older when compared to normal (Table 1). At maximum exercise, VO2max (% predicted), Qtmax (% predicted), RVEF, P(A-a)O2, mPAP and PVR were highest in resting PAH, lowest in normals and intermediate in exercise-induced PAH, while PCWP and RAP were not different. There was no difference in ventilatory efficiency, as measured by VE/VCO2 at the ventilatory threshold, between the normal and exercise-induced PAH groups, while the resting PAH group had a significantly higher value then both.
Table 1. Demographic and CPET Variables.
| Normal (n=16) | EIPAH (n=78) | RPAH (n=15) | |
|---|---|---|---|
| Age (years) | 45.9±14.9 | 58.8±15.1 * | 58.5±15.7 * |
| Female gender (%) | 68.8 | 65.8 | 46.7 |
| BMI | 25.5±4.2 | 30.2±5.3 * | 28.1±6.2 |
| Work max (watts) | 155.5±43.1 | 90.3±41.7 * | 70.0±41.5 * |
| VO2 max (ml/min) | 2022±468 | 1284±58 * | 1127±507 * |
| VO2 max (% predicted) | 91.7±13.7 | 66.5±16.3 * | 55.8±20.3 *, † |
| P(A-a)O2 max (mmHg) | 14.7±7.6 | 32.0±18.0 * | 52.7±17.3 *, † |
| CaO2 (mg/ml) | 19.0±1.2 | 18.0±2.5 | 16.8±3.3 |
| PaCO2 max (mmHg) | 32.9±4.4 | 35.1±6.1 | 37.1±7.6 |
| VCO2 max (ml/min) | 2380±722 | 1561±705 * | 1310±626 * |
| mPAP rest (mmHg) | 13.9±2.9 | 18.6±3.2 * | 30.9±8.9 *, † |
| mPAP max (mmHg) | 27.4±3.7 | 36.6±5.7 * | 48.4±11.1 *, † |
| PCWP max (mmHg) | 14.8±4.5 | 15.0±2.4 | 15.2±3.1 |
| Qt max (l/min) | 15.5±3.2 | 11.4±3.0 * | 10.4±3.6 * |
| Qt max (% predicted) | 99.4±11.1 | 83.1±18.9 * | 71.8±22.4 *, † |
| PVR rest (dynes-sec/cm5) | 154±61 | 223±82 * | 352±141 *, † |
| PVR max (dynes-sec/cm5) | 62±20 | 161±60 * | 294±158 *, † |
| RAP max (mmHg) | 9.1±3.5 | 9.6±3.0 | 11.0±6.1 |
| RVEF max | 0.58±0.06 | 0.53±0.08 * | 0.43±0.11 *, † |
| VE/VCO2 @ AT | 36.0±8.9 | 37.8±8.0 | 43.1±6.9 * |
p < 0.05 vs. Normal;
p < 0.05 vs. EIPAH
Legend: EIPAH: exercise-induced pulmonary arterial hypertension; RPAH: resting pulmonary arterial hypertension; BMI: body mass index; VO2: oxygen consumption; P(A-a)O2: alveolar-arterial difference in partial pressure of O2; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; Qt: cardiac output; PVR: pulmonary vascular resistance; RAP: right atrial pressure; RVEF: right ventricular ejection fraction; VE: minute ventilation; VCO2: carbon dioxide production
Takeoff vs. Plateau Patterns of mPAP vs. VO2
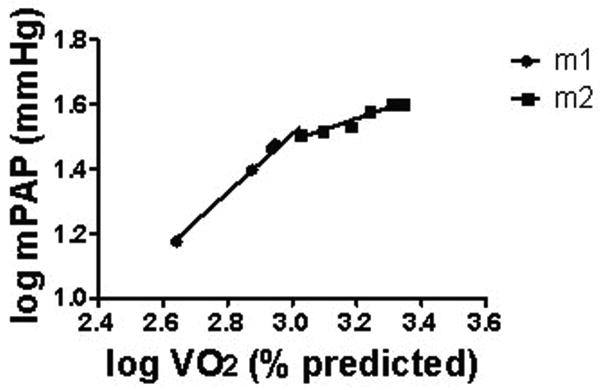
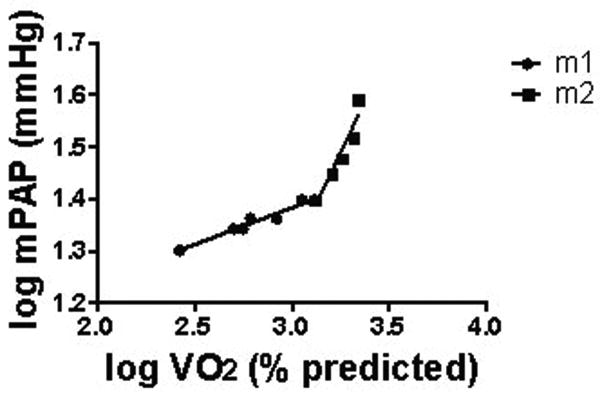
Figures 2 and 3 depict representative plateau and takeoff patterns of mPAP vs.VO2, respectively. For normals, of 15 interpretable mPAP vs. VO2 log-log plots, 14 demonstrated a takeoff and one a plateau pattern. Of the 78 patients with exercise-induced PAH, 32 had a plateau, 40 had takeoff pattern, and six were uninterpretable. In the resting PAH group, 9 demonstrated plateau pattern, 2 demonstrated takeoff pattern, and four were uninterpretable. mPAP vs. Qt log-log patterns were highly concordant with mPAP vs. VO2 log-log patterns for all groups.
Figure 2.
Representative plateau pattern of log-log plot of mPAP vs. VO2 in EIPAH.
Figure 3.
Representative takeoff pattern of log-log plot of mPAP vs. VO2 in EIPAH.
For the exercise-induced PAH group, a plateau pattern was associated with a reduced maximum exercise work, VO2, and Qt. There was no difference in mPAP, RVEF or P(A-a)O2 at peak exercise. The resting PVR was higher, with a trend towards higher PVR at peak exercise, in those with a plateau pattern (Table 2).
Table 2. EIPAH: mPAP Takeoff vs. Plateau Patterns.
| Plateau (n=32) | Takeoff (n=40) | P value | |
|---|---|---|---|
| Age (years) | 57.7±15.9 | 59.6±13.9 | NS |
| Female gender (%) | 69 | 63 | NS |
| BMI (kg/m2) | 31.5±5.9 | 29.4±5.2 | NS |
| Work max | 79.2±38.3 | 101.7±41.0 | <0.05 |
| VO2 max (ml/min) | 1207±507 | 1373±516 | NS |
| VO2 max (% predicted) | 60.6±15.1 | 72.0±16.1 | <0.01 |
| P(A-a)O2 max (mmHg) | 36.1±16.9 | 28.8±18.1 | 0.09 |
| PvO2 (mmHg) | 23.8±3.7 | 22.6±4.2 | NS |
| pHv | 7.29±0.04 | 7.28±0.05 | NS |
| mPAP rest (mmHg) | 19.1±3.5 | 17.8±2.7 | NS |
| mPAP max (mmHg) | 35.9±4.2 | 37.1±6.8 | NS |
| PCWP max (mmHg) | 15.1±2.8 | 15.2±2.7 | NS |
| Qt max (l/min) | 10.8±3.3 | 12.0±2.8 | 0.10 |
| Qt max (% predicted) | 78.2±17.1 | 87.8±18.3 | <0.05 |
| PVR rest (dynes-sec/cm5) | 247±101 | 199±56 | <0.05 |
| PVR max (dynes-sec/cm5) | 174±76 | 150±45 | 0.12 |
| RAP max (mmHg) | 9.1±3.1 | 9.9±2.9 | NS |
| RVEF max | 0.54±0.08 | 0.53±0.07 | NS |
| RV/LV stroke counts ratio | 0.8±0.2 | 0.8±0.1 | NS |
Legend: BMI: body mass index; VO2: oxygen consumption; P(A-a)O2: alveolar-arterial difference in partial pressure of O2; PvO2: mixed venous pO2; pHv: mixed venous pH; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; Qt: cardiac output; PVR: pulmonary vascular resistance; RAP: right atrial pressure; RVEF: right ventricular ejection fraction; RV: right ventricle; LV: left ventricle
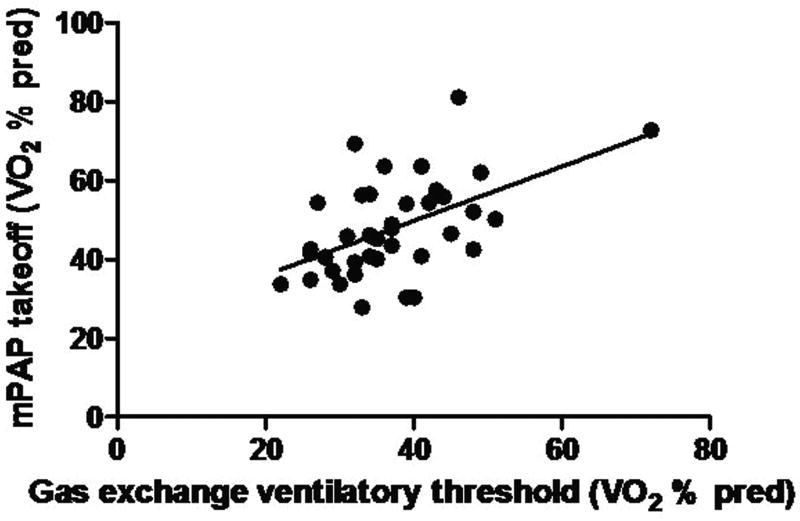
For those with takeoff patterns, there was a significant relationship between the VO2 at the mPAP breakpoint and that for the ventilatory threshold (p<0.05, r = 0.52, Fig. 4). No such relationship was found for those with plateau patterns (p=0.40, r = 0.17).
Figure 4.
mPAP takeoff vs. ventilatory threshold, p < 0.05, r = 0.52
Takeoff and plateau patterns of RAP vs. VO2 were equally represented in the two groups. There was no difference in RVEF or RV/LV stroke counts ratio.
Follow-up data
There were five subjects with CPET physiologic diagnoses of exercise-induced PAH who underwent a repeated clinically-indicated invasive exercise test following the same protocol. The time to re-test was 29.8±10.7 months. Both diagnoses and treatment regimens were heterogeneous (Table 3). At peak exercise, there was a non-significant decrease in VO2max (69.8±20.2 to 61.2±21.9 % predicted) that was associated with a similar change in Qtmax (86.4±25.6 to 80.0±23.8 % predicted, p>0.05 for both), but with no change in central hemodynamics (mPAP: 38.4±4.3 to 37.2±7.5 mmHg; PVR: 175±79 to 131±29 dynes-sec/cm5, p>0.05 for both).
Table 3. Patient Diagnosis and Treatment at times of 1st and 2nd CPETs.
| Pt | Diagnosis | CPET-1 | Between CPETs | CPET-2 |
|---|---|---|---|---|
| 1 | Congenital heart disease | None | None | None |
| 2 | Idiopathic | None | Bosentan → Sildenafil | Sildenafil |
| 3 | Idiopathic | None | None | None |
| 4 | Mitral valve disease | None | None | None |
| 5 | Idiopathic | None | Bosentan → Sildenafil | Sildenafil |
Legend: CPET: Cardiopulmonary exercise test
Discussion
We have described a large subset of symptomatic patients who meet the NIH registry criteria for PAH during exercise yet do not have an elevated mean pulmonary artery pressure at rest. At maximum exercise, their overall aerobic capacity and central hemodynamics lie between those of the normal subject and the patient with resting pulmonary artery hypertension. In these patients, it is only under the stress of incremental cycling exercise that evidence of a pulmonary vasculaopthy is uncovered.
Prior Studies
Most previous studies describing exercise-induced PAH have utilized noninvasive techniques, particularly stress Doppler trans-thoracic echocardiography7-10. Although echocardiography has emerged as a useful screening modality for resting PAH11, it has not been well validated during exercise, where there are significant methodological concerns. For instance, during incremental exercise, right atrial pressure normally rises well beyond the usual assumed five mmHg18, 22. Right atrial pressure has been estimated at rest by trans-thoracic echocardiography based on inspiratory changes in inferior vena cava caliber23, but it has never been validated during exercise when venous compliance is known to decrease24. Second, the contribution of the pulmonary capillary wedge pressure to an exercise-induced RVSP rise cannot be assessed directly with trans-thoracic echocardiography. Pulmonary capillary wedge pressure has been estimated by echocardiography at rest25 but not during exercise. The former may be critical given that many suspected cases of PAH based on echocardiography actually have pulmonary venous hypertension, especially in the elderly26. In the current study, exercise-induced PAH and left-ventricular diastolic dysfunction represented the two largest categories of unexplained exertional intolerance. At the present time, careful distinction of the two is important, as resulting treatment is usually quite different. Finally, the influence of cardiac output on the right-ventricular systolic pressure, and therefore the pulmonary vascular resistance response to exercise, cannot be directly measured by echocardiography. Estimates of cardiac output and pulmonary vascular resistance have been recently made by trans-thoracic echocardiography at rest 25, but they have not yet been validated during exercise. The latter is important given a wide range of normal right-ventricular systolic pressure at peak exercise, especially in the well-trained athlete27. In such individuals, both right-ventricular systolic pressure and mPAP may be high at peak exercise, but the fall in pulmonary vascular resistance remains totally normal18. Thus, although stress echocardiography holds promise in the diagnosis of exercise-induced PAH, it remains to be validated by direct measurements of central hemodynamics.
Non-invasive cardiopulmonary exercise testing has also been shown to be useful in the diagnosis and assessment the severity of resting PAH28-30. Certain noninvasive exercise testing parameters correlate with an exercise-induced rise in right-ventricular systolic pressure by trans-thoracic echocardiography in breathless patients31. Our laboratory has recently validated a non-invasive CPET diagnostic algorithm for PAH with direct central hemodynamic measurements32. We and others13, 33 have described CPET-induced exaggerated rise of pulmonary arterial pressure in resting PAH.
We know of three recent studies in the English literature employing pulmonary arterial catheters that describe possible exercise-induced PAH. Raeside, et al.3, 13, reported exercise-induced PAH in two of a total of 16 patients with connective tissue disease or idiopathic PAH who cycled at 30% of VO2max. They did not, however, include measurements of pulmonary capillary wedge pressure during exercise, and pulmonary vascular resistance is therefore unknown. James and co-workers12 described three patients with unexplained exertional dyspnea who had normal resting mPAP and an exaggerated rise at peak cycling exercise. The mean pulmonary capillary wedge pressure at peak exercise in that study was > 20mmHg, however, raising the possibility that some of the patients had diastolic heart failure.
Clinical Significance
The current study confirms, with direct measurements of central hemodynamics during maximum cardiopulmonary exercise testing, that exercise-induced PAH exists and is associated with exertional symptoms. As noted above, the two most common CPET diagnoses in patients referred for dyspnea of uncertain etiology were exercise-induced PAH and left-ventricular diastolic dysfunction. Because all of our patients were referred for symptoms, we can shed no light on whether there are additional patients with exercise-induced PAH who may be asymptomatic or preclinical5, 6.
Our data support the notion that exercise-induced PAH represents a mild, intermediate physiologic stage of PAH. At maximum exercise, there was evidence of intermediate exercise capacity in exercise-induced PAH, in terms of VO2max (% predicted), and measurements of cardiac function, as measured by Qtmax (% predicted) and RVEFmax. Likewise, central hemodynamics, namely mPAP and pulmonary vascular resistance at both rest and peak exercise, and peak alveolar-arterial PO2 difference, a hallmark of abnormal diffusion in diseased pulmonary vasculature, were highest in resting PAH, lowest in normals and intermediate in exercise-induced PAH.
The takeoff pattern of mPAP vs. VO2 during exercise testing is most commonly seen in the normal and less severe exercise-induced PAH patients, as measured by maximum exercise VO2 and cardiac output. Conversely, the plateau pattern of mPAP is typical of the more severely affected exercise-induced PAH patients and of those with resting PAH. Interestingly, Wonisch and associates34 found a similar plateau of invasively measured right-ventricular systolic pressure in resting PAH. This suggests a continuum of pulmonary vascular responses to exercise, beginning with the normal takeoff pattern, moving through two stages of exercise-induced PAH, and finally reaching the plateau pattern of resting PAH.
If exercise-induced PAH is an early phase of pulmonary arterial hypertension, screening and early detection might facilitate treatment aimed at preventing progression to resting PAH. In our study, exercise parameters, such as VE/VCO2 at the ventilatory threshold and peak P(A-a)O2, were not sufficiently sensitive in to distinguish EIPAH from normal. Likewise, resting mPAP, including those in an “indeterminate” range of 21-25mmHg, did not reliably predict exercise-induced PAH. Thus, at the moment, screening for exercise-induced PAH seems best accomplished with invasive exercise testing.
We do not yet have systematic longitudinal follow-up of our exercise-induced PAH patients. However, our limited longitudinal data suggest that exercise-induced PAH patients may remain relatively stable from a clinical and hemodynamic standpoint over several years. Conversely, two patients have been described elsewhere with progressive systemic sclerosis and echocardiographically-diagnosed exercise-induced PAH progressing to resting PAH over a two year period10. Clearly, long-term clinical and hemodynamic follow-up is much needed.
Potential Mechanisms
In the normal human, the increase in cardiac output from rest to maximum exercise far outweighs the slight widening of input and outflow pressure difference across the pulmonary vascular bed; i.e., pulmonary vascular resistance falls35, as a result of both passive and active pulmonary vascular recruitment and distension. Despite being referred for clinical symptoms, the normal subjects in this study were found to have normal oxygen uptake, central hemodynamics, and fall in pulmonary vascular resistance at peak exercise. These results are similar to those of two recent studies of healthy, asymptomatic, physically active males22, 36.
In long-standing pulmonary hypertension, intimal proliferation and fibrosis, medial hypertrophy, and in-situ thrombosis characterize the pathologic findings in the pulmonary vasculature, although at an earlier stage, changes may be confined to the small pulmonary arteries37-40. These changes, as well as the upstream sequelae such as right ventricular dysfunction, are time dependent and result in progressive symptoms41 and impairment of exercise tolerance28. Thus, it seems biologically plausible that patients with PAH of varying duration and severity will exhibit very different mPAP responses to exercise.
In this study, the takeoff pattern of mPAP suggests pulmonary vasoconstriction late during incremental exercise in normals and those with mild exercise-induced PAH. The phenomenon is similar to other well-described “thresholds” described during incremental exercise, including those of arterial blood lactate concentration, ventilation, CO2 output16, and humoral catecholamines. The correlation between the VO2 at mPAP takeoff and the ventilatory threshold in the current study suggests either a causal relationship or a shared underlying mechanism. Examples of the former include pulmonary arterial vasoconstrictive effects of desaturated and acid mixed-venous blood, neither of which was related to mPAP pattern in the current study. Alternatively, catecholamines have been postulated to both drive skeletal muscle glycolysis42 and potentiate pulmonary vascular resistance43. Interestingly, IL-6 is the one humoral cytokine that rises measurably in proportion to exercise intensity, has been related to catecholamines44, and may play a role in the genesis of pulmonary hypertension45. Alternatively, intimal proliferation and decreased compliance46 of the pulmonary vasculature could cause a takeoff of mPAP. However, given that normals most often exhibit a takeoff pattern and resting PAH patients do not, pulmonary vascular stiffness is an unlikely unifying explanation.
Conversely, the plateau pattern of mPAP was typical of more severely compromised exercise-induced PAH and of resting PAH patients. Potential mechanisms include exercise-induced right-ventricular dysfunction with or without tricuspid regurgitation. Lower cardiac output in the exercise-induced PAH plateau vs. takeoff groups, and more severe PAH with a depressed RVEF in resting PAH (dominated by the plateau pattern) support the former. RAP and LV/RV stroke count ratios did not suggest exercise-induced tricuspid regurgitation was responsible for the plateau pattern, however.
Limitations of the study
The principal CPET indication at our institution is unexplained dyspnea, which might make our results generally applicable. A very active pulmonary vascular clinic at this institution, however, likely results in the referral of more PAH patients than would be expected in a general medical clinic. Thus, any inferences concerning prevalence of exercise-induced PAH must be made with caution. Mechanisms underlying the two mPAP patterns during CPET can only be indirectly addressed in this study, but they have served to generate several testable hypotheses. This study is largely cross-sectional, and systematic longitudinal studies of exercise-induced PAH, with and without treatment, remain to be done.
Summary
Using invasive maximum cardiopulmonary exercise testing, we have for the first time fully phenotyped the patient with exercise-induced PAH from a large cohort of symptomatic patients, and have lent support to the hypothesis that exercise-induced PAH is a mild and clinically-relevant phase of the PAH spectrum. If exercise-induced PAH is an early form of a progressive disease, screening and early intervention may prevent the progression of vascular remodeling and development of established PAH in much the same way that we currently approach the diagnosis and treatment of systemic hypertension.
Acknowledgments
The authors would like to acknowledge Shannon Burnham and McKenzie Wessen for their assistance with data collection.
Funding Sources This work was supported by the following grant: NIH K24HL04022-05.
Footnotes
Clinical Summary We provide the first large invasive characterization of symptomatic patients with exercise induced pulmonary arterial hypertension In contrast to previous studies that utilized stress-echocardiography, our use of in-dwelling pulmonary arterial catheters during maximum incremental cardiopulmonary exercise testing allows the appropriate exclusion of patients whose exertional pulmonary hypertension is due to either elevated left-sided filling pressures or increased cardiac output (and normal pulmonary vascular resistance). We further demonstrate that the patient with exercise-induced pulmonary arterial hypertension has physiologic characteristics that are intermediate between those with e normal and those with resting PAH. We conclude that exercise-induced pulmonary arterial hypertension is a mild, but clinically relevant form of PAH. Given the progressive nature of PAH, our findings may have important implications in its early recognition and treatment.
Conflict of Interest Disclosures Dr. Waxman has received other research support from Gilead Pharmaceuticals and Epix Pharmaceuticals. He has also served on speakers' bureaus and consultant/advisory boards for Gilead Pharmaceuticals and United Therapeutics. Drs. Tolle and Systrom, and Ms. Van Horn and Mr. Pappagianopoulos, have no disclosures.
Contributor Information
James J. Tolle, Email: jjtolle@yahoo.com.
Aaron B. Waxman, Email: abwaxman@partners.org.
Teresa L. Van Horn, Email: tvanhorn@partners.org.
Paul P. Pappagianopoulos, Email: ppappagianopoulos@partners.org.
David M. Systrom, Email: dsystrom@partners.org.
References
- 1.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, Williams GW. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 2.Proudman SM, Stevens WM, Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: the need for early detection and treatment. Intern Med J. 2007;37:485–494. doi: 10.1111/j.1445-5994.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 3.Raeside DA, Chalmers G, Clelland J, Madhok R, Peacock AJ. Pulmonary artery pressure variation in patients with connective tissue disease: 24 hour ambulatory pulmonary artery pressure monitoring. Thorax. 1998;53:857–862. doi: 10.1136/thx.53.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGoon M, Gutterman D, Steen V, Barst R, McCrory DC, Fortin TA, Loyd JE. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1suppl):14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 5.Grunig E, Janssen B, Mereles D, Barth U, Borst MM, Vogt IR, Fischer C, Olschewski H, Kuecherer HF, Kubler W. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation. 2000;102:1145–1150. doi: 10.1161/01.cir.102.10.1145. [DOI] [PubMed] [Google Scholar]
- 6.Pignone A, Mori F, Pieri F, Oddo A, Galeota G, Fiori G, Del Rosso A, Perfetto F, Becucci A, Livi R, Tempestini A, Benvenuti C, Gramigna L, Fedi R, Generini S, Minelli M, Cinelli M, Guiducci S, Arcangeli C, Conforti ML, Bernardo P, Cerinic MM. Exercise Doppler echocardiography identifies preclinic asymptomatic pulmonary hypertension in systemic sclerosis. Ann N Y Acad Sci. 2007;1108:291–304. doi: 10.1196/annals.1422.031. [DOI] [PubMed] [Google Scholar]
- 7.Cotrim C, Simoes O, Loureiro MJ, Cordeiro P, Lopes L, Almeida S, Iala M, Miranda R, Carrageta M. Stress echocardiography in the evaluation of exercise physiology in patients with severe arterial pulmonary hypertension. New methodology. Rev Port Cardiol. 2005;24:1451–1460. [PubMed] [Google Scholar]
- 8.Cotrim C, Cordeiro A, Loureiro MJ, Santos MJ, Simoes O, Cordeiro P, da Silva JC, Carrageta M. Exercise stress echocardiography for detection of pulmonary arterial hypertension in a patient with systemic sclerosis. Rev Port Cardiol. 2006;25:199–203. [PubMed] [Google Scholar]
- 9.Collins N, Bastian B, Quiqueree L, Jones C, Morgan R, Reeves G. Abnormal pulmonary vascular responses in patients registered with a systemic autoimmunity database: Pulmonary Hypertension Assessment and Screening Evaluation using stress echocardiography (PHASE-I) Eur J Echocardiogr. 2006;7:439–446. doi: 10.1016/j.euje.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Alkotob ML, Soltani P, Sheatt MA, Katsetos MC, Rothfield N, Hager WD, Foley RJ, Silverman DI. Reduced exercise capacity and stress-induced pulmonary hypertension in patients with scleroderma. Chest. 2006;130:176–181. doi: 10.1378/chest.130.1.176. [DOI] [PubMed] [Google Scholar]
- 11.Bossone E, Bodini BD, Mazza A, Allegra L. Pulmonary arterial hypertension: the key role of echocardiography. Chest. 2005;127:1836–1843. doi: 10.1378/chest.127.5.1836. [DOI] [PubMed] [Google Scholar]
- 12.James KB, Maurer J, Wolski K, Lutton SR, Haas G, Schilz R, Rubin D, Young JB. Exercise hemodynamic findings in patients with exertional dyspnea. Tex Heart Inst J. 2000;27:100–105. [PMC free article] [PubMed] [Google Scholar]
- 13.Raeside DA, Smith A, Brown A, Patel KR, Madhok R, Cleland J, Peacock AJ. Pulmonary artery pressure measurement during exercise testing in patients with suspected pulmonary hypertension. Eur Respir J. 2000;16:282–287. doi: 10.1034/j.1399-3003.2000.16b16.x. [DOI] [PubMed] [Google Scholar]
- 14.Maroni JM, Oelberg DA, Pappagianopoulos P, Boucher CA, Systrom DM. Maximum cardiac output during incremental exercise by first-pass radionuclide ventriculography. Chest. 1998;114:457–461. doi: 10.1378/chest.114.2.457. [DOI] [PubMed] [Google Scholar]
- 15.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 Pt 2):S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 16.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. 4th. Philadelphia, Pennsylvania: Lipincott Williams & Wilkins; 2005. p. 216. [Google Scholar]
- 18.Reeves JT, Moon RE, Grover RF, Groves BM. Increased wedge pressure facilitates decreased lung vascular resistance during upright exercise. Chest. 1988;93(3 Suppl):97S–99S. doi: 10.1378/chest.93.3_supplement.97s. [DOI] [PubMed] [Google Scholar]
- 19.Groves BM, Reeves JT, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen. J Appl Physiol. 1987;63:521–530. doi: 10.1152/jappl.1987.63.2.521. [DOI] [PubMed] [Google Scholar]
- 20.Medoff BD, Oelberg DA, Kanarek DJ, Systrom DM. Breathing reserve at the lactate threshold to differentiate a pulmonary mechanical from cardiovascular limit to exercise. Chest. 1998;113:913–918. doi: 10.1378/chest.113.4.913. [DOI] [PubMed] [Google Scholar]
- 21.Orr GW, Green HJ, Hughson RL, Bennett GW. A computer linear regression model to determine ventilatory anaerobic threshold. J Appl Physiol. 1982;52:1349–1352. doi: 10.1152/jappl.1982.52.5.1349. [DOI] [PubMed] [Google Scholar]
- 22.Stickland MK, Welsh RC, Petersen SR, Tyberg JV, Anderson WD, Jones RL, Taylor DA, Bouffard M, Haykowsky MJ. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol. 2006;100:1895–1901. doi: 10.1152/japplphysiol.01485.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 24.Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. AJP - Heart and Circulatory Physiology. 1998;275:H767–H775. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 25.Selimovic N, Rundqvist B, Bergh CH, Andersson B, Petersson S, Johansson L, Bech-Hanssen O. Assessment of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2007;26:927–934. doi: 10.1016/j.healun.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest. 2007;131:94–100. doi: 10.1378/chest.06-1571. [DOI] [PubMed] [Google Scholar]
- 27.Bossone E, Rubenfire M, Bach DS, Ricciardi M, Armstrong WF. Range of tricuspid regurgitation velocity at rest and during exercise in normal adult men: implications for the diagnosis of pulmonary hypertension. J Am Coll Cardiol. 1999;33:1662–1666. doi: 10.1016/s0735-1097(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 28.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104:429–435. doi: 10.1161/hc2901.093198. [DOI] [PubMed] [Google Scholar]
- 29.Deboeck G, Niset G, Lamotte M, Vachiery JL, Naeije R. Exercise testing in pulmonary arterial hypertension and in chronic heart failure. Eur Respir J. 2004;23:747–751. doi: 10.1183/09031936.04.00111904. [DOI] [PubMed] [Google Scholar]
- 30.Oudiz RJ. The role of exercise testing in the management of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2005;26:379–384. doi: 10.1055/s-2005-916152. [DOI] [PubMed] [Google Scholar]
- 31.Chenivesse C, Rachenne V, Fournier C, Leroy S, Neviere R, Le Tourneau T, Wallaert B. Cardiopulmonary exercise testing in exercise-induced pulmonary hypertension. Rev Mal Respir. 2006;23(2 Pt 1):141–148. doi: 10.1016/s0761-8425(06)71477-2. [DOI] [PubMed] [Google Scholar]
- 32.Markowitz DH, Systrom DM. Diagnosis of pulmonary vascular limit to exercise by cardiopulmonary exercise testing. J Heart Lung Transplant. 2004;23:88–95. doi: 10.1016/s1053-2498(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 33.Laskey WK, Ferrari VA, Palevsky HI, Kussmaul WG. Pulmonary artery hemodynamics in primary pulmonary hypertension. J Am Coll Cardiol. 1993;21:406–412. doi: 10.1016/0735-1097(93)90682-q. [DOI] [PubMed] [Google Scholar]
- 34.Wonisch M, Fruhwald FM, Maier R, Watzinger N, Hodl R, Kraxner W, Perthold W, Klein WW. Continuous haemodynamic monitoring during exercise in patients with pulmonary hypertension. Int J Cardiol. 2005;101:415–420. doi: 10.1016/j.ijcard.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 35.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288:L419–L425. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 36.Eldridge MW, Podolsky A, Richardson RS, Johnson DH, Knight DR, Johnson EC, Hopkins SR, Michimata H, Grassi B, Feiner J, Kurdak SS, Bickler PE, Wagner PD, Severinghaus JW. Pulmonary hemodynamic response to exercise in subjects with prior high-altitude pulmonary edema. J Appl Physiol. 1996;81:911–921. doi: 10.1152/jappl.1996.81.2.911. [DOI] [PubMed] [Google Scholar]
- 37.Rich S. Clinical insights into the pathogenesis of primary pulmonary hypertension. Chest. 1998;114(3 Suppl):237S–241S. doi: 10.1378/chest.114.3_supplement.237s. [DOI] [PubMed] [Google Scholar]
- 38.Fishman AP. Etiology and pathogenesis of primary pulmonary hypertension: a perspective. Chest. 1998;114(3 Suppl):242S–247S. doi: 10.1378/chest.114.3_supplement.242s. [DOI] [PubMed] [Google Scholar]
- 39.Fesler P, Pagnamenta A, Vachiery JL, Brimioulle S, Abdel Kafi S, Boonstra A, Delcroix M, Channick RN, Rubin LJ, Naeije R. Single arterial occlusion to locate resistance in patients with pulmonary hypertension. Eur Respir J. 2003;21:31–36. doi: 10.1183/09031936.03.00054202. [DOI] [PubMed] [Google Scholar]
- 40.Kafi SA, Melot C, Vachiery JL, Brimioulle S, Naeije R. Partitioning of pulmonary vascular resistance in primary pulmonary hypertension. J Am Coll Cardiol. 1998;31:1372–1376. doi: 10.1016/s0735-1097(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 41.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352:719–725. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 42.Walsh ML, Banister EW. Possible mechanisms of the anaerobic threshold. A review. Sports Med. 1988;5:269–302. doi: 10.2165/00007256-198805050-00001. [DOI] [PubMed] [Google Scholar]
- 43.Salvi SS. Alpha-1-adrenergic hypothesis for pulmonary hypertension. Chest. 1999;115:1708–1719. doi: 10.1378/chest.115.6.1708. [DOI] [PubMed] [Google Scholar]
- 44.Steensberg A, Toft AD, Schjerling P, Halkjar-Kristensen J, Pedersen BK. Plasma interleukin-6 during strenuous exercise: role of epinephrine. AJP - Cell Physiology. 2001;281:C1001–C1004. doi: 10.1152/ajpcell.2001.281.3.C1001. [DOI] [PubMed] [Google Scholar]
- 45.Golembeski SM, West J, Tada Y, Fagan KA. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest. 2005;128(6 Suppl):572S–573S. doi: 10.1378/chest.128.6_suppl.572S-a. [DOI] [PubMed] [Google Scholar]
- 46.Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008 Mar 17; doi: 10.1093/eurheartj/ehn103. epub ahead of print. [DOI] [PubMed] [Google Scholar]