Abstract
Interest has recently been rekindled in receptors that are activated by low molecular weight, non-catecholic, biogenic amines that are typically found as trace constituents of various vertebrate and invertebrate tissues and fluids. The timing of this resurgent focus on receptors activated by the ‘trace amines’ (TAs) β-phenylethylamine (PEA), tyramine (TYR), octopamine (OCT), synephrine (SYN), and tryptamine (TRYP) is the direct result of two publications that appeared in 2001 describing the cloning of a novel G protein-coupled receptor (GPCR) referred to by their discoverers as TA1 (Borowsky et al., 2001) and TAR1 (Bunzow et al., 2001). When heterologously expressed in Xenopus laevis oocytes and various eukaryotic cell lines recombinant rodent and human TA receptors dose-dependently couple to the stimulation of cAMP production. Structure-activity profiling based on this functional response has revealed that in addition to the TAs, other biologically active compounds containing a 2 carbon aliphatic side chain linking an amino group to at least one benzene ring are potent and efficacious TA receptor agonists with amphetamine, methamphetamine, 3-iodothyronamine, thyronamine, and dopamine among the most notable. Almost 100 years after the search for TA receptors began numerous TA1/TAR1-related sequences, now called Trace Amine-Associated Receptors (TAARs), have been identified in the genome of every species of vertebrate examined to date. Consequently, even though heterologously expressed TAAR1 fits the pharmacological criteria established for a bona fide TA receptor a major challenge for those working in the field is to discern the in vivo pharmacology and physiology of each purported member of this extended family of GPCRs. Only then will it be possible to establish whether TAAR1 is the family archetype or an iconoclast.
Keywords: trace amine, phenethylamine, tyramine, iodothyronamine, orphan receptor, psychostimulant, schizophrenia, mental health
1. Introduction
The “trace amines” (TAs; Usdin & Sandler, 1976; Baldessarini & Fischer, 1977), also referred to in the literature as “false transmitters” (Kopin et al., 1964), “microamines” (Boulton, 1976a), and noncatecholic phenylethylamines (Mosnaim & Wolf, 1980), form a small collection of chemically related, low molecular weight, naturally occurring aromatic aliphatic compounds with potent sympathomimetic actions, as originally defined by Barger, Dale, and Dixon (Dale & Dixon, 1909; Barger & Dale, 1910). Traditionally included among this group of compounds are: β-phenylethylamine (PEA), para-hydroxyphenylethylamine (p-tyramine; p-TYR), octopamine (OCT), synephrine (SYN), and tryptamine (TRYP). Figure 1 depicts the structures for each of the compounds discussed in the text.
Figure 1.
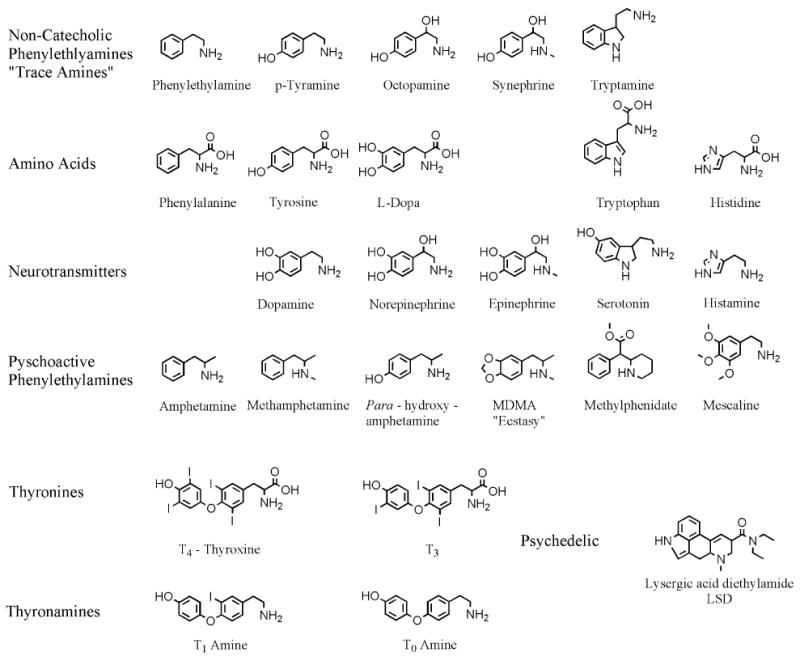
Structural formulae of the compounds discussed in the text.
Structurally these compounds are closely related to the classic vertebrate biogenic amine neurotransmitters dopamine (DA), norepinephrine (NE), epinephrine (EPI), and serotonin (5-HT). The TAs are also close relatives of the synthetic phenylethylamine psychostimulant amphetamine (AMPH) and its numerous analogs (e.g. methamphetamine, METH; Cho and Segal, 1994; Sulzer et al., 2005). In addition to being physically related the TAs are similar to the vertebrate biogenic amine neurotransmitters in terms of their biosynthesis, cellular localization, anatomic distribution, degradation, and elimination (Boulton & Quan, 1970; Boulton & Wu, 1972; Boulton & Wu, 1973; Wu & Boulton, 1973).
Given so many similarities with the biogenic amine neurotransmitters most working in the field were surprised by the technical challenges they encountered while attempting to demonstrate functional receptors that mediate the biological effects of TAs.
The TAs are produced by many, if not all genera of Prokaryotes and Eukaryotes. In the kingdom Animalia endogenously produced TAs have been detected in all invertebrate and vertebrate species examined to date, including humans (Philips et al., 1978; D'Andrea et al., 2003a; Berry, 2004). As a result of their widespread occurrence in both the plant and animal kingdoms, foodstuffs can contain appreciable amounts of TAs either inherently (e.g. cacao from which chocolate is produced), as an unintentional consequence of bacterial action (e.g. food spoilage; Nencki, 1876; Jeanneret, 1877; Gale, 1940; Geornaras et al., 1995), as an intentional consequence of bacterial action (e.g. cheese and wine; Lonvaud-Funel, 2001), or through fungal infestation of grain stuffs (e.g. ergot-infected rye; Barger & Dale, 1909). Additionally, TAs are generated in the gastrointestinal tract of vertebrates by the action of bacterial aromatic amino acid decarboxylase during the normal course of digesting a protein-rich meal (Jansen et al., 2003).
Under most circumstances dietary TAs absorbed in the gastrointestinal system are rapidly catabolized to harmless compounds by the oxidative deamination capabilities of monoamine oxidase (MAO) B (McCabe and Tsuang, 1982; McCabe, 1986). However, this reaction also results in the production of hydrogen peroxide (H2O2) and other reactive oxygen species that can subsequently have detrimental effects (Sandri et al., 1990). Furthermore, abnormal blood levels of TAs can build up in certain pathological conditions (e.g. phenylketonuria) in individuals receiving inhibitors of MAO, and in acute ergotism poisining during which toxic levels of TAs can occur resulting in dramatic behavioral changes including repetitive movements (stereotypy), psychosis, and life-threatening circulatory disturbances that can lead to gangrene. Historically it has been these notable manifestations of extracts prepared from ergot and putrfied meat that originally motivated physiologists, pharmacologists, and medicinal chemists to better understand the mechanism(s) by which the TAs act. The recent cloning of vertebrate metabotropic G protein-coupled receptors (GPCRs) activated by TAs provides an ecixting new opportunity that promises to expand our comprehension of the biological roles these simple but fascinating compounds play (reviewed in Premont et al., 2001; Kim & von Zastrow, 2001; Branchek & Blackburn, 2003; Davenport, 2003; Berry, 2004; Lindemann et al., 2005; Lindemann & Hoener, 2005; Burchett & Hicks, 2006; Lewin, 2006; Zucchi et al., 2006).
2. Natural history of the trace amines
2.1. Discovery of the prototypical trace amines
Of the TAs, PEA is structurally the simplest with the molecular formula C8H11N. PEA is also the most studied TA and is widely considered prototypical. The first mention of a compound with this composition can be traced back to a presentation given by the physiological chemist Professor M. Nencki (1876) during a Festschrift in honor of the 40-year career of a Prof. Valentin of Bern, Switzerland.
It was in the course of his own long research effort to understand the processes of putrefaction and fermentation that Nencki, a contemporary of Louis Pasteur, isolated from decomposing gelatin an aryl akyl amine with the chemical composition of phenylethylamine: C8H11N.
Nencki's protégé Jules Jeanneret shared his mentor's interest in understanding the relationship between these two economically important processes: fermentation and putrefaction. In Bern, during the fall of 1876 and the winter of 1877, Jeanneret conducted a series of studies in Nencki's laboratory where he confirmed the presence of PEA in decomposing gelatin and in addition, discovered the same substance in putrid egg white (Jeanneret, 1877).
By 1879 it was appreciated by Schulze and Barbieri (Guggenheim, 1951) that PEA can be produced by bacterial decarboxylation of the amino acid phenylalanine (F) under anaerobic conditions. In 1882 and 1883 Gautier and Etard demonstrated the presence of PEA in decomposing mackerel (Guggenheim, 1951). PEA was reported in 1896 to be a byproduct of decomposing fibrin by Emmerling (Guggenheim, 1951). Ten years later Winterstein and Bisegger made the interesting observation that ripe Emmanthaler cheese can have a high PEA content (Guggenheim, 1951). It is also now known that PEA can be found at varying concentrations in other products of fermentation including wines, beers, and cheeses (Da Prada et al., 1988; Skerritt et al., 2000).
PEA has also been isolated from several plant species and was reported by White to constitute as much as 1% of an extract prepared from Acacia floribunda blossoms (Guggenheim, 1951).
Although the advent of the 20th century saw PEA accepted as a natural byproduct of fermentation, its biological properties were not yet appreciated. This was to change when in 1906 Abelous and colleagues demonstrated that organic extracts of putrefied horsemeat could dramatically raise arterial blood pressure.
Among the investigators who followed up on this observation were two chemists, Barger and Walpole. They have the distinction of being the first to isolate PEA and TYR (historically the term ergotamine was originally used to refer to the latter compound; see Clark, 1911 but today refers to a different compound) from a biological source (i.e. putrefied horsemeat) and in collaboration with the physiologist H.H. Dale to demonstrate its ability to cause a robust physiological response: a rapid rise in arterial pressure when injected intravenously (Barger & Walpole, 1909; Barger & Dale, 1910; Clark, 1911).
Organic chemists had been familiar with PEA and TYR for years prior to their isolation from decomposing animal (Barger & Walpole, 1909) and ergot fungus-infested cereals (Barger & Dale, 1909). But not until the pioneering work of Barger, Clark, Dale, Dixon, and Walpole was it firmly established that the biogenic amines pTYR and PEA were, in fact, the mysterious substances present in aqueous extracts of decomposing animal matter (Barger & Walpole, 1909) and preparations used in obstetrics made from ergot fungus extracts that possessed potent “adrenine-like” pressor-inducing and uterus mobilizing capabilities (Barger & Dale, 1909; Dale & Dixon, 1909; Barger & Dale, 1910).
Although these ground breaking studies unquestionably demonstrated the ability of naturally derived pTYR and PEA to produce significant physiological responses in vertebrates, their work left unanswered important questions including: How are endogenous TAs synthesized? Do endogenous TAs serve important biological roles? If endogenous TAs have a biological function how then are their actions mediated and terminated?
2.2. Biosynthesis and turnover of the trace amines
Some have referred to the TAs as members of the “first family” (PEA, TYR, OCT, DA, NE, and phenylethanolamine) of neurotransmitters (Walker & Kerkut, 1978; Boulton, 1983) and in the context of catecholamine biosynthesis this is literally true.
The enzymatic pathways that generate the TAs PEA, TYR, OCT, TRYP have always been of considerable interest because they also participate in the biosynthesis of the catecholamines DA, NE, and EPI (Kopin, 1968). Given that the absence of an alpha carboxyl moiety is all that distinguishes these compounds from the aromatic amino acids tyrosine (Y), phenylalanine (F), and tryptophan (W) respectively, early biochemists attempted to experimentally establish that the latter's enzymatic decarboxylation was the most likely route to the former (Barger & Dale, 1909; Dale & Dixon, 1909). Immediately prior to this time it had been shown that TYR and PEA could be formed from Y and F via decarboxylation reactions performed by bacteria and other unicellular organisms (Jeanneret, 1877; Guggenheim, 1951).
Although simple in concept it proved difficult in practice to unquestionably demonstrate that a similar aromatic amino acid decarboxylase (AADC) enzymatic activity was endogenous to animal tissues (Blaschko, 1942; Blaschko & Chrusciel, 1960; Lovenberg et al., 1962) and can yield both PEA (Sabelli et al., 1974; Mosnaim et al., 1974) and TYR (Boulton & Quan, 1970; Boulton & Wu, 1973) although other routes of synthesis certainly exist (Boulton & Quan, 1970; Sabelli et al., 1975).
It is important to note that AADC enzymatic activity is tightly regulated. Sensory stimuli (e.g. light, Hadjiconstantinou et al., 1988) increase retinal AADC activity as do pharmacologic agents that antagonize alpha2-adrenergic receptors and DA D1 receptors in the retina where their agonists decrease AADC activity (Rossetti et al., 1989; Rossetti et al., 1990). In the striatum AADC mRNA is increased in response to chronic inhibition of DA receptors (Buckland et al., 1992) and its enzymatic activity is elevated in response to antagonists of DA D1 and D2 receptors (Zhu et al., 1992; Zhu et al., 1993; Hadjiconstantinou et al., 1993; Cho et al., 1997) while DA receptor agonists depress AADC activity (Hadjiconstantinou et al., 1993; Zhu et al., 1994; Cho 1997). In this context it is interesting to note that Reith et al. (1994) found elevated AADC activity in patients with psychosis while more recently Lasko et al. (2005) found evidence that an allele of the human DA D2 receptor gene (A1 allele) is associated with activity of striatal AADC in healthy subjects.
Changes in AADC activity are known to influence TA accumulation with DA antagonists increasing striatal PEA (Juorio et al., 1991a) and TRYP (Juorio, 1982) while causing behavioral supersensitivity to PEA (Stoff et al., 1984). Electrical stimulation of midbrain neurons in the substantia nigra results in a decrease in accumulation of PEA (Juorio et al, 1991b) and TYR (Jones et al., 1983).
Not surprisingly, within 60 min of an acute dose of AMPH striatal PEA levels are reduced (Borison et al., 1974; Borison et al., 1975; Juorio et al, 1991a) concomitant with a decrease in striatal synaptosomal AADC enzyme activity (Zhu et al., 1994). Chronic AMPH exposure results in a reduction in detectable AADC mRNA (Buckland et al., 1996). The molecular mechanisms by which AADC activity is regulated have been shown to include post-translational phosphorylation of the protein by both protein kinase-A (PKA) (Duchemin et al., 2000) and cGMP-dependent protein kinase (Hadjiconstantinou, et al., 2003).
Since AADC is widely expressed in vertebrate brain a given cell's biogenic amine neurotransmitter phenotype (i.e. histamine, HIS; 5-HT; or the catecholamines DA and NE) is going to be determined by the other enzymes it expresses. In this context it is interesting to note that immunohistochemical studies have revealed the existence of a unique type of neuron-like cell, “D”-cells, that are AADC-positive but lack tyrosine hydroxylase (TH), tryptophan hydroxylase, and 5-HT immunoreactivity (Jaeger et al., 1984a,b; Kitahama et al., 1990; Beltramo et al., 1993). There is also evidence for endogenous TRYP producing “B”-cells that are indolamine-containing but distinct from 5-HT neurons in their microspectrofluorometric and pharmacological properties (Björklund et al., 1976).
Following its decarboxylation by AADC and in the presence of dopamine β-hydroxylase TYR is now known to be converted to octopamine (OCT; Brandau & Axelrod, 1972), first discovered in the salivary glands of the octopus (Erspamer & Boretti, 1951). Synephrine (SYN) is then generated by methylation of OCT through the action of phenylethanolamine-N-methyl transferase (Axelrod & Saavedra, 1977). In an enzymatic reaction analogous to the decarboxylation of Y and F the amino acid tryptophan (W) is decarboxylated to yield TRYP (Saavedra & Axelrod, 1972a; Saavedra & Axelrod, 1972b; Saavedra & Axelrod. 1974).
Concurrent with the studies that demonstrated the in vivo enzymatic decarboxylation and methylation of these aromatic amino acids Axelrod, Saavedra, Durden, Phillips, and others (Saavedra & Axelrod, 1972; Durden et al. 1973; Saavedra & Axelrod, 1974b; Tallman et al., 1976a; Tallman et al., 1976b; Axelrod & Saavedra, 1977; Danielson et al., 1977; Philips et al., 1978; Durden & Phillips, 1980; Parker & Cubeddu, 1988; Durden & Davis, 1993) were developing quantitative analytical detection methods that documented the presence, quantitated the abundance, monitored the turnover, and described the heterogeneous distribution of endogenously synthesized TYR, PEA, OCT, and TRYP in the central nervous system and numerous peripheral tissues including salivary gland, heart, and kidney of several animal species. In the brain at least, PEA is found at a concentration many hundred-fold lower than DA, NE, or 5-HT in the extracellular space of (i.e. 2 – 15 nM, Henry et al., 1988; Scarr et al., 1994) likely having diffused to its site of synthesis in the cytoplasm and through the plasma membrane (Oldendorf 1971; Boulton & Baker, 1975) given its lipophilic nature (Mack & Bonsich, 1979; Paterson et al., 1990).
Even though the concentration of the noncatecholic phenylethylamines is generally very low, hence the moniker “trace amines,” they appear to be synthesized at rates equivalent to the catecholamines (Durden & Philips, 1980; Paterson et al., 1990), a disparity that has been interpreted to be the consequence of their rapid turnover with a half life on the order of 30 sec (Durden & Philips, 1980).
The principle route of TA catabolism is via the monoamine oxidases A and B (MAO-A, MAO-B) except in the case of PEA, which is preferentially if not exclusively degraded by MAO-B (Yang & Neff, 1973; Philips & Boulton, 1979; Durden & Philips, 1980). Other routes of metabolism have also been proposed but they are thought to contribute in only minor ways or become significant only under special circumstances (Saavedra, 1974; Danielson et al., 1977; Paterson et al., 1990; Yu et al., 2003).
2.3. Storage & release of trace amines
It has been difficult to establish exact sites of TAs synthesis and the same holds true for their storage. The TAs are detectable in synaptosomes (Boulton & Baker, 1975; Baldessarini & Vogt, 1972) prepared from brain but to date they have not been shown to be stored in vesicles. Evidence against the storage of TAs in catecholamine-like vesicles has been reported by Dyck (1988) and Henry et al. (1988) who were unable to demonstrate K+-induced release of PEA (Dyck, 1988; Henry et al., 1988). Instead, the amount of PEA “released” is proportional to the tissue level of PEA. This observation may reflect the fact that PEA readily crosses cell membranes (Boulton et al., 1990). Reserpine pretreatment also has no effect on tissue levels of PEA (Boulton et al., 1977; Juorio et al., 1988). In contrast to PEA there is some evidence for activity-dependent release of m- and p-TYR from striatal slices following veratidine-induced depolatization (Dyck 1988, 1989).
2.4. Biological actions of trace amines
Guided by the philosophy that evolutionary pressure selects inheritable characteristics confering a reproductive advantage it has been proposed that the TAs confer a selective advantage on certain species of plants due to their ability to deter animal foragers (Smith, 1977; Kawano et al., 2000a; Kawano et al., 2000b; Enan, 2005) in addition to their cultivation by human consumers (Furst, 1972; Furst, 1976; Booth, 1996).
2.4.1. Classic physiology of the trace amines
Traditionally the scientific literature (Kopin et al., 1964; Kopin, 1968; Baldessarini & Fischer, 1977; Baldessarini & Fischer, 1978; Baldessarini, 1978) and medical pharmacology texts (e.g. Goodman & Gilman's 10th edition, 2005; Katzung 10th edition, 2007) refer to the TAs as ‘false’ transmitters or, at best, physiologic neuromodulators with indirect sympathomimetic effects (Berry, 2004). The intention of this designation is to convey that they these molecules do not fulfill the criteria established for acetylcholine and the biogenic amines HIS, DA, NE, and 5-HT. However, it should be remembered that TA physiology has been conducted in bioassays developed to study the actions of acetylcholine and the catecholamines. Other assays might be more revealing.
The physiological actions of TAs are relatively weak in the intact animal compared to the cathecholamines. However, under conditions where MAO activity is inhibited TA levels can become significantly elevated displacing more efficacious ‘true’ neurotransmitters from their vesicles (Ibrahim et al., 1985). At even higher concentrations TAs enhance DA release, and to a lesser extent NE and 5-HT, into synapses. The effects evoked at these high TA concentrations have been referred to as “amphetamine-like” (Berry, 2005; Burchett & Hicks, 2006).
2.4.1.a. Invertebrates
The physiology of TAs has been best studied in invertebrates. Secretion from the posterior gland of the octopus, where OCT was discovered, is under its control (Ghiretti, 1953). OCT has a unique distribution in the lobster (Livingstone et al., 1981) where it strongly influences the heart, exoskeletal muscle (Battelle & Kravitz, 1978), and the animal's posture (Harris-Warrick et al., 1984). OCT also acts as a neurohormone in this species (Kravitz et al., 1980). In another ancient species, Limulus the horseshoe crab, Battelle et al., (1979) found evidence of TA synthesis in ventral nerve photoreceptors.
Other species of the phylum Arthropoda, of the class Insecta, also depend on OCT signaling for survival. In what is perhaps the first report of an important physiological action of OCT in insects Robertson & Steele (1972) demonstrated that low concentrations stimulate the activity of the glycogen phosphorylase enzyme present in the ventral nerve cord of the cockroach Periplaneta americana. This finding is important for several reasons, not the least of which being it demonstrates an animal's changing energy requirements can be met via glycogenolysis modulated by TA signaling.
The work of Robertson and Steele, as well as others, stimulated further investigations into the physiological actions of OCT. The following year Nathanson & Greengard (1973) reported low concentrations of OCT stimulate a novel adenylyl cyclase present in thoracic ganglia of P. americana that is not responsive to either DA or 5-HT. Subsequent investigations revealed the cockroach brain expresses an adenylyl cyclase that is stimulated by OCT (Harmar & Horn, 1977) as well as inhibited by OCT (Uzzan & Dudai, 1982).
OCT-induced changes in second messengers control the firefly's mating flash (Nathanson et al., 1979). OCT also stimulates the contraction of locust skeletal muscle (Evans, 1987; Evans et al., 1988) while TYR is active in the oviduct of this species (Donini et al., 2004). The terminal abdominal ganglion of the female gypsy moth Lymantria dispar is stimulated by OCT, responding with an increase in cAMP formation and nerve cell firing (Olianas et al., 2006). Overall, the effects of TAs in invertebrate systems, in particular OCT, resemble the classic actions of NE in vertebrates (Roberston & Juorio, 1976; Roeder, 1999).
2.4.1.b. Vertebrates
The first demonstration in vertebrates of a physiological effect of chemically pure PEA and TYR was reported by Barger & Walpole (1909) in collaboration with H.H. Dale (Barger & Dale, 1910) and later expanded upon by Clark (1911). Barger and Walpole were chemists working at Wellcome's Herne Hill Physiological Research Laboratories in southeast London. One of them had become interested in the report by Abelous and colleagues of a chloroform-soluble pressor principle they extracted from rotten meat (Abelous et al., 1906). Back in London this observation was quickly reproduced in the pithed cat bioassay in which a purified organic extract of putrid ox-heart tails was given intravenously producing a dramatic, rapid, and long lasting rise in carotid blood pressure. Having validated that their approach worked on a small scale, ox-hearts were abandoned for horsemeat in an attempt to increase yield.
Their efforts resulted in the identification and characterization of three amines: one that is soluble in chloroform – isoamylamine - and two that are water soluble: p-hydroxyphenylethylamine (also known as p-tyramine; TYR) and PEA. When injected intravenously the pithed cat responds within 10 minutes with a rapid rise in arterial blood pressure. Of the three compounds isolated TYR had the greatest pressor effect while PEA was somewhat less efficacious and isoamylamine was the weakest. This paper is remarkable in that Barger and Walpole establish a powerful paradigm in which a robust biological assay is used to analyze chemical principles, both natural and synthetic. Furthermore, by using a bacteriological preparation to convert vertebrate structures (e.g. muscle protein) into their most basic molecules relatively large amounts of “natural” products with powerful physiological effects could be produced for experimental study and as medicines. Finally, their work laid the foundation of what became a fertile field of study that continues to be extremely active a century later.
The next 40 years saw the biogenic amines continue to enjoy considerable attention although the focus gradually shifted more to the catecholamines and the enzymes that synthesize them. The identification and characterization of AADC and MOA-A and MAO-B led to the development of highly selective inhibitors that proved to have benefit in some clinical settings. Just as drugs such as iproniazid, an irreversible MAO inhibitor, provided a new means of perturbing catecholamine physiology, they also provide a means to probe the physiology of the TAs.
In 1952 Zeller and Barsky and Griesemer et al. (1953) were the first to report that iproniazid dramatically potentiates the CNS-stimulating effects of PEA in guinea pigs evoking behavioral excitation and convulsions (Rebhun et al., 1954). These powerful CNS effects were further elaborated on the following year by Fleckenstein and Stöckle (1955).
Several years later Spector et al. (1963) published a distribution of TYR in mammalian tissues. Nakajima et al (1964) reported perhaps the first evidence of endogenous PEA and its effect on motor tissue in the mouse while Fuxe et al. (1967) found that PEA releases catecholamines from central and peripheral monoamine-synthesizing neurons. Though these findings generated some interest others remained skeptical as to the relevance of the animal studies to human physiology.
This attitude began to change in the early 1960's as explanations were sought for the hypertensive crisis also referred to as the tyramine pressor effect (Da Prada et al., 1988) that some foods can produce in patients taking MAO inhibitors for depression (McCabe, 1986); the hypotensive effect of MAO inhibitors experienced by patients on long term antiangina medication; and the hypotensive effect of alpha-methylated analogs of m-TYR and DOPA (dihydroxyphenylalanine). The hypertensive response is widely thought to be due to TYR-activated NE release. The hypotensive effect seen in chronically medicated patients develops because TYR levels become too elevated. Under these conditions TYR is not metabolized by MAO-B so it can be transported into synaptic vesicles where it is converted to OCT by the action of vesicular dopamine-beta-hydroxylase. This nascent OCT co-occupies the vesicle with NE such that when depolarization occurs there is less NE released. Furthermore, the OCT that is released is less potent at stimulating post synaptic alpha and beta adrenergic receptors. Thus, according to the false-transmitter hypothesis build up of the less physiologically active TAs at the expense of the more active catecholamine transmitters contributes to the clinical presentation (Kopin et al., 1964; Kopin, 1968). As analytical methods became more sophisticated TAs were unequivocally demonstrated to be present in rat tissue (Majer & Boulton, 1970), and in mouse (Mosnaim & Sabelli, 1971), rabbit (Sabelli et al., 1973; Zeller et al. 1976), and finally human brain (Inwang et al., 1973).
Evidence that the actions of TAs in the CNS are distinct from those of the catecholamines was reported by Sabelli et al. (1976) who made microelectrode recordings using iontophoretic techniques to demonstrate that PEA had opposite effects on cortical neuron stimulation compared to the inhibitory effects of DA and NE at the same concentration (0.5M). Interestingly these authors then compared the behavioral effects of an intraperitoneal injection of PEA (10 mg/kg) to those of epinephrine (1-5 mg/kg), NE (1-5 mg/kg), and DA (10-50 mg/kg) in newly hatched chicks. Whereas the catcholamines induced sleep, PEA induced a prolonged AMPH-like excitement characterized by increased locomotor activity, chirping, and aggressive fighting behavior. However, depending on the tissues these agents can have similar effects (e.g. TYR inhibition of prolactin from rat pituitary) albeit through distinct mechanisms of action (Becu-Villalobos et al., 1987).
Besides PEA and TYR, OCT has been found to affect the synaptosomal transport of NE (Raiteri et al., 1977) as well asneuronal activity in the rat dorsal horn (Hicks et al., 1978a), cerebral cortex (Hicks & McLennan, 1978b), and other regions of the CNS (Dao et al., 1980).
2.4.2. Trace amines as modulators of neurotransmission
Neuromodulators can be defined as compounds present in the CNS that can alter the sensitivity of neurons to other neurotransmitters but have no effect on their own.
It has long been appreciated that TAs, in particular OCT and TYR, are likely neurotransmitters, and not neuromodulators, that substitute for NE in invertebrates (Saavedra et al., 1974; Saavedra & Axelrod, 1976; Roberston & Juorio, 1976; Axelrod & Saavedra, 1977; Evans & O'Shea; 1977; Roeder, 1999) although Nagaya et al., (2002) found TYR to function as a neuromodulator in Drosophila melanogaster and Donini et al. (2004) reported evidence that TYR functions as both a neurotransmitter and neuromodulator in the locust oviduct.
In mammals it has been suggested that OCT might be a neuromodulator involved in some of the physiological effects ascribed to MAO inhibitors (Kakimoto & Armstrong, 1962). In a series of papers Baldessarini and Vogt (Baldessarini, 1971; Baldessarini & Vogt, 1971; Baldessarini & Vogt, 1972) reported TA involvement in the release of aromatic amines in rat brain, uptake, and subcellular distribution of aromatic amines in rat brain. In 1975 Boulton proposed that the TAs have “direct” and “indirect” effects on synaptic transmission involving DA, NE, and 5-HT (Boulton, 1976b). The direct effects are the result of the TA being released or diffusing to a receptive site of action. The term indirect refers to the downstream consequences of a TA interfering with catecholamine uptake and/or release. Boulton (1976b) hypothesized that TAs would achieve their direct modulatory effects by altering the sensitivity of a post synaptic target (1976b).
By 1982 a lively debate surrounded the role of TAs in the CNS and could be summed up in the question posed by Jones (1982): Does TRYP act as a neuromodulator or neurotransmitter in mammalian brain? Boulton's group asked the same question about PEA (Paterson et al., 1990) and seems to have fully embraced the idea that PEA is a neuromodulator of catecholamine neurotransmission in the CNS (Boulton et al., 1990).
Contrary to this view Baud et al. (1985) provided compelling evidence that TYR and PEA can act independently of DA to inhibit acetylcholine release in striatal slices. Parker & Cubeddu, (1988) reported observing effects of PEA and AMPH on DA efflux, DA uptake and mazindol binding.
Paterson (1993) showed that PEA potentiates cortical neuron responses to NE independent of any endogenous NE. The ability of PEA to modulate DA neurotransmission in the nigrostriatal pathway was reported by Barroso & Rodriguez (1996). Ishida et al. (2005) found that PEA stimulates acetylcholine release by activating glutamatergic signaling pathways. More recently Geracitano et al. (2004), Federici et al. (2005), and Berretta et al. (2005) demonstrated that the TAs depress GABAB responses in dopaminergic neurons by inhibiting G-βγ-gated inwardly rectifying potassium channels.
Additional studies suggest that at high concentrations TAs interact and interfere with biogenic amine transporter function in ways similar to AMPH and METH but different from DA, NE, cocaine, and methylphenidate (Hirano et al., 1989; Janssen et al., 1999; Mundorf et al., 1999; Berry, 2004; Sulzer et al., 2005). Classically then, the TAs have been shown to inhibit DA uptake and to a lesser extent NE and 5-HT (Horn & Snyder, 1972; Raiteri et all., 1977; Dyck, 1983; Bailey et al., 1987), as well as exert an AMPH-like effect (Janssen et al., 1999) on presynaptic monoamine transporters causing them to reverse their normal direction of transport (Stamford et al., 1986; Parker & Cubeddu, 1988). This results in the displacement of DA from intracellular vesicles elevating cytoplasmic concentrations, and ultimately elevating neurotransmitter concentrations in the synaptic space (Amara & Sonders, 1998; Sulzer et al., 2005).
In their review of the subject Burchett & Hicks (2006) suggest that when the classical actions of TAs are considered together with the recent discovery of vertebrate receptors activated by TAs (Borowsky et al., 2001; Bunzow et al., 2001) the evidence suggests four kinds of TA activity in the CNS: co-transmitters released with DA, NE, and 5-HT; transmitters in their own right with their own receptors; false transmitters at DA-and NE-selective receptors; and neuromodulators hence the name protean neurotransmitters.
2.4.3. Behavioral manifestations of trace amines
Depending on the dose, PEA is capable of producing dramatic increases in animal behavior or significant decreases in behavior. In rodents PEA elicits AMPH-like behavior (Hirano et al., 1989; Janssen et al., 1999) at doses of ∼50 mg/kg (intraperitoneal) characterized by hyperactivity, occasional walking backward, rearing, sniffing, gnawing, and licking (Dourish, 1982; Boulton, 1982; Lapin, 1996). At higher doses (75-100 mg/kg) repetitive, stereotypical behaviors, predominate including ‘wet dog’ shaking, excessive grooming and head movement, seizures, labored breathing, salivation, and straub tail.
Further evidence of important interactions between TAs and DA signaling with behavioral consequences is the work of Barroso and Rodriguez (1996). When PEA (1.75 mg/kg) is administered intravenously to rats with a unilateral 6-OH DA lesion of the nigrostriatal DA system they begin to display rotation behavior within seconds ipsilateral (same as) to the lesion.
In nonhuman primates PEA (o.3-1.0 mg/kg), in the presence of the MAO-B inhibitors R-(-)-deprenyl or MDL 72974 (each at 0.3 mg/kg), fully substitutes for an intramuscular injection of METH (0.3 mg.kg) in squirrel monkeys trained to discriminate METH from saline (Bergman et al., 2001). Such psychostimulant effects of PEA are thought to be dependent upon intact nigrostriatal and mesolimbic DA pathways Boulton et al., 1990).
The availability of mice that completely lack the DA transporter (DAT; Giros et al., 1996) provides an opportunity to dissociate the behavioral, neurochemical, and molecular effects of PEA, and other TAs as well, that are DAT-independent from those that are DAT-dependent. When wild type and DAT-deficient animals were administered PEA at a dose that was effective at producing hyperlocomotion (50 mg/kg, intraperitoneally) only the wild type mice responded with a transient elevation (6.5 fold) in striatal extracellular DA levels, as determined by microdialysis (Sotnikova et al., 2004).
In behavioral experiments wild type mice respond to PEA (50 mg/kg, intraperitoneally) with a short-lived (10-15 min) burst of hyperactivity compared to saline-injected controls. Surpsingly, mice genetically engineered to lack the DAT display spontaneous hyperlocomotion in a novel environment, a response that is supressed by all doses (10, 30, 50, 70, 100 mg/kg) of PEA administered i.p. (Giros et al., 1996). At the highest doses of PEA wild type mice exhibit stereotypies including headweaving, padding, sniffing rearing, grooming, and licking whereas no dose of PEA produces stereotypies in mice lacking DAT.
To explain these findings Sotnikova et al. (2004) in a follow-up study proposed that DA-dependent locomotor activity is probably influenced by a balance struck between activity of stimulatory 5-HT1B and 5-HT2A receptors and inhibitory 5-HT1A and 5-HT2C receptors (Gainetdinov et al. 1999; Spielewoy et al. 2001; Powell et al. 2004; Barr et al. 2004).
2.4.4. Trace amines in human health & disease
With the availability of reliable quantitative methods for determining TA content in tissues and fluids data quickly accumulated on the abundance and tissue distribution of various TAs in healthy individuals as well as those afflicted with somatic (e.g. phenylketonuria, Wolf & Mosnaim, 1983; liver failure, Fischer & Baldessarini,1971) and mental ailments (Huebert & Boulton, 1979; Boulton, 1980; Wolf & Mosnaim, 1983).
The results from these studies, often but not always replicated (Anderson et al., 1984), correlated levels of PEA, TYR, TRYP, or their metabolites in blood and/or urine with methylphenidate and AMPH exposure (Borison et al., 1975), hypertension (Andrew et al., 1993), and hepatic encephalopathy (Manghani et al., 1975) as well as mental conditions including schizophrenia (Boulton et al., 1967; Vogel, 1967; Faurbye, 1968; Zeller et al., 1976; Sandler & Reynolds, 1976; Boulton, 1980; Boulton, 1982; Szymnanski et al., 1987; Myojin et al., 1989;O'Reilly et al., 1991; O'Reilly & Davis, 1994; Buckland et al., 1997), Tourette's syndrome (Baker et al., 1993), attention deficit hyperactivity disorder (i.e. ADHD; Baker et al. 1991; Kusaga, 2002), migraine (Hannington, 1967; Smith et al., 1970; Sever, 1979; D'Andrea et al., 2003b) and other headache (D'Andrea et al., 2004; Aridon et al., 2004), and depression (Mosnaim et al., 1973; Reynolds, 1979; Sandler et al., 1980; Chance et al., 1985; Davis & Boulton, 1994).
Certain forms of depression are unresponsive to available treatments and have been proposed to be manifestations of TA insufficiency (Mosnaim et al., 1973; Wolf & Mosnaim, 1983). The development of TA-like enhancer substances similar to selegiline (Deprenyl), widely used in treating movement (e.g. Parkinson's disease) and cognitive (e.g. Alzheimer's disease) deficits, remains an active area of research (Shimazu & Miklya, 2004; Gaszner & Miklya, 2006).
Another anti-Parkinson's disease medication, L-dopa, affects TA metabolism (Edwards et al., 1981). Interest in the possibility that the TAs play an important part in the etiology of Parkinson'e disease as well as the beneficial and adverse effects produced by L-dopa pharmacotherapy has been rekindled by the discovery of receptors that are activated by these biogenic amines and the work of Mercuri, Bernardi, and their colleagues (Geracitano et al., 2004; Mercuri & Bernardi, 2005;)
Many of the earliest attempts to link TAs and human health came at a time when the psychiatric and neuroscience communities were awash in the paradigm-shifting realization that small molecules, such as chlorpromazine (Thuillier, 1999), could produce significant improvements in certain mentally ill individuals. Encouraged by early successes substantial effort went into developing small molecule-based medications for treating cognitive deficits, psychosis, and mood disorders that continue to this day.
Of course the potential for altering mood and cognition through pharmacologic means has long been appreciated by humans (Furst, 1972; Furst, 1976; Booth, 1996). The use of naturally occurring mind-altering, psychoactive compounds present in certain plants (e.g. mescaline from the peyote cactus) and fungi (e.g. psilocybin from mushrooms) has been ritualized for millennia. Most of the psychoactive principles extracted from these natural sources are structurally related to PEA and TRYP and have inspired the synthesis of a plethora of substituted phenylethylamines and tryptamines of varying degrees of psychoactive potential (Shulgin & Shulgin, 1991; Shulgin & Shulgin, 1997).
The simple structure of PEA has made it a natural point of departure for many medicinal chemists. Over the years their efforts have led to the creation of many clinically useful derivative compounds. Arguably the most important of these are the AMPHs. Not surprisingly AMPH and METH bind the same cellular sites and display many of the same sympathomimetic properties as the naturally occurring TAs (Sulzer et al., 2005). What is significantly different about them though is that AMPH and METH produce profound psychomotor and anorectic effects that are accompanied by a high abuse potential in humans. In contrast high doses of exogenous PEA are tolerated by healthy volunteers with no apparent risk potential.
The disparate efficacies and diversity of effects produced by such chemically similar molecules as PEA and METH likely reflects differences in their pharmacokinetic and pharmacodynamic properties. Of these two the latter has recently become one of the exciting revelations to come from the discovery of a putative vertebrate TA receptor; METH is a potent and efficacious TA receptor agonist (Bunzow et al., 2001; Reese et al., 2007).
3. Receptors for trace amines
3.1. Historic context
The large body of biochemical, pharmacological, and physiological evidence collected over the course of almost 100 years convincingly demonstrates the presence of endogenous TAs in all species of invertebrates (de Rome et al., 1980; Degen et al., 2000; Dudai, 1982; Dudai & Zvi, 1984; Guillen et al., 1989; Hashemzadeh et al., 1985; Rex & Komuniecki, 2002) and vertebrates that have been examined (Molinoff & Axelrod, 1969; Molinoff & Axelrod, 1972; Durden et al., 1973; Philips et al., 1974a; Philips et al., 1974b; Saavedra et al., 1974; Boulton et al., 1975; Juorio, 1976; Juorio & Philips, 1976; Juorio & Robertson, 1977; Philips et al., 1978; Reynolds et al., 1980; Juorio & Kazakoff, 1984; Williams et al., 1987; Juorio & Sloley, 1988; Downer et al., 1993).
Furthermore, pharmacologic manipulations (Borison et al., 1974; Borison et al., 1975; Boulton, 1976a; Philips & Boulton, 1979; Stoff et al., 1984; Boulton et al., 1990; Juorio et al. 1991a; Juorio et al. 1991b) and lesioning studies (Boulton et al., 1977; Juorio & Jones, 1981; Greenshaw et al., 1985; Juorio & Greenshaw, 1986; Greenshaw et al., 1986; Greenshaw et al., 1986; Juorio et al., 1987; Juorio, 1988) can significantly influence TA turnover and levels with physiological (Becu-Villalobos, 1987; Cheng, 1990; Hirashima, 1999; Lee et al., 2003) and behavioral (Dourish, 1982; Lapin, 1996; Rex et al., 2004; Suo et al., 2006) consequences.
The thesis that TAs act as endogenous signaling molecules in human brain as well as other organs is a logical one reinforced by the recognition that in animal models of drug seeking behavior TAs are self-administered (Shannon & Degregorio, 1982; Bergman et al., 2001) while in humans the chemically related compounds mescaline, AMPH, and METH produce intense intoxication, sensitization, and a profoundly altered psychotic state (Faurbye, 1968; Furst, 1972; Furst, 1976; Titeler et al., 1988; Sato, 1992; Ujike & Sato, 2004) in addition to their cardiovascular and thermic effects.
However, for any molecule, including a TA, to achieve the status of a bona fide neurotransmitter (NT) several criteria must be met. Of these the principle requirements are that: (1) an organism possesses the biosynthetic and catabolic capabilities to produce and inactivate the putative NT substance; (2) the putative NT must be found in the terminals of neurons; (3) the putative NT must be stored in vesicles and released upon stimulation; (4) once released the putative NT must be capable of binding to a specific, saturable, and functionally active receptive site that in turn couples to a measurable biological effect; and (5) application of exogenously prepared NT substance mimics the biological effect(s) of the endogenous material.
Of the traditional TAs OCT was the first to meet essentially all of these requirements albeit in an invertebrate species (Saavedra & Axelrod, 1976; Axelrod & Saavedra, 1977), including the demonstration of “true” OCT receptors (Carpenter & Gaubatz, 1974). However, the existence of functional mammalian TA receptors remained the subject of considerable debate until recently (Borowsky et al., 2001; Bunzow et al., 2001).
Since TAs are present in every vertebrate and invertebrate species that has been examined it was anticipated by most of those pioneers working in the area that TA receptors (TARs) would be pharmacologically defined and biochemically characterized apace with the receptors for the biogenic amines DA, NE, 5-HT, and histamine (HIS).
This expectation was met in several species of invertebrates. In stark contrast the demonstration of membrane-bound receptors specific for the TAs proved much more challenging in vertebrates. As no vertebrate TAR candidate emerged with each passing year the initial excitement that accompanied the prospect of establishing the TAs as bona fide neurotransmitters in vertebrates began to wane and the concept that these ‘false’ transmitters played primarily a neuromodulatory role gained widespread acceptance. With the recent cloning and characterization of vertebrate GPCRs functionally activated by TAs this area of research has been rejuvenated and efforts to define their biological roles have a promising future.
3.2. Trace amine binding sites
Binding sites are similar to receptors in that both selectively interact with ligands and at some concentration become saturated. However, binding sites are importantly distinguished from receptors in that no functional (i.e. biological/physiological) consequence of their interaction with ligand is implied.
The search for specific and saturable TA recognition sites in brain can be traced back in the literature to the early 1970's when Baldessarini and Vogt (1971) explored the uptake and subcellular distribution of PEA and TYR while Boulton and his colleagues, using 14C-PEA, 14C-TYR, and 14C-TRYP, demonstrated the labeling of myelin containing ‘complexes’ present in rat brain homogenates associated with synaptosomes (Boulton et al., 1972; Boulton & Baker, 1975).
The first demonstration of specific and saturable binding sites for 3H-PEA was reported for homogenized rat forebrain by Hauger et al. (1982). Using 100 mM cold PEA to define the nonspecific binding of the labeled compound (3H-PEA; ∼44 Ci/mmol), these investigators were able to perform a Scatchard analysis and estimate the number of PEA binding sites (Bmax = 1078 fmol/mg protein) and determine a dissociation constant (Kd = 55 nM). That the 3H-PEA was associating with sites composed at least partially of protein was demonstrated by the ability of proteases and heat to disrupt them.
With access to relatively high specific activity (∼32 Ci/mmol) 3H-TYR Vaccari (1986) found that synaptosomal membranes prepared from rat brain (e.g. striatum, hypothalamus, cortex, pons-medulla and cerebellum) and incubated in the presence of the MAO inhibitor pargyline (10 mM) bound 3H-TYR in a temperature-dependent, sodium ion-dependent, and saturable manner with a low dissociation constant (Kd = ∼10 nM). Besides TYR reserpine, DA, and several DA reuptake inhibitors (e.g. nomifensine, methylphenidate, d-AMPH) were potent competitors of 3H-TYR binding. With respect to DA this profile was consistent with a presynaptic site of interaction, a hypothesis supported by lesioning of the nigrostriatal pathway (Vaccari, 1986; Vaccari, 1993).
Prior to the cloning of a rat GPCR activated by TAs (Borowski et al., 2001; Bunzow et al., 2001) the presence of saturable OCT binding sites in membranes had only been convincingly documented in several invertebrate species. Using 10 mM phentolamine to define nonspecific binding Dudai (1982) and Dudai & Zvi (1984) demonstrated saturable [3H]p-OCT labeling of membrane preparations made from the heads of Drosophila melanogaster with a Kd of 5-6 nM while Hashemzadeh et al. (1985) found both high (Kd = 1 nM) and low (60 nM) affinity labeling in the light organ of the firefly that was displaceable by 10 mM p-OCT. In both species of fly the addition of guanosine-5′-triphosphate (GTP) significantly reduced the number of [3H]p-OCT binding sites providing the first evidence in support of the hypothesis that insect tissues possess functional OCT GPCRs. In their efforts to isolate an OCT receptor protein Nathanson and his colleagues (Nathanson et al., 1989) took advantage of the apparent high density of OCT receptors in this tissue.
Specific and saturable binding sites for the TA TRYP, defined in the presence of excess cold TRYP, were first demonstrated in rat brain membranes by Kellar and Cascio (1982) and Perry et al. (1982). Soon thereafter the uneven distribution of specific high affinity (Kd = 1.5 - 5 nM) sites labeled by [3H]TRYP were also identified in other tissue homogenates (Cascio & Kellar, 1983; Wood et al., 1984; Bruning & Romelspacher, 1984; Martin et al., 1986; Graham & Langer, 1987) and sections (Perry, 1986; McCormack et al., 1986; Kaulen et al., 1986). Taken together these studies were consistent with the interpretation that TRYP binding was likely associated with a plasma membrane-bound site primarily located in synaptosomes but with a pattern of anatomic distribution distinct from that of 5-HT (Nguyen & Juorio, 1989).
3.3. Invertebrate trace amine-activated receptors
By the early 1970's it was becoming widely accepted, in vertebrates at least, that many of the physiological consequences of neurotransmitters such as DA are mediated by membrane-bound receptive proteins that coupled to and activated intracellular G proteins. Once activated these G proteins could directly modulate adenylyl cyclase activity to stimulate or depress intracellular levels of adenosine 3′, 5′-monophosphate (cAMP). As early as 1972 Walker et al. had demonstrated that species of snail possess neurons that are hyperpolarized by OCT due to an increased conductance of potassium while Robertson and Steele reported that insect nerve cord phosphorylase activity is stimulated by both OCT and cAMP (1972). OCT responses were soon reported in species of Aplysia (Saavedra et al., 1974).
That same year it was firmly established that OCT stimulates the accumulation of cAMP in insects. Using homogenates and intact preparations of thoracic ganglia from the cockroach Periplaneta americana Nathanson and Greengard (1974) were able to demonstrate the presence of an adenylyl cyclase that could be stimulated by low doses of OCT but was otherwise insensitive to either DA or 5-HT. Compelling pharmacological evidence that OCT-stimulated cAMP accumulation in the ventral nerve of P. americana was receptor-mediated followed in 1976 (Nathanson, 1976). In 1977 Harmar and Horn published that cockroach brain contained an adenylyl cyclase that is sensitive to OCT.
In 1978 Dougan and Wade published a report in which they had used the DA receptor antagonists clozapine and metoclopramide to demonstrate the likelihood of OCT receptors in the mollusk Tapes' ventricle that are pharmacologically distinct from DA receptors present in the same tissue.
The following year Bodnaryk discovered an OCT-stimulated adenylyl cyclase activity in the moth Mamestra configurata (Bodnaryk, 1979 a, 1979b). Subsequently, the ability of low OCT concentrations to raise cAMP levels with a distinctive pharmacological profile was reported in preparations of Drosophila melanogaster heads (Uzzan & Dudai, 1982) and flight muscle of Locusta migratoria (Lafon-Cazal et al., 1985).
The notion that OCT's effect on invertebrate tissue preparations might be simple was dispelled when Evans published pharmacological evidence of what appeared to be at least 3 distinct receptor subtypes in the locust extensor-tibiae muscle (Evans, 1981). He proposed 3 subtypes and organized them into 2 major classes. OCT-1 receptors coupled to increases in intracellular calcium fluxes, a similar physiological response displayed by other insects (Jahagirdar, 1987). OCT-2 receptors were of two pharmacologically distinguishable subtypes, 2A and 2B, but both coupled to increases in cAMP (Evans, 1981; Evans, 1984; Evans & Robb, 1993).
Based on studies of OCT's effects on intact locust neurons Roeder and colleagues proposed the existence of another class of OCT receptor, OA3, that also coupled to the stimulation of cAMP production (Roeder & Gewecke, 1990; Roeder, 1992; Roeder & Nathanson, 1993; Roeder, 1995; Roeder et al., 1995). However, due to its similarities with OCT-2A receptors it eventually came to be considered a member of the OCT-2 class and was designated OCT-2C.
Concomitant with the more traditional approaches to studing receptors in the 1970's and 1980's several powerful biochemical and molecular biological techniques were being developed that would revolutionize receptor research. One of these allowed investigators to reliably determine the sequence of almost any polypeptide as long as a few picomoles (10-12 mol) of it were available for analysis. At the same time advances in solid-phase nucleotide chemistry were making the efficient synthesis of custom oligonucleotides, or ‘oligos,’ routine and the cost affordable by most laboratories.
The ability to sequence proteins and then custom design an oligonucleotide meant that if one could obtain a partial receptor amino acid sequence then a sequence of possible codons could be deduced and used to design an oligonucleotide specific for the mRNA, cDNA, or gene from which that protein was derived. Such an oligo would serve as a powerful tool in the identification of putative receptor-encoding cDNAs or genes using strategies based on nucleic acid hybridization. By subjecting these putative receptor-encoding clones to automated DNA sequencing and computer-aided analyses the most promising of them could be quickly and reliably identified. The chosen clones could then be heterologously expressed in cells that typically lack them. Thus, for the first time, it would be possible to characterize a receptor protein in an environment far less complex than a whole tissue homogenate.
In spite of the overwhelming evidence that OCT receptors existed as true proteinaceous entities they proved difficult to characterize biochemically because of their relatively low abundance and functional lability during purification. Furthermore, the ligands that activate them are agonists of relatively low affinity. In an attempt to overcome these technical hurdles Nathanson developed a potent OCT receptor agonist, NC-5Z, that could be photoactivated and irreversibly bound to a glycoprotein in the light organ of the firefly Photimus pyralis in a manner consistent with the protein being an OCT receptor (Nathanson, 1989; Nathanson & Kaugars, 1989). From this NC-5Z-labeled material a short stretch of N-terminal amino acid sequence was determined (Nathanson et al., 1989). This same photoaffinity reagent allowed Roeder and Nathanson (1994) to identify a protein with similar biochemical and pharmacological characteristics in the desert locust Schistocerca gregaria. It was understandably a great disappointment when these investigators came to realize that the peptide fragment they had so painstakingly obtained did not arise from an OCT receptor in spite of their best efforts to avoid artifactual labeling.
The distinction of being the first to report the successful cloning and expression of an invertebrate TA receptor goes to Arakawa et al. (1990). With a clone of the human beta2 adrenergic receptor as their hybridization probe these investigators screened a Drosophila melanogaster genomic library under ‘low stringency’ conditions that allow nucleic acids to form stable duplexes in spite of extensive mis-matched base-pairing. The hybridizing DNAs were sequenced and in one reading frame a polypeptide was deduced whose amino acid sequence was homologous to several mammalian adrenergic receptors. A probe containing part of this putative receptor sequence was made and used to identify a full-length cDNA in a library constructed from Drosophila head mRNA.
The longest open reading frame in their cDNA coded for a protein that shared extensive sequence homology, sites for post-translational modification such as glycosylation, and a similar hydropathy profile with several vertebrate receptors that are activated by biogenic amines including dopamine, epinephrine, norepinephrine, and 5-HT. However, this putative receptor sequence shared the highest degree of sequence identity with vertebrate alpha2 adrenergic receptors.
The availability of a new cDNA provides a sensitive and precise means by which to investigate the tissue distribution of its corresponding mRNA. Interestingly, this particular putative receptor-coding sequence was exclusively expressed in the head of Drosophila.
The challenge confronting the investigator who has cloned a novel nucleotide sequence is figuring out what its product does. In the case of Arakawa et al. their thinking was guided considerably by the extensive homology between their putative receptor and other G protein-coupled receptors (GPCRs) that had already been cloned. However, to establish that the protein is a receptor its pharmacological and functional attributes have to be characterized. Since Chinese hamster ovary (CHO) cells are easy to culture and do not express the Drosophila mRNA under study this cell line was chosen to be co-transfected with two plasmids: a eukaryotic expression vector containing the putative receptor-coding sequence and a plasmid carrying a drug resistance gene. Clonal populations derived from drug-resistant CHO cells maintained under Geneticin selection were identified that expressed high levels of the putative receptor mRNA by Northern blotting, expanded in size, and used in subsequent membrane binding assays.
Given the extensive sequence conservation between the putative Drosophila ‘orphan’ receptor and the alpha2 adrenergic receptors binding studies with [3H]yohimbine and a series of unlabeled neurotransmitter agonists and antagonists were conducted. In addition, since there was considerable evidence for the existence of OCT-coupled adenylyl cyclases in several invertebrate species, cAMP accumulation was also investigated.
Despite a higher than expected affinity for [3H]yohimbine, a slightly higher affinity for synephrine, and the lack of any TYR binding data the authors concluded they had cloned the cDNA for a G protein-coupled OCT receptor of the type 1 subtype based on the rank order of affinity it displays for noncatecholic phenylethylamines and its ability to couple OCT exposure to the inhibition of forskolin-stimulated cAMP production in a pertussis toxin- sensitive manner.
Although confident in assigning their new receptor to the OCT-1 class, the authors noted there were important discrepancies in the effective concentration of OCT observed versus expected. Also, the relative potencies of some compounds at the Drosophila OCT-1 receptor were not the same as had been reported for the prototypical OCT-1 in the locust flight muscle preparation.
In the discussion of possible explanations for their observations the authors identify a number of important parameters that still must be considered when attempting to draw conclusions from studies involving receptors expressed in an atypical environment. Paramount among these influences are the nature of the cellular milieu in which the putative receptor is expressed and the number of receptors that are actually expressed.
Six months later Saudou et al. (1990) reported the success of their cloning efforts using degenerate oligonulceotide probes based on conserved amino acid residues present in the highly conserved, putative transmembrane domains VI and VII of several biogenic amine responsive vertebrate receptors. The cDNA they identified turned out to code for a GPCR identical to the one reported by Arakawa et al. (1990). Membranes prepared from Cos-7 cells transiently expressing the putative receptor displayed a similar affinity (Kd∼= 4.45 nmol) for [3H]yohimbine and a comparable competition binding profile. However, to their credit Saudou et al. included TYR in their binding assays and found that it was about 30 times more potent in displacing [3H]yohimbine than was OCT. The stable expression of their clone in cultures of mouse NIH-3T3 cells allowed them to functionally characterize the receptor which was found to inhibit forskolin-stimulated cAMP when exposed to TYR (EC50=2.4 μM) and OCT (EC50=35 μM). Based on their pharmacological and physiological findings the authors suggested that the receptor they had expressed was in fact more appropriately referred to as a TYR receptor.
Though the authors acknowledged there was substantially more experimental evidence in support of insect receptors for OCT, they pointed out the literature was not silent with respect to the possible existence of invertebrate TYR receptors. In fact, there were several compelling pieces of evidence that supported the hypothesis that TYR and OCT played physiologically distinct roles. As examples they cited the observation that OCT can stimulate glyconeolysis in cockroach nerve cord and fat bodies whereas TYR causes a decrease (Downer, 1979). In addition, the tissue distribution of OCT differs from TYR (Maxwell et al., 1978;Juorio & Sloley, 1988) with the latter more abundant than the former in insect brains.
The availability of a cDNA coding for a Drosophila OCT/TYR receptor gave Evans and colleagues an opportunity to further explore its pharmacology and physiology in isolation and in different cellular milieus (Robb et al., 1994). Interestingly, these authors found that in competition binding assays PEA was 10 times more potent than TYR at displacing [3H]yohimbine binding from OCT/TYR-containing CHO membranes while TYR was about 2 orders of magnitude more potent than OCT. The same rank order was found in the functional assay where they inhibited forskolin-stimulated cAMP production in CHO cells. However, in contrast to the differences they displayed with respect to potency in competition binding and cAMP assays the effects of TYR and OCT on the level of intracellular calcium in transfected CHO cells were insensitive to pertussis toxin and nearly identical in their response demonstrating that the same receptor protein was capable of interacting with multiple G proteins and multiple signaling pathways in the same cell. In conclusion these authors suggested based on its pharmacological profile that this particular OCT/TYR receptor was most likely a Drosophila homolog of the locust OCT-2/skeletal muscle class (Evans, 1981; Evans, 1984; Arakawa et al., 1990).
Soon thereafter Davis's group reported their discovery of OAMB, a novel Drosophila OCT GPCR-coding cDNA enriched in mushroom bodies. They based this assignment on the putative receptor's deduced amino acid sequence, its unique tissue distribution, its pharmacology, and its distinctive ability to couple to the stimulation of cAMP production and intracellular calcium mobilation in transfected Drosophila S2 and human embryonic kidney (HEK) cells (Han et al., 1998). The EC50 for OCT-stimulated cAMP accumulation was 190 nM while TYR appeared to be a partial agonist with an EC50 ∼100 fold less potent than OCT. In the presence of OCT calcium mobilization was stimulated in OAMB-expressing HEK cells. The restriction of OAMB's expression to structures associated with Drosophila's olfactory system together with evidence for biogenic amines influencing insect beavior prompted these investigators to conclude that the receptor they identified would be involved in olfactory conditioning presaging the finding of Farooqui et al. (2003) in the honeybee and the recent speculation that receptors for trace amines expressed in the olfactory epithelium of the mouse may recognize odorants in urine that serve as social cues (Liberles & Buck, 2006).
In the course of pursuing their interest in determining a biological function for TYR in Drosophila distinct from OCT Kutsukake et al. (2000) demonstrated that they had differing effects on excitatory junction potentials (EJPs) in dorsal acute muscles with OCT concentration-dependently enhancing, while TYR depressed, the amplitude of neurally evoked EJPs. These observations were extended by Nagaya et al. (2002) who studied the actions of TYR and OCT in a Drosophila mutant known as honoka (hono). This mutant fly carries a P-element inserted 100 bases upstream of the OCT/TYR receptor gene cloned by Arakawa et al. (1990) and Saubou et al. (1990). The presence of this P-element not only interferes with the expression of this gene but also their normal avoidance response to various odorants. Interestingly, OCT's effect on stimulating larval EJPs remained intact in wild type and mutant larvae whereas TYR's inhibitory effect on EJPs was completely absent from hono larvae thus demonstrating almost unequivocally the existence of two separate receptor entities with distinct physiological properties.
In addition to providing the means by which to further characterize Drosophila OCT/TYR GPCRs these clones served as nucleic acid hybridization probes that were used to identify and isolate homologous receptor sequences from several invertebrate species (Vanden Broeck et al., 1995; von Nickisch-Rosenegk et al., 1996; Gerhardt et al., 1997a; Gerhardt et al., 1997b; Reale et al., 1997; Baxter & Barker, 1999; Blenau et al. 2000; Chang et al., 2000; Poels et al., 2001; Rex & Komuniecki, 2002; Ohta et al., 2003;Farooqui et al., 2003; Farooqui et al., 2004; Grohmann et al., 2003; Bischof & Enan, 2004; Balfanz, 2005; Molaei et al., 2005; Mustard et al. 2005; Rex et al., 2005; Dacks et al., 2006). These clones have also been instrumental in identifying the important structural features that particpate in the binding of ligand and coupling of the the receptor to its effectors (Chatwin et al., 2003; Huang, 2003; Ohta et al., 2004).
Given the extensive evidence for OCT stimulation of cAMP production in invertebrates and the likely involvement of at least 3 distinct receptors in the process (Evans, 1981; Evans, 1993) it was surprising to many that cDNAs for only two invertebrate OCT-stimulating receptors had been cloned by the end of the millennium: one from Drosophila (Han et al., 1998) and the other from Aplysia (Chang et al., 2000).
Perhaps no one was more aware of this fact than P.D. Evans. Not surprisingly then, as the Drosophila genome became more completely characterized, Evans and his colleagues scoured the databases for putative GPCR-coding open reading frames that predicted polypeptides more closely related to vertebrate beta-adrenergic receptors. The fruit of these labors was the identification of three candidate receptors that bestowed on cells expressing them a greater sensitivity to OCT than TYR in terms of stimulating the production of cAMP (Maquiera et al., 2005) and ultimately resulted in a new classification scheme for invertebrate OCT receptors (Evans & Maqueira, 2005).
Another benefit of being able to express a putative receptor at will is that it makes direct pharmacological and physiological analyses of expected (Evans, 1980; Evans, 1981) and unexpected novel receptor subtypes (Venter et al., 1988) more straightforward, with certain limitations, as mentioned above (Hiripi et al., 1994; Hirashima et al., 2003; Lee et al., 2003; Rex et al., 2004; Cazzamali et al., 2005;, Klaerke & Grimmelikhuijzen, 2005; Ohta et al.2005).
3.4. Discovery of vertebrate trace amine-associated receptors
In the pre-genomic era of molecular neuroscience the cloning of rare vertebrate transcripts, in particular those that code for novel GPCRs, was in many ways an art form whose practioners eagerly embraced the latest molecular technique while participating in the development of new ones. The most successful of these efforts were those that began with a firm biological foundation. Typically this would consist of well-documented observations of pharmacological agents producing physiological and/or behavioral effects that could not be explained by known mechanisms. Not suprisingly then given their long history and importance in terms of human health, receptors selective for the putative biogenic amine neurotransmitters were early targets of great interest to molecular neuroscientits.
In the years before the advent of molecular biology traditional biochemical strategies, used successfully to isolate soluble proteins (e.g. enzymes), failed for the most part to yield purified receptor proteins. Among the explanations suggested to explain this outcome are their relatively small size, limited abundance, and hydrophobic nature. Hard won, some receptor protein sequence information eventually began to emerge that led to the successful cloning of cDNAs and genes for the opsins (Nathans & Hogness, 1983; Nathans & Hogness, 1984; Nathans et al., 1986) and the hamster beta2-adrenergic receptor (Dixon et al., 1986; Kobilka et al., 1987). Even with the limited number of examples available at the time molecular neurobiologists following these developments latched on immediately to the remarkable extent of amino acid sequence and structural conservation, in particular the presence of 7 putative transmembrane (TM) domains, shared by these integral membrane proteins. The hypothesis to emerge from this observation was: All membrane-bound receptors that activate second messenger systems by way of stimulating G protein activity are structurally related.
This prediction was immediately put to the test experimentally in the form of “cloning by homology.” Through the efforts of many individual laboratories this approach rapidly yielded cDNA and genomic clones for receptors of the major biogenic amine neurotransmitters. Aligning the sequences of these receptors led to the construction of families whose members share extensive sequence with one another. In addition to identifying receptors predicted by years of physiology and pharmacology, this approach also led to the identification of sequences that appeared to code for novel or so-called “orphan” receptors in search of endogenous ligands.
3.4.1. Cloning of a rat trace amine receptor
3.4.1.a. Borowsky et al
In the course of their efforts to identify additional members of the 5-HT receptor family, scientists at Synaptic Pharmaceutical Corporation (Paramus, NJ) designed degenerate oligonucleotides complementary to conserved 5-HT receptor sequences located in putative TMs VI and VII. These primers were used in polymerase chain reactions (PCRs) to amplify similar sequences from a rat genomic library (Borowsky et al., 2001). The nucleotide sequence of one of the resulting amplification products contained an open reading frame that predicted a novel protein not present in the databases of the day. This polypeptide fragment was between 42-48% identical to sequences in the 5-HT4, DA D2, and beta-adrenergic receptors. Borowsky et al. used this fragment of genomic sequence to eventually obtain a full-length clone from rat testes cDNA. In an attempt to discover an agonist for this orphan receptor the sequence was expressed in Xenopus oocytes along with mRNA coding for the cystic fibrosis transporter regulator (CFTR), a chloride channel that is activated by cAMP.
With this functional assay Borowsky et al. screened a large panel of compounds and found that among them 100 μM OCT, and to lesser extent DA, and 5-HT, evoked significant inward currents only in oocytes that had been injected with the mRNA produced from their novel cDNA. This finding led them to try TYR which they found to be almost 20 times more potent than OCT. Taken together these data suggested that this orphan receptor was activated by TAs and so the authors referred to it thereafter as TA1.
3.4.1.b. Bunzow et al
With the cloning of the DA D2 receptor (Bunzow et al., 1989) the race was on to clone the DA D1 receptor, the second pharmacologically and physiologically defined receptor for DA. In the course of their efforts to clone this receptor scientists working at Oregon Health & Science University (Portland, OR) developed a set of degenerate oligonulceotide PCR primers based on conserved amino acid sequences found in putative TM domains III and VI of several catecholamine GPCRs. Subsequent to their use in the successful cloning of rat and human D1 receptor cDNAs and genes (Zhou et al., 1990) these primers were employed in a search for additional GPCRs that might be activated by DA or other related biogenic amines.
To this end these investigators performed reverse transcriptase-PCR (RT-PCR) on cDNAs they had prepared from a panel of cell lines derived from vertebrate peripheral tissues known to receive sympathetic innervation or be sites of catecholamine synthesis. One of the cell lines screened in this way, designated ARJ42 (American Type Culture Collection), was derived from a rat pancreatic tumor. When Bunzow et al. (2001) used their degenerate primers in a RT-PCR with mRNA prepared from ARJ42 as the template they amplified a cDNA fragment whose nucleotide sequence predicted a novel polypeptide, ost closely related to GPCRs known to be activated by catecholamines.
Efforts to express the full-length rat receptor-coding clone in a number of cellular backgrounds were frustrated for many years until it was determined that this receptor was primarily localized in the cytoplasm. Others had shown that modifying a GPCR's amino terminal domain can improve its expression (Guan et al., 1992). Consequently, Bunzow et al. added a 16-amino acid signal sequence from the influenza hemagglutinin virus followed by an 8-amino acid M1-“Flag” epitope and a “MetGly” spacer to the N-terminus of their putative receptor's cDNA. This modified sequence was then cloned into a eukaryotic expression vector containing a drug resistance marker and transiently expressed in HEK293 and COS-7 cells. Under G418 selection these cells gave rise to populations that stably expressed orphan receptor immunoreactivity.
Although the anatomic distribution of the putative receptor's mRNA did not provide insight regarding the nature of its endogenous ligand, the deduced amino acid sequence did. Consequently, based on the extensive conservation found between the novel sequence and catecholamine receptors Bunzow et al. assembled a large panel of compounds and began screening them for activity in a functional assay that monitored drug-induced changes in cAMP. In this functional assay 10 μM DA was able to elevate cAMP production but TYR and PEA were considerably more potent with nanomolar concentrations as efficacious as 10 μM forskolin. Subsequently they found that TRP, SYN, and OCT were also more potent than DA but less potent than either TYR or PEA, in that order. The pharmacological profile of this functional activity was interpreted by these investigators as being consistent with that of a TA receptor and hence it was named TAR1.
An extensive structure activity profiling effort revealed that the rat TAR1 was unusual in that it can be activated by a wide assortment of compounds, some of which were traditionally considered to be biologically inactive products of catecholamine metabolism (e.g. 3-methoxytyramine). Equally thought provoking was their demonstration that the synthetic phenylethylamine amphetamine (AMPH) is a potent full agonist at heterologously expressed rat TAR1. This observation was recently extended by Reese et al. (2007) who reported that S(+)-METH is a potent TAR1 agonist with EC50s of 0.89 μM, 0.92 μM, and 4.44 μM for rat, mouse, and the human-rat chimeric receptors, respectively (see below). Reese et al. (2007) also showed that PEA is a potent and full agonist at each species of TAR1, and that TYR is a full agonist for the rodent receptors but only a partial agonist at the human-rat chimera.
While the manuscript by Bunzow et al. (2001) was under review the findings of Borowsky et al. (2001) were published. A comparison of both group's nucleotide and deduced amino acid sequences revealed that they were one and the same receptor.
3.4.2. Cloning of a human trace amine-activated receptor
Borowsky et al. (2001) and Bunzow et al. (2001) were well aware of the considerable interest there would be in the identification of a human homolog to the rat TA receptor. Consequently, both groups pursued and reported cloning a human TA receptor homolog. When the putative human TA1 was co-expressed with CFTR in Xenopus oocytes Borowsky et al. (2001) demonstrated the activation of an inward chloride current in response to 100 nM TYR. The physiological and pharmacological characterization of this protein was then extended using COS-7 cells that transiently expressed the human TA1 receptor clone. PEA, TYR, and to a lesser extent OCT and DA stimulated the production of cAMP in these cells. Furthermore, their membranes displayed saturable and high affinity binding of [3H]TYR that could be displaced in the rank order of: PEA > TYR > DA >OCT > TRP.
Due to difficulty establishing a population of tissue culture cells that stably expressed their human receptor clone Bunzow et al. (2001) were unable to pharmacologically or functionally characterize their human TAR1. Interestingly, they were able to overcome this problem using a recombinant TAR1 receptor that consisted of human sequences with the exception of short stretches at the N-terminus, C-terminus, and in third intracellular loop. In these places the human sequences were replaced with the corresponding rat TAR1 sequences. The design of this chimera was intended to maintain all of the human putative TMs and the proposed ligand binding domain (Kratochwil et al., 2005). When a line of HEK cells that stably express this construct was generated and characterized it displayed pharmacological and physiological profiles more like that of cells stably expressing the mouse TAR1 with PEA being a more potent agonist than TYR (Lindemann et al., 2005; Lindemann & Hoener, 2005), in contrast to the report of Borowsky et al. (2001). Recently, Bunzow, Reese, and Grandy have found that METH is a potent and full agonist of transiently expressed wild type human TAR1 heterologously expressed in HEK cells (unpublished observations).
3.4.3. Cloning of a mouse trace amine-activated receptor
In their original paper the Synaptic scientists reported the cloning of a mouse brain cDNA by virtue of the sequence identity it shared with rat TA1 (Borowsky et al., 2001). Although they did not report the characterization of the protein coded by this clone they successfully used it to begin mapping the anatomic distribution of the receptor's mRNA in mouse brain.
Hybridization of an antisense TA1 riboprobe in tissue sections suggests that the mRNA is expressed in several brain regions including the mitral cell layer of the olfactory bulb, the arcuate nucleus of the hypothalamus, ventral tegmental area, locus coeruleus, Purkinje cells in the cerebellum, and ventral horn of the spinal column. In the report by Winsky et al. (2006) a more complete pharmacological description of the Synaptic mouse TAAR1 clone transiently expressed in HEK293 cells is presented.
Grandy's laboratory also cloned the mouse TAR1 and stably expressed it in HEK293 cells. With this cell line Reese et al. (2007) demonstrated that it displays a characteristic pharmacologic profile with respect to cAMP production in response to the stereoisomers of AMPH and METH (see below).
3.4.4. Cloning of a nonhuman primate trace amine-activated receptor
The cloning of a non-human primate TA receptor was reported for the rhesus monkey by Miller et al. (2002). Relying on human TA receptor sequence information oligonucleotide primers were designed and used to amplify rhesus genomic DNA by PCR. Nucleotide sequencing confirmed that the amplified product was the rhesus ortholog of human TAR1. In the paper that followed (Miller et al., 2005) demonstrated the transient expression of the rhesus TAR1 in HEK293 cells. Aware that ligand activation of rat and human TAR1 results in the stimulation of cAMP production, Miller and colleagues developed a cAMP Response Element-Luciferase (CRE-Luc) reporter assay to monitor the stimulation of rhesus TAR1. In spite of an issue with high background levels of luminescence, TYR and PEA could be shown to produce a response only in those cells cotransfected with receptor and CRE-Luc constructs.
An attempt to convincingly demonstrate specific [3H]PEA labeling of rhesus TAR1-expressing HEK293 cells or their homogenates proved difficult to achieve in spite of employing multiple buffering systems with and without sodium. This outcome was likely due to a combination of factors including low levels of receptor expression, high nonspecific binding of labeled PEA, and the fact that the ligand is an agonist. The development of a robust filtration-binding assay is likely going to require a selective TAR1 antagonist labeled to high specific activity.
Familiar with the observation by Bunzow et al. (2001) and Borowsky et al. (2001) that the majority of TAR1 immunoreactivity is intracellular, Miller et al. (2005) constructed and expressed a recombinant rhesus TAR1 expression vector that covalently coupled the receptor's N-terminus to enhanced green fluorescent protein (EGFP). In the CRE-Luc assay this construct still appeared to confer PEA sensitivity to cells that transiently express it albeit the response was notably less than with wild type receptors. Confocal fluorescence microscopy of cells transfected with the EGFP-rhesus TAR1 chimera revealed that most of the fluorescence was intracellular.
3.4.5. Zebra fish trace amine-associated receptors
It is often the case that comparative physiological and phylogenetic approaches provide important insights on biological function. With this long term objective in mind Gloriam et al. (2005a,b) undertook a computer-assisted scan of the published vertebrate genomes with the intention of identifying every human TA1 receptor-like sequence present in humans, mice, rats, and two species of teleost - fugu (Takifugu rubripes) and zebra fish (Danio rerio).
Unexpectedly these efforts revealed the genome of zebra fish to have experienced a very large expansion in its repertoire of TA receptor sequences compared to all others. Based on sequence analyses alone 57 putative TA receptors were identified as were another 40 containing the hallmarks associated with pseudogenes (i.e. premature stop codons interrupting the reading frame). To date none of these putative zebra fish TA receptor sequences have been shown to be expressed in vivo nor have any of them been validated in vitro, pharmacologically or physiologically. However, the very fact that the genome of Danio contains orthologs of the human and rodent TA receptors provides a compelling rationale for further use of this model organism in efforts ultimately understand the biological role(s) of the TAs in humans.
3.4.6. A comment on nomenclature
As the number of laboratories characterizing these receptors grew so did the number of related genes reported in the literature. It soon became apparent that a uniform nomenclature would be preferable to the confusing literature that was emerging. At the same time it was being realized that other members of this family displayed very different pharmacological profiles than TA1/TAR1. As a consequence Lindemann et al. (2005) proposed a naming algorithm based solely on the genomic and phylogenetic relationships between members of this large receptor gene family that importantly implies nothing specific about their pharmacology. Beginning with the receptor gene whose physical position defines one end of the chromosomal region that contains the entire family as a contiguous cluster in humans, mice, and rats TA1/TAR1 becomes: “Trace Amine-Associated Receptor #1,” or TAAR1 for short, (Lindemann et al., 2005). This convention has been accepted by the Human Genome Organization Gene Nomenclature Committee and has been adopted throughout the remainder of this review.
4. Biology of vertebrate trace amine-associated receptors
4.1. Expression of vertebrate trace amine-associated receptors
One strength of the molecular pharmacological approach to the characterization of GPCRs is the ability to establish immortalized, clonal cell lines whose cytoplasmic milieus support the functional coupling of stably expressed, heterologous receptors. Such model cell lines not only provide an endless supply of cells expressing predominantly the receptor of interest but importantly the vector alone-transfected control cells as well.
4.1.1. In vitro expression
The stable in vitro expression of the vertebrate TAARs has proved to be a challenge. From the first it was difficult to reliably express any TAAR clone in tissue culture in spite of the fact that a number of human, rodent, and non-human primate cell types were tested.
After repeated attempts failed to demonstrate coupling of any second messenger system to their transfected receptor Bunzow et al. (2001) engineered a rat TAAR1 expression construct such that the receptor's N-terminus carried an epitope tag. The confocal images immediately provided a possible explanation for their inability to functionally couple the receptor to a signaling pathway: the immunoreactivity was essentially all intracellular (Bunzow et al., 2001).
Based on this information these investigators engineered another expression vector that provided the nascent TAAR1 with a a cleavable signal sequence; a modification that had been previously used to augment the expression of some GPCRs (Guan et al., 1992). This modified form of the rat receptor achieved some stable cell surface expression in HEK293 cells and responded to various TAs with an increase in cAMP. Still, the majority of the immunoreactivity appeared to be in the cytoplasm (Bunzow et al., 2001). Borowsky et al. (2001) also commented that their TA1 was predominantly localized in the cytoplasm of the COS-7 cells transiently expressing it. Eventually Grandy's laboratory also succeeded in establishing a HEK293 cell line that stably expresses the mouse TAAR1. However, all attempts by Grandy's laboratory to stably express the human TAAR1 in tissue culture were unsuccessful. They overcame this inconvenience by expressing a hybrid receptor consisting of mostly human sequence but whose N-terminus, C-terminus, and third intracellular loop were replaced with the corresponding rat sequences. This particular chimeric design was chosen to preserve the proposed ligand binding pocket formed by the juxtaposition of the 7 putative TMs. Interestingly, the pharmacological profile of this chimeric receptor resembled that of the mouse TAAR1 more than the rat (Lindemann et al., 2005).
Recently a few laboratories have reported the success of a brute force approach to establishing cell lines that stably express the wild type human TAAR1 (Navarro et al., 2006; Wainscott et al., 2006). Unfortunately these rare successes have yet to shed much light on the molecular determinants of human TAAR1 expression. It is hoped that the identification of cell lines that express TAARs endogenously, such as ARJ42 and RIN 5, will provide additional opportunities to crack the expression enigma.
More widespread success has been reported with transient expression (Borowsky et al., 2001; Miller et al., 2005; Liberles & Buck, 2006) although assays using these preparations typically suffer from low signal to noise ratios and variable receptor densities since the proportion of cells expressing the receptor varies from transfection to transfection. Given these limitations the experience of Liberles and Buck (2006) is particularly remarkable since they apparently expressed all 15 mouse genes identified by Lindemann et al. (2005). Interestingly, transient expression of the epitope-tagged rat TA2 (TAAR4 according to the revised nomenclature of Lindemann et al., 2005; Borowsky et al., 2001) and the rhesus monkey TAR1 (TAAR1 according to the revised nomenclature of Lindemann et al., 2005; Miller et al., 2005) recapitulate the intracellular distribution observed by Bunzow et al. (2001) in HEK293 cells stably expressing rat TAR1.
4.1.2. In vivo expression
Determination of the anatomic distribution of the TAARs is a work in progress that will take considerable time to complete. This situation is largely a technical one because low levels of TAAR mRNAs are expressed, members of this extensive family share considerable stretches of sequence, and it is inherently difficult to generate antibodies against GPCRs.
Borowsky et al. (2001) using quantitative RT-PCR found evidence for TAAR1 in human brain tissue and several peripheral tissues including stomach, kidney, lung, small intestine, liver, pancreas, prostate, skeletal muscle, and spleen. Transcripts for TAAR 6, TAAR8, & TAAR 9 (formerly TA4, TA5, & TA3, respectively) were also detected by this method in the kidney. A systematic survey of TAAR1's mRNA distribution throughout the mouse brain was conducted using antisense riboprobes complementary to mouse TAAR1. Strong hybridization signals were found in numerous brain structures including the olfactory bulb and others rich in monoamine-producing neurons (i.e. ventral tegmental area, DA; locus coeruleus, NE; dorsal raphe, 5-HT).
Efforts by the Grandy laboratory to characterize the distribution of TAAR1 in rat tissues by Northern blotting and in situ hybridization were unsuccessful, most likely because of low expression of its mRNA. The more sensitive method of RT-PCR was successfully employed to amplify TAAR1 sequences from oligo-dT-primed RNA prepared from several brain regions and peripheral tissues. Most notably the highest level of TAAR1 expression was found in the olfactory bulb, nucleus accumbens, olfactory tubercle, prefrontal cortex, ventraltegmental/substantia nigra, cerebellum and pons. Of the peripheral tissues examined highest levels of expression were found in the liver, kidney, gastrointestinal tract, spleen, pancreas, and heart.
Recently, the expression of mRNA for TAAR1, TAAR6, TAAR8, and TAAR9 (formerly TA1, TA4, TA5, and TA3, respectively) in human leukocytes was demonstrated by RT-PCR while HPLC-coupled electrochemical detection revealed the presence of TYR, OCT, and SYN in platelets (D'Andrea et al, 2003). Vanti et al. (2003) find expression of the human TAAR9 gene in pituitary and skeletal muscle. Chiellini et al. (2006) reported that mRNA extracted from rat heart contained transcripts for TAAR1, TAAR2, TAAR3, TAAR4, and TAAR8a that could be amplified by RT-PCR using oligonucleotide primers specific for individual TAARs.
In an interesting recent study Liberles and Buck (2006) surprisingly failed to detect TAAR1 transcripts in mouse brain or olfactory epithelium. However, they did report finding transcripts for each of the remaining mouse TAARs in olfactory neurons. Further analysis of tissue sections subjected to dual labeling suggests each TAAR subtype is expressed independently of all other TAARs and odorant receptors. The surprising discrepancy between the reports by Liberles and Buck (2006) and Borowsky et al. (2001) and Bunzow et al. (2001) remains to be resolved and underscores the technical difficulties associated with studying ythis family of receptors.
4.2.Trafficking of vertebrate trace amine-associated receptors
The trafficking of vertebrate TA receptors is an aspect of their biology that warrants further systematic study and most certainly will hold many surprises. The fact that immunoreactivity associated with epitope-tagged TAAR1 protein appears to be mostly intracellular already is an indication that something is special about this sequence that distinguishes it from other catecholamine receptors such as the DA D1 receptor that typically trafficks to the plasma membrane (Bunzow et al., 2001). Other GPCRs such as for vasopressin and cannabinoids have also been reported to display characteristic cytoplasmic distributions (Bohn, 2007; Fenton et al., 2007) while some, such as the oxytocin receptor (Kinsey et al., 2007) and receptors for bioactive lipoids (Gobeil et al., 2006), are associated with the nucleus.
The poor expression of TAARs in vitro is another hallmark of this receptor family. Although transient expression has been reported for many members of the family (Bunzow et al., 2001; Borowsky et al., 2001; Liberles & Buck, 2006) cells that stably express heterologous TAAR receptors have been difficult to establish. As a family the TAARs have been difficult to study in vitro. The identification of cell lines that “naturally” express members of the TAAR family would be one way to overcome this obstacle.
Unfortunately, one of the frustrations encountered when working with novel receptors is the lack of selective reagents and the TAARs are no exception. Consequently the development of labeling methods selective for TAARs such as high affinity, radiolabeled antagonists, novel photoaffinity ligands, and traditional antibodies will contribute considerably to our experimental efforts to understand the life cycle of each TAAR subtype in vivo and in vitro.
One question that could be addressed with the reagents currently available is: Do the potential endogenous agonists PEA, TYR and the thyronamines (Scanlan et al., 2004) differ from the synthetic agonists AMPH and METH in their ability to promote TAAR1 internalization and possibly desensitization? The answer to this question could have important implications for understanding behavioral sensitization and other effects of chronic psychostimulant abuse.
Other questions related to TAAR trafficking include the nature of their intracellular localization. Are they associated with vesicles or some other organelle? What are the molecular determinants of their distribution? What would be the nature of the advantage conferred on cells that sequester TAARs intracellularly? Do TAARs form dimers or higher order complexes with themselves and/or other GPCRs? If so, how is trafficking affected? Answers to these questions should shed light on the biological functions of these receptors. Interestingly, similar questions are also being asked by those studying GPCR-mediated pheromone signaling in yeast. In that model organism endosomes are emerging as an important platform on which second messenger signaling events take place (Slessareva & Dohlman, 2006).
4.3. Pharmacology of the vertebrate trace amine-associated receptors
The pharmacology of the TAs has been a topic of considerable interest since TYR and PEA they were first shown by Barger and Walpole (1909) and Barger and Dale (1910) to be potent pressor agents. The assays that have been developed over time to explore the physiology and pharmacology of these biogenic amines have necessarily employed complex tissue preparations or whole animal models. The complexity of these systems makes the study of a single receptor subtype challenging at best, if not impossible in practice, even if so-called receptor “selective” antagonists are available. Consequently, the discovery of TAAR1 and the ability to express it in vitro independently of other TAARs has rendered the pharmacologic analysis of this complex family of related sequences tractable.
4.3.1. Structure-activity profiling of vertebrate TAARs
The first extensive study published on the pharmacology of any TAAR was that completed by Bunzow et al. (2001). Using a line of HEK293 cells they engineered to stably express rat TAAR1 they were able to screen a large number of compounds for their effect on cAMP accumulation compared to PEA, TYR, and forskolin.
Rank order potencies in this functional assay revealed that a hydroxyl group in the meta position of PEA analogs or the 5-position on TRYP diminishes a compound's potency at rat TAAR1 in contrast to catecholamine receptors. A possible explanation for this difference is revealed by amino acid sequence analysis. Although putative TM V of the rat TAAR1 contains the serine residue deep in the putative ligand binding pocket that is thought to H-bond with the para-hydroxyl of catecholamines, rat TAAR1 TM V lacks the shallower, conserved serine residues that are thought to H-bond with the meta-hydroxyl of the catechol moiety (Liapakis, 2000).
Another interesting finding to emerge from Bunzow et al.'s extensive quantitative structure activity profiling was that the meta-O-methyl metabolites 3-methoxytyramine, normetanephrine, and metanephrine are significantly more potent than their precursors DA, NE, and epinephrine. This result is intriguing since it is widely accepted that these metabolites of catechol-O-methyl transferase (COMT) activity are biologically inactive (Langer & Rubio, 1973; Seeman, 1980). The data of Bunzow et al. (2001) demonstrated that increasing the lipophilicity of catecholamines by O-methylation of the meta-hydroxyl increases potency and most likely their affinity as well, though this remains to be demonstrated. In further support of the conjecture that endogenous agonists of rat TAAR1 might be O-methylated derivatives of the catecholamines is the finding that rat TAAR1 mRNA appears to be most heavily expressed in liver, kidney, gastrointestinal tract, and brain which are areas where COMT is expression is reported to be highest (Männistö & Kaakkola, 1999).
The structural similarities between the endogenous TAs PEA and TYR and a number of widely abused psychostimulants including mescaline, AMPH, METH, and methylenedioxymethamphetamine (MDMA) led Bunzow et al. (2001) to test the ability of these drugs to activate rat TAAR1. Over the limited range of doses tested all of them were found to be potent agonists.
In a recently published report Reese et al. (2007) extend this observation by first establishing that PEA is the most potent and a full agonist at each species of TAAR1 while TYR is a potent full agonist for both rodent receptors but less potent and only a partial agonist at the human-rat chimera. Reese et al. (2007) then went on to show that mouse, rat, and the human-rat chimera display species-dependent selectivity for the stereoisomers of METH, AMPH, and POHA when compared to PEA. Both stereoisomers of METH were full mouse- and chimera TAAR1 agonists while R(-)METH was a partial agonist at the rat receptor. S(+)-METH was the most potent TAAR1 agonist tested with EC50s of 0.89 μM, 0.92 μM, and 4.44 μM for rat, mouse, and the chimeric receptor, respectively. When 2006 came to an end Wainscott et al. (2006) reported the wild type human TAAR1 they stably expressed in AV12-664 cells displays stereoselectivity for isomers of AMPH with EC50 values of 994 nM and 1.7 μM for the S(+) and R(-) forms, respectively.
Traditionally the biological effects of the AMPHs are thought to principally involve the monoamine transporters although other sites of action have been proposed (Shi et al., 2000). In fact AMPH and METH have been shown to be “substrate-type releasers” (Rothman and Baumann, 2006). They promote DA release from synaptic storage vesicles into the cytoplasm (Partilla et al., 2006) and then into the extracellular space via DAT with an EC50 of 25 nM and a Ki uptake in rat synaptosomes of 34 nM (Rothman et al., 2001).
These are drug concentrations approximately 20-30 fold lower than the EC50 values Reese et al. (2007) calculated for eliciting an in vitro functional response from rTAAR1 (0.8 μM). However, experienced METH users can typically consume gram quantities of drug per day (Kramer et al., 1967) and achieve peak blood concentrations of 100 μM (Derlet et al., 1989; Baselt, 2002; Peters et al., 2003). METH's high bioavailability, low protein binding, and long half life contribute to its achieving high micromolar plasma concentrations (Drummer, 2001; Baselt, 2002; Peters et al., 2003).
The extracellular free concentration of METH surrounding relevant human dopaminergic synapses in the brain presumably is the relevant pharmacodynamic parameter, at least in part, underlying the desirable effects of the drug. Although this value is not known with certainty for humans METH serum levels typically represent one tenth of what is found in rat brain (Riviere et al., 2000). Consequently, when considered together with the human forensic evidence the in vitro results of Reese et al. (2007) are consistent with the interpretation that in vivo the human TAAR1 is likely to be a mediator of at least some of METH's effects.
That TAAR1 might mediate some of the actions of AMPH in vivo is supported by studies involving mice descended from a founder in which one of its two TAAR1 alleles was deleted. By selective breeding the descendents of this founder, homozygous for the targeted TAAR1 allele, can be generated that are completely deficient in TAAR1 from conception (see below; Wolinsky et al., 2006).
Since TRYP acts as a potent (EC50=∼300 nM) and fully efficacious agonist at the rat TAAR1 several ergot alkaloids were tested for their ability to stimulate cAMP production in the receptor-expressing HEK cells (Bunzow et al., 2001). This study revealed that dihydroergotamine, ergometrine, dopamine, the hallucinogen d-lysergic acid diethylamide (LSD), and the antiparkinsonian drugs bromocriptine and lisuride are also potent stimulators of rat TAAR1.
An additional interesting feature of rat TAAR1's pharmacological profile is that several biogenic amine receptor antagonists and transporters are rat TAAR1 agonists. Included among these are: the adrenergic receptor antagonists phentolamine and tolazoline; the serotonergic antagonists cyproheptadine, ihydroergotamine, and metergoline; and the nonsubstrate of DAT nomifensine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Chlorpromazine, a DA D2 receptor antagonist and the original antipsychotic medication (Thuillier, 1999), also has an agonist's effect on the rat TAAR1. Unfortunately, none of the biogenic amine receptor antagonists behaved as rat TAAR1 antagonists. Even though a large number of naturally occurring compounds tested activate rat TAAR1 many do not including acetylcholine, GABA, glutamate, HIS, cocaine, and morphine.
After Bunzow et al. (2001) the most extensive pharmacologic characterization of the rat and human TAAR1 was conducted by scientists working in the Lilly Research Laboratories (Eli Lilly & Company, Indianapolis, ID). Wainscott et al. (2006) successfully co-transfected the cell line AV12-664 with a rat G alpha s expression construct and either the wild type human TAAR1 epitope-tagged with hemagglutinin or the rat TAAR1. Receptor stimulation by various ligands was assessed by the accumulation of cAMP after precautions were taken to block the endogenous alpha2 and beta-adrenergic receptors endogenously expressed in the AV12-664 cells. In their assay PEA has an EC50 0f ∼100 nM while congeners with substitutions on the ring at positions 3 and/or 4 tended to have diminished potencies. Interestingly, compounds with ring substitutions of small atomic dimensions, such as chlorine, at the 2 position, displayed EC50s equal to or exceeding that of PEA.
Similar to the experience of Bunzow et al. (2001), Wainscott et al. (2006) failed to identify a candidate TAAR1 antagonist but did find distinct species differences between the rat and human TAAR1 that were in agreement with Navarro et al. (2006) and Reese et al. (2007). Perhaps most striking is that all 3 groups find that PEA is a more potent agonist than TYR at the human and mouse TAAR1 whereas TYR is more potent than PEA at the rat TAAR1.
4.3.2. Novel endogenous ligands of vertebrate TAARs
Although rat, mouse, and human TAAR1 are activated by the endogenously produced TAs PEA, TYR, SYN, TRP, and OCT it remains to be shown that any of these compounds act as a TAAR1 ligand in vivo. The structure-activity profiling carried out by Bunzow et al. (2001) already revealed that the pharmacology of this receptor was going to be complex with the demonstration that O-methoxy derivatives of the catecholamines were potent agonists at TAAR1.
Far from futile, the search for additional endogenous TAAR1 ligands has already led to the discovery of 3-iodothyronamine (T1AM) and its deiodinated relative thyronamine (T0AM), molecules that are closely related to thyroid hormone (Scanlan et al., 2004; Hart et al., 2006; Tan et al., 2007). Based on the structure activity profile compiled by Bunzow et al. (2001) and the fact that the catecholamines and para-tyramine are all derivatives of the amino acid tyrosine (Y), Grandy and Scanlan conjectured that decarboxylated metabolites of thyroid hormone would be TAAR1 agonists. Through the combined efforts of a synthetic organic chemistry group and a group of molecular pharmacologists and physiologists, epitomized by the historic collaboration between Barger and Dale almost exactly a century before, Scanlan and Grandy were able to demonstrate that T1AM, and its enantiomer thryonamine (T0AM), are potent full agonists at rat, mouse (Scanlan et al., 2004), and human (unpublished observations) TAAR1 expressed in vitro with EC50s in the low to middle nM range. This observation immediately prompted additional in vitro and in vivo studies that continue to generate exciting results (see below).
4.4. Physiology of the vertebrate trace amine-associated receptors
4.4.1. Cellular signaling
The majority of studies directly investigating TAAR1 physiology have been conducted in vitro. This is out of necessity since crucial reagents, such as TAAR1-selective agonists and antagonists, remain to be developed. As has already been pointed out immortalized cells have many advantages but they also suffer from disadvantages. Not the least of these is that the cell most likely does not provide a cytoplasmic milieu best suited to the heterologous receptor. This is the reason any conclusion drawn about the physiology of a receptor studied under such conditions should be considered suspect until corroborating evidence can be obtained by in vivo experimentation.
There is no better example of the need for this degree of caution than the universal acceptance that TAAR1 activation results in the stimulation of cAMP production. It is satisfying that the predictions made by Bunzow et al. (2001) concerning possible new ligands and signaling pathways based on the receptor's putative structure were correct. However, it remains to be firmly established whether or not the stimulation of cAMP production is relevant to TAAR1 biology. Investigations into other second messenger pathways involving kinases, calcium, and perhaps even volatile signals including nitric oxide (NO), should be encouraged and pursued.
An interesting approach to understanding the cellular biology of TAAR1 as it relates to DA signaling has been taken by Drs. Miller, Madras and their colleagues (Miller et al., 2005; Madras et al., 2006). Based on the ventral midbrain localization of rodent TAAR1 transcripts reported by Borowsky et al., (2001) and Bunzow et al. (2001) plus the ability of DA, AMPH, and METH to functionally stimulate the receptor it was of obvious interest to explore possible interactions between TAAR1 and the DAT.
Given their productive studies of nonhuman primates in the past these investigators naturally chose to clone the TAAR1 from a species of nonhuman primate, the rhesus monkey (see above). Subsequent to its cloning the identity of the putative rhesus TAAR1 receptor was confirmed by sequence analysis as well as its in vitro pharmacological and functional profiles. They then went on to investigate the effect co-expression of human DAT has on rhesus TAAR1 function. They found that in the presence of DAT the ability of rhesus TAAR1 to stimulate cAMP production in response to PEA, AMPH, and MDMA was increased. In contrast, TYR might interfere with rhesus TAAR1 to stimulate cAMP (Miller et al., 2005). Consistent with the findings of others they noted that substantial TAAR1 protein remains in the cytoplasm of the cells transiently expressing both constructs and that one explanation for their observations is that the DAT provided an additional route for agonists to access the receptor (Xie et al., 2007; Xie and Miller, 2007).
4.4.2. Thermoregulation & metabolism
The foray into establishing a biological role for TAAR1, let alone other member of the family, has been understandably tentative. Many of the experimental reagents required to conduct meaningful experiments such as high affinity antagonists, selective agonists, and receptor antibodies have yet to be developed. Furthermore, it has only been in the recent past that a line of mouse, lacking TAAR1 expression, has been described (Wolinsky et al., 2006). In spite of these limitations, exploration of TAAR1's role in animal physiology promises to be extremely exciting if initial reports are confirmed (Scanlan et al., 2004; Liberles & Buck, 2006; Wolinsky et al., 2006; Zucchi et al., 2006).
As previously mentioned (see above) the existence of a novel thyroid hormone-like compound that would also be a TAAR1 agonist was hypothesized by Grandy and Scanlan. Chemically synthesized T1AM and T0AM both were found to be potent full agonists at mouse and rat TAAR1 heterologously expressed in vitro. Intrigued by the possibility that these two molecules could have biological effects in vivo, and thereby shed some light on possible roles of TAAR1, Scanlan's group synthesized enough T1AM and T0AM to allow Grandy's group to investigate the actions of these thyronamines in mice.
Within minutes of receiving an intraperitonal injection of synthetic T1AM or T0AM mice rapidly enter what appears to be a torpor-like state of inactivity. Simultaneously the animals display a profound bradycardia, hypotension, and hypothermia that are dose-dependent and fully reversible.
Encouraged by these findings a mass spectrometry method using artificial standards was developed that unequivocally detects picomol quantities of both T1AM and T0AM in biological material. With the synthesis of a deuterium-substitued T1AM standard the method is now quantitative. Coupled with a simple organic extraction procedure the means were available for Grandy, Scanlan, and their colleagues to look for the presence of both compounds which they have found in the brains of mice, rats, and guinea pigs. In subsequent studies both compounds were also found in human plasma and serum (J. Douglass, unpublished observations).
Although many details remain to be confirmed, a conservative interpretation of these results is that the thyronamines T1AM and T0AM are naturally occurring relatives of TH that might be generated by the concerted actions of aromatic acid decarboxylase and the three TH deiodinases that have been described (Visser et al., 1994; Kuiper et al., 2000; Kalsbeek et al., 2005; Köhrle, 2005; Moreno, et al., 2005). However, the possibility that alternative routes of thyronamine biosynthesis exist must also be explored. Furthermore, the rapid time frame in which their effects manifest and the fact that invariably responses to thyronamines are in a direction opposite that of TH led Grandy and Scanlan to hypothesize that the thyronamines represent a second arm of thyroid hormone biology that provides rapid feed back to the organism so that it can more efficiently fine-tune its physiology and metabolism in response to ever-changing environmental conditions. Whether or not T1AM and/or T0AM elicit their effects by activating TAAR1, or some other TAAR, in the brain and/or the periphery, remains to be established. However, the very existence of these naturally occurring, novel thyroid hormone-like compounds means an exciting new dimension to animal physiology has been revealed as a direct consequence of efforts to pharmacologically profile TAAR1 in vitro.
4.4.3. Heart & circulation
The rapid pressor effect produced by TYR and PEA in mammals has been known for a century. In fact it was through the monitoring of this response in the pithed cat that Dale was able to physiologically characterize and rank the compounds present in the watery extracts of ergot and putrified meat that Barger purified and chemically identified (Barger & Dale, 1907; Barger & Dale, 1909; Barger & Walpole, 1909; Dale & Dixon, 1909; Barger & Dale, 1910). These pioneering studies were followed up nearly eighty years later in dogs by Shannon et al. (1982) who examined the cardiovascular and behavioral effects of PEA administered intravenously and found it to produce a short-lived dose-dependent biphasic effect on heart rate besides slightly elevating body temperature. Around the same time human and animal studies designed to characterize TYR's robust pressor action in the context of hyperetension and MAO inhibutor therapy were conducted by Bianchetti et al. (1982) and later by Simpson and de Leon (1989).
Although the pressor effects of the TAs are usually explained solely in terms of their ‘false transmitter’ properties (Kopin et al., 1964; Kopin, 1968), the discovery that TAAR1 mRNA is expressed in the heart (Bunzow et al., 2001) and synthetic T1AM and T0AM have such dramatic effects on the cardiovascular system gave rise to an international effort, led by Dr. R. Zucchi at the University of Pisa, to explore the cardiovascular biology of thyronamines and TAARs.
Subsequent to the initial report of Scanlan et al. (2004), Zucchi's group (Chiellini et al., 2006) explored the actions of T1AM on the isolated working rat heart and in rat cardiomyocytes. In their recently published report T1AM is seen to produce both a reversible, dose dependent negative chronotropic effect as well as a reversible and dose dependent negative inotropic effect (27±5%, 51±3%, and 65±2% decrease in cardiac output at 19 μM, 25 μM, and 38 μM, respectively).
The hemodynamic effects of T1AM were notably increased in the presence of the tyrosine kinase inhibitor genistein but decreased in the presence of the tyrosine phosphatase inhibitor vanadate. No effect was produced by a panel of protein kinase A, protein kinase C, calcium-calmodulin kinase II, phosphatidylinositol-3-kinase, or MAP kinase inhibitors. The results of Western blotting experiments with rat ventricular protein and anti-phosphotyrosine antibodies suggests that phosphorylation of microsomal and cytosolic proteins is reduced following perfusion with synthetic T1AM.
To begin addressing whether the effects of T1AM on the heart are mediated by TAAR, or some other member of the family, Chiellini et al. (2006) conducted a survey using RT-PCR that revealed the presence of transcripts for at least 5 rat TAAR subtypes (TAAR1, TAAR2, TAAR3, TAAR4, & TAAR8a) in rat ventricle mRNA.
In an attempt to demonstrate T1AM binding in the heart, cardiac membranes were labeled with [125I]T1AM in the absence or presence of increasing concentrations of cold T1AM. A specific and saturable binding site is reported with a calculated KD in the low micromolar range (5 μM). Finally, they completed the picture using tandem mass spectrometry to show that T1AM is endogenous to the heart.
It is noteworthy that the effects of the classic TAs PEA and TYR produce a stimulatory effect on the cardiovascular system whereas in contrast the thyronamines have the opposite effect. If the TAs and the thyronamines are evoking their cardiovascular responses by activating TAAR1 then an interesting paradox presents itself. However, given the multiplicity of TAAR genes, their complex expression in the myocardium, and the lack of information about their pharmacology and second messenger signaling a resolution to this enigma will most likely have to wait until more is known about each member of this receptor family.
4.4.4. Behavior involving trace amine-associated receptors
Mention has already been made (see above) of the rich literature that implicates TAs in a number of human mental conditions including schizophrenia, ADHD, and depression. The lack of highly selective TAAR1 ligands that lack any confounding catecholamine-releasing actions has made it impossible to examine the physiology of TAAR1 in the central nervous system. However, one way to circumvent the paucity of pharmacological tools and still study the in vivo physiology of TAAR1 is to generate a line of mice that do not express TAAR1.
Wolinsky et al. (2006) report having accomplished this objective by targeted mutagenesis in an embryonic stem cell line that eventually gave rise to a founder animal that was able to pass on the mutant TAAR1 allele to its progeny. Descendents of this mouse, homozygous for the missing TAAR1 allele, and their wild type littermates have now been evaluated in several behavioral paradigms AMPH is known to influence.
The total lack of any TAAR1 expression had no apparent effect on the behavior of mice evaluated for overall activity or anxiety, basal temperature, or stress-induced hyperthermia assay. Wild type mice and mice completely lacking TAAR1 performed at the same level in the Y-maze, working memory task. This is perhaps not surprising given that at least 15 TAAR1-related genes have been documented in the mouse (Lindemann et al, 2005; Gloriam et al., 2005).
Intriguingly, mice that completely lack TAAR1 throughout their development have a significant deficit in sensorimotor gating that manifests as a deficit in prepulse inhibition. The TAAR-deficient animals also differ from their wild type littermates in that they display supersensitivity to the locomotor stimulating effect of AMPH. In addition, microdialysis probes placed in the dorsal striatum revealed that 60 minutes after the mutant mice were injected intraperitoneally with AMPH (2.9 mg/kg) they respond by releasing significantly more DA (910%) in their dorsal striata than do their wild type litter mates (410%). Similarly, NE levels were significantly elevated in the TAAR1-deficient mice (837%) compared to wild type mice (424%).
Familiar with the work of Seeman et al. (see Seeman et al., 2005) that documents increased proportions of DA D2 receptors in the high-affinity state (i.e. DA D2High) in essentially all animal models displaying DA-supersensitivity to AMPH, Wolinsky et al. (2006) sought to determine whether a similar condition existed in the brains of their supersensitive TAAR1- deficient mice. They found that in membranes prepared from the striata of adult mice lacking TAAR1 the proportion of DA D2 receptors in the D2High state was significantly elevated 2.6-fold (% D2High in wild type mice, 18.5; TAAR1-/-, 48% representing a 262% increase). Thus it appears that the phenomenon Seeman has described in other contexts continues to correlate with DA-mediated behavioral supersensitivity. What remains to be established is the connection between this phenomenon, behavior, and TAAR1-mediated signaling in the brain and perhaps in the periphery, as well.
4.4.5. Olfaction
It was noted by both Borowsky et al. (2001) and Bunzow et al. (2001) that TAAR1 transcripts are expressed at a relatively low copy number compared to most biogenic amine GPCRs. Despite this Borowsky et al. (2001) were successful in their use of in situ hybridization to map the brain distribution of TAAR1 transcripts in the mouse.
Bunzow et al. (2001) used their rat TAAR1 clone to probe Northern blots of RNA prepared from rat brain and peripheral tissues as well as sections of rat brain but these efforts failed to convincing reveal any TAAR1 message. These results prompted them to use a more sensitive technique and so they turned to RT-PCR. Always the primary concern with this approach when applied to the amplification of TAAR1 transcripts is that the signal might be the product of amplifying genomic DNA contaminating the cDNA template since the gene's coding region is uninterrupted by introns (Lindemann et al., 2005; see below).
Controlling for possible artifacts, Bunzow et al. (2001) collected convincing RT-PCR evidence for the wide-spread expression of TAAR1 transcripts in the central nervous system and peripheral organs of mice, rats, and humans. In a striking example of simultaneous discovery and corroboration of independent observations both groups found that the densest expression of TAAR1 transcripts was in the olfactory bulb.
With these two initial reports it seemed the way was clear for those with an interest in GPCR receptors expressed in the olfactory circuitry to become involved. Surprisngly however, the scent laid down by Borowsky et al. (2001) and Bunzow et al. (2001) was not immediately picked up by the olfactory field. Instead, the olfactory TAARs were “rediscovered” by Liberles and Buck (2006) in the course of their efforts to identify novel chemosensory GPCRs in the olfactory epithelium.
Using cDNA made from olfactory sensory neuron (OSN) mRNA as the template in real-time quantitative RT-PCRs primed with oligonucleotides designed to amplify putative GPCRs “…not previously implicated in odour, pheromone or taste detection…,” Liberles and Buck (2006) initially identified TAAR7 and TAAR8 as abundant GPCR transcripts in mouse OSNs. Subsequent attempts to look for the presence of other members of the mouse TAAR family in OSNs revealed the expression of transcripts corresponding to each of the mouse TAAR genes - except TAAR1 – at levels comparable to bona fide odorant receptors. Surprisingly, they “…obtained no evidence for Taar gene expression in any tissue – including the brain – apart from the olfactory epithelium…” What accounts for this discrepancy between the findings of Borowsky et al. (2001), Bunzow etal. (2001), and Miller et al. (2005) remains to be determined.
Although mouse TAARs are not close relatives of the odorant receptors their expression pattern raised the question: What chemosensory stimuli activate TAARs expressed in the OSN? Populations of HEK293 cells, each transiently expressing a different mouse TAAR subtype, were screened with respect to their sensitivity to mixtures of odorants chosen to cover a broad range of chemical structures and odor qualities at concentrations of 2–5 μM. In addition to compounds such as PEA that had previously been shown to be TAAR1 agonists (Borowsky et al., 2001; Bunzow et al., 2001; Miller et al., 2005), this screening strategy identified several novel TAAR agonists.
Transiently expressed mouse TAAR3 is activated by primary amines such as isoamylamine (EC50 = 10 μM) whereas tertiary amines are 30 times more potent at stimulating mouse TAAR5 and TAAR7 transiently expressed in HEK293 cells with EC50s of 0.3 μM and 20 μM, respectively. Similar in sequence to mouse TAAR1, mouse TAAR4 is also activated by PEA but is 10 fold less potent with an EC50 of 1 μM at the latter vs 0.1 μM at the former.
Many TAAR ligands are derived from amino acids, but neither F (amino acid precursor to PEA) nor leucine (precursor to isoamylamine) proved to be TAAR agonists. Furthermore, five known mouse pheromones, each at a concentration of 0.2 μM, as well as a major histocompatibility (MHC) peptide at a concentration of 0.5 μM failed to stimulate any of the mouse TAARs. As the authors correctly point out, one plausible alternative explanation for this negative result is that pheromone-responsive TAARs do not express well, if at all, in HEK293 cells.
Of the TAAR agonists identified by these investigators 3 are known to be present in mouse urine: PEA, isoamylamine, and trimethylamine. Interestingly the PEA content of urine has been shown to be elevated in animals under stress while only male mice excrete both trimethylamine and isoamylamine, the latter molecule ostensibly a pheromone that accelerates the onset of puberty in female mice.
A search for additional TAAR agonists in mouse urine was also attempted. Using a complex functional assay, Liberles and Buck (2006) were able to show with some difficulty that a potent mTAAR5 agonist is present in urine secreted by post-pubertal male BALB/c and C57BL/6 mice that can produce similar physiological responses in cells transiently expressing members of the TAAR gene family. This observation was interpreted as supporting the conclusion that the activating ligand(s) is/are it/they is/are not MHC-linked cues related to self-recognition.
Finally, whatever compound in male mouse urine is responsible for stimulating TAAR5, it appears to be volatile as evidenced by the stimulation of surrounding cultures of HEK293 cells transiently expressing mTAAR5. The authors go so far as to suggest trimethylamine may be the candidate activator because of its volatile nature.
4.5. Genomics & genetics of trace amine–associated receptors
The successful sequencing and annotation of several complete model organism genomes has generated a wealth of information and opportunities that previously could only be dreamed of. For those interested in the evolution and phylogeny of vertebrate gene families these databases are a treasure trove with the potential to reveal all of an organism's protein coding sequences. Theoretically then, it is now possible to organize all of an organism's protein coding genes into families of related sequences and map them onto their respective chromosomes with a high degree of precision.
4.5.1. Chromosomal localization of trace amine-associated receptors
Prior to the human genome initiative the physical mapping of a gene to its chromosome was a difficult and tedious process that at best resulted in a rough localization with poor resolution in terms of open reading frame orientation and neighboring genes. In spite of these limitations it was a worthwhile endeavor to map every new protein coding sequence because it would then be in a context that could be related to the findings of classic genetic linkage and association approaches used to identify causal factors of human disease.
Consequently, whenever a new protein-coding sequence was identified there was an immediate interest in mapping it to its human chromosomal location. The cloning of TAAR1 was no exception. Borowsky et al. (2001) reported the use of radiation hybrids in conjunction with primers unique to four related human TAAR sequences. Based on this approach they were able to assign all four of the genes to essentially the same region on the long arm of human chromosome 6 referred to as q23.2.
Independently, Bunzow et al. (2001) took a different approach that involved fluorescence in situ hybridization of a recombinant genomic lambda phage containing the human TAAR1 to human metaphase chromosomes. Robust hybridization signals were detected over the long arms of both chromosome 6 chromatids in a region consistent with a localization of 6q23.2.
The mapping of TAAR1 to 6q23.2 is interesting for several reasons. First, at least two other potential orphan GPCRs related in sequence to TAAR1 had already been mapped to this region thus presaging the later finding that all TAAR genes are clustered in this region. Second, this region of chromosome 6 has been repeatedly associated with schizophrenia in linkage/association studies, suggesting the possibility of TAAR1's involvement in the etiology of psychosis (Bunzow et al., 2001).
4.5.2. Phylogeny of trace amine-associated receptors
The GPCRs represent the largest family of related proteins coded by the human genome. Consequently, there has been a long-standing interest in trying to organize all of their genes into families according to conserved sequence motifs. In addition, the rarity of some GPCR transcripts and the restricted developmental expression of others means that a genomic approach is the only way to insure a complete collection of GPCR coding genes has been assembled.
In addition to the cloning and characterization of rat and human TAAR1 Borowsky et al. (2001) also reported a large number of related human and rat genomic sequences that they had amplified by PCR using degenerate oligonucleotide primers. In addition to rat and human TAAR1 they identified an additional 13 rat TAAR1-related genes and 3 novel human sequences.
The evolutionary relationship between these putative receptors was visualized by constructing a brancing phylogenetic tree on whose limbs the genes are arranged to reflect the extent to which they share sequence identity. Based on this analysis Borowsky et al. (2001) proposed that the TAARs form a branch distinct from other mammalian biogenic GPCRs as well as the invertebrate TA receptors. Based on their analysis they also proposed that this large family of genes was composed of two subfamilies. One of these consists of TAAR1, TAAR2, TAAR3, TAAR4, TAAR5 and the 5-HT4 pseudogene. The second subfamily consists of TAAR6, 7a, 7b, 7d, 7e, 7f, 7g, 8a, 8b, 8c, and 9 (according to the now accepted nomenclature; see below and Lindemann et al., 2005).
The availability of complete genomic sequences for several model organisms provided two groups, working independently and unknown to each other, another opportunity to study the evolutionary origins and phylogeny of the TAAR family of genes. In the process a number of sequences that code for previously unknown TAARs were identified.
Gloriam et al. (2005) approached the phylogeny of TAARs from the perspective of evolutionary biologists. Aware of the extensive literature suggesting the clinical importance of TAs in human health and disease they noted that no definite function had yet been attributed to these long-studied molecules. Furthermore, essentially nothing was known about the evolution of their receptors. Their thesis then was that a comparative approach to the evolution and phylogenic organization of TAARs would provide important new insights regarding the biological functions of these proteins.
Using published TAAR1 sequences to query publicly accessible genomic databases for several vertebrate species including humans, mice, rats, and teleost fish, Gloriam et al. (2005) perfomed an in silico search for TAAR1-related genes. The remarkable outcome of this investigation was the discovery of 14 previously unpublished mouse sequences related to TAAR1 and genes with open reading coding for 57 putative TAAR-like receptors and an additional 40 putative pseudogenes in the genome of the zebra fish Danio rerio. Mapping these sequences onto human, mouse, and rat chromosomes revealed that in all three species TAAR family members are tightly clustered together. In the human every TAAR gene is located on chromosome 6 at q23.2. In the mouse all 15 genes identified are clustered together on chromosome 10. In the rat its 18 TAAR1-like genes are found on chromosome 1.
These authors also performed a phylogenetic analysis and, although much more complicated in appearance than the tree published by Borowsky et al. (2001) given the inclusion of the large zebra fish contingent, two main subfamilies of mammalian TAAR1-like sequences were predicted.
In an independent effort Lindemann et al. (2005) initially used a comprehensive in silico approach to that of Gloriam et al. (2005). Their examination of the literature and publicly accessible databases uncovered many different strategies were being used to name the TA-sensitive GPCRs and their related sequences. This created an environment wherein it was cumbersome and often confusing to relate reports from laboratories that used different descriptors for their receptors. Consequently it was thought desirable to propose a uniform nomenclature for these receptor sequences that was not only based on their sequence/structure but their function as well.
According to their proposal the name of the entire family would be changed to “trace amine-associated receptors” in recognition that some TAARs do not appear to be activated by TAs (although the difficulty with which they are expressed in tissue culture suggests possible alternative explanations) in agreement with a prediction based on the putative pharmacophore binding pockets predicted for each of the mammalian TAARs (Kratochwil et al., 2005). In addition, the number assigned to each TAAR gene would reflect its sequential position along the chromosome and their purported phylogenetic relationships. This proposed nomenclature is now used by most investigators.
Lindemann et al. (2005) went on to complete the inventory of human, chimpanzee (Pan troglydytes), rat, and mouse TAARs by identifying 53 TAAR1-like genes: 9 human, 3 of which are predicted to be pseudogenes; 9 in chimpanzee, 6 of which are predicted to be pseudogenes; 19 rat, including 2 putative pseudogenes; and 16 mouse that includes 1 possible pseudogene. All of the genomic sequences identified were cloned and sequenced providing confirmation of most but also revealing discrepancies in some of the published sequences. Interestingly, all of the TAAR1-like subtypes, with the exception of TAAR2, are coded for by genes whose protein-coding regions are uninterrupted by intervening, nonreceptor-coding sequences and are of approximately the same length.
Equipped with what appears to represent the complete collection of human, rat, and mouse TAAR sequences, Lindemann et al. (2005) proceeded to assign each gene to its respective chromosome and determine the orientation of expression. This exercise revealed that human TAARs 6, 8, and 9 are oriented on their chromosome in such a way as to be transcribed in the opposite direction of TAARs 1-5. Further analysis failed to reveal a functional human ortholog of the mouse and rat TAAR7, a subtype that accounts for almost half the TAAR genes in these rodent species. Instead the human genome contains a pseudogene (TAAR7P). Similarly, the rodent TAAR 3 and TAAR4 genes code for functional receptors but these are pseudogenes in the human.
The chromosomal clustering and extensive sequence identity between TAAR genes from multiple mammals suggested that a phylogenetic approach might yield important clues as to the biological roles of these receptors. The results of their phylogenetic analysis of human, rat, and mouse TAARs suggest that the ancestral TAAR sequence has undergone eight gene duplication events. These events gave rise to a group of nine genes prior to the divergence of the rodent and primate lineages. The rodent TAAR7 and TAAR8 paralogues may have evolved by recent independent duplications within the mouse and rat lineages. Based on the chromosomal localization of each species' family of TAARs, the identification of a carboxy-terminal peptide “fingerprint” sequence that is only found associated with TAARs, the structure of the phylogenetic tree of human, rat, and mouse TAARs, and predicted ligand binding pockets (see below), Lindemann et al. (2005) concluded that the collection of TAAR sequences constitutes a new receptor family, distinct from any other.
Every member of the TAAR family of GPCRs shares structural features that are characteristic of the rhodopsin/beta-adrenergic receptor superfamily. Collectively their deduced amino acid sequences predict a protein that contains 7 putative TMs with relatively short amino- and carboxy-termini, 23-49 and 27-33 amino acids in length, respectively. Based on the X-ray crystal structure of bovine rhodopsin and mutational studies of other GPCRs activated by biogenic amines a highly conserved 3-dimensional structure emerges in which the putative seven TMs fold in such a way that their constituent amino acid residues are positioned to form a ligand binding pocket. An analysis of all biogenic amine-responsive GPCRs, including the TAARs, was conducted by Kratochwil et al. (2005) who identified 35 essential TM amino acids that they predict constitute a “ligand pocket vector,” or LPV, that is theoretically directly responsible for coordinating ligand binding. Interestingly, this analysis predicts three pharmacologically, and possibly functionally, distinct subgroups of TAARs: group I contains human TAARs 1-4; group II contains TAAR 5; and group III contains TAAR6-9.
Based on their LPV analysis these authors predicted that the pharmacology of the human TAAR1 would be more similar to that of mouse TAAR1 than its rat ortholog, which is the case for many of the compounds tested. The LPVs of TAAR4, 5, and 9 are nearly identical in all species evaluated. They also predict that TAAR1 and TAAR4 may well be the only TAARs that are activated by TAs.
The alignment of TAAR sequences also allowed Lindemann et al. (2005) to identify a unique TAAR-identifying peptide motif: NSXXNPXX[YH]XXX[YF]XWF, that overlaps the sequence that constitutes putative TM VII.
4.6. Mental Health
There is a rich literature that implicates TAs in normal and abnormal human brain functioning. However, the reports of Borowsky et al. (2001) and Bunzow et al. (2001) at first seemed not to have stimulated much interest among psychiatric geneticists. This is obviously no longer the situation based on the number of publications that have recently appeared in the literature.
4.6.1. Psychiatric genetics of human TAARs
The first to begin the systematic study of TAAR genes in the context of human disease was Vanti et al. (2003) who identified a single nucleoptide polymorphism (SNP) resulting in a null mutation in what is now referred to as the human TAAR9 gene (formerly TA3; Borowsky et al., 2001) but no association of of the SNP with ADHD or bipolar disorder. This report was followed by Duan et al. (2004) who reported the identification of polymorphisms in the human TAAR4 gene that appeared to be associated with susceptibility to schizophrenia. However, Ikeda et al. (2005) reported that they could find no association between TAAR4-linked single nucleotide polymorphisms and schizophrenia in a population of Japanese patients. A similar negative result was also reported by Amann et al. (2006) and in 2006 Duan et al. failed to find an association between TAAR4 polymorphisms and schizophrenia in a population of Chinese Han.
In 2005 Abou et al. published a family-based, case-control association study of bipolar affective disorder and TAAR polymorphisms. Pae et al. (2006) found an association of polymorphisms in human TAAR6 with schizophrenia and bipolar disorder in a Korean case control sample but Venken et al. (2006) could find no such evidence of an association of with bipolar disorder in a northern Swedish population.
To date Bly (2005) is the only one to examine human TAAR2 gene polymorphisms in the context of schizophrenia. While searching the 6q23 region, previously identified as a region of interest associated with schizophrenia (Schwab et al., 2000; Levinson et al., 2000), the TAAR2 was amplied by PCR from 56 (43 males and 13 females) unrelated normal (i.e. with no diagnosable psychiatric illness) individuals and 23 individuals (18 males and 5 females) diagnosed with schizophrenia.
A SNP has also been found that results in a mutation (G368A) that codes for a premature stop codon (W123STOP) after putative TM III thus rendering any protein made from the resulting transcript inactive. This mutation was documented in 10.7% of the schizophrenics and only 5.5% of the control group. Since the number of individuals examined in this study was small no significant association of the SNP with schizophrenia was observed screening a larger sample population will have to be undertaken.
However, one interesting observation is that of the three possible genotypes not one of the individuals examined was homozygous for the premature stop codon. This raises the possibility that in humans, the complete lack of a functional TAAR2 receptor is a developmentally lethal condition.
5. Current challenges & future directions
In the short time since the TAARs were first cloned (Borowsky et al., 2001; Bunzow et al., 2001) many rapid advances in our understanding of TA biology have been made. Although new questions have arisen and some old questions remain the future of the field is bright.
5.1. Pharmacology
The genome of every species examined so far contains a family of receptor-coding sequences that are related to TAAR1. Most of these new targets remain to be pharmacologically characterized in large part due to difficulties that have been experienced in the course of heterologously expressing them in vitro. The ability to reliably and stably express every member of the mammalian TAARs is of primary importance if a complete understanding of their biology is to be achieved, including the identification of their cognate endogenous ligands.
5.1.1 Development of trace amine-associated receptor binding assays
The major impediment currently facing molecular pharmacologists interested in characterizing the TAARs is in vitro expression. Although transient expression of all mouse TAARs has been reported (Liberles & Buck, 2006) it remains to be seen if this success can be transferred to other species of TAAR, especially the human orthologs.
Once routine, stable expression of TAARs is attainable in an immortalized cell line these cells can be grown and used as a replenishable source of receptor for binding studies. Although levels of receptor expression do change over time, cells stably expressing a GPCR offer a more predictable starting material than is obtainable from transiently expressing cells.
Traditional radioligand filtration binding assays involving TAAR1 have been attempted but none have been reported (Grandy et al., unpublished observations). There are likely to be several reasons for this. At present the most relevant commercially available radioligand is [3H]TYR. This is unfortunate since TYR is an agonist and a better one at the rat than the mouse or human TAAR1 (Reese et al., 2007). Consequently, given that TAARs are GPCRs it is to be expected that TYR will have both high and low affinity states. Also a likely negative factor is the apparent low level of receptor expressed on the surface of transfected cells (Boroskwy et al., 2001; Bunzow et al., 2001; Miller et al., 2005; Reese et al., 2007).
Low signal to noise plagues every attempt to demonstrate specific binding. The development of a high affinity, selective TAAR antagonist will help ameliorate this problem as well as provide a more suitable radioligand for characterizing the pharmacodynamics of TAs and the TAARs.
As no TAAR antagonist is commercially available recent efforts in Grandy's laboratory have turned to developing a centrifugation binding assay using [3H]TYR as radioligand. At the present time, using membranes prepared from HEK293 cells stably expressing the rTAAR1, this binding assay displays excellent signal to noise characteristics. Although somewhat cumbersome in execution if it can provide reliable results heretofore unattainable kinetic data should be forthcoming.
5.1.2. Identification of novel endogenous TAAR ligands
The availability of a reliable binding assay together with a functional assay of receptor activation or antagonism (e.g. cAMP accumulation) provide the means by which to screen for additional endogenous substances that have activity at the various TAARs.
Caution should be consulted however, before concluding that the TAs are endogenous TAAR agonists. Simply because an endogenously synthesized compound acts as an agonist is not sufficient proof it is an endogenous ligand for a given receptor subtype. Indeed, it has already been shown that other endogenous compounds, such as the novel thyronamine T1AM and its congener T0AM, can have robust biological activity in rodents with potencies that are predicted by their rank order potency in functional assays of activation involving recombinant, heterologously expressed TAAR1. However, The question yet to be answered remains: Does TAAR1 mediate any of the biological effects evoked by any of the TAs, T1AM, or T0AM? As Barger, Walpole, and Dale recognized a century ago the combined efforts of biologists and chemists offer the most promise for understanding complex biological systems of great medical importance.
5.2. Physiology
Future efforts to comprehend the nature and extent of the physiology evoked by the TAs and TAARs will necessarily benefit from studies conducted both in vitro and in vivo. Among the advantages of working in vitro is the extent to which the experimental parameters can be controlled. The major drawback though, especially when heterologous expression of a GPCR is concerned, is the unnatural cellular environment the receptor finds itself in. One must always consider the possibility that even if a response in the assay is reproducible, it could be a reproducible artifact that does not convey anything about the receptor's typical behavior in terms of in vivo trafficking, signaling, formation of heterodimers, and therefore to some extent its pharmacological profile, as well.
The advantage of in vivo work is that one is assured the context in which the receptor is typically expressed remains intact unless efforts to the contrary have been undertaken (i.e. “knock out” mice). The disadvantage is the complexity inherent to the tissues of interest, especially the brain, that makes arriving at a firm interpretation risky. In the case of the TAARs, efforts to study the biological consequences of their activation continue to be hindered since no selective TAAR antagonists have as yet been reported. While waiting for the identification of TAAR-selective antagonists other approaches should be considered including approaches that knock down the expression of the receptor of interest through antisense or siRNA technologies.
5.2.1. Trafficking & second messenger signaling
As was just mentioned caution is always advisable when interpreting the results of in vitro assays involving the heterologous expression of a GPCR. Consequently, confidence in a finding can be raised if it can be demonstrated in another cell line that naturally expresses the receptor of interest.
A PCR screen of tissue culture collections is likely to provide the fastest means of identifying cell lines that endogenously express TAARs. Once identified these lines, representing multiple organ types, could provide the experimental means of identifying genetic and cellular elements that determine TAAR expression, post-translational modification, trafficking, cellular localization, and second messenger signaling (Cornea-Hebert et al., 1999; Cornea-Hebert et al., 2002). They could also be useful in testing the hypothesis that functional TAARs exist as homo- and/or heteromeric complexes.
5.2.2. Organ function
The distribution of TAAR transcripts has been best studied in rodents. Interestingly, with one exception, the experience of those who have attempted to map TAAR1 expression have found it to varying degrees in essentially every tissue examined. A definitive description of the anatomic distribution of mRNA and protein corresponding to each TAAR will be a valuable and necessary resource for designing rationale experimental approaches to understanding TAAR physiology. Currently this effort is most advanced with respect to TAAR expression in the mouse brain (www.brainatlas.org/aba).
Although a complete expression atlas is not yet commercially available the anatomic and physiologic evidence collected so far indicates that TAAR-mediated signaling is likely to influence all organ systems but perhaps most importantly among them the brain, heart, skeletal muscle, pancreas, kidney, liver, and gastrointestinal tract. A better understanding of TAAR-mediated signaling in response to natural and synthetic agonists and antagonists in the tissues of these organs is likely to provide important new opportunities for better pharmacological management of human disease. Already the profound effects of the novel TAAR1 agonist T1AM on rodent physiology appear to affect several organ systems in a coordinated manner. The nature of these responses is such that if they occur in humans important new medical uses may be the result.
5.3. Genetics
Completion of several genome-sequencing projects has provided the unprecedented opportunity for investigators to potentially identify all genes that comprise a given family of sequences in a species. The success of this approach has been particularly impressive with respect to identifying members of the TAAR gene family. Now it appears probable that all of the human TAAR1-like genes have been identified (Gloriam et al., 2005; Lindemann et al., 2005). Equipped with this knowledge a number of laboratories have already successfully begun to identify interesting polymorphisms, many of which should be useful in human genetic studies attempting to uncover association with or linkage to a particular disease. Although the attention of human geneticists has been focused on the involvement of TAARs in diseases of the brain it is likely that in the future our understanding of human maladies afflicting other organ systems including the heart, kidney, liver, pancreas, gastrointrestinal tract, and skeletal muscle will benefit from a similar analysis.
5.4. Animal models & behavior
One of the most exciting aspects of identifying and cloning all members of the TAAR family of genes is the unprecedented opportunity to explore each receptor's contribution to an animal's biology. The surprisingly large contingent of related sequences, especially in the genomes of teleost fish and rodents (Gloriam et al., 2005; Lindemann et al., 2005), necessitates the development of many new reagents, such as receptor-selective antagonists, and animal models.
The recent demonstration that mice lacking the TAAR1 gene are viable and display distinct behavioral phenotypes underscores the power of this approach (Wolinsky et al., 2006). Unfortunately, the large number of TAAR genes that have been found in the mouse genome means that many more lines of mice still have to be generated, a long and expensive proposition. However, in the end such an effort will prove to have been worthwhile since together with novel TAAR-selective pharmacologic agents genetically engineered mice provide the only certain way to sort out the role of each TAAR gene product in normal and abnormal development, physiology, and behavior involving olfaction, compulsive drug use, movement disorders, various brain pathologies, and metabolic homeostasis.
6. Conclusions
The cloning of the TAAR1 gene and the subsequent identification of all related sequences in several mammalian species, including homo sapiens, represents a major step toward comprehending the biology of these receptors and the TAs, as well. The ability to routinely and stably express each TAAR subtype in vitro will provide an unprecedented opportunity to define its pharmacology and explore its cellular (second messenger signaling, desensitization, and internalization) and whole animal physiology. Already such efforts have led to the provocative realization that rodent and human TAAR1s are activated by METH and related AMPHs making them important novel targets for the development of medications to treat and combat METH abuse. From these efforts new molecules, especially subtype-selective antagonists, will be identified and become valuable reagents in efforts to understand the functional consequences of TAAR activation, and inactivation, in increasingly more complex biological systems.
Although the TAs are endogenously synthesized agonists of heterologously expressed TAAR1, their status as the endogenous ligands for any receptor remains to be established. It is to be expected that in the search for each TAAR subtype's putative endogenous ligand new signaling pathways will be revealed. The discovery of T1AM and T0AM being the first examples of such an outcome, is already forcing a re-examination of thyroid hormone signaling as it relates to metabolism, behavioral activation, and mood. That certain TAAR gene products are expressed in cells constituting the mouse olfactory epithelium and are activated by volatile non-TAs present in mouse urine raises the intriguing possibility that a role for TAARs in discriminating social cues can be anticipated in other species, including humans.
Already human psychiatric geneticists have made important discoveries with respect to the possible contribution of TAARs to mental illness and other pathologies of the brain such as Parkinson's disease. As more studies are undertaken and data is collected the extent to which any one of these genes contributes to predisposing an individual to disease will be established and hopefully provide insights on and new options for treating medical conditions for which no therapy currently exists.
Receptors for TAs have long been sought in efforts to establish the innate biological significance of these fascinating noncatecholic biogenic amines. Now that candidate genes for their putative receptor proteins have been found the task at hand is to discern the pharmacology and physiology of each purported member of this extended family of G protein-coupled receptors. Only then will it be possible to know whether TAAR1 is the family archetype or its iconoclast.
Acknowledgments
The author heartily thanks: M.T. Ackermans, S.G. Amara, S. Arttamangkul, J. Axelrod, L.J. Braulke, J.R. Bunzow, A. Carlsson, L. Crawshaw, D.A. Crossley, II, A. DeBarber, T. Darland, J. Douglass, K.P. Doyle, J. Douglass, E. Fliers, G. Giraud, D. Hatton, G. Heldmaier, M. Hoener, R. Hohimer, C. Jimenez, A. Kalsbeek, E. Kaufman, M. Kaufmann, T.M. Keck, M.J. Kelly, J.L. Kennedy, L.P. Klieverik, J. Köhrle, P.J. Kruzich, M. Litt, R.E. Magenis, E.K. Morris, S.B. Olson, S. Parameswaran, S.J. Patton, D.I. Quigley, N. Rediske, E.A. Reese, O. Rønnekleiv, B.L. Roth, T.S. Scanlan, S.-L. Shyng, L.L. Simon, G. Smith, K. Smith, A. Snead, M.S. Sonders, M.P. Stenzel-Poore, K.L. Suchland, E. Tan, K. Thornburg, T.J. Visser, W. Woodward, F.-F. Yan, A. Yonkin, G. Zhang, and R. Zucchi for thought-provoking discussions. The author is also very appreciative of the assistance received from C. Jimenez who produced the figure and A. Nilsen for assistance with depicting the structural formulae; W. Wood who helped the author search the early organic chemistry literature; and C. Lorentz who assisted the author with his translation of the original chemistry literature from German into English. Finally, the author wishes to express his heartfelt gratitude to R. Baldessarini and A.D. Mosnaim for sharing their personal recollections about the formative period when vertebrate non-catecholic phenylethylamines came of age. The author is supported in part by the Methamphetamine Abuse Research Center, the National Institute on Drug Abuse, and the National Institute for Mental Health.
Abbreviations
- AADC
aromatic amino acid decarboxylase
- ADHD
attention deficit hyperactivity disorder
- d-AMPH
amphetamine
- ATCC
American Type Culture Collection
- cAMP
adenosine 3′, 5′-monophosphate
- COMT
catechol-O-methyl transferase
- CFTR
cystic fibrosis transporter regulator
- CRE-Luc
cAMP Responsive Element-Luciferase
- DA
dopamine
- DAT
dopamine transporter
- DNA
deoxyribonucleic acid
- L-DOPA
EGFP, enhanced green fluorescent protein
- EPI
epinephrine
- F
phenylalanine
- GABA
gamma amino butyric acid
- GPCR
Guanosine triphosphate binding protein-coupled receptor
- HIS
histamine
- i.p.
intraperitoneally
- L-dopa
dihydroxyphenylalanine
- LPV
ligand pocket vector
- LSD
d-lysergic acid diethylamide
- MDMA
methylenedioxymethamphetamine
- METH
methamphetamine
- NE
norepinephrine
- NET
norepinephrine transporter
- NSXXNPXX[YH]XXX[YF]XWF
asparagine-serine-X-X-asparagine-proline-X-X-[tyrosine-histidine]-X-X-X-[tyrosine-phenylalanine]-X-tryptophan-phenylalanine
- NT
neurotransmitter
- OCT
octopamine
- OSN
olfactory sensory neuron
- PCR
polymerase chain reaction
- PEA
β-phenylethylamine
- POHA
para-hydroxy-amphetamine
- RT-PCR
reverse transcriptase polymerase chain reaction
- SERT
serotonin transporter
- SNP
single nucleotide polymorphism
- SYN
synephrine
- TA
trace amine
- T1AM
3-iodothyronamine
- TAR
trace amine receptor
- TAAR
trace amine-associated receptor
- TM
transmembrane
- TRYP
tryptamine
- TYR
tyramine
- VMAT
vesicular monoamine transporter
- W
tryptophan
- Y
tyrosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelous, Ribaut, Soulie, Toujan C R Soc de Biol I. 1906:463–530. [Google Scholar]
- Abou Jamra R, Sircar I, Becker T, Freudenberg-Hua Y, Ohlraun S, Freudenberg J, Brockschmidt F, Schulze TG, Gross M, Spira F, Deschner M, Schmal C, Maier W, Propping P, Rietschel M, Cichon S, Nothen MM, Schumacher J. A family-based and case-control association study of trace amine receptor genes on chromosome 6q23 in bipolar affective disorder. Mol Psychiatry. 2005;10:618–620. doi: 10.1038/sj.mp.4001665. [DOI] [PubMed] [Google Scholar]
- Amann D, Avidan N, Kanyas K, Kohn Y, Hamdan A, Ben-Asher E, Macciardi F, Beckmann JS, Lancet D, Lerer B. The trace amine receptor 4 gene is not associated with schizophrenia in a sample linked to chromosome 6q23. Mol Psychiatry. 2006;11:119–121. doi: 10.1038/sj.mp.4001752. [DOI] [PubMed] [Google Scholar]
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Gerner RH, Cohen DJ, Fairbanks L. Central tryptamine turnover in depression, schizophrenia, and anorexia: measurement of indoleacetic acid in cerebrospinal fluid. Biol Psychiatry. 1984;19:1427–1435. [PubMed] [Google Scholar]
- Andrew R, Best SA, Watson DG, Midgley JM, Reid JL, Squire IB. Analysis of biogenic amines in plasma of hypertensive patients and control group. Neurochem Res. 1993;18:1179–1182. doi: 10.1007/BF00978371. [DOI] [PubMed] [Google Scholar]
- Arakawa S, Gocayne JD, McCombie WR, Urquhart DA, Hall LM, Frazer CM, Venter JC. Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron. 1990;4:343–354. doi: 10.1016/0896-6273(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Aridon P, D'Andrea G, Rigamonti A, Leone M, Casari G, Bussone G. Elusive amines and cluster headache: mutational analysis of trace amine receptor cluster on chromosome 6q23. Neurol Sci. 2004;3:S279–280. doi: 10.1007/s10072-004-0309-1. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Saavedra JM. Octopamine, phenylethanolamine, phenylethylamine and tryptamine in the brain. In: Wolstenholme GEW, Fitzsimons DW, editors. Aromatic Amino Acids in the Brain. Ciba Foundation Symposium 22 (new series); held 15-17th May, 1973; New York: Elsevier; 1974. pp. 51–66. [Google Scholar]
- Axelrod J, Saavedra JM. Octopamine. Nature. 1977;265:501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- Bailey BA, Philips SR, Boulton AA. In vivo release of endogenous dopamine, 5-hydroxytryptamine and some of their metabolites from rat caudate nucleus by phenylethylamine. Neurochem Res. 1987;12:173–178. doi: 10.1007/BF00979534. [DOI] [PubMed] [Google Scholar]
- Baker G, Bornstein RA, Rouget AC, Schton SE, van Meyden JC, Couts RT. Phenylethylaminergic mechanisms in attention-deficit disorder. Biol Psychiatry. 1991;29:15–22. doi: 10.1016/0006-3223(91)90207-3. [DOI] [PubMed] [Google Scholar]
- Baker GB, Bornstein RA, Yeragani VK. Trace amines and Tourette's syndrome. Neurochem Res. 1993;18:951–956. doi: 10.1007/BF00966752. [DOI] [PubMed] [Google Scholar]
- Baker GB, Coults RT, Greenshaw AJ. Neurochemical and metabolic aspects of antidepressants: an overview. J Psychitry Neurosci. 2000;25:481–496. [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ. Release of aromatic amines from brain tissue of the rat in vitro. J Neurochem. 1971;18:2509–2518. doi: 10.1111/j.1471-4159.1971.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. Trace amines and alternative neurotransmitters in the central nervous system. Biochem Pharmacol. 1978;27:621–626. doi: 10.1016/0006-2952(78)90495-1. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Vogt M. The uptake and subcellular distribution of aromatic amines in the brain of the rat. J Neurochem. 1971;18:2519–2533. doi: 10.1111/j.1471-4159.1971.tb00208.x. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Vogt M. Regional release of aromatic amines from tissues of the rat brain in vitro. J Neurochem. 1972;19:755–761. doi: 10.1111/j.1471-4159.1972.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Fischer JE. Substitute and alternative neurotransmitters in neuropsychiatric illness. Arch Gen Psychiatry. 1977;34:958–964. doi: 10.1001/archpsyc.1977.01770200096013. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Fischer JE. Trace amines and alternative neurotransmitters in the central nervous system. Biochem Pharmcol. 1978;27:621–626. doi: 10.1016/0006-2952(78)90495-1. [DOI] [PubMed] [Google Scholar]
- Balfanz S, Strunker T, Frings S, Baumann A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem. 2004;93:440–451. doi: 10.1111/j.1471-4159.2005.03034.x. [DOI] [PubMed] [Google Scholar]; J Neurochem. 2005;94:1168. doi: 10.1111/j.1471-4159.2005.03105.x. Erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger G, Dale HH. Ergotoxine and some other constiuents of ergot. Biochem J. 1907;2:240–299. doi: 10.1042/bj0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger G, Dale HH. The water-soluble active principles of ergot. J Physiol. 1909;38:77–79. [Google Scholar]
- Barger G, Walpole GS. Isolation of the pressor principles of putrid meat. J Physiol. 1909;38:343–352. doi: 10.1113/jphysiol.1909.sp001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger G, Dale HH. Chemical structure and sympathomimetic action of amines. J Physiol. 1910;41:19–59. doi: 10.1113/jphysiol.1910.sp001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Lehmann-Masten V, Paulus M, Gainetdinov RR, Caron MG, Geyer MA. The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology. 2004;29:221–228. doi: 10.1038/sj.npp.1300343. [DOI] [PubMed] [Google Scholar]
- Barroso N, Rodriguez M. Action of β-phenylethylamine and related amines on nigrostriatal dopamine neurotransmission. Eur J Pharmacol. 1996;297:195–203. doi: 10.1016/0014-2999(95)00757-1. [DOI] [PubMed] [Google Scholar]
- Baselt R. Disposition of Toxic Drugs and Chemicals in Man. Chemical Toxicology Institute; Foster City, CA, London: 2002. [Google Scholar]
- Battelle B, Kravitz EA. Targets of octopamlne action in the lobster: cyclic nucleotide changes and physiological effects in hemolymph, heart and exoskeletal muscle. J Pharmacol Exp Ther. 1978;205:438–448. [PubMed] [Google Scholar]
- Battelle B, Kravitz EA, Stieve H. Neurotransmitter synthesis in Limulus ventral nerve photoreceptors. Experientia. 1979;35:778. doi: 10.1007/BF01968242. [DOI] [PubMed] [Google Scholar]
- Baud P, Arbilla S, Cantrill RC, Scatton B, Langer SZ. Trace amines inhibit the electrically evoked release of [3H]acetylcholine from slices of rat striatum in the presence of pargyline: similarities between β-phenylethylamine and amphetamine. J Pharmacol Exp Ther. 1985;235:220–229. [PubMed] [Google Scholar]
- Baxter GD, Barker SC. Isolation of a cDNA for an octopamine-like, G-protein coupled receptor from the cattle tick, Boophilus microplus. Insect Biochem Mol Biol. 1999;29:461–467. doi: 10.1016/s0965-1748(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Becu-Villalobos D, Vacas MI, Libertun C. Prolactin inhibition by p-tyramine in the male rat: site of action. Endocrinology. 1987;120:2297–2301. doi: 10.1210/endo-120-6-2297. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Krieger M, Calas A, Franzoni MF, Thibault J. Aromatic amino acid decarboxylase (AADC) immunohistochemistry in vertebrate brainstem with an antiserum raised against AADC made in E. coli. Brain Res Bull. 1993;32:123–132. doi: 10.1016/0361-9230(93)90066-k. [DOI] [PubMed] [Google Scholar]
- Bergman J, Yasar S, Winger G. Psychomotor stimulant effects of β- phenylethylamine in monkeys treated with MAO-B inhibitors. Psychopharmacology (Berl) 2001;159:21–30. doi: 10.1007/s002130100890. [DOI] [PubMed] [Google Scholar]
- Berretta N, Giustizieri M, Bernardi G, Mercuri NB. Trace amines reduce GABA(B) receptor-mediated presynaptic inhibition at GABAergic synapses of the rat substantia nigra pars compacta. Brain Res. 2005;1062:175–178. doi: 10.1016/j.brainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Bianchetti MG, Minder I, Beretta-Piccoli C, Meier A, Weidmann P. Effects of tyramine on blood pressure and plasma catecholamines in normal and hypertensive subjects. Klin Wochenschr. 1982;60:465–470. doi: 10.1007/BF01720361. [DOI] [PubMed] [Google Scholar]
- Bischof LJ, Enan EE. Cloning, expression and functional analysis of an octopamine receptor from Periplaneta americana. Insect Biochem Mol Biol. 2004;34:511–521. doi: 10.1016/j.ibmb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Björklund A, Axelsson S, Falck B. Intraneural indolamines in the central nervous system. Adv Biochem Psychopharmcol. 1976;15:87–94. [PubMed] [Google Scholar]
- Blaschko H. The activity of l(-)-DOPA decarboxylase. J Physiol. 1942;101:337–349. doi: 10.1113/jphysiol.1942.sp003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H, Chrusciel TL. The decarboxylation of amino acids related to tyrosine and their awakening action in reserpine-treated mice. J Physiol. 1960;151:272–284. doi: 10.1113/jphysiol.1960.sp006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenau W, Balfanz S, Baumann A. Amtyr1: characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. J Neurochem. 2000;74:900–908. doi: 10.1046/j.1471-4159.2000.0740900.x. [DOI] [PubMed] [Google Scholar]
- Bly M. Examination of the trace amine-associated receptor 2 (TAAR2) Schizophr Res. 2005;80:367–368. doi: 10.1016/j.schres.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bodnaryk RP. Identification of specific dopamine- and octopamine-sensitive adenylate cyclases in the brain of Mamestra configurata Wlk. Insect Biochem. 1979a;9:155–162. doi: 10.1139/o79-028. [DOI] [PubMed] [Google Scholar]
- Bodnaryk RP. Basal, dopamine- and octopamine-stimulated adenylate cyclase activity in the brain of the moth, Mamestra configurata, during its metamorphosis. J Neurochem. 1979b;33:275–282. doi: 10.1111/j.1471-4159.1979.tb11729.x. [DOI] [PubMed] [Google Scholar]
- Bohn LM. Constitutive trafficking-more than just running in circles? Mol Pharmacol. 2007;71:957–958. doi: 10.1124/mol.107.034223. [DOI] [PubMed] [Google Scholar]
- Booth M. Opium: A History. St. Martin's Griffin; New York, NY: 1996. [Google Scholar]
- Borison RL, Mosnaim AD, Sabelli H. Biosynthesis of brain 2-phenylethylamine: influence of decarboxylase inhibitors and D-amphetamine. Life Sci. 1974;15:1837–1848. doi: 10.1016/0024-3205(74)90185-4. [DOI] [PubMed] [Google Scholar]
- Borison RL, Mosnaim AD, Sabelli H. Brain 2-phenylethylamine as a major mediator for the central actions of amphetamine and methylphenidate. Life Sci. 1975;17:1331–1344. doi: 10.1016/0024-3205(75)90147-2. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Brachek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci, USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AA. Amines and theories in psychiatry. Lancet. 1974;2:52. doi: 10.1016/s0140-6736(74)91390-7. [DOI] [PubMed] [Google Scholar]
- Boulton AA. Identification, distribution, metabolism and function of meta-and para-tyramine, phenylethylamine and tryptamine in brain. Adv Biochem Psychopharmacol. 1976a;15:57–67. [PubMed] [Google Scholar]
- Boulton AA. Cerebral aryl alkyl aminergic mechanisms. In: Usdin E, Sandler M, editors. Trace Amines and the Brain. Marcel Dekker; New York: 1976b. pp. 22–39. [Google Scholar]
- Boulton AA. Trace amines and mental disorders. Can J Neurol Sci. 1980;7:261–263. doi: 10.1017/s0317167100023313. [DOI] [PubMed] [Google Scholar]
- Boulton AA. Some aspects of basic psychopharmacology: the trace amines. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:563–570. doi: 10.1016/s0278-5846(82)80150-4. [DOI] [PubMed] [Google Scholar]
- Boulton AA. Trace amines and the neurosciences: an overview. In: Boulton AA, Baker GB, Dewhurst WG, Sandler M, editors. Neurobiology of the Trace Amines. Humana Press; Clifton: 1983. pp. 13–24. [Google Scholar]
- Boulton AA. Trace amines: comparative and clinical neurobiology. In: Ed Juorio AV, Downer RGH, editors. Experimental and Clinical Neuroscience. New Jersey: Humana; 1988. [Google Scholar]
- Boulton AA, Quan L. Formation of p-tyramine from DOPA and DOPamine in rat brain. Can J Biochem. 1970;48:1287–1291. doi: 10.1139/o70-199. [DOI] [PubMed] [Google Scholar]
- Boulton AA, Wu PH. Biosynthesis of of cerebral phenolic amines. I. In vivo formation of p-tyramine, octopamine, and synephrine. Can J Biochem. 1972;50:261–267. doi: 10.1139/o72-037. [DOI] [PubMed] [Google Scholar]
- Boulton AA, Wu PH. Biosynthesis of cerebral phenolic amines. II. In vivo regional formation of p-tyramine and octopamine from tyrosine and dopamine. Can J Biochem. 1973;51:428–435. doi: 10.1139/o73-050. [DOI] [PubMed] [Google Scholar]
- Boulton AA, Baker GB. The subcellular distribution of b-phenylethylamine, p-tyramine and tryptamine in rat brain. J Neurochem. 1975;25:477–481. doi: 10.1111/j.1471-4159.1975.tb04353.x. [DOI] [PubMed] [Google Scholar]
- Boulton AA, Pollitt RJ, Majer JR. Identity of a urinary “pink spot” in schizophrenia and Parkinson's disease. Nature. 1967;215:132–134. doi: 10.1038/215132a0. [DOI] [PubMed] [Google Scholar]
- Boulton AA, Wu PH, Philips S. Binding of some primary aromatic amines to certain rat brain particulate fractions. Can J Biochem. 1972;50:1210–1218. doi: 10.1139/o72-164. [DOI] [PubMed] [Google Scholar]
- Boulton AA, Juorio AV, Philips SR, Wu PH. Some arylalkylamines in rabbit brain. Brain Res. 1975;96:212–216. doi: 10.1016/0006-8993(75)90600-9. [DOI] [PubMed] [Google Scholar]
- Boulton AA, Juorio AV, Philips SR, Wu PH. The effects of resperpine and 6-hydroxydopamine on the concentrations of some arylalkylamines in rat brain. Br J Pharmacol. 1977;59:209–214. doi: 10.1111/j.1476-5381.1977.tb06996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AA, Juorio AV, Paterson IA. Phenylethylamine in the CNS: effects of monoamine oxidase inhibiting drugs, deuterium substitution and lesions and its role in the neuromodulation of catecholaminergic neurotransmission. J Neural Transm Suppl. 1990;29:119–129. doi: 10.1007/978-3-7091-9050-0_12. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Current Opinion. 2003;3:90–97. doi: 10.1016/s1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Brandau K, Axelrod J. The biosynhthesis of octopamine. Naunyn—Schmiederberg's Arch Pharmacol. 1972;273:123–133. doi: 10.1007/BF00508085. [DOI] [PubMed] [Google Scholar]
- Bruning G, Rommelspacher H. High affinity [3H]tryptamine binding sites in various organs of the rat. Life Sci. 1984;34:1441–1446. doi: 10.1016/0024-3205(84)90058-4. [DOI] [PubMed] [Google Scholar]
- Buckland PR, O'Donovan MC, McGuffin P. Changes in dopa decarboxylase mRNA but not tyrosine hydroxylase mRNA levels in rat brain following antipsychotic treatment. Psychopharmacology. 1992;108:98–102. doi: 10.1007/BF02245292. [DOI] [PubMed] [Google Scholar]
- Buckland PR, Spurlock G, McGuffin P. Amphetamine and vigabatrin down regulate aromatic L-amino acid decarboxylase mRNA levels. Mol Brain Res. 1996;35:69–76. doi: 10.1016/0169-328x(95)00182-r. [DOI] [PubMed] [Google Scholar]
- Buckland PR, Marshall R, Watkins P, McGuffin P. Does phenylethylamine have a role in schizophrenia? LSD and PCP up-regulate aromatic amino acid decarboxylase mRNA levels. Brain Res Mol Brain Res. 1997;49:266–270. doi: 10.1016/s0169-328x(97)00160-5. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1989;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4 methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Gaubatz GL. Octopamine receptors on Aplysia neurones mediate hyperpolarisation by increasing membrane conductance. Nature. 1974;252:483–485. doi: 10.1038/252483a0. [DOI] [PubMed] [Google Scholar]
- Cascio CS, Kellar KJ. Effect of pargyline, reserpine, and neurotoxin lesions on [3H]tryptqamine binding sites in rat brain. Eur J Pharmacol. 1986;120:101–105. doi: 10.1016/0014-2999(86)90646-1. [DOI] [PubMed] [Google Scholar]
- Cazzamali G, Klaerke DA, Grimmelikhuijzen CJ. A new family of insect tyramine receptors. Biochem Biophys Res Commun. 2005;338:1189–1196. doi: 10.1016/j.bbrc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Chance WT, Bernardini AP, James JH, Edwards LL, Minnema K, Fischer JE. Behavioral depression after intraventricular infusion of octopamine in rats. Am J Surg. 1985;150:577–584. doi: 10.1016/0002-9610(85)90441-6. [DOI] [PubMed] [Google Scholar]
- Chang DJ, Li XC, Lee YS, Kim HK, Kim US, Cho NJ, Lo X, Weiss KR, Kandel ER, Kaang BK. Activation of a heterologously expressed octopamine receptor coupled only to adenylyl cyclase produces all the features of presynaptic facilitation in Aplysia sensory neurons. Proc Natl Acad Sci, USA. 2000;97:1829–1934. doi: 10.1073/pnas.97.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatwin HM, Rudling JE, Patel D, Reale V, Evans PD. Site-directed mutagenesis studies on the Drosophila octopamine/tyramine receptor. Insect Biochem Mol Biol. 2003;33:173–184. doi: 10.1016/s0965-1748(02)00188-1. [DOI] [PubMed] [Google Scholar]
- Cheng JT, Shen CL, Jou TC. Inhibitory effect of octopamine on dopamine D-1 receptor in striatal homogenates of the rat. Neurosci Res. 1990;9:202–207. doi: 10.1016/0168-0102(90)90005-y. [DOI] [PubMed] [Google Scholar]
- Chiellini G, Frascarelli S, Ghelardoni S, Carnicelli V, Tobias SC, DeBarber A, Brogioni S, Ronca-Testoni S, Cerbai E, Grandy DK, Scanlan TS, Zucchi R. Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function. FASEB J. 2007 Dec; doi: 10.1096/fj.06-7474com. epub. [DOI] [PubMed] [Google Scholar]
- Cho S, Neff NH, Hadjiconstantinou M. Regulation of tyrosine hydroxylase and aromatic L-amino acid decarboxylase by dopaminergic drugs. Eur J Pharmacol. 1997;323:149–157. doi: 10.1016/s0014-2999(97)00037-x. [DOI] [PubMed] [Google Scholar]
- Clark A. The clinical application of ergotamine (tyramine) Biochem J. 1911;5:236–242. doi: 10.1042/bj0050236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Watkins KC, Roth BL, Kroeze WK, Gaudreau P, Leclerc N, Descarries L. Similar ultrastructural distribution of the 5-HT(2A) serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience. 2002;113:23–35. doi: 10.1016/s0306-4522(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Dacks AM, Dacks JB, Christensen TA, Nighorn AJ. The cloning of one putative octopamine receptor and two putative serotonin receptors from the tobacco hawkmoth, Manduca sexta. Insect Biochem Mol Biol. 2006;36:741–747. doi: 10.1016/j.ibmb.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH, Dixon WE. The action of pressor amines produced by putrefaction. J Physiol. 1909;39:25–44. doi: 10.1113/jphysiol.1909.sp001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea GD, Terrazzino S, Fortin D, Farruggio A, Rinaldi L, Leon A. HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci Letts. 2003a;346:89–92. doi: 10.1016/s0304-3940(03)00573-1. [DOI] [PubMed] [Google Scholar]
- D'Andrea G, Terrazzino S, Fortin D, Cocco P, Balbi T, Leon A. Elusive amines and primary headaches: historical background and prospectives. Neurol Sci. 2003b;24:S65–S67. doi: 10.1007/s100720300044. [DOI] [PubMed] [Google Scholar]
- D'Andrea G, Terrazzino S, Leon A, Fortin D, Perini F, Granella F, Bussone G. Elevated levels of circulating trace amines in primary headaches. Neurology. 2004;62:1701–1705. doi: 10.1212/01.wnl.0000125188.79106.29. [DOI] [PubMed] [Google Scholar]
- Danielson TJ, Boulton AA, Robertson HA. m-Octopamine, p-octopamine and phenylethanolamine in mammalian brain: a sensitive, specific assay and effect of drug. J Neurochem. 1977;29:1131–1135. doi: 10.1111/j.1471-4159.1977.tb06519.x. [DOI] [PubMed] [Google Scholar]
- Dao WPC, Walker RJ. Effect of cyproheptadine on the octopamine-induced responses in the mammalian central nervous system. Experientia. 1980;36:584–585. doi: 10.1007/BF01965816. [DOI] [PubMed] [Google Scholar]
- Da Prada M, Zurcher G, Wuthrich I, Haefely WE. On tyramine, food, beverages and the reversible MAO inhibitor moclobemide. J Neural Transm Suppl. 1988;26:31–56. [PubMed] [Google Scholar]
- Davenport AP. Peptide and trace amine orphan receptors: prospects for new therapeutic targets. Current Opinion. 2003;3:127–134. doi: 10.1016/s1471-4892(03)00003-1. [DOI] [PubMed] [Google Scholar]
- Davis BA, Boulton AA. The trace amines and their acidic metabolites in depression-an overview. Progress Neuropsychopharmacology Biol Psychiatry. 1994;18:17–45. doi: 10.1016/0278-5846(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Degen J, Gewecke M, Roeder T. Octopamine receptors in the honey bee and locust nervous system: pharmacological similarities between homologous receptors of distantly related species. Br J Pharmacol. 2000;130:587–594. doi: 10.1038/sj.bjp.0703338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derlet RW, Rice P, Horowitz BZ, Lord RV. Amphetamine toxicity: experience with 127 cases. J Emerg Med. 1989;7:157–161. doi: 10.1016/0736-4679(89)90263-1. [DOI] [PubMed] [Google Scholar]
- de Rome PJ, Jamieson DD, Taylor KM, Davies LP. Ligand-binding and pharmacological studies on dopamine and octopamine receptors in the heart of the bivalve mollusc, Tapes watlingi. Comp Biochem Physiol C. 1980;67C:9–16. doi: 10.1016/0306-4492(80)90051-9. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE, Mumford RA, Slater EE, Sigal IS, Caron MG, Lefkowitz RJ, Strader CD. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Donini A, Lange AB. Evidence for a possible neurotransmitter/neuromodulator role of tyramine on the locust oviducts. J Insect Physiol. 2004;50:351–361. doi: 10.1016/j.jinsphys.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Dougan DF, Wade DN. Differential blockade of octopamine and dopamine receptors by analogues of clozapine and metoclopramide. Clin Exp Pharmacol Physiol. 1978;5:341–349. doi: 10.1111/j.1440-1681.1978.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Dourish CT. A pharmacological analysis of the hyperactivity syndrome induced by β-phenylethylamine in the mouse. Br J Pharmacol. 1982;77:129–139. doi: 10.1111/j.1476-5381.1982.tb09278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer RG. Trehalose production in isolated fat body of the American cockroach, Periplaneta americana. Comp Biochem Physiol C. 1979;62C:31–34. doi: 10.1016/0306-4492(79)90096-0. [DOI] [PubMed] [Google Scholar]
- Downer RGH, Hiripi L, Juhos S. Characterization of the tyraminergic system in the central nervous system of the locust, Locusta migratoria migratoides. Neurochem Res. 1993;18:1245–1248. doi: 10.1007/BF00975042. [DOI] [PubMed] [Google Scholar]
- Drummer O, Odell M. The Forensic Pharmacology of Drugs of Abuse. Oxford University Press; New York: 2001. [Google Scholar]
- Duan J, Martinez M, Sanders AR, Hou C, Saitou N, Kitano T, Mowry BJ, Crowe RR, Silverman JM, Levinson DF, Gejman PV. Polymorphisms in the trace amine receptor 4 (TRAR4) gene on chromosome 6q23.2 are associated with susceptibility to schizophrenia. Am J Hum Genet. 2004;75:624–638. doi: 10.1086/424887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Du J, Xu Y, Xing Q, Wang H, Wu S, Chen Q, Li X, Li X, Shen J, Feng G, He L. Failure to find association between TRAR4 and schizophrenia in the Chinese Han population. J Neural Transm. 2006;113:381–385. doi: 10.1007/s00702-005-0335-z. [DOI] [PubMed] [Google Scholar]
- Duchemin AM, Berry MD, Neff NH, Hadjiconstantinou M. Phosphorylation and activation of brain aromatic L-amino acid decarboxylase by cyclic AMP-dependent protein kinase. J Neurochem. 2000;75:725–731. doi: 10.1046/j.1471-4159.2000.0750725.x. [DOI] [PubMed] [Google Scholar]
- Dudai Y. High-affinity octopamine receptors revealed in Drosophila by binding or [3H]octopamine. Neurosci Lett. 1982;28:163–167. doi: 10.1016/0304-3940(82)90146-x. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Zvi S. High-affinity [3H]octopamine-binding sites in Drosophila melanogaster: interaction with ligands and relationship to octopamine receptors. Comp Biochem Physiol C. 1984;77:145–151. doi: 10.1016/0742-8413(84)90143-9. [DOI] [PubMed] [Google Scholar]
- Durden DA, Phillips SR. Kinetic measurements of the turnover rates of phenylethylamine and tryptamine in vivo in the rat brain. J Neurochem. 1980;34:1725–1732. doi: 10.1111/j.1471-4159.1980.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Durden DA, Davis BA. Determination of regional distributions of phenylethylamine and meta- and para-tyramine in rat brain regions and presence in human and dog plasma by an ultra-sensitive negative chemical ion gas chromatography-mass spectrometric (NCI—GC—MS) method. Neurochemical Research. 1993;18:995–1002. doi: 10.1007/BF00966759. [DOI] [PubMed] [Google Scholar]
- Durden DA, Phillips SR, Boulton AA. Identification and distribution of beta-phenylethylamine in the rat. Can J Biochem. 1973;51:995–1002. doi: 10.1139/o73-129. [DOI] [PubMed] [Google Scholar]
- Dyck LE. Release of monoamines from striatal slices by phenelzine and β-phenylethylamine. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:797–800. doi: 10.1016/0278-5846(83)90069-6. [DOI] [PubMed] [Google Scholar]
- Dyck LE. Release of some endogenous trace amines from rat striatal slices in the presence and absence of a monoamine oxidase inhibitor. In: Boulton AA, Juorio AV, Downer RGH, editors. Trace Amines: Comparative and Clinical Neurobiology. Humana Press; New Jersey: 1988. pp. 223–237. [DOI] [PubMed] [Google Scholar]
- Dyck LE. Release of some endogenous trace amines from rat striatal slices in the presence and absence of a monoamine oxidase inhibitor. Life Sci. 1989;44:1149–1156. doi: 10.1016/0024-3205(89)90309-3. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Rizk M, Spiker DG. Effects of L-DOPA on the excretion of alcoholic metabolites of catecholamines and trace amines in rat and human urine. Biochem Med. 1981;25:135–148. doi: 10.1016/0006-2944(81)90070-3. [DOI] [PubMed] [Google Scholar]
- Enan EE. Molecular response of Drosophila melanogaster tyramine receptor cascade to plant essential oils. Insect Biochem Mol Biol. 2005;35:309–321. doi: 10.1016/j.ibmb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Erspamer V, Boretti G. Identification and characterization by paper chromatography of enteramine, octopamine, tyramine, histamine and allied substances in extracts of posterior salivary glands of octopoda and in other tissue extracts of vertebrates and invertebrates. Arch Int Pharmacodyn. 1951;88:296–332. [PubMed] [Google Scholar]
- Evans PD, O'Shea M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature. 1977;270:257–258. doi: 10.1038/270257a0. [DOI] [PubMed] [Google Scholar]
- Evans PD. Biogenic amines in the insect nervous system. Adv Insect Physiol. 1980;15:317–473. [Google Scholar]
- Evans PD. Multiple receptor types for octopamine in the locust. J Physiol. 1981;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD. Studies on the mode of action of octopamine, 5.hydroxytryptamine and proctolin on a myogenic rhythm in the locust. J Exp Biol. 1984;110:231–251. doi: 10.1242/jeb.110.1.231. [DOI] [PubMed] [Google Scholar]
- Evans PD. Phenyliminoimidazolidine derivatives activate both octopamine, and octopamine, receptor subtypes in locust skeletal muscle. J Exp Biol. 1987;129:239–250. [Google Scholar]
- Evans PD, Thonoor M, Midgeley JM. Activities of octopamine and synephrine stereoisomers on octopaminergic receptor subtypes in locust skeletal muscle. J Pharm Pharmacol. 1988;40:855–861. doi: 10.1111/j.2042-7158.1988.tb06288.x. [DOI] [PubMed] [Google Scholar]
- Evans PD. Molecular studies on insect octopamine receptors. EXS. 1993;63:286–296. doi: 10.1007/978-3-0348-7265-2_16. [DOI] [PubMed] [Google Scholar]
- Evans PD, Robb S. Octopamine receptor subtypes and their modes of action. Neurochem Res. 1993;18:869–874. doi: 10.1007/BF00998270. [DOI] [PubMed] [Google Scholar]
- Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Farooqui T, Robinson K, Vaessin H, Smith BH. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci. 2003;23:5370–5380. doi: 10.1523/JNEUROSCI.23-12-05370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui T, Vaessin H, Smith BH. Octopamine receptors in the honeybee (Apis mellifera) brain and their disruption by RNA-mediated interference. J Insect Physiol. 2004;50:701–713. doi: 10.1016/j.jinsphys.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Faurbye A. The role of amines in the etiology of schizophrenia. Comp Psychiatry. 1968;9:155–177. doi: 10.1016/s0010-440x(68)80051-3. [DOI] [PubMed] [Google Scholar]
- Federici M, Geracitano R, Tozzi A, Longone P, Di Angelantonio S, Bengtson CP, Bernardi G, Mercuri NB. Trace amines depress GABA B response in dopaminergic neurons by inhibiting G-βγ-gated inwardly rectifying potassium channels. Mol Pharmacol. 2005;67:1283–1290. doi: 10.1124/mol.104.007427. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Brond L, Nielsen S, Praetorius J. Cellular and subcellular distribution of the type II vasopressin receptor in kidney. Am J Physiol Renal Physiol. 2007 doi: 10.1152/ajprenal.00316.2006. Epub Jun 6. [DOI] [PubMed] [Google Scholar]
- Fischer E, Heller B, Miro AH. Phenylethylamine in the urine of mental patients. Prensa Med Argent. 1968;55:1193–1195. [PubMed] [Google Scholar]
- Fischer JE, Baldessarini RJ. False transmitters and hepatic failure. Lancet. 1971;2:75–80. doi: 10.1016/s0140-6736(71)92048-4. [DOI] [PubMed] [Google Scholar]
- Fischer E, Spatz H, Heller B, Reggiani H. Phenethylamine content of human urine and rat brain, its alterations in pathological conditions and after drug administration. Experientia. 1972;28:307–308. doi: 10.1007/BF01928707. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A, Stöckle D. Zum Mechanismus der Wirkungs-Verstarkung und Wirkungs-Abschwachung sympathomimetischer Amine durch Cocain und andere. Pharmaka Arch Exp Pathol Pharmakol. 1955;224:401–410. [PubMed] [Google Scholar]
- Furst PT. Flesh of the Gods: The Ritual Use of Hallucinogens. Praeger Publishers; 1972. [Google Scholar]
- Furst PT. Hallucinogens and Culture. Chandler & Sharp Publishers, Inc.; San Francisco, CA: 1976. [Google Scholar]
- Fuxe K, Grobecker H, Jonsson J. The effect of β-phenylethylamine on central and peripheral monoamine-containing neurons. Eur J Pharmacol. 1967;2:202–207. doi: 10.1016/0014-2999(67)90088-x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Gale EF. The production of amines by bacteria: The production of tyramine by Streptococcus faecalis. Biochem J. 1940;34:846–852. doi: 10.1042/bj0340846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner P, Miklya I. Major depression and the synthetic enhancer substances, (-)-deprenyl and R-(-)-1-(benzofuran-2-yl)-2-propylaminopentane. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:5–14. doi: 10.1016/j.pnpbp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Geornaras I, Dykes GA, von Holy A. Biogenic amine formation by poultry-associated spoilage and pathogenic bacteria. Lett Appl Microbiol. 1995;21:164–166. doi: 10.1111/j.1472-765x.1995.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Geracitano R, Federici M, Prisco S, Bernardi G, Mercuri NB. Inhibitory effects of trace amines on rat midbrain dopaminergic neurons. Neuropharmacology. 2004;46:807–814. doi: 10.1016/j.neuropharm.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, Bakker RA, Piek GJ, Planta RJ, Vreugdenhil E, Leysen JE, van Heerikhuizen H. Molecular cloning and pharmacological characterization of a molluscan octopamine receptor. Mol Pharmacol. 1997;51:293–300. doi: 10.1124/mol.51.2.293. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, Lodder HC, Vincent M, Bakker RA, Planta RJ, Vreugdenhil E, Kits KS, van Heerikhuizen H. Cloning and expression of a complementary DNA encoding a molluscan octopamine receptor that couples to chloride channels in HEK293 cells. J Biol Chem. 1997;272:6201–6207. doi: 10.1074/jbc.272.10.6201. [DOI] [PubMed] [Google Scholar]
- Ghiretti F. Enteramine, octopamine, and tyramine in external and internal secretion of the posterior salivary gland in octopus. Arch Sci Biol (Bologna) 1953;37:435–441. [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gloriam DE, Bjarnadottir TK, Schioth HB, Fredriksson R. High species variation within the repertoire of trace amine receptors. Ann N Y Acad Sci. 2005a;1040:323–327. doi: 10.1196/annals.1327.052. [DOI] [PubMed] [Google Scholar]
- Gloriam DE, Bjarnadottir TK, Yan YL, Postlethwait JH, Schioth HB, Fredriksson R. The repertoire of trace amine G-protein-coupled receptors: large expansion in zebrafish. Mol Phylogenet Evol. 2005b;35:470–482. doi: 10.1016/j.ympev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol. 2006;84:287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE, editors. Goodman and Gillman's The Pharmacologic Basis of Therapeutics. 10th. McGraw-Hill; New York: 2005. [Google Scholar]
- Graham D, Langer SZ. [3H]tryptamine binding sites of rat cerebral cortex: Pharmcological profile and plasticity. Neuropharmacology. 1987;26:1093–1097. doi: 10.1016/0028-3908(87)90253-x. [DOI] [PubMed] [Google Scholar]
- Greenshaw AJ, Juorio AV, Boulton AA. Behavioral and neurochemical effects of deprenyl and β-phenylethylamine in Wistar rats. Brain Res Bull. 1985;15:183–189. doi: 10.1016/0361-9230(85)90134-0. [DOI] [PubMed] [Google Scholar]
- Greenshaw AJ, Juorio AV, Nguyen TV. Depletion of striatal β-phenylethylamine following dopamine but not 5-HT denervation. Brain Res Bull. 1986;17:477–484. doi: 10.1016/0361-9230(86)90214-5. [DOI] [PubMed] [Google Scholar]
- Griesemer EC, Barsky J, Dragstedt CA, Wells JA, Zeller EA. Potentiating effect of iproniazid on the pharmacological action of sympathomimetic amines. Proc Soc Exp Biol Med. 1953;84:699–701. doi: 10.3181/00379727-84-20757. [DOI] [PubMed] [Google Scholar]
- Grohmann L, Blenau W, Erber J, Ebert PR, Strunker T, Baumann A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J Neurochem. 2003;86:725–735. doi: 10.1046/j.1471-4159.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- Guan XM, Kobilka TS, Kobilka BK. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J Biol Chem. 1992;267:21995–21998. [PubMed] [Google Scholar]
- Guggenheim M. Die biogenen Amine und ihre Bedeutung für die Physiologie und Pathologie des pflanzlichen und tierischen Stoffwechsels. Basel: Karger; 1951. VII. Phenylalkylamine und Oxyphenylalkylamine; pp. 514–516. [Google Scholar]
- Guillen A, Haro A, Municio AM. A possible new class of octopamine receptors coupled to adenylate cyclase in the brain of the dipterous Ceratitis capitata. Pharmacological characterization and regulation of 3H-octopamine binding. Life Sci. 1989;45:655–662. doi: 10.1016/0024-3205(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Rossetti Z, Silvia C, Krajnc D, Neff NH. Aromatic L-amino acid decarboxylase activity of the rat retina is modulated in vivo by environmental light. J Neurochem. 1988;51:1560–1564. doi: 10.1111/j.1471-4159.1988.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Wemlinger TA, Silvia CP, Hubble JP, Neff NH. Aromatic L-amino acid decarboxylase activity of mouse striatum is modulated via dopamine receptors. J Neurochem. 1993;60:2175–2180. doi: 10.1111/j.1471-4159.1993.tb03503.x. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Duchemin AM, Neff NH. Cyclic GMP-dependent protein kinase phosphorylates and activates brain aromatic L-amino acid decarboxylase. J Neurochem. 2003;85(Suppl 1):40. doi: 10.1046/j.1471-4159.2000.0750725.x. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannington E. Preliminary report on tyramine headache. Br Med J. 1967;2:550–551. doi: 10.1136/bmj.2.5551.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Horn AS. Octopamine-sensitive adenylate cyclase in cockroach brain: effects of agonists, antagonists and guanylyl nucleotides. Mol Pharmacol. 1977;13:512–520. [PubMed] [Google Scholar]
- Harris-Warrick RM, Kravitz EA. Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart ME, Suchland KL, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. Trace amine-associated receptor agonists: synthesis and evaluation of thyronamines and related analogues. J Med Chem. 2006;49:1101–1112. doi: 10.1021/jm0505718. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Skolnick P, Paul SM. Specific [3H]-beta-phenylethylamine binding sites in rat brain. Eur J Pharmacol. 1982;83:147–148. doi: 10.1016/0014-2999(82)90301-6. [DOI] [PubMed] [Google Scholar]
- Hashemzadeh H, Hollingworth RM, Voliva A. Receptors for 3H-octopamine in the adult firefly light organ. Life Sci. 1985;37:433–440. doi: 10.1016/0024-3205(85)90405-9. [DOI] [PubMed] [Google Scholar]
- Heller B, Fischer E, Martin R. Therapeuic action of β-phenylalanine in Parkinson's disease. Arzneimittelforschung. 1976;26:577–579. [PubMed] [Google Scholar]
- Henry DP, Russell WL, Clemens JA, Plebus LA. Phenylethylamine and p-tyramine in the extracellular space of the rat brain: quantification using a new radioenzymatic assay and in situ microdialysis. In: Boulton AA, Juorio AV, Downer RGH, editors. Trace Amines: Comparative and Clinical Neurobiology. Humana Press; New Jersey: 1988. pp. 239–250. [Google Scholar]
- Hicks TP, McLennan H. Actions of octopamine upon dorsal horn neurones of the spinal cord. Brain Res. 1978a;757:402–406. doi: 10.1016/0006-8993(78)90050-1. [DOI] [PubMed] [Google Scholar]
- Hicks TP, McLennan H. Comparison of the actions of octopamine and catecholamines on single neurones of the rat cerebral cortex. Br J Pharmacol. 1978b;64:485–491. doi: 10.1111/j.1476-5381.1978.tb17309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Uchimura H, Shiraishi A, Kuroki T, Matsumoto T, Tsutsumi T. β-Phenylethylamine and amphetamine: similar aspects in their behavior, pharmacological and neurochemical characteristics. Japanese. Yakubutsu Seishin Kodo. 1989;9:335–348. [PubMed] [Google Scholar]
- Hirashima A, Rafaeli A, Gileadi C, Kuwano E. Three-dimensional pharmacophore hypotheses of octopamine receptor responsible for the inhibition of sex-pheromone production in Helicoverpa armigera. Mol Graph Model. 1999;17:43–50. 53–54. doi: 10.1016/s1093-3263(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Morimoto M, Kuwano E, Eto M. Octopaminergic agonists for the cockroach neuronal octopamine receptor. J Insect Sci. 2003;3:10. doi: 10.1093/jis/3.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiripi L, Juhos S, Downer RG. Characterization of tyramine and octopamine receptors in the insect (Locusta migratoria migratorioides) brain. Brain Res. 1994;633:119–126. doi: 10.1016/0006-8993(94)91530-x. [DOI] [PubMed] [Google Scholar]
- Horn AS, Snyder SH. Steric requirements for catecholamine uptake by rat brain synaptosomes: studies with rigid analogs of amphetamine. J Pharmacol Exp Ther. 1972;180:523–530. [PubMed] [Google Scholar]
- Huang ES. Construction of a sequence motif characteristic of aminergic G protein-coupled receptors. Protein Sci. 2003;12:1360–1367. doi: 10.1110/ps.0305603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebert ND, Boulton AA. Longiytudinal urinary trace amine esxcretion in a human male. J Chromatog. 1979;162:169–176. doi: 10.1016/s0378-4347(00)81910-6. [DOI] [PubMed] [Google Scholar]
- Ibrahim KE, Couch MW, Williams CM, Fregly ML, Midgley JM. m-Octopamine: normal occurrence with p-octopamine in mammalian sympathetic nerves. J Neurochem. 1985;44:1862–1967. doi: 10.1111/j.1471-4159.1985.tb07180.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Inada T, Ozaki N. No association of haplotype-tagging SNPs in TRAR4 with schizophrenia in Japanese patients. Schizophr Res. 2005;78:127–130. doi: 10.1016/j.schres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Inwang EE, Mosnaim AD, Sabelli HC. Isolation and characterization of phenylethylamine and phenylethanolamine from human brain. J Neurochem. 1973;20:1469–1473. doi: 10.1111/j.1471-4159.1973.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Ishida K, Murata M, Kato M, Utsunomiya I, Hoshi K, Taguchi K. β-phenylethylamine stimulates striatal acetylcholine release through activation of the AMPA glutamatergic pathway. Biol Pharm Bull. 2005;28:1626–1629. doi: 10.1248/bpb.28.1626. [DOI] [PubMed] [Google Scholar]
- Jacob MS, Presti DE. Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine. Med Hypotheses. 2005;64:930–937. doi: 10.1016/j.mehy.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Jaeger CB, Ruggiero DA, Albert VR, Joh TH, Reis DJ. Immunocytochemical localization of aromatic-L-amino acid decarboxylase. In: Björkland A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy, Vol. 2, Classical Transmitters in the CNS, Part 1. Elsevier; Amsterdam: 1984a. pp. 387–408. [Google Scholar]
- Jaeger CB, Ruggiero DA, Albert VR, Park DH, Joh TH, Reis DJ. Aromatic L-amino acid decarboxylase in the rat brain: immunocytochemical localization in neurons of the brain stem. Neuroscience. 1984b;11:691–713. doi: 10.1016/0306-4522(84)90053-8. [DOI] [PubMed] [Google Scholar]
- Jahagirdar AP, Milton C, Viswanatha T, Downer RCH. Calcium involvement in mediating the action of octopamine and hypertrehalosemic peptides on insect haemocytes. FEBS Lett. 1987;219:83–87. [Google Scholar]
- Janssen PA, Leysen JE, Megens AA, Awouters FH. Does phenylethylamine act as an endogenous amphetamine in some patients? Int J Neuropsychopharmacol. 1999;2:229–240. doi: 10.1017/S1461145799001522. [DOI] [PubMed] [Google Scholar]
- Jansen SC, van Dusseldorp M, Bottema KC, Dubois AEJ. Intolerance to dietary biogenic amines: a review. Ann Allergy Asthma Immunol. 2003;91:233–241. doi: 10.1016/S1081-1206(10)63523-5. [DOI] [PubMed] [Google Scholar]
- Jeanneret J. Untersuchen über die Zersetzung von Gelatine und Eiweiss durch die geformten Pankreasfermente bei Luftausschluss. J prak Chem. 1877;15:353–390. [Google Scholar]
- Jones RSJ. Tryptamine: a neuromodulator or neurotransmitter in mammalian brain? Prog Neurobiol. 1982;19:117–139. doi: 10.1016/0301-0082(82)90023-5. [DOI] [PubMed] [Google Scholar]
- Jones RSG, Juorio AV, Boulton AA. Changes in levels of dopamine and tyramine in the rat caudate nucleus following alterations in impulse flow in the nigrostriatal pathway. J Neurochem. 1983;40:396–401. doi: 10.1111/j.1471-4159.1983.tb11295.x. [DOI] [PubMed] [Google Scholar]
- Juorio AV. Presence and metabolism of β-phenethylamine, p-tyramine, m-tyramine, and tryptamine in the brain of the domestic fowl. Brain Res. 1976;111:442–445. doi: 10.1016/0006-8993(76)90792-7. [DOI] [PubMed] [Google Scholar]
- Juorio AV. The effects of some antipsychotic drugs, D-amphetamine, and reserpine on the concentration and rate of accumulation of tryptamine and 5-hydroxytryptamine in the mouse striatum. Can J Physiol Pharmacol. 1982;60:376–380. doi: 10.1139/y82-054. [DOI] [PubMed] [Google Scholar]
- Juorio AV. Brain trace amines: mapping studies and effects of mesencephalic lesions. In: Boulton AA, Juorio AV, Downer RGH, editors. Trace Amines: Comparative and Clinical Neurobiology. Humana Press; New Jersey: 1988. pp. 157–174. [Google Scholar]
- Juorio AV, Philips SR. Arylalkylamies in Octopus tissues. Neurochem Res. 1976;1:501–509. doi: 10.1007/BF00964211. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Roberston HA. Identification and distribution of some monoamines in tissues of the sunflower star, Pycnopodia heliianthoides (echinodermata) J Neurochem. 1977;28:573–579. doi: 10.1111/j.1471-4159.1977.tb10428.x. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Jones RSG. The effect of mesencephalic lesions on tyramine and dopamine in the caudate nucleus of the rat. J Neurochem. 1981;36:1898–1903. doi: 10.1111/j.1471-4159.1981.tb10813.x. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Kazakoff CW. The presence of β-phenylethylamine, p-tyramine m-tyramine and tryptamine in ganglia and foot muscle of the garden snail (Helix aspersa) Experientia. 1984;40:549–551. [Google Scholar]
- Juorio AV, Greenshaw AJ. Tryptamine depletion in the rat striatum following electrolytic lesions of the substantia nigra. Brain Res. 1986;371:385–389. doi: 10.1016/0006-8993(86)90381-1. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Sloley BD. The presence of tyramine and related monoamines iin the nerve cord and some other tissues of the lobster, Homarus americanus. Brain Res. 1988;444:380–382. doi: 10.1016/0006-8993(88)90951-1. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Greenshaw AJ, Nguyen TV. Effect of intranigral administration of 6-hydroxydopamine and 5,7-dihydroxytryptamine on rat brain tryptamine. J Neurochem. 1987;48:1346–1350. doi: 10.1111/j.1471-4159.1987.tb05669.x. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Greenshaw AJ, Wishart TB. Reciprocal changes in striatal dopamine and β-phenylethylamine induced by reserpine in the presence of monoamine oxidase inhibitors. Naunyn- Schmiedeberg's Arch Pharmacol. 1988;338:644–648. doi: 10.1007/BF00165628. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Greenshaw AJ, Zhu MY, Paterson IA. The effects of some neuroleptics and D-amphetamine on striatal 2-phenylethylamine in the mouse. Gen Pharmacol. 1991a;22:407–413. doi: 10.1016/0306-3623(91)90473-j. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Paterson IA, Zhu MY, Matte G. Electrical stimulation of the substantia nigra substantia nigra and changes of 2-phenylethylamine synthesis in the rat striatum. J Neurochem. 1991b;56:213–220. doi: 10.1111/j.1471-4159.1991.tb02583.x. [DOI] [PubMed] [Google Scholar]
- Kakimoto Y, Armstrong MD. On the identification of octopamine in mammals. J Biol Chem. 1962;237:422–427. [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, van Schaik R, Kaptein E, Visser TJ, Doulabi BZ, Fliers E. Daily variations in type II iodothyronine deiodinase activity in the rat brain as controlled by the biological clock. Endocrinology. 2005;146:1418–1427. doi: 10.1210/en.2004-0763. [DOI] [PubMed] [Google Scholar]
- Katzung BG. Basic and Clinical Pharmacology. 10th. McGraw-Hill; New York: 2007. [Google Scholar]
- Kaulen P, Bruning G, Rommelspacher H, Baumgarten HG. Characterization and quantitative autoradiography of [3H]tryptamine binding sites in rat brain. Brain Res. 1986;366:72–88. doi: 10.1016/0006-8993(86)91282-5. [DOI] [PubMed] [Google Scholar]
- Kawano T, Pinontoan R, Uozumi N, Miyake C, Asada K, Kolattukudy PE, Muto S. Aromatic monoamine-induced immediate oxidative burst leading to an increase in cytosolic Ca2+ concentration in tobacco suspension culture. Plant Cell Physiol. 2000a;41:1251–1258. doi: 10.1093/pcp/pcd052. [DOI] [PubMed] [Google Scholar]
- Kawano T, Pinontoan R, Uozumi N, Morimitsu Y, Miyake C, Asada K, Muto S. Phenylethylamine-induced generation of reactive oxygen species and ascorbate free radicals in tobacco suspension culture, mechanism for oxidative burst mediating Ca2+ influx. Plant Cell Physiol. 2000b;41:1259–1266. doi: 10.1093/pcp/pcd053. [DOI] [PubMed] [Google Scholar]
- Kellar KJ, Cascio CS. [3H]Tryptamine: High affinity binding sites in rat brain. Eur J Pharmacol. 1982;78:475–478. doi: 10.1016/0014-2999(82)90492-7. [DOI] [PubMed] [Google Scholar]
- Kim KA, von Zastrow M. Old drugs learn new tricks: insights from mammalian trace amine receptors. Mol Pharmacol. 2001;60:1165–1167. doi: 10.1124/mol.60.6.1165. [DOI] [PubMed] [Google Scholar]
- Kinsey CG, Bussolati G, Bosco M, Kimura T, Pizzorno MC, Chernin MI, Cassoni P, Novak JF. Constitutive and ligand-induced nuclear localization of oxytocin receptor. J Cell Mol Med. 2007;11:96–110. doi: 10.1111/j.1582-4934.2007.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahama K, Denoyer M, Raynaud B, Borri-Voltattorni C, Weber M, Jouvet M. Aromatic L-amino acid decarboxylaseimmunohistochemistry in the cat lower brainstem and midbrain. J Comp Neurol. 1990;302:935–953. doi: 10.1002/cne.903020418. [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Frielle T, Collins S, Yang-Feng T, Kobilka TS, Francke U, Lefkowitz RJ, Caron MG. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987;329:75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Kopin IJ, Fischer JE, Musacchio J, Horst WD. Evidence for a false neurochemical transmitter as a mechanism for the hypotensive effect of monoamine oxidase inhibitors. Proc Natl Acad Sci, U S A. 1964;52:716–721. doi: 10.1073/pnas.52.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ. False adrenergic transmitters. Annu Rev Pharmacol. 1968;8:377–394. doi: 10.1146/annurev.pa.08.040168.002113. [DOI] [PubMed] [Google Scholar]
- Köhrle J. The deiodinase family: selenoenzymes regulating thyroid hormone availability and action. Thyroid. 2005;15:899–903. doi: 10.1007/PL00000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JC, Fischman VS, Littlefield DC. Amphetamine abuse. Pattern and effects of high doses taken intravenously. JAMA. 1967;201:305–309. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- Kratochwil NA, Malherbe P, Lindemann L, Ebeling M, Hoener MC, Muhlemann A, Porter RH, Stahl M, Gerber PR. An automated system for the analysis of G protein-coupled receptor transmembrane binding pockets: alignment, receptor-based pharmacophores, and their application. J Chem Inf Model. 2005;45:1324–1336. doi: 10.1021/ci050221u. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Clusman S, Harris-Warrick RM, Livingstone MS, Schwarz T, Coy MF. Amines and a peptide as neurohormones in lobsters: actions on neuromuscular preparations and preliminary behavioural studies. J Exp Biol. 1980;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Kester MH, Peeters RP, Visser TJ. Biochemical mechanisms of thyroid hormone deiodination. Cell Mol Life Sci. 2000;57:1853–1863. doi: 10.1089/thy.2005.15.787. [DOI] [PubMed] [Google Scholar]
- Kusaga A. Decreased beta-phenylethylamine in urine of children with attention deficit hyperactivity disorder and autistic disorder. No To Hattatsu (Japanese) 2002;34:243–248. [PubMed] [Google Scholar]
- Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/s0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, Syvalahti E, Hietala J. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenet Genomics. 2005;15:387–391. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Bockaert J. Pharmacological characterization of octopamine-sensitive adenylate cyclase in the flight muscle of Locusta migratoria L. Eur J Pharmacol. 1985;119:53–59. doi: 10.1016/0014-2999(85)90321-8. [DOI] [PubMed] [Google Scholar]
- Langer SZ, Rubio MC. Effects of the noradrenaline metabolites on the adrenergic receptors. Naunyn Schmiedeberg's Arch Pharmacol. 1973;276:71–88. doi: 10.1007/BF00500780. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Antagonism by CPP, (±)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid, of β-phenylethylamine (PEA)-induced hypermotility in mice of different strains. Pharmacol Biochem Behav. 1996;55:175–178. doi: 10.1016/0091-3057(95)02230-9. [DOI] [PubMed] [Google Scholar]
- Lee HG, Seong CS, Kim YC, Davis RL, Han KA. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 2003;264:179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Holmans P, Straub RE, et al. Multicenter linkage study of schizophrenia candidate regions on chromosomes 5q, 6q, 10p, and 13q: Schizophrenia Linkage Collaborative Group 111. Am J Hum Genet. 2000;67:652–663. doi: 10.1086/303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liapakis G, Ballesteros JA, Papachristou S, Chan WC, Chen X, Javitch JA. The forgotten serine. A critical role for Ser-2035.42 in ligand binding to and activation of the beta 2-adrenergic receptor. J Biol Chem. 2000;275:37779–37788. doi: 10.1074/jbc.M002092200. [DOI] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine associated receptors form structurally and functionally distinct subfamilies of novel G-protein coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci. 2005;26:274–281. doi: 10.1016/j.tips.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Schaeffer SF, Kravitz EA. Biochemistry and ultrastructure of serotonergic nerve endings in the lobster: serotonin and octopamineare contained in different nerve endings. J Neurobiol. 1981;72:27–54. doi: 10.1002/neu.480120104. [DOI] [PubMed] [Google Scholar]
- Lonvaud-Funel A. Biogenic amines in wines: role of lactic acid bacteria. FEMS Microbiol Lett. 2001;199:9–13. doi: 10.1111/j.1574-6968.2001.tb10643.x. [DOI] [PubMed] [Google Scholar]
- Lovenberg W, Weissbach H, Udenfriend S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962;237:89–93. [PubMed] [Google Scholar]
- Mack F, Bonisch H. Dissociation constants and lipophilicity of catecholamines and related compounds. Naunyn-Schmiedeberg's Arch Pharmacol. 1979;310:1–9. doi: 10.1007/BF00499868. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Majer JR, Boulton AA. Absolute, unambiguous ultramicroanalysis of metabolites present in complex biological extracts. Nature. 1970;225:658–60. doi: 10.1038/225658a0. [DOI] [PubMed] [Google Scholar]
- Manghani KK, Lunzer M, Billing BH, Sherlock S. Urinary and serum octopamine in patients with portal systemic encephalopathy. Lancet. 1975;2:943–946. doi: 10.1016/s0140-6736(75)90359-1. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94:547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- Martin LL, Roland DM, Neale RF, Wood PL. Structural relationships in the inhibition of [3H]tryptamine binding to rat brain membranes in vitro by phenylethylamines. Eur J Pharmacol. 1986;132:53–55. doi: 10.1016/0014-2999(86)90008-7. [DOI] [PubMed] [Google Scholar]
- Maxwell GD, Tait JF, Hildebrand JG. Regional synthesis of neurotransmitter candidates in the CNS of the moth Manduca sexta. Comp Biochem Physiol C. 1978;61C:109–119. doi: 10.1016/0306-4492(78)90120-x. [DOI] [PubMed] [Google Scholar]
- McCabe B, Tsuang MT. Dietary consideration in MAO inhibitor regimens. J Clin Psychiatry. 1982;43:178–181. [PubMed] [Google Scholar]
- McCabe BJ. Dietary tyramine and other pressor amines in MAOI regimens: a review. J Am Diet Assoc. 1986;86:1059–1064. [PubMed] [Google Scholar]
- McCormack JK, Beitz AJ, Larson AA. Autoradiographic localization of tryptamine binding sites in the rat and dog central nervous system. J Neurosci. 1986;6:94–101. doi: 10.1523/JNEUROSCI.06-01-00094.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Bernardi G. The ‘magic’ of L-dopa: why is it the gold standard Parkinson's disease therapy? Trends Pharmacol Sci. 2005;26:341–344. doi: 10.1016/j.tips.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Miller GM, Madras BK. Cloning of rhesus monkey TAR-1, a novel G-protein-linked receptor for ‘trace’ amines. Soc Neurosci Abstr Programme number 10.1 2002 [Google Scholar]
- Miller GM, Verrico CD, Jassen A, Konar M, Yang H, Panas H, Bahn M, Johnson R, Madras BK. Primate trace amine receptor 1 modulation by the dopamine transporter. J Pharmacol Exp Ther. 2005;313:983–994. doi: 10.1124/jpet.105.084459. [DOI] [PubMed] [Google Scholar]
- Molaei G, Paluzzi JP, Bendena WG, Lange AB. Isolation, cloning, and tissue expression of a putative octopamine/tyramine receptor from locust visceral muscle tissues. Arch Insect Biochem Physiol. 2005;59:132–149. doi: 10.1002/arch.20067. [DOI] [PubMed] [Google Scholar]
- Molinoff PB, Axelrod J. Octopamine: normal occurrence in sympathetic nerves of rats. Science. 1969;164:428–429. doi: 10.1126/science.164.3878.428. [DOI] [PubMed] [Google Scholar]
- Molinoff PB, Axelrod J. Distribution and turnover of octopamine in tissues. J Neurochem. 1972;19:157–163. doi: 10.1111/j.1471-4159.1972.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Moreno M, Berry MJ, Horst C, Thoma R, Goglia F, Harney JW, Larsen PR, Visser TJ. Activation and inactivation of thyroid hormone by type I iodothyronine deiodinase. Thyroid. 2005;15:787–798. doi: 10.1016/0014-5793(94)00365-3. [DOI] [PubMed] [Google Scholar]
- Mosnaim AD, Sabelli HC. Quantitative determination of the brain levels of a β-phenylethylamine-like substance in control and drug-treated mice. Pharmacologist. 1971;13:283. [Google Scholar]
- Mosnaim AD, Inwang EE, Sugerman JH, DeMartini WJ, Sabelli HC. Ultraviolet spectrophotometric determination of 2-phenylethylamine in biological samples and its possible correlation with depression. Biol Psychiatry. 1973;6:235–257. [PubMed] [Google Scholar]
- Mosnaim AD, Inwang EE, Sabelli HC. The influence of psychotropic drugs on the levels of endogenous 2-phenylethylamine in rabbit brain. Biol Psychiatry. 1974;8:227–234. [PubMed] [Google Scholar]
- Mosnaim AD, Wolf ME. Noncatecholic Phenylethylamines Parts 1 & 2. Marcel Dekker; New York: 1980. [Google Scholar]
- Mundorf ML, Hochstetler SE, Wightman RM. Amine weak bases disrupt vesicular storage and promote exocytosis in chromaffin cells. J Neurochem. 1999;73:397–405. doi: 10.1046/j.1471-4159.1999.0732397.x. [DOI] [PubMed] [Google Scholar]
- Mustard JA, Kurshan PT, Hamilton IS, Blenau W, Mercer AR. Developmental expression of a tyramine receptor gene in the brain of the honey bee, Apis mellifera. J Comp Neurol. 2005;483:66–75. doi: 10.1002/cne.20420. [DOI] [PubMed] [Google Scholar]
- Myojin T, Taga C, Tsuji M. Concentrations of beta-phenylethylamine in plasma and plateletes of schizophrenics. Jpn J Psychiatry Neurol. 1989;43:171–176. doi: 10.1111/j.1440-1819.1989.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kakimoto Y, Sano I. Formation of β-phenylethylamine in mammalian tissue and its effect on motor activity in the mouse. J Pharmacol Exp Ther. 1964;143:319–325. [PubMed] [Google Scholar]
- Nathans J, Hogness DS. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983;34:807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nathans J, Hogness DS. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984;81:4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Nathanson JA. In: Trace Amines and the Brain. Usdin E, Sandler M, editors. Dekker; New York: 1976. pp. 161–190. [Google Scholar]
- Nathanson JA. Octopamine receptors, adenosine 3′,5′- monophosphate, and neural control of firefly flashing. Science. 1979;203:65–68. doi: 10.1126/science.214856. [DOI] [PubMed] [Google Scholar]
- Nathanson JA. Development of a photoaffinity ligand for octopamine receptors. Mol Pharmacol. 1989;36:34–43. [PubMed] [Google Scholar]
- Nathanson JA, Greengard P. Octopamine-sensitive adenylate cyclse: evidence for a biological role of octopamine in nervous tissue. Science. 1973;180:308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- Nathanson JA, Kaugars G. A probe for octopamine receptors: synthesis of 2-[(4-azido-2,6-diethylphenyl)imino]imidazolidine and its tritiated derivative, a potent reversible-irreversible activator of octopamine-sensitive adenylate cyclase. J Med Chem. 1989;32:1795–1799. doi: 10.1021/jm00128a022. [DOI] [PubMed] [Google Scholar]
- Nathanson JA, Kantham L, Hunnicutt EJ. Isolation and N-terminal amino acid sequence of an octopamine ligand binding protein. FEBS Lett. 1989;259:117–120. doi: 10.1016/0014-5793(89)81508-x. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Gilmour BP, Lewin AH. A rapid functional assay for the human trace amine-associated receptor 1 based on the mobilization of internal calcium. J Biomol Screen. 2006;11:688–693. doi: 10.1177/1087057106289891. [DOI] [PubMed] [Google Scholar]
- Nencki M. Festchrift für 40 jähriges Jubiläum des Prof Valentin. Bern: 1876. [Google Scholar]
- Nguyen TV, Juorio AV. Binding sites for brain trace amines. Cell Mol Neurobiol. 1989;9:297–311. doi: 10.1007/BF00711411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Nickisch-Rosenegk E, Krieger J, Kubick S, Laage R, Strobel J, Strotmann J, Breer H. Cloning of biogenic amine receptors from moths (Bombyx mori and Heliothis virescens) Insect Biochem Mol Biol. 1996;26:817–827. doi: 10.1016/s0965-1748(96)00031-8. [DOI] [PubMed] [Google Scholar]
- Ohta H, Utsumi T, Ozoe Y. B96Bom encodes a Bombyx mori tyramine receptor negatively coupled to adenylate cyclase. Insect Mol Biol. 2003;12:217–223. doi: 10.1046/j.1365-2583.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- Ohta H, Utsumi T, Ozoe Y. Amino acid residues involved in interaction with tyramine in the Bombyx mori tyramine receptor. Insect Mol Biol. 2004;13:531–538. doi: 10.1111/j.0962-1075.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- Ohta H, Khan MA, Nagai I, Umemoto N, Hamasaki T, Ozoe Y. Responses of the silkworm tyramine receptor to 2-phenylethylamines and 5-phenyloxazoles. Arch Insect Biochem Physiol. 2005;59:150–160. doi: 10.1002/arch.20066. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Solari P, Garau L, Liscia A, Crnjar R, Onali P. Stimulation of cyclic AMP formation and nerve electrical activity by octopamine in the terminal abdominal ganglion of the female gypsy moth Lymantria dispar. Brain Res. 2006;1071:63–74. doi: 10.1016/j.brainres.2005.11.096. [DOI] [PubMed] [Google Scholar]
- O'Reilly R, Davis BA, Durden DA, Thorpe L, Machnee H, Boulton AA. Plasma phenylethylamine in schizophrenic patients. Biol Psychiatry. 1991;30:145–150. doi: 10.1016/0006-3223(91)90168-l. [DOI] [PubMed] [Google Scholar]
- O'Reilly RL, Davis BA. Phenylethylamine and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:63–75. doi: 10.1016/0278-5846(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Pae CU, Yu HS, Amann D, Kim JJ, Lee CU, Lee SJ, Jun TY, Lee C, Paik IH, Patkar AA, Lerer B. Association of the trace amine associated receptor 6 (TAAR6) gene with schizophrenia and bipolar disorder in a Korean case control sample. J Psychiatr Res. 2006 Nov 8; doi: 10.1016/j.jpsychires.2006.09.011. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Parker EM, Cubeddu LX. Comparative effects of amphetamine, phenylethylamine and related drugs on dopamine efflux, dopamine uptake and mazindol binding. J Pharmacol Exp Ther. 1988;245:199–210. [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319:237–246. doi: 10.1124/jpet.106.103622. [DOI] [PubMed] [Google Scholar]
- Paterson IA, Juorio AV, Boulton AA. 2-Phenylethylamine: A Modulator of Catecholamine Transmission in the Mammalian Central Nervous System? J Neurochem. 1990;55:1827–1837. doi: 10.1111/j.1471-4159.1990.tb05764.x. [DOI] [PubMed] [Google Scholar]
- Paterson IA. The potentiation of cortical neurone responses to noradrenaline by 2-phenylethylamine is independent of endogenous noradrenaline. Neurochem Res. 1993;12:1329–1336. doi: 10.1007/BF00975055. [DOI] [PubMed] [Google Scholar]
- Perry DC, Manning DC, Snyder SH. In vitro autoradiographic localization of [3H]tryptamine binding sites in rat brain. Neurosci Abstr. 1982;8:783. [Google Scholar]
- Perry DC. [3H]Tryptamine autoradiography in rat brain and choroid plexus reveals two distinct sites. J Pharmacol Exp Ther. 1986;236:548–559. [PubMed] [Google Scholar]
- Peters FT, Samyn N, Wahl M, Kraemer T, De Boeck G, Maurer HH. Concentrations and ratios of amphetamine, methamphetamine, MDA, MDMA, and MDEA enantiomers determined in plasma samples from clinical toxicology and driving under the influence of drugs cases by GC-NICI-MS. J Anal Toxicol. 2003;27:552–559. doi: 10.1093/jat/27.8.552. [DOI] [PubMed] [Google Scholar]
- Philips SR, Durden DA, Boulton AA. Identification and distribution of p-tyramine in the rat. Can J Biochem. 1974a;52:366–373. doi: 10.1139/o74-055. [DOI] [PubMed] [Google Scholar]
- Philips SR, Durden DA, Boulton AA. Identification and distribution of tryptamine in the rat. Can J Biochem. 1974b;52:447–451. doi: 10.1139/o74-068. [DOI] [PubMed] [Google Scholar]
- Philips SR, Boulton AA. The effect of monoamine oxidase inhibitors on some arylalkylamines in rat striatum. J Neurochem. 1979;33:159–167. doi: 10.1111/j.1471-4159.1979.tb11718.x. [DOI] [PubMed] [Google Scholar]
- Philips SR, Rozdilsky B, Boulton AA. Evidence for the presence of m-tyramine, p-tyramine, tryptamine, and phenylethylamine in the rat brain and several areas of the human brain. Biol Psychiatry. 1978;13(1):51–57. [PubMed] [Google Scholar]
- Poels J, Suner MM, Needham M, Torfs H, De Rijck J, De Loof A, Dunbar SJ, Vanden Broeck J. Functional expression of a locust tyramine receptor in murine erythroleukaemia cells. Insect Mol Biol. 2001;10:541–548. doi: 10.1046/j.0962-1075.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Karoum F, Chuang LW, Cannon-Spoor HE, Phillips I, Wyatt RJ. Phenylethylamine in paranoid chronic schizophrenia. Science. 1979;206:470–471. doi: 10.1126/science.504988. [DOI] [PubMed] [Google Scholar]
- Powell SB, Lehmann-Masten VD, Paulus MP, Gainetdinov RR, Caron MG, Geyer MA. MDMA ‘ecstasy’ alters hyperactive and perseverative behaviors in dopamine transporter knockout mice. Psychopharmacology. 2004;173:310–317. doi: 10.1007/s00213-003-1765-7. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc Natl Acad Sci, U S A. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M, Del Carmine R, Bertollini A, Levi G. Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol. 1977;41:133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- Reale V, Hannan F, Midgley JM, Evans PD. The expression of a cloned Drosophila octopamine/tyramine receptor in Xenopus oocytes. Brain Res. 1997;769:309–320. doi: 10.1016/s0006-8993(97)00723-3. [DOI] [PubMed] [Google Scholar]
- Rebhun J, Feinberg SM, Zeller EA. Potentiating effect of iproniazid on action of some sympathicomimetic amines. Proc Soc Exp Biol Med. 1954;87:218–220. doi: 10.3181/00379727-87-21339. [DOI] [PubMed] [Google Scholar]
- Reese EA, Bunzow JR, Arttamangkul S, Sonders MS, Grandy DK. Trace amine–associated receptor 1 displays species dependent stereoselectivity for isomers of metamphetamine, amphetamine and para-hydroxyamphetamine. J Pharmacol Exptl Ther. 2007 doi: 10.1124/jpet.106.115402. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve SE, Etienne P, Evans AC, Lal S, Shevell M, Savard G, Wong DF, Chouinard G, Gjedde A. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci, USA. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. J Neurochem. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- Rex E, Molitor SC, Hapiak V, Xiao H, Henderson M, Komuniecki R. Tyramine receptor (SER-2) isoforms are involved in the regulation of pharyngeal pumping and foraging behavior in Caenorhabditis elegans. J Neurochem. 2004;91:1104–1115. doi: 10.1111/j.1471-4159.2004.02787.x. [DOI] [PubMed] [Google Scholar]
- Rex E, Hapiak V, Hobson R, Smith K, Xiao H, Komuniecki R. TYRA-2 (F01E11.5): a Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J Neurochem. 2005;94:181–191. doi: 10.1111/j.1471-4159.2005.03180.x. [DOI] [PubMed] [Google Scholar]
- Reynolds GP. Phenethylamine- a role in mental illness? Trend in Neurosci. 1979;2:265–268. [Google Scholar]
- Reynolds GP, Sandler M, Hardy J, Bradford H. The determination of 2-phenylethylamine in sheep brain. J Neurochem. 1980;34:1123–1125. doi: 10.1111/j.1471-4159.1980.tb09950.x. [DOI] [PubMed] [Google Scholar]
- Riviere GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292:1042–1047. [PubMed] [Google Scholar]
- Robb S, Cheek TR, Hannan FL, Hall LM, Midgley JM, Evans PD. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 1994;13:1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HA, Steele JE. Activation of insect nerve cord phosphorylase by octopamine and adenosine 3′,5′-monophosphate. J Neurochem. 1972;19:1603–1606. doi: 10.1111/j.1471-4159.1972.tb05105.x. [DOI] [PubMed] [Google Scholar]
- Robertson HA, Juorio AV. Octopamine and some related noncatecholic amines in invertebrate nervous systems. Int Rev Neurobiol. 1976;19:173–224. doi: 10.1016/s0074-7742(08)60704-7. [DOI] [PubMed] [Google Scholar]
- Roeder T. A new octopamine receptor class in locust nervous tissue, the octopamine 3 (OA3) receptor. Life Sci. 1992;50:21–28. doi: 10.1016/0024-3205(92)90193-s. [DOI] [PubMed] [Google Scholar]
- Roeder T. Pharmacology of the octopamine receptor from locust central nervous tissue (OAR3) Br J Pharmacol. 1995;114:210–216. doi: 10.1111/j.1476-5381.1995.tb14927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59:533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Roeder T, Gewecke M. Octopamine receptors in locust nervous tissue. Biochem Pharmacol. 1990;39:1793–1797. doi: 10.1016/0006-2952(90)90127-7. [DOI] [PubMed] [Google Scholar]
- Roeder T, Nathanson JA. Characterization of insect neuronal octopamine receptors (OA3 receptors) Neurochem Res. 1993;18:921–925. doi: 10.1007/BF00998278. [DOI] [PubMed] [Google Scholar]
- Roeder T, Nathanson JA. Photoaffinity labeling of a neuronal octopamine receptor. J Neurochem. 1994;63:1516–1521. doi: 10.1046/j.1471-4159.1994.63041516.x. [DOI] [PubMed] [Google Scholar]
- Roeder T, Degen J, Dyczkowski C, Gewecke M. Pharmacology and molecular biology of octopamine receptors from different insect species. Prog Brain Res. 1995;106:249–258. doi: 10.1016/s0079-6123(08)61221-2. [DOI] [PubMed] [Google Scholar]
- Rossetti Z, Krajnc D, Neff NH, Hadjiconstantinou M. Modulation of retinal aromatic L-amino acid decarboxylase via a2 adrenoceptors. J Neurochem. 1989;52:647–652. doi: 10.1111/j.1471-4159.1989.tb09169.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Silvia CP, Krajnc D, Neff NH, Hadjiconstantinou M. Aromatic L-amino acid decarboxylase is modulated by D1dopamine receptors in rat retina. J Neurochem. 1990;54:787–791. doi: 10.1111/j.1471-4159.1990.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic potential of monoamine transporter substrates. Curr Top Med Chem. 2006;6:1845–1859. doi: 10.2174/156802606778249766. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potentl than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Enzymatic isotopic assay for and presence of β-phenylethylamine in brain. J Neurochem. 1974;22:211–216. doi: 10.1111/j.1471-4159.1974.tb11581.x. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Axelrod J. Psychotomimetic N-methylated tryptamines: formation in brain in vivo and in vitro. Science. 1972a;175:1365–1366. doi: 10.1126/science.175.4028.1365. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Axelrod J. A specific and sensitive enzymatic assay for tryptamine in tissues. J Pharmacol Exp Ther. 1972b;182:363–369. [PubMed] [Google Scholar]
- Saavedra JM, Axelrod J. Brain tryptamine and the effects of drugs. Adv Biochem Psychopharmacol. 1974a;10:135–139. [PubMed] [Google Scholar]
- Saavedra JM, Axelrod J. Enzymatic-isotopic micromethods for the measurement of biogenic amines in brain tissue and body fluids. J Psychiatr Res. 1974b;11:289–291. doi: 10.1016/0022-3956(74)90108-3. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Axelrod J. Octopamine as a putative neurotransmitter. Adv Biochem Psychopharmacol. 1976;15:95–110. [PubMed] [Google Scholar]
- Saavedra JM, Brownstein MJ, Carpenter DO, Axelrod J. Octopamine: presence in single neurons of Aplysia suggests neurotransmitter function. Science. 1974;185:364–365. doi: 10.1126/science.185.4148.364. [DOI] [PubMed] [Google Scholar]
- Sabelli HC, Giardina WJ, Mosnaim AD, Sabelli NH. A comparison of the functional roles of norepinephrine, dopamine, and phenylethylamine in the central nervous system. Acta Physiol Pol. 1973;24:33–40. [PubMed] [Google Scholar]
- Sabelli HC, Mosnaim AD, Vazquez AJU. Phenylethylamine: Possible role in depression and antidepressive drug action. In: Drucker-Colin RR, Myers RD, editors. Advances in Behavioral Biology, Vol. 10: Neurohumoral Coding of Brain Function. Plenum Press; New York: 1974. pp. 331–357. [Google Scholar]
- Sabelli HC, Mosnaim AD, Vazquez AJ, Giardina WJ, Borison RL, Pedemonte WA. Biochemical plasticity of synaptic transmission: a critical review of Dale's Principle. Biol Psychiatry. 1976;11:481–524. [PubMed] [Google Scholar]
- Sabelli H, Fink P, Fawcett J, Tom C. Sustained antidepressant effect of PEA replacement. J Neuropsychciatry Clin Neurosci. 1996;8:168–171. doi: 10.1176/jnp.8.2.168. [DOI] [PubMed] [Google Scholar]
- Sandler M, Reynolds GP. Does phenylethylamine cause schizophrenia? Lancet. 1976;1:70–71. doi: 10.1016/s0140-6736(76)90156-2. [DOI] [PubMed] [Google Scholar]
- Sandler M, Ruthven CR, Goodwin BL, Reynolds GP, Rao VA, Coppen A. Trace amine deficit in depressive illness: the phenylalanine connexion (sic) Acta Psychiatr Scand Suppl. 1980;280:29–39. [PubMed] [Google Scholar]
- Sandri G, Panfili E, Ernster L. Hydrogen peroxide production by monoamine oxidase in isolated rat-brain mitochondria: its effect on glutathione levels and Ca2+ efflux. Biochim Biophys Acta. 1990;1035:300–305. doi: 10.1016/0304-4165(90)90092-b. [DOI] [PubMed] [Google Scholar]
- Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654:160–170. doi: 10.1111/j.1749-6632.1992.tb25965.x. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amlaiky N, Plassat JL, Borrelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- Scarr E, Wingerchuk DM, Juorio AV, Paterson IA. The effects of monoamine oxidase B inhibition on dopamine metabolism in rats with nigro-striatal lesions. Neurochem Res. 1994;19:153–159. doi: 10.1007/BF00966810. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Hallmayer J, Albus M, et al. A genomewide autosomal screen for schizophrenia susceptibility loci in 71 families with affected siblings: support for loci on chromosome 10p and 6. Mol Psychiatry. 2000;5:638–649. doi: 10.1038/sj.mp.4000791. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980;32:230–313. [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava L, Bhardwaj SK, Grandy DK, Premont R, Sotnikova T, Boksa P, El-Ghundi M, O'Dowd BF, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci, USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever PS. False transmitters and migraine. Lancet. 1979;1:333. doi: 10.1016/s0140-6736(79)90754-2. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Cone EJ, Yousefnejad D. Physiologic effects and plasma kinetics of beta-phenylethylamine and its N-methyl homolog in the dog. J Pharmacol Exp Ther. 1982;223:190–196. [PubMed] [Google Scholar]
- Shannon HE, Degregorio CM. Self-administration of the endogenous trace amines β-phenylethylamine, N-methyl phenylethylamine and phenylethanolamine in dogs. J Pharmacol Exp Ther. 1982;222:52–60. [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20(9):3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu S, Miklya I. Pharmacological studies with endogenous enhancer substances: beta-phenylethylamine, tryptamine, and their synthetic derivatives. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:421–427. doi: 10.1016/j.pnpbp.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- Shulgin A, Shulgin A. TiHKAL: The Continuation. Transform Press; Berkeley, CA: 1997. [Google Scholar]
- Simpson GM, de Leon J. Tyramine and new monoamine oxidase inhibitor drugs. Br J Psychiatry Suppl. 1989 Oct;(6):32–37. [PubMed] [Google Scholar]
- Skerritt JH, Guihot SL, McDonald SE, Culvenor RA. Development of immunoassays for tyramine and tryptamine toxins of Phalaris aquatica L. J Agric Food Chem. 2000;48:27–32. doi: 10.1021/jf990452z. [DOI] [PubMed] [Google Scholar]
- Slessareva JE, Dohlman HG. G protein signaling in yeast: new components, new connections, new compartments. Science. 2006;314:1412–1413. doi: 10.1126/science.1134041. [DOI] [PubMed] [Google Scholar]
- Smith TA. Phenethylamine and related compounds in plants. Phytochemistry. 1977;16:9–18. [Google Scholar]
- Smith I, Kellow AH, Hanington E. A clinical and biochemical correlation between tyramine and migraine headache. Headache. 1970;10:43–52. doi: 10.1111/j.1526-4610.1970.hed1002043.x. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Budygin EA, Jones SR, Dykstra LA, Caron MG, Gainetdinov RR. Dopamine transporter-dependent and -independent actions of trace amine β-phenylethylamine. J Neurochem. 2004;91:362–373. doi: 10.1111/j.1471-4159.2004.02721.x. [DOI] [PubMed] [Google Scholar]
- Spector S, Melmon K, Lovenberg W, Sjoerdsma A. The presence and distribution of tyramine in mammalian tissues. J Pharmacol Exptl Therap. 1963;140:229–235. [PubMed] [Google Scholar]
- Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros B. Hypolocomotor effects of acute and daily D-amphetamine in mice lacking the dopamine transporter. Psychopharmacology. 2001;159:2–9. doi: 10.1007/s002130100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. An in vivo voltammetric comparison of the effects of three psychomotor stimulants on electrically evoked neostriatal dopamine release. Brain Res. 1986;366:350–353. doi: 10.1016/0006-8993(86)91317-x. [DOI] [PubMed] [Google Scholar]
- Stoff DM, Jeste DV, Gillin JC, Moja EA, Cohen L, Stauderman KA, Wyat RJ. Behavioral supersensitivity to β-phenylethylamine after chronic administration of haloperidol. Biol Psychiat. 1984;19:101–106. [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Suo S, Kimura Y, Van Tol HH. Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J Neurosci. 2006;26:10082–10090. doi: 10.1523/JNEUROSCI.0819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski HV, Naylor EW, Karoum F. Plasma phenylethylamine and phenylalanine in chronic schizophrenic patients. Biol Psychiatry. 1987;22:194–198. doi: 10.1016/0006-3223(87)90230-7. [DOI] [PubMed] [Google Scholar]
- Tallman JF, Saavedra JM, Axelrod J. A sensitive enzymatic-isotopic method for the analysis of tyramine in brain and other tissues. J Neurochem. 1976a;27:465–469. doi: 10.1111/j.1471-4159.1976.tb12269.x. [DOI] [PubMed] [Google Scholar]
- Tallman JF, Saavedra JM, Axelrod J. Biosynthesis and metabolism of endogenous tyramine and its normal presence in sympathetic nerves. J Pharmacol Exp Ther. 1976b;199:216–221. [PubMed] [Google Scholar]
- Tan ES, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. Exploring the structure-activity relationship of the ethylamine portion of 3-iodothyronamine for rat and mouse trace amine-associated receptor 1. J Med Chem. 2007;50(12):2787–2798. doi: 10.1021/jm0700417. [DOI] [PubMed] [Google Scholar]
- Thuillier J. In: Ten Years that Changed the Face of Mental Illness. Hickish G, translator. Martin Dunitz Ltd.; London: 1999. [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025:279–287. doi: 10.1196/annals.1316.035. [DOI] [PubMed] [Google Scholar]
- Usdin E, Sandler M. Trace Amines and the Brain: Proceedings of a Study Group Held at the Fourteenth Annual Meeting of the American College of Neuropsychopharmacology; New York: Dekker; 1976. [Google Scholar]
- Uzzan A, Dudai Y. Aminergic receptors in Drosophila melanogaster: responsiveness of adenylate cyclase to putative neurotransmitters. J Neurochem. 1982;38:1542–1550. doi: 10.1111/j.1471-4159.1982.tb06631.x. [DOI] [PubMed] [Google Scholar]
- Vaccari A. High affinity binding of [3H]-tyramine in the central nervous system. Br J Pharmacol. 1986;89:15–25. doi: 10.1111/j.1476-5381.1986.tb11116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari A. The tyramine binding site in the central nervous system: an overview. Neurochem Res. 1993;18:861–868. doi: 10.1007/BF00998269. [DOI] [PubMed] [Google Scholar]
- Vanden Broeck J, Vulsteke V, Huybrechts R, De Loof A. Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. J Neurochem. 1995;64:2387–2395. doi: 10.1046/j.1471-4159.1995.64062387.x. [DOI] [PubMed] [Google Scholar]
- Vanti WB, Muglia P, Nguyen T, Cheng R, Kennedy JL, George SR, O'Dowd BF. Discovery of a null mutation in a human trace amine receptor gene. Genomics. 2003;82:531–536. doi: 10.1016/s0888-7543(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Venken T, Alaerts M, Adolfsson R, Broeckhoven CV, Del-Favero J. No association of the trace amine-associated receptor 6 with bipolar disorder in a northern Swedish population. Psychiatr Genet. 2006;6:1–2. doi: 10.1097/01.ypg.0000180682.18665.a6. [DOI] [PubMed] [Google Scholar]
- Venter JC, DiPorzio U, Robinson DA, Shreeve SM, Lai J, Kerlavage AR, Fracek SP, Jr, Lentes KU, Fraser CM. Evolution of neurotransmitter receptor systems. Prog Neurobiol. 1988;30:105–169. doi: 10.1016/0301-0082(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaptein E, Terpstra OT, Krenning EP. Deiodination of thyroid hormone by human liver. FEBS Lett. 1994;344:143–146. doi: 10.1210/jcem-67-1-17. [DOI] [PubMed] [Google Scholar]
- Vogel WH, Ahlberg CD, Di Carlo V, Horwitt MK. Pink spot, p-tyramine and schizophrenia. Nature. 1967;216:1038–1039. doi: 10.1038/2161038a0. [DOI] [PubMed] [Google Scholar]
- Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL. Pharmacologic Characterization of the Cloned Human Trace Amine-Associated Receptor 1 (TAAR1) and Evidence for Species Differences with the Rat TAAR1. J Pharmacol Exp Ther. 2006;320:475–485. doi: 10.1124/jpet.106.112532. [DOI] [PubMed] [Google Scholar]
- Walker RJ, Ramage AG, Woodruff GN. The presence of octopamine in the brain of Helix aspersa and its action on specific snail neurones. Experientia. 1972;28:1173–1174. doi: 10.1007/BF01946151. [DOI] [PubMed] [Google Scholar]
- Walker RJ, Kerkut GA. The first family (adrenaline, noradrenaline, dopamine, octopamine, tyramine, phenylethanolamine and phenylethylamine) Comp Biochem. 1978;67C:261–266. doi: 10.1016/0306-4492(78)90051-5. [DOI] [PubMed] [Google Scholar]
- Williams CM, Couch MW, Thonoor CM, Midgley JM. Isomeric octopamines: their occurrence and functions. J Pharm Pharmacol. 1987;39:153–157. doi: 10.1111/j.2042-7158.1987.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Wolf M, Mosnaim AD. Phenylethylamine in neuropsychiatric disorders. Gen Pharmacol. 1983;14:385–390. doi: 10.1016/0306-3623(83)90020-4. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2006 Dec 21; doi: 10.1111/j.1601-183X.2006.00292.x. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wood PL, Pilapil C, LaFaille F, Nair PV, Glennon RA. Unique [3H]tryptamine binding sites in rat brain: Distribution and pharmacology. Arch Int Pharmacodyn. 1984;268:194–201. [PubMed] [Google Scholar]
- Wu PH, Boulton AA. Distribution and metabolism of tryptamine in rat brain. Can J Biochem. 1973;51:1104–1112. doi: 10.1139/o73-144. [DOI] [PubMed] [Google Scholar]
- Xie Z, Madras BK, Miller G. Brain distribution of rhesus monkey trace amine receptors TA1 and TA4. Program No 451.14. Society for Neuroscience; Washington, DC: 2005. Online. [Google Scholar]
- Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM. Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther. 2007;321:116–127. doi: 10.1124/jpet.106.116863. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. Trace amine-associated receptor 1 is a modulator of the dopamine transporter. J Pharmacol Exp Ther. 2007;321:128–136. doi: 10.1124/jpet.106.117382. [DOI] [PubMed] [Google Scholar]
- Yang HY, Neff NH. Beta-phenylethylamine: a specific substrate for type B monoamine oxidase of brain. J Pharmacol Exp Ther. 1973;187:365–371. [PubMed] [Google Scholar]
- Yu AM, Granvil CP, Haining RL, Krausz KW, Corchero J, Kupfer A, Idle JR, Gonzalez FJ. The relative contribution contribution of monoamine oxidase and cytochrome P450 isozymes to the metabolic deamination of the trace amine tryptamine. J Pharmacol Exp Ther. 2003;304:539–546. doi: 10.1124/jpet.102.043786. [DOI] [PubMed] [Google Scholar]
- Zeller EA, Barsky J. In vivo inhibition of liver and brain monoamine oxidase by 1-Isonicotinyl-2-isopropyl hydrazine. Proc Soc Exp Biol Med. 1952;81:459–461. doi: 10.3181/00379727-81-19910. [DOI] [PubMed] [Google Scholar]
- Zeller EA, Mosnaim AD, Huprikar SV. Phenylethylamine: Studies on the mechanism of its physiological action. Adv Biochem Psychopharmacol. 1976;15:75–86. [PubMed] [Google Scholar]
- Zhu MY, Juorio AV, Paterson IA, Boulton AA. Regulation of aromatic L-amino acid decarboxylase by dopamine receptors in the rat brain. J Neurochem. 1992;58:636–641. doi: 10.1111/j.1471-4159.1992.tb09765.x. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV, Paterson IA, Boulton AA. Regulation of striatal aromatic L-amino acid decarboxylase: effects of blockade or activation of dopamine receptors. Eur J Pharmacol. 1993;238:157–164. doi: 10.1016/0014-2999(93)90843-7. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV, Paterson IA, Boulton AA. Regulation of aromatic L-amino acid decarboxylase in rat striatal synaptosomes: effects of dopamine receptor agonists and antagonists. Br J Pharmacol. 1994;112:23–30. doi: 10.1111/j.1476-5381.1994.tb13023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Grandy DK, Thambi L, Kushner JA, Van Tol HH, Cone R, Pribnow D, Salon J, Bunzow JR, Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990;347:76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]


