Abstract
The interactions between adipocytes and endothelial cells in adipose tissue development are poorly understood. In this study, we characterized the growth and differentiation of 3T3-L1 preadipocytes and human umbilical vein endothelial cells (HUVECs) in planar and collagen gel cocultures. In planar coculture, preadipocyte proliferation was up to three times as great as in the control culture with only preadipocytes, where the increase was proportional to the HUVEC fraction in the seeding mixture. In the collagen gel coculture, triglyceride (TG) content (per adipocyte) was up to 3.4 times as much as in the control with only adipocytes. This effect depended on the total density and composition of the seeding mixture, with the largest increase observed at the highest density (2 × 106 cells/mL collagen) and preadipocyte:HUVEC ratio (90:10) tested in this study. Immunostaining showed that the collagen gel coculture also supported the elongation of endothelial cells. Blockade of vascular endothelial growth factor receptor 2 (VEGFR2) abolished the adipogenesis- and neovascularization-related effects of the coculture. Taken together, our results indicate that endothelial cell–mediated enhancement of adipocyte differentiation requires the activation of VEGFR2.
Introduction
In vitro models have played a vital role in elucidating the molecular basis of adipose tissue development.1 The most widely used experimental models have been planar cultures of preadipocytes and adipocytes. More recently, several groups have begun to explore the use of three-dimensional (3-D) scaffolds to more closely mimic in vivo adipose tissue geometry.2–4 Little work has been done to characterize the interactions between adipocytes and other adipose tissue components. In larger adult mammals, body fat consists predominantly of white adipose tissue (WAT). In vivo, the bulk of WAT is a loose association of white adipocytes held in a collagen matrix. The tissue is also highly vascularized.5 The vascular fraction contains locally resident precursor cells, including preadipocytes, and endothelial cells that form the microcirculation. Normalizing for active protoplasm, the capillary bed of adipose tissue is denser than that of muscle.6 Each adipocyte is in contact with at least one capillary, ensuring that there is sufficient nutrient supply and waste removal.7 The endothelial cells contribute directly to the metabolic function of the WAT, which is the major site of energy storage in the body. During periods of nutrient excess and deprivation, respectively, the WAT stores and mobilizes triglycerides (TGs). TG synthesis involves the uptake of free fatty acids from the circulation and their esterification with glucose-derived glycerol phosphate. Diet- or liver-derived fatty acids are trafficked to the adipose tissue through the circulatory system packaged as TGs in lipoproteins such as chylomicrons. Unloading the free fatty acids requires lipoprotein lipase (LPL), which is synthesized in adipocytes but needs to be expressed along the luminal surface of the capillaries.8
In this light, an improved in vitro model could be developed by considering the coculture of preadipocytes and adipocytes with vascular endothelial cells. Such a coculture system should better capture the cell–cell interactions found in vivo and provide a new platform to establish models of WAT-related diseases. One obvious application involves obesity, which has become a leading health problem in the United States. Obesity results from excessive expansion of WAT and is a risk factor for many diseases, including type 2 diabetes, cardiovascular disease, and some forms of cancer.9 In obese adults, body fat mass correlates with the volume of WAT microcirculation.5,10 A recent study found that treating genetically obese mice with angiogenesis inhibitors resulted in a dose-dependent, selective ablation of adipose tissue.11 Another in vivo study showed that over-expressing a dominant-negative form of the adipocyte differentiation regulatory factor peroxisome proliferator activated receptor-γ not only inhibited adipogenesis, but also impaired angiogenesis.12 These findings underscore the importance of neovascularization in adipose tissue development and the need for controlled experiments on cell–cell interactions in isolation from confounding systemic influences. In this study, we have characterized the growth and differentiation of endothelial cells and preadipocytes in coculture. The goal was to determine whether the proliferation of endothelial cells and differentiation of adipocytes were enhanced by the presence of the other cell type.
Materials and Methods
Materials
3T3-L1 cells were obtained from ATCC (Manassas, VA). The 3T3-L1 preadipocyte cell line has been used extensively to study adipocyte development, because it can be consistently differentiated under defined conditions.13 Adipocytes derived from 3T3-L1 cells exhibit all of the major biochemical characteristics of fat cells in vivo, including insulin sensitivity and formation of intracellular lipid droplets.1 Human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex (Walkersville, MD). Tissue culture reagents including Dulbecco's modified Eagle medium (DMEM), calf serum (CS), fetal bovine serum (FBS), human insulin, and penicillin/ streptomycin were purchased from Invitrogen (Carlsbad, CA). Endothelial cell growth supplement from bovine brain extract (ECGS), AlexaFluor 647–conjugated mouse antihuman CD31, and rat tail type 1 collagen were purchased from BD Biosciences (Bedford, MA). ProLong Gold Antifade reagent was purchased from Molecular Probes (Eugene, OR). Monoclonal antihuman vascular endothelial growth factor receptor 2 (VEGFR2) antibody was purchased from R & D Systems (Minneapolis, MN). Unless otherwise noted, all other chemicals were purchased from Sigma (Saint Louis, MO).
Stable transfection of 3T3-L1 preadipocytes with enhanced green fluorescent protein
We generated a 3T3-L1 cell line that expresses green fluorescent protein (GFP) to distinguish the preadipocytes and adipocytes from the endothelial cells and to quantify their amount in the cocultures. GFP expression was constitutive and not tied to the promoter of a differentiation-related gene. Plasmids pRevTet-Off and pRevTRE were purchased from BD Biosciences (Mountain View, CA). Dr. Kenneth Wieder (Shriners Hospital for Children, Boston, MA) kindly donated gull-length complementary DNA (cDNA) for GFP. The GFP cDNA was excised and subcloned into the SalI and XbaI sites of plasmid pSP72 (Promega, Madison, WI) to generate pSP72-GFP. The GFP cDNA was subsequently excised out of pSP72-GFP and cloned into the SalI and ClaI sites of pRevTRE to generate pRevTRE-GFP. The pRevTet-Off and pRev-TRE-GFP plasmids were sequentially introduced into 3T3-L1 preadipocytes using electroporation. Approximately 10 μg of linearized plasmid (using ScaI for pRevTet-Off and BsaA I for pRev-TRE-GFP) was used for each electroporation. Low-passage preadipocytes (2.5 × 106) were trypsinized and electroporated with pRevTet-Off at 950 μF, 240 V, in a 0.4-mm cuvette using a Bio-Rad Gene Pulser II (Hercules, CA). After a 10-min recovery at room temperature, the cells were seeded into T-75 flasks and incubated at 37°C. After 48 h, 700 μg/mL of G418-sulfate was applied, and the cells were incubated for 8 days to select for preadipocytes that had pRevTet-Off stably integrated into the genome. All surviving cells were pooled, cultivated in a T-75 flask, and electroporated again with linearized pRevTRE-GFP under the same conditions as described above, except that 200 μg/mL of hygromycin was used to select for cells that contained pRevTRE-GFP in addition to pRevTet-Off.
Planar coculture
The GFP-expressing 3T3-L1 cells were expanded and maintained in a preadipocyte growth medium consisting of DMEM supplemented with 10% CS (v/v), 200 U/mL penicillin, and 200 μg/mL streptomycin. HUVECs were expanded and maintained in a basal medium (MCDB131, Sigma) containing 50 μg/mL ECGS, 50 μg/mL heparin, 20% FBS (v/v), penicillin, and streptomycin. Separately expanded GFP-expressing preadipocytes and HUVECs were mixed and seeded together into 6-well culture plates. An initial total seeding density of 105 cells per well was kept constant for all experiments. Medium was replaced 1 day after the initial seeding and every other day thereafter. Table 1 summarizes the media used for seeding and subsequent coculture. On day 5, cells were detached using trypsin–ethylenediaminetetraacetic acid (EDTA), gently centrifuged at low speed, washed, resuspended, and then placed on ice for immediate analysis. Total cell number was determined using a hemocytometer. The number of preadipocytes was quantified by measuring the GFP fluorescence of the cell suspensions using a microplate reader (GeminiEX, Molecular Devices, Sunnyvale, CA). The number of HUVECs was calculated by subtracting the number of GFP-expressing cells from the total cell number.
Table 1.
Coculture Media
| Medium | Components |
|---|---|
| Basal medium | 50% M199 + 50% Dulbecco's modified Eagle medium |
| Coculture growth medium | Basal medium + 5% calf serum, 10% FBS, 30 μg/mL ECGS, and 10 μg/mL heparin |
| Coculture differentiation medium 1 | Basal medium + 15% FBS, 30 μg/mL ECGS, 10 μg/mL heparin, 1 μg/mL insulin, 1 μM dexamethasone, and 0.5 mM isobutylmethylxanthine |
| Coculture differentiation medium 2 | Basal medium + 15% FBS, 30 μg/mL ECGS, 10 μg/mL heparin, and 1 μg/mL insulin |
| Coculture maintenance medium | Basal medium + 15% FBS, 30 μg/mL ECGS, and 10 μg/mL heparin |
FBS, fetal bovine serum; ECGS, endothelial cell growth supplement from bovine brain extract.
Collagen gel coculture
The GFP-expressing 3T3-L1 cells were plated onto 6-well plates and expanded in the preadipocyte growth medium. On day 2 postconfluence, the cells were induced to differentiate using a standard protocol.14 The first induction medium (DM1) consisted of a basal medium (DMEM with 10% FBS and penicillin/streptomycin) supplemented with an adipogenic cocktail (1 μg/mL insulin, 0.5 mM isobutylmethylxanthine and 1 μM dexamethasone). After 48 h, DM1 was replaced with a second induction medium (DM2) consisting of the basal adipocyte medium supplemented with only insulin. After another 24 h, the induced cells were detached, concentrated, and mixed into an ice-cold collagen prepolymer solution (2 mg/mL) with or without HUVECs. The collagen cell suspensions were added into 12-well plates (0.5 mL per well) and allowed to gel at 37°C. After 1.5 h, 0.5 mL fresh DM2 was added to each well. On day 1 after seeding, another round of adipogenic differentiation was induced by repeating the DM1–DM2 cycle. Beginning on day 5, cultures were kept in a maintenance medium (Table 1) until they were sacrificed on day 13. During this time, medium was replaced every other day. On day 13, cell samples were collected using a collagenase solution (0.1% w/v) digest followed by in situ lysis with a sodium dodecyl sulfate (SDS) buffer (0.1% w/v). The lyzed samples were briefly sonicated and then immediately analyzed for GFP fluorescence, TGs, and total DNA. TGs were measured using an enzymatic assay as described previously.15 DNA content was measured using the Hoechst dye.
VEGFR2 inhibition
To neutralize VEGF activity, HUVECs were incubated overnight in growth medium supplemented with 100 ng/mL antihuman VEGFR2 antibody. The following day, the preincubated HUVECs were mixed with GFP-expressing preadipocytes into a collagen prepolymer solution at a 1:9 ratio. This collagen–cell mixture was seeded and cultured as described above. The culture media were supplemented with 50 ng/mL anti-VEGFR2 antibody. The inhibition targeted an endothelial cell receptor for VEGF to prevent disruption of potential autocrine interactions between VEGF and the adipocytes and to account for the presence of serum-derived VEGF in the culture medium. The working concentration of the receptor blocking antibody was selected based on the results of preliminary dose-response experiments. The selected dose is comparable with those used in other studies reporting nearly complete inhibition of VEGFR2-mediated signaling.16
Calibration of GFP activity
The GFP-expressing preadipocytes grown in a T75 flask were transferred into the wells of microtiter plates at varying densities. The plates were quickly read (within 0.5 h of cell detachment) at 484/510 nm excitation/emission. The resulting calibration curve was applied to fluorescence readings of detached, centrifuged, and resuspended cells collected on day 5 from the planar cocultures. Cell-free medium was used as the blank control. Similar steps were used to calibrate the GFP fluorescence of 3T3-L1 cells in collagen gel culture against the sample DNA content. Preadipocytes expressing GFP were grown in a T75 flask, washed with 1 × PBS (phosphate buffered saline), detached with trypsin-EDTA, centrifuged, and resuspended in an ice-cold collagen prepolymer solution. Collagen suspensions of varying cell densities were placed into 12-well plates, allowed to gel, and incubated in the coculture growth medium. After 24 h, cell samples were collected using a collagenase digest, lyzed in situ, and briefly sonicated. The crude extracts were analyzed for GFP fluorescence and DNA content. The blank control was a solution of collagenase-digested cell-free collagen in SDS buffer.
Immunostaining
On day 13 of the collagen gel coculture, the medium was removed, and the collagen gel was washed with 1 × PBS for 10 min. The gel was fixed with 10% formalin and then washed with a 0.15-M glycine solution in PBS. After another wash in PBS, a 0.02% Triton X-100 solution in PBS (w/v) was added to the fixed gel. The gel was blocked overnight at 4°C with a 5% milk/1% bovine serum albumin solution in PBS (w/v). The following day, the gel was incubated overnight at 4°C with AlexaFlour 647-conjugated mouse antihuman CD31 antibody (1:100 dilution). The gel was subsequently washed three times in PBS for 10 min each and mounted with Antifade solution for imaging. The distribution of the endothelial cell markers was recorded using a confocal laser scanning microscope (DMIRE2, Leica Microsystems, Bannockburn, IL 60015) at 633 nm excitation.
Image analysis
Cellular morphology in the collagen gel cocultures was recorded using phase-contrast microscopy (TE300, Nikon-US, Melville, NY). Images were obtained for five representative but randomly chosen areas from different wells in each experimental group (n = 6 per experiment). The images were analyzed using the Simple PCI (Compix, Cranberry Township, PA) and Image J17 software packages. Adipocyte sizes were estimated by averaging the diameters of randomly selected cells with visible lipid droplets.13 Ten cells were selected from each of the five images recorded for each group per experiment. The number of differentiated adipocytes was estimated based on the counts of lipid containing cells averaged across 10 randomly selected regions of each image.
Statistics
All experiments were performed at least twice in triplicate cultures unless otherwise stated. Comparisons between two experimental groups were performed using the Student t-test. Group means were deemed to be statistically significantly different when p < 0.05.
Results
Correlation between GFP fluorescence and 3T3-L1 cell number
The calibration experiments showed a linear correlation between GFP fluorescence and 3T3-L1 cell number or DNA content with coefficients of determination of 0.998 and 0.977, respectively (Supplementary Fig. 1, available online at www.liebertonline.com/ten). The auto-fluorescence of HUVECs at the GFP excitation/emission wavelengths was negligible (<20% of the value for the lower limit of the calibration range) up to 105 cells per well. The highest HUVEC concentration in assayed samples was 0.9 × 105 per well. In these samples, the 3T3-L1 cell concentration was 3.4 × 105 per well. The GFP-DNA correlation derived for the preadipocytes also fit the data for adipocytes (Supplementary Fig. 1B), indicating that differentiation did not significantly alter the forced expression of GFP in these cells.
Proliferation
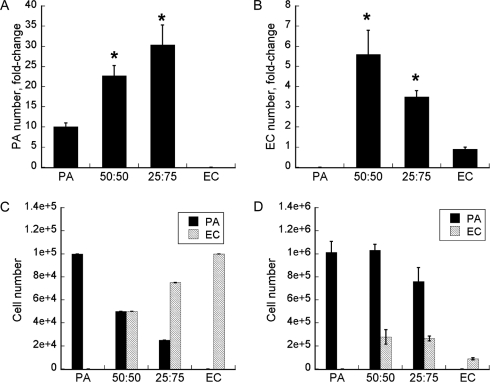
We first assessed the effects of HUVECs on the proliferation of 3T3-L1 preadipocytes in planar coculture. From the time of seeding (day 0) to day 5, the preadipocyte numbers in the control, 50:50, and 25:75 preadipocyte:HUVEC cocultures increased 10, 23, and 30 times, respectively (Fig. 1A). Here, control refers to cultures consisting of only preadipocytes. The increases in the 50:50 and 25:75 cocultures were two to three times as great, respectively, as in this preadipocyte control. Similarly, the coculture enhanced the increase in the HUVEC number. This effect was again proportional to the fraction of the other cell type in the seeding mixture. The increases in the 50:50 and 25:75 cocultures were six and four times as great, respectively, as in the HUVEC control. From day 0 to day 5, the HUVEC number increased 5.6 and 3.5 times in the 50:50 and 25:75 cocultures, respectively, but decreased 10% in the HUVEC control (Fig. 1B). The compositions of the 50:50 and 25:75 cocultures favored the preadipocytes, consistent with their faster doubling time than HUVECs (23 vs 36 h).18 On day 5, the preadipocytes accounted for 79% and 74% of the 50:50 and 25:75 cocultures, respectively (Fig. 1D). The total cell number was substantially higher in the 50:50 coculture (1.31 × 106) than in the 25:75 coculture (1.02 × 106) or the preadipocyte control (1.01 × 106). No expansion was noted for the HUVEC control in the coculture medium; rather, there was a net decrease in total cell number from day 0 to 5 (Fig. 1C, D, from 1 × 105 to 0.88 × 105).
FIG. 1.
(A) Preadipocyte (PA) and (B) endothelial cell (EC) proliferation in planar coculture. (C) Initial (day 0) and (D) day-5 culture compositions. Data shown are means ±standard deviations (SDs) of six independent replicate experiments. The total initial seeding density was kept constant at 105 cells per well (of a 6-well plate) for all cultures. The coculture ratios refer to PA:EC in the seeding mixture. Data shown are fold-changes of PA or EC number at day 5 relative to day 0. Error bars represent SDs of six independent replicate experiments. *Statistically significantly different from PA or EC control at p < 0.003.
Adipogenic differentiation
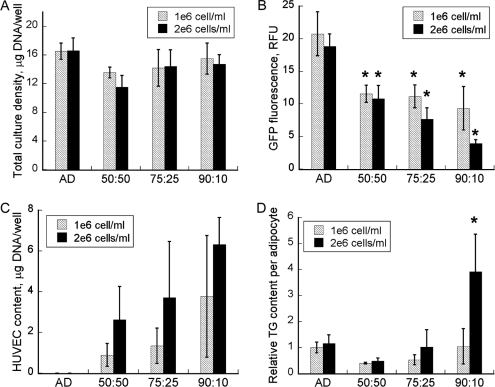
Prior work by others,15,19,20 as well as our own preliminary experiments, had shown that the collagen gel matrix promoted adipogenic differentiation and endothelial cell network formation. The collagen gel cultures were seeded at high densities to lessen proliferation-driven effects. Analyses were performed on samples obtained 16 days after the first adipogenic induction (day 13 of coculture), when the 3T3-L1 cells were expected to have achieved the fully differentiated phenotype. Adipogenic differentiation was assessed using morphological and biochemical analyses. Morphologically, preadipocytes resemble fibroblasts, whereas adipocytes are rounded and contain intracellular lipid droplets. The size of an adipocyte directly reflects the total volume of the intracellular lipid droplets and thus provides an important measure of the cell's ability to synthesize and store fatty acids. Adipocyte sizes are generally larger in vivo than in vitro.13 The lipid droplets were quantified using a TG assay on crude cell lysates. Neither preadipocytes nor endothelial cells exhibit significant lipogenic activity. Increasing the total seeding density from 5 × 105 to 106 cells per well (in 0.5 mL collagen gel) had no effect on total cell density on day 13 (Fig. 2A). A more significant effect was noted for the composition of the cocultures at the time of seeding. Overall, cell density generally increased with the proportion of preadipocytes in the seeding mixture. The adipocyte content as determined using GFP fluorescence decreased as the preadipocyte:HUVEC seeding ratio increased (Fig. 2B). This trend was similar for high and low seeding densities but more pronounced at higher density. The trend for HUVEC content with respect to seeding ratio was the opposite (Fig. 2C). The largest HUVEC content (∼43% of the total DNA) was observed for the high-density 90:10 coculture.
FIG. 2.
Growth and differentiation of collagen gel cocultures. (A) Total cell content; (B) 3T3-L1 cell (differentiated adipocyte + preadipocyte) content and (C) human umbilical vein endothelial cell (HUVEC) content on day 13. In panel (B), the green fluorescent protein (GFP) fluorescence is directly proportional to number of 3T3-L1 cells in the coculture (see also Supplementary Figure 1A, B). The amount of DNA from HUVECs (EC) was calculated by subtracting the DNA estimate for 3T3-L1 cells (AD) from the coculture total. AD refers to the control culture seeded with only the induced 3T3-L1 cells. The coculture ratios indicate AD:EC proportions in the seeding mixture. (D) Relative triglyceride (TG) content per adipocyte. Culture TG contents were first normalized with the corresponding GFP intensities and then divided by the normalized TG of the control (AD) seeded at the lower (106 cells/mL collagen) density. Data shown are means ± standard deviations of six independent replicate experiments. *Statistically significantly different from control (AD) or low density control (for panel D only) at p < 0.002.
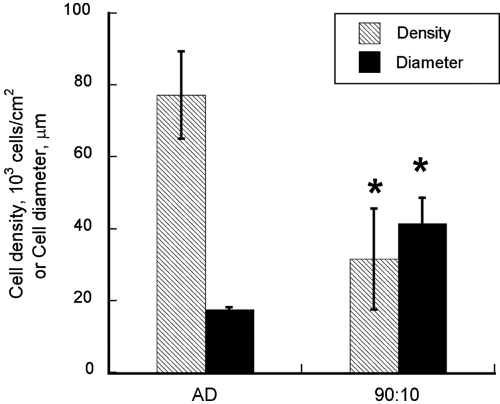
Seeding cell density and ratio also dramatically affected TG accumulation. Normalized to the adipocyte content, TG level increased with preadipocyte:HUVEC seeding ratio (Fig. 2D). At the lower total seeding density, the 50:50 coculture achieved only 40% of the normalized TG content in the corresponding control. Raising the seeding ratio to 75:25 and 90:10 increased the normalized TG content to 75% and 100% of the control, respectively. A similar trend was observed for the higher seeding density, with quantitatively larger differences between the cocultures. The normalized TG levels in the higher-density control, 50:50, 75:25, and 90:10 cocultures were 115%, 49%, 103%, and 391%, respectively of those of the control culture seeded at the lower density. The increase observed for the 90:10 coculture was statistically significant when compared with the low- or high-density control (p < 0.002). Morphological analysis confirmed that the cocultures contained fewer adipocytes with visible lipid droplets. The number of adipocytes in the 90:10 coculture was 59% less per culture area than in the corresponding high-density control, but the average size was 2.4 times as large (Fig. 3).
FIG. 3.
Adipocyte density and average diameter. Data shown are means ± standard deviations of five images randomly selected from different replicate cultures. *Statistically significantly different from control (AD) at p < 0.001.
VEGFR2 inhibition
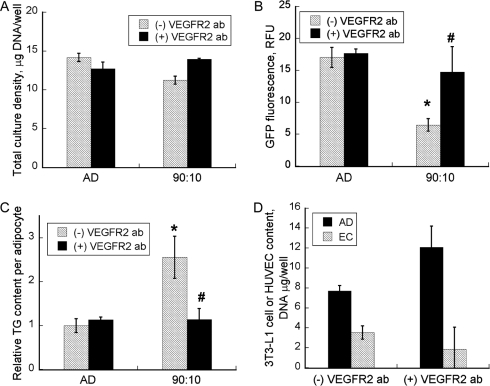
To assess the role of VEGF in mediating the observed synergy between adipogenic differentiation and endothelial cell growth, we examined the effects of human anti-VEGFR2 antibody treatment on the high-density 90:10 coculture. The positive control was the 90:10 coculture without the antibody treatment. Negative controls were the 3T3-L1 cell-only control cultures with and without the antibody treatment. There were no significant differences between the two negative controls in DNA content, GFP intensity, or TG accumulation (Fig. 4A-C), indicating that the antibody treatment had no direct effect on cell proliferation and adipogenic differentiation in the absence of the HUVECs. For the 90:10 coculture, treatment with the VEGFR2 antibody almost completely abolished the increase in per-cell TG content. When treated with the antibody, the GFP intensity and per-cell TG content of the 90:10 coculture were not significantly different from those of the two negative controls (Fig. 4B, C). The day-13 composition of the 90:10 coculture treated with the antibody was 13% HUVECs and 87% 3T3-L1 cells, close to the composition of the seeding mixture (Fig. 4D). As expected, withholding the antibody treatment essentially reproduced the adipocyte-only control and 90:10 coculture results shown in Figure 2.
FIG. 4.
(A) Total culture density (B) 3T3-L1 cell (differentiated adipocyte + preadipocyte) content and (C) relative triglyceride (TG) content per adipocyte. Plus and minus symbols in the figure legends refer to cultures with and without antibody treatment, respectively. (D) 3T3-L1 cell (AD) and human umbilical vein endothelial call (EC) contents of the 90:10 cocultures on day 13. Data shown are means ± standard deviations of six independent replicate experiments. *Significantly different from control (3T3-L1 only) with antibody treatment at p < 0.002; #Significantly different from 90:10 coculture without antibody treatment at p < 0.002.
Discussion
In this study, we found enhanced proliferation of preadipocytes and lipid accumulation of adipocytes in planar and collagen gel coculture with vascular endothelial cells. The coculture setting also stimulated proliferation of endothelial cells. This discussion will compare our results with other published findings and present a paracrine mechanism for adipocyte–endothelial cell interaction in adipose tissue development.
There have been only a handful of studies on the effects of endothelial cell–derived factors on preadipocyte growth in a controlled in vitro setting. One earlier work showed that medium conditioned with freshly isolated human microvascular endothelial cells stimulate the proliferation of cultured preadipocytes.21 Another conditioned-medium study found that differentiated 3T3-F442A adipocytes secreted a mitogen that potently stimulated the proliferation of cultured bovine aortic endothelial cells.22 Conditioning the medium with undifferentiated 3T3-F442A cells significantly weakened the potency, suggesting that the mitogen is a product of adipocyte differentiation. In contrast, the coculture setting of our study dramatically enhanced endothelial cell growth (up to 6.2 times more than in controls in the same serum-containing medium) even when adipocyte differentiation was not induced (Fig. 1B). A more-direct comparison with the conditioned medium study is not possible, because different cell lines and basal culture media were used. If the coculture indeed stimulated endothelial cell growth to a greater degree, the difference could reflect a bidirectional interaction between the two cell types that would be absent when medium is conditioned using one cell type and applied to the other.
Conditioned medium experiments have also been used to study the effects of endothelial cell–derived factors on adipogenic differentiation. Lau and coworkers found that extracellular matrix components secreted by microvascular endothelial cells stimulated the differentiation of cultured preadipocytes.23 Applying an exogenous induction cocktail completely masked this effect, indicating that the cocktail components overwhelmed the relatively weak “natural” signal. Here, we found that the exogenously induced preadipocytes accumulated significantly more TG (on a per-cell basis) in coculture with HUVECs. Other recent reports have also found important differences between the effects of coculture and conditioned medium treatment. For example, Aoki and coworkers found that rat lung endothelial cells (RLEs) triggered the de-differentiation of freshly isolated mature adipocytes in coculture, which was not observed when the adipocytes were treated with RLE-conditioned media or RLE-derived cytokines.24 A recent study by Borges and coworkers showed that a fibrin matrix–supported spheroid coculture increased the proliferation of preadipocytes and endothelial cells.25 When the preadipocytes were absent, the endothelial cells in the fibrin matrix underwent apoptosis. In the present study, we found that endothelial cell growth in the coculture medium was compromised in the absence of the preadipocytes (Fig. 1B).
A key advantage of the coculture is that it allows cyclical interactions between the cell types. In this light, the following mechanism could explain the hypertrophy of adipocytes in coculture. First, the adipogenic cocktail induced an initial round of adipogenic differentiation. Second, the differentiating adipocytes then secreted a vascular endothelial cell growth factor. Third, the endothelial cell growth factor in turn stimulated the production of an adipogenic factor by the HUVECs. The following three observations support this sequence. First, significant more TG than in control (on a per-cell basis) was found only when the 3T3-L1 cells constituted the initial bulk of the coculture (Fig. 2D). Preadipocytes and endothelial cells do not exhibit any significant lipogenic activity. Thus, greater lipid accumulation demonstrates greater adipogenesis. Second, the increase in cellular TG correlated with the increase in HUVEC content (Fig. 2C). Third, seeding at a higher total density promoted TG accumulation and endothelial cell growth. In the 50:50 coculture, adipogenic differentiation and endothelial cell growth were limited. On day 13, 3T3-L1 cells constituted the bulk of the coculture. Because mature adipocyte generally do not divide, it is likely that a majority of these cells were preadipocytes. In the 90:10 coculture, the amount of TG and the average diameter of an adipocyte were 3.4 and 2.4 times as great, respectively, as in the control culture with only induced 3T3-L1 cells. At the higher seeding density, endothelial cells, which initially constituted only 10% of the coculture, constituted 43% of the coculture on day 13. The in vivo composition in mice is similar, where the ratio of endothelial cells to mature adipocytes in subcutaneous fat pads is approximately 1.1.26 Taken together, these observations suggest that the adipocytes secreted one or more growth factors that triggered endothelial cell growth, which in turn stimulated adipocyte differentiation.
In addition to greater cell growth, the enhanced adipogenic differentiation in the 90:10 coculture also appeared to correlate with morphological changes of the endothelial cells. To visualize the spatial organization of HUVECs, we treated the cocultures with an immunostain for CD31 (platelet endothelial cell adhesion molecule). At the lower seeding density, we did not observe any significant elongation or organized structures regardless of the initial preadipocyte:HUVEC ratio. At the higher seeding density, the endothelial cells in the 90:10 coculture exhibited greater elongation and appeared to connect with each other (Supplementary Fig. 2, available online at www.liebertonline.com/ten), although extensive network formation was not observed. Additional studies will be needed, possibly with alternative matrices, to determine whether the endothelial cells will further differentiate and organize into a vascular network.
The literature has suggested a number of candidates for the adipocyte-derived growth factor, including VEGF-A, VEGF-B, VEGF-C, angiopoietin-1 and -2, transforming growth factor-beta, leptin, and metalloproteinase.26,27 Corresponding endothelial cell receptors have been identified for several of these factors, including VEGF, leptin, and angiopoietin.12,28–30 In this study, we have confirmed the involvement of VEGF in mediating the interplay of adipogenesis and angiogenesis in vitro. An earlier in vivo study, in which inhibiting blood vessel formation with a VEGFR-blocking antibody reduced the adipogenic conversion of preadipocytes subcutaneously injected into mice, corroborate our results.12 Nevertheless, it is possible that additional factors are involved. In vitro studies have shown that leptin is a potent angiogenic factor that promotes endothelial cell tube formation. This effect was also observed in an in vivo study on corneal neovascularization.28 The angiopoietin-1 receptor tyrosine kinase Tie-2 is another vascular endothelial cell signaling system that contributes to vessel maintenance, growth, and stabilization in adipose tissue. Folkman and coworkers reported that angiopoietin-1 messenger RNA levels in mice inversely correlated with the rate of body weight change.30
Although significant progress has been made in identifying these and other angiogenic factors that likely contribute to adipose tissue development, additional studies are needed to elucidate the specific contributions of each of these factors. It remains an open question whether and which of these signaling pathways are the most promising target(s) for therapeutic intervention to treat obesity. Our coculture model represents a new, controlled setting to investigate the details of adipocyte–endothelial cell interactions in adipose tissue development. We have demonstrated that this experimental model can be used to clearly delineate adipocyte VEGF production as an upstream event in the interplay between angiogenesis and adipogenesis. Additional, related studies are warranted to identify and characterize other, for example, downstream events, such as secretion of lipogenic factors by the endothelial cells. With regard to clinical applications such as soft tissue repair, one current limitation of the coculture system investigated in this study involves the use of model cell systems. The adipocytes were derived from a well characterized and widely used murine cell line. The endothelial cells were of human origin but were not freshly isolated from adipose tissue. Thus, further studies are warranted, preferably with freshly isolated human cells, to explore clinical applications, for example, in soft tissue repair.31 One attractive approach is to isolate cells from the stromal vascular fraction (SVF) of a specific adipose tissue depot. There is increasing evidence that the SVF contains stem cell–like progenitor cells that can proliferate and possess the capacity to differentiate into multiple lineages,32 including adipocytes and endothelial cells.33 In this light, methods to coculture these two cell types should be useful in evaluating the SVF of human WAT depots as potentially renewable and autologous cell sources for a variety of tissue-engineering applications.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. Irene Georgakoudi for the use of confocal laser scanning microscope and William Rice for technical assistance. This work was in part supported by a grant from the National Institutes of Health (1 R21 DK67228) and the National Science Foundation (CBET-0651963) to K.L. and A.J.
References
- 1.Gregoire F.M. Smas C.M. Sul H.S. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 2.Kang X. Xie Y. Kniss D.A. Adipose tissue model using three-dimensional cultivation of preadipocytes seeded onto fibrous polymer scaffolds. Tissue Eng. 2005;11:458. doi: 10.1089/ten.2005.11.458. [DOI] [PubMed] [Google Scholar]
- 3.Neubauer M. Hacker M. Bauer-Kreisel P. Weiser B. Fischbach C. Schulz M.B. Goepferich A. Blunk T. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 2005;11:1840. doi: 10.1089/ten.2005.11.1840. [DOI] [PubMed] [Google Scholar]
- 4.Patel P.N. Gobin A.S. West J.L. Patrick C.W., Jr. Poly(ethylene glycol) hydrogel system supports preadipocyte viability, adhesion, and proliferation. Tissue Eng. 2005;11:1498. doi: 10.1089/ten.2005.11.1498. [DOI] [PubMed] [Google Scholar]
- 5.Crandall D.L. Hausman G.J. Kral J.G. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 6.Frye C.A. Wu X. Patrick C.W. Microvascular endothelial cells sustain preadipocyte viability under hypoxic conditions. In Vitro Cell Dev Biol Anim. 2005;41:160. doi: 10.1290/0502015.1. [DOI] [PubMed] [Google Scholar]
- 7.Hausman D.B. DiGirolamo M. Bartness T.J. Hausman G.J. Martin R.J. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 8.Beigneux A.P. Davies B.S. Gin P. Weinstein M.M. Farber E. Qiao X. Peale F. Bunting S. Walzem R.L. Wong J.S. Blaner W.S. Ding Z.M. Melford K. Wongsiriroj N. Shu X. de Sauvage F. Ryan R.O. Fong L.G. Bensadoun A. Young S.G. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway B. Rene A. Obesity as a disease: no lightweight matter. Obes Rev. 2004;5:145. doi: 10.1111/j.1467-789X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 10.Bouloumie A. Lolmede K. Sengenes C. Galitzky J. Lafontan M. Angiogenesis in adipose tissue. Ann Endocrinol. 2002;63:91. [PubMed] [Google Scholar]
- 11.Rupnick M.A. Panigrahy D. Zhang C.Y. Dallabrida S.M. Lowell B.B. Langer R. Folkman M.J. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumura D. Ushiyama A. Duda D.G. Xu L. Tam J. Krishna V. Chatterjee K. Garkavtsev I. Jain R.K. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93. 2003:e88. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song H. O'Connor K.C. Papadopoulos K.D. Jansen D.A. Differentiation kinetics of in vitro 3T3-L1 preadipocyte cultures. Tissue Eng. 2002;8:1071. doi: 10.1089/107632702320934164. [DOI] [PubMed] [Google Scholar]
- 14.Green H. Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 15.Si Y. Yoon J. Lee K. Flux profile and modularity analysis of time-dependent metabolic changes of de novo adipocyte formation. Am J Physiol Endocrinol Metab. 2007;292:E1637. doi: 10.1152/ajpendo.00670.2006. [DOI] [PubMed] [Google Scholar]
- 16.Gavrilovskaya I.N. Gorbunova E.E. Mackow N.A. Mackow E.R. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol. 2008;82:5797. doi: 10.1128/JVI.02397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasband W.S. Image J. Bethesda, MD: U. S. National Institutes of Health; 1997-2007. [Google Scholar]
- 18.Pierantoni G.M. Battista S. Pentimalli F. Fedele M. Visone R. Federico A. Santoro M. Viglietto G. Fusco A. A truncated HMGA1 gene induces proliferation of the 3T3-L1 pre-adipocytic cells: a model of human lipomas. Carcinogenesis. 2003;24:1861. doi: 10.1093/carcin/bgg149. [DOI] [PubMed] [Google Scholar]
- 19.Daya S. Loughlin A.J. Macqueen H.A. Culture and differentiation of preadipocytes in two-dimensional and three-dimensional in vitro systems. Differentiation. 2007;75:360. doi: 10.1111/j.1432-0436.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanzawa S. Endo H. Shioya N. Improved in vitro angiogenesis model by collagen density reduction and the use of type III collagen. Ann Plast Surg. 1993;30:244. doi: 10.1097/00000637-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Hutley L.J. Herington A.C. Shurety W. Cheung C. Vesey D.A. Cameron D.P. Prins J.B. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol Endocrinol Metab. 2001;281:E1037. doi: 10.1152/ajpendo.2001.281.5.E1037. [DOI] [PubMed] [Google Scholar]
- 22.Castellot J.J., Jr. Karnovsky M.J. Spiegelman B.M. Potent stimulation of vascular endothelial cell growth by differentiated 3T3 adipocytes. Proc Natl Acad Sci U S A. 1980;77:6007. doi: 10.1073/pnas.77.10.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varzaneh F.E. Shillabeer G. Wong K.L. Lau D.C. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism. 1994;43:906. doi: 10.1016/0026-0495(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 24.Aoki S. Toda S. Sakemi T. Sugihara H. Coculture of endothelial cells and mature adipocytes actively promotes immature preadipocyte development in vitro. Cell Struct Funct. 2003;28:55. doi: 10.1247/csf.28.55. [DOI] [PubMed] [Google Scholar]
- 25.Borges J. Muller M.C. Momeni A. Stark G.B. Torio-Padron N. In vitro analysis of the interactions between preadipocytes and endothelial cells in a 3D fibrin matrix. Minim Invasive Ther Allied Technol. 2007;16:141. doi: 10.1080/13645700600935398. [DOI] [PubMed] [Google Scholar]
- 26.Voros G. Maquoi E. Demeulemeester D. Clerx N. Collen D. Lijnen H.R. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146:4545. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouloumie A. Drexler H.C. Lafontan M. Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 29.Cao R. Brakenhielm E. Wahlestedt C. Thyberg J. Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dallabrida S.M. Zurakowski D. Shih S.C. Smith L.E. Folkman J. Moulton K.S. Rupnick M.A. Adipose tissue growth and regression are regulated by angiopoietin-1. Biochem Biophys Res Commun. 2003;311:563. doi: 10.1016/j.bbrc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Patrick C.W., Jr. Tissue Eng strategies for adipose tissue repair. Anat Rec. 2001;263:361. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 32.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wosnitza M. Hemmrich K. Groger A. Graber S. Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12. doi: 10.1111/j.1432-0436.2006.00110.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.