Abstract
Tobacco use is predicted to result in over 1 billion deaths worldwide by the end of the 21st century. How genetic variation contributes to the observed differential predisposition in the human population to drug dependence is unknown. The zebrafish (Danio rerio) is an emerging vertebrate model system for understanding the genetics of behavior. We developed a nicotine behavioral assay in zebrafish and applied it in a forward genetic screen using gene-breaking transposon mutagenesis. We used this method to molecularly characterize bdav/cct8 and hbog/gabbr1.2 as mutations with altered nicotine response. Each have a single human ortholog, identifying two points for potential scientific, diagnostic, and drug development for nicotine biology and cessation therapeutics. We show this insertional method generates mutant alleles that are reversible through Cre-mediated recombination, representing a conditional mutation system for the zebrafish. The combination of this reporter-tagged insertional mutagen approach and zebrafish provides a powerful platform for a rich array of questions amenable to genetic-based scientific inquiry, including the basis of behavior, epigenetics, plasticity, stress, memory, and learning.
Keywords: behavior, addiction
Tobacco use is a worldwide epidemic. By 2030, approximately 8 million annual deaths attributable to tobacco use will occur globally, and tobacco use is predicted to result in over one billion cumulative deaths by the end of the 21st century (1). Insights into how nicotine from tobacco causes profound physiological and neurological effects include a large number of diverse genetic variations associated with subsets of the human population with differential predisposition to addiction (2). Notably, parallel human genetic studies recently identified sequence variations near a nicotinic receptor locus associated with predisposition to lung cancer (3–5), suggesting that changes in nicotine response biology can play an important role in the major deleterious clinical outcomes of nicotine dependency. However, the molecular assignment of the genes and the pathways they change that contribute to differential human responses to nicotine dependency are largely unknown (6).
Much of our current understanding of behavior has come from extensive pharmacological model organism work, for example identifying the central dopaminergic neural signaling network in addiction (7–9). Reverse genetic studies in the mouse have complemented extensive environmental impact work that has historically used mammalian model systems (10, 11). Pioneering papers demonstrating that zebrafish (Danio rerio) readily respond to addictive drugs (12–18) has set the stage for forward genetic approaches using this vertebrate model system. We report here an insertional mutagenesis method suitable for diverse forward genetic screening applications including the biology of behavior, epigenetics, environment/gene interactions, as well as traditional developmental genetic research. The conditional nature of this mutagenesis tool is particularly noteworthy due to its potential to greatly reduce the impact of confounding variables on the genetics of behavioral processes.
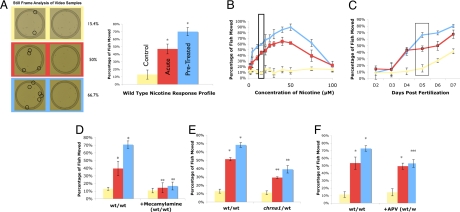
We developed a simple zebrafish nicotine behavioral response assay suitable for use in large-scale forward genetic screening work (Fig. 1). We assessed locomotor activity by analogy to prior work with rodent models (19–21). Locomotor activity (Fig. 1A and Movies S1–S4) was measured using a digital imaging station in a time window where, 30 s following a stimulus of water, approximately 15% of a testing population moved (Fig. 1A, yellow framed images; yellow lines and bars in all graphs in Fig. 1; and Movie S1). Locomotor activity was assessed at different nicotine doses; movement as a function of added nicotine is shown in red bars and graphs in Fig. 1. Note the characteristic “inverted-U” shape of the dose-response curve (22), where higher doses of nicotine result in reduced, rather than increased, movement (Fig. 1B). The attenuation in locomotor effects at high doses is known to occur in rodents and depends on the particular complement of nicotinic acetylcholine receptor types activated by these doses (22). Based on this dose–response curve, we selected 10 μM nicotine for subsequent experiments (box, Fig. 1B). Next, we tested multiple developmental time-points, demonstrating a functional nicotine response in 4-day-old, but not 3-day-old, zebrafish (Fig. 1C).
Fig. 1.
Nicotine response profiling of zebrafish larvae. Locomotion of zebrafish larvae was assessed using sibling animals. Yellow datasets (Control) indicate basal locomotion rates (no stimulation). Red datasets (Acute) demonstrate locomotion rates during a single exposure to nicotine. Blue datasets (Pretreated) indicate locomotion rates during nicotine exposure in fish that had been pretreated with a single, brief exposure to nicotine 8 h prior (see Experimental Procedures). All data points represent at least three independent measurements on independent biological samples with at least 10 animals measured for each replicate. (A) Representative data for standard nicotine response profile conditions. Video imaging of a single behavioral analysis is shown at left. Movement rates were determined as a function of differential location (circled fish) during a standard time window of 0.25 s (middle). A standard wild-type response profile of 5-day-old zebrafish is shown (right); this profile examines normal movement rates (Control), movement as a function of a single nicotine treatment (Acute), and shows the increased locomotor activity over an acute response after receiving multiple doses of nicotine (Pretreated). (B) Dose–response of 5-day-old zebrafish to nicotine. Zebrafish show an increase in movement for doses from 2.5–50 μM doses of nicotine. The greatest difference in movement between the acute and pretreated fish occurs in doses between 10 and 25 μM; the main dose used for the subsequent work (10 μM) is highlighted by the black box. Note the inverted-U-shape of the curve, with animals showing reduced movement enhancement at doses above 50 μM nicotine. (C) Zebrafish respond to nicotine at 4 days of age and not before. Zebrafish can be sensitized to nicotine at 5 days of age and not before, highlighted by the black box. (D) Mecamylamine, a central nervous system specific nicotinic competitive inhibitor, quenches the overall nicotine response when added before the testing dose of nicotine without affecting the basal locomotion rate in the absence of nicotine. (E) chrna1 heterozygous fish exhibit a reduced nicotine response when compared to their wild-type siblings. (F) APV, an NMDAR competitive inhibitor, quenches the development of the sensitization response when added concurrently with initial nicotine dose. *, P < 0.05 when comparing to control or acute. **, P < 0.05 when comparing to corresponding treatment group. ***, P < 0.05 when comparing to control, but not acute. Also P < 0.05 when comparing to the corresponding treatment group.
One additional characteristic of the behavioral response to nicotine is sensitization, an increase in response upon prior exposure to nicotine; the sensitization response is shown in blue lines and bars in Fig. 1. As demonstrated in Fig. 1A, fish that had been previously exposed to nicotine became sensitized, yielding a marked increase in activity. This level of sensitization is comparable to that noted in rodents (23–25). Five- and six-day-old, but not 4-day-old, zebrafish were capable of being sensitized (Fig. 1C), demonstrating the development of competency for sensitization was delayed compared to the ability to respond to acute nicotine exposure.
We assessed whether this locomotor response is specific for and subject to perturbation of nicotine receptor signaling in zebrafish (Fig. S1). First, we examined the effect of mecamylamine, a nicotinic receptor antagonist (26). Mecamylamine administered to the embryos 4 h before nicotine treatment blocked locomotor activation but did not change the basal level of movement of the fish (Fig. 1D). Second, we took advantage of the preexisting alpha polypeptide 1 nicotinic cholinergic receptor mutation [chrna1; (27)]. Compared to their wild-type siblings, chrna1 heterozygous animals demonstrate reduced total (both acute and sensitized) locomotor activation due to an initial exposure and to repeated exposures (Fig. 1E). This altered response could be due to fewer total nicotinic receptors present in heterozygote animals. We cannot exclude the possible contribution of a neural circuitry change from reduced chrna1 in these heterozygote animals. The zebrafish thus represents a vertebrate system for genetic testing of nicotine response.
An emerging theme in the study of the neural substrates for initiation of behavioral sensitization is the role for glutamate receptor subtypes, in particular the NMDA-type receptor (NMDAR). NMDAR inhibition blocks the development of sensitization in rodents (28, 29). The coadministration of nicotine with the competitive NMDAR antagonist APV prevented the development of nicotine sensitization (Fig. 1F).
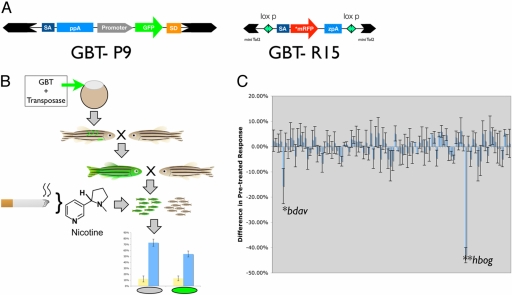
We took advantage of the quantitative nature of the nicotine behavioral response and its ability to detect dominant phenotypes (Fig. 1E) in a forward genetic screen for loci that impact the nicotine response (Fig. 2B). We used variants of the gene-breaking transposon [GBT; (30); Fig. 2A] named P9 and R15 to induce genetic variation in zebrafish families and tested for alterations in the nicotine response profile of mutant carriers compared to their siblings. In this gene-trapping related approach (31), GBT insertions induce truncations in the mutated proteins encoded by the tagged loci by introducing a terminal exon to the mutated gene (30). Genotyping of mutant versus normal siblings is facilitated by the expression of a fluorescent protein cassette in GBT vectors.
Fig. 2.
Zebrafish behavioral screen for nicotine response mutants. (A) Diagram of gene-breaking transposons (GBTs) deployed in this study. GBT-P9 [this Tol2-based vector is adapted from the GBT-P6 vector from (28)] harbors a transcriptional terminator cassette in cis with a strong ubiquitous promoter driving the green fluorescent protein (GFP) reporter upstream of a splice-donor sequence. Intronic insertions in the sense orientation activate the GFP reporter by providing a functional poly(A) signal from the nearby genetic locus. GBT-R15 contains a modified transcriptional terminator cassette that harbors an AUG-free monomeric red fluorescent protein (mRFP) reporter cassette. mRFP expression is only detected after an in-frame integration event. GBT-R15 also deploys the miniTol2 gene vector backbone and is flanked by loxP sites for downstream genetic causality analyses. (B) Behavioral genetic screening paradigm using GBTs as insertional mutagens. Standard transposase-mediated transgenesis protocols (20) generate a pool of mosaic F0 founder animals. These are outcrossed to generate stable transgenic F1 animals that are then outcrossed to generate mixed clutches of wild-type and mutant siblings. Mutant animals are segregated from their genetically wild-type siblings using fluorescent tags in these GBTs (GFP fluorescence is diagrammed as noted in the GBT-P9 screen; for the GBT-R15 screen, red fluorescence was used to sort). Nicotine response profile is obtained on each subpopulation (fluorescently tagged vs. wild-type). Nicotine was administered at the standard dosage of 10 μM. (C) Differential nicotine response profile between wild-type and fluorescently tagged siblings are diagrammed. Response bars are derived by subtracting the pretreated response of fluorescently tagged GBT-mutants from the pretreated response of their wild-type siblings. This analysis identified two nicotine response mutants (*, P ≪ 0.05), betty davis (bdav) and (**, P ≪ 0.05) humphrey bogart (hbog), out of a total of 102 lines screened: 89 GBT-P9 representing an estimated 178 expressed loci and 23 GBT-R15 representing an estimated 35 expressed loci.
For each expression-tagged candidate mutant locus, we initially examined two main aspects of their behavioral profile. First, we eliminated any loci with changes in the basal movement rate to avoid mutations that overtly altered the mechanics of swimming (yellow bars, Fig. 2B). We next examined the differential nicotine response profile (blue bars, Fig. 2C) of a series of GBT-P9-mutated zebrafish, identifying one locus out of an estimated 178 screened GBT-P9-induced loci with inherited reduction in the nicotine response. A second locus was similarly identified (Fig. 2C) using GBT-R15, a vector that allowed the preselection of mutant loci based on endogenous gene expression (an estimated 35 prioritized GBT-R15 loci were screened). GBT-R15 also includes flanking loxP sites for Cre-mediated excision of the mutagenic cassette, permitting reversal of the phenotype for proof of causality (see Fig. 4). We named these mutant zebrafish with reduced nicotine response for celebrities that suffered from tobacco-related cancers bette davis (bdav) and humphrey bogart (hbog).
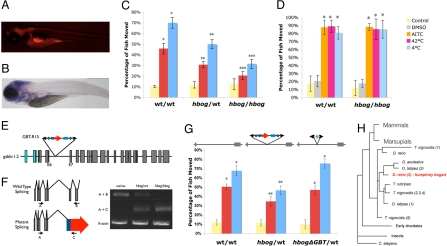
Fig. 4.
humphrey bogart (hbog) Nicotine response mutation is encoded by the zebrafish Gaba-B receptor 1.2 (gabbr1.2) locus. (A) Sagittal fluorescent imaging (anterior to left) shows a general neural expression of mRFP-gabbr1.2 fusion protein at 5 days pf. (B) Sagittal imaging (anterior to left) of a whole mount in situ hybridization for gabbr1.2 at 5 days pf (bottom) shows high levels of neural expression. (C) Full nicotine response profile of the hbog mutant comparing homozygous and heterozygous mutant animals to wild-type siblings at the standard nicotine dosage of 10 μM. Reduction from wild-type response is seen in both acute and sensitized groups. Reduction of homozygote phenotype is greater than heterozygote phenotype (P = 0.05). (D) hbog heterozygous fish show no difference in response to noxious chemicals (allyl isothiocyanate) or temperatures (4 and 42 °C) when compared to wild-type siblings. (E) Schematic representation of the GBT-R15 GBT insertion in intron 6 of the zebrafish gabbr1.2 gene. Location of exons 4 and 9 of the gabbr1.2 gene and primers A, B, and C used for RT-PCR analysis are indicated. (F) RT-PCR analysis of gabbr1.2 transcript in wild-type, heterozygous, and homozygous hbog fish. Primers in exons 4 and 9 were used to detect wild-type transcript of the gabbr1.2 gene. RT-PCR using primers in β-actin were performed as an internal control. (G) Germline propagation of Cre-mediated reversion of GBT-induced hbog mutant shows a nicotine response profile indistinguishable from wild-type sibling animals. (H) Simplified homology of the gabbr1/hbog locus shown in red. Humans and other mammals encode a single gabbr1 locus. Derived from Ensembl homology engine. *, P < 0.05 when comparing to control or acute. **, P < 0.05 when comparing to corresponding wild-type group. ***, P < 0.05 when comparing to corresponding heterozygote group and wild-type group.
Bette Davis.
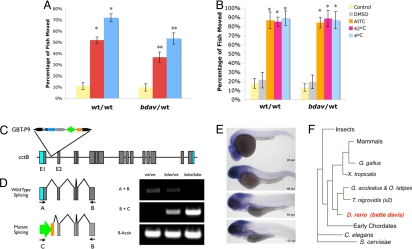
In the GBT-P9 screen, the initial pool of GBT-mutagenized fish contained multiple expressed GBT chromosomes; upon outcrossing and subsequent selection, we established a zebrafish line with only a single expressed GBT whose offspring exhibited a markedly reduced nicotine response profile linked to GFP expression from the GBT (bdav) (Fig. 3A). Interestingly, we note that the reduction in the total (acute and sensitized) nicotine response of bdav heterozygous fish is comparable to that noted in the cholinergic receptor mutant, chrna1 (compare to Fig. 1E). In contrast to the reproducible differences in the nicotine response, bdav mutant fish react normally to other tested behavioral stimulatory processes including locomotor activation, due to external physical stimulation, to the noxious chemical AITC (mustard oil) or to extreme temperatures (Fig. 3B). This noted altered nicotine, but normal stimulatory response profile, in bdav animals does not exclude yet-to-be determined effects from other physical or pharmacological stimuli (see more complete description in Discussion below). The onset of the reduced nicotine response in bdav heterozygous fish is also not delayed compared to their wild-type siblings (Fig. S2A).
Fig. 3.
bette davis (bdav) Nicotine response mutation is linked to a GBT insertion in zebrafish chaperone containing protein 8 (cct8). (A) The founder F1 animal (Fig. 2C) harbored three independently expressed GBTs. Upon out-crossing and further analysis, animals with altered nicotine response profile (A) harboring only a single GFP locus were isolated and propagated for six generations formally isolating the bdav nicotine response locus. Nicotine was administered at the standard dose of 10 μM. (B) bdav heterozygous fish show no difference in response to allyl isothiocyanate or extreme temperatures when compared to their wild-type siblings. (C) The single expressed GFP locus is encoded by a GBT integration in predicted intron two of zebrafish chaperone containing protein 8 (cct8). (D) RT-PCR analysis of cct8 transcript in wild-type, heterozygous, and homozygous bdav embryos. Primers in exons 1 and 5 were used to detect wild-type transcript of the cct8 gene. Primers in exon 5 and the GFP portion of the transposon were used to determine expression of transposon mRNA. RT-PCR using primers in β-actin were performed as an internal control. (E) Sagittal imaging of a whole mount in situ hybridization for cct8 at 1 day post fertilization (pf) (top) through 5 days pf (bottom) shows high levels of neural expression. (F) Phylogenetic analysis (Ensembl) indicates cct8 is an evolutionarily ancient gene and is encoded by a single ortholog in humans and other mammals. *, P < 0.05 when comparing to control or acute. **, P < 0.05 when comparing to corresponding treatment group.
The transposon-based molecular tag was used to follow genetic linkage over six generations to date, demonstrating dominant Mendelian inheritance. Molecular analysis identified a single, sense strand GBT-P9 insertion in bdav fish in the zebrafish Chaperonin Containing Protein 8 (cct8; Fig. 3C). RT-PCR analysis demonstrates this GBT insertion results in reduced wild-type cct8 transcript levels in bdav heterozygous animals and a greatly attenuated level of wild-type cct8 RNA in bdav homozygous animals (Fig. 3D). cct8 RNA expression is found in specific neural tissue during embryonic and larval stages of development (Fig. 3E) similar to the pattern previously reported (32). Phylogenetic analysis demonstrates that cct8 is an evolutionarily ancient gene with only a single human ortholog (Fig. 3F).
Humphrey Bogart.
We added three technical advances for the GBT-R15 screen. First, we used the miniTol2 transposon vector (33) to facilitate the addition of complex genetic elements in subsequent GBT vectors. We then modified the critical transcriptional termination cassette with a red fluorescent protein (mRFP) reporter that lacks an initiation codon requiring fusion to the nascent peptide to yield information regarding temporal and spatial regulation of the trapped locus. Third, the core mutagenesis module was flanked by loxP sites for reversion via Cre-mediated recombination as formal proof of causality.
GBT-R15 mutagenized fish were screened for mRFP expression before subsequent nicotine response profiling with the goal of enriching our screening efforts for genes more likely to be involved in neural responses. Thus, unlike the largely unbiased GBT-P9-based screening approach, the GBT-R15 screen allows for a preselection screening step. hbog fish display neurally restricted mRFP expression (Fig. 4A). hbog heterozygous fish exhibit a strong reduction in the nicotine response profile (Fig. 4C). Homozygous fish are adult viable, and in contrast to wild-type fish, homozygous larvae display a 65% reduction in the pretreated total nicotine response as determined by comparing pretreated movement to control movement rates (Fig. 4C). Homozygous hbog mutant fish, however, react normally to other tested behavioral stimulatory processes including locomotor activation due to external physical stimulation, to the noxious chemical AITC (mustard oil) or to extreme temperatures (Fig. 4D). This noted altered nicotine but normal stimulatory response profile in hbog animals excludes yet-to-be determined effects from other physical or pharmacological stimuli (see more complete description in Discussion below). The onset of the reduced nicotine response in hbog heterozygous fish is also not delayed compared to their wild-type siblings (Fig. S2B).
Molecular analysis shows that hbog is due to a single GBT-R15 insertion in the sense GBT orientation of the sixth intron of the zebrafish gabbr1.2 locus (Fig. 4E) that encodes one zebrafish ortholog of the GABA(B) receptor seven-pass transmembrane subunit 1. RT-PCR analysis demonstrates this GBT insertion results in reduced wild-type transcript levels in heterozygous animals and a greatly attenuated level of wild-type RNA in homozygous animals (Fig. 4F). This insertion generates a severely truncated chimeric mRNA (Fig. 4F) that encodes for an mRFP protein fused to the first 135 amino acids of the gabbr1.2-encoded protein, deleting 830 amino acids in this G protein coupled receptor. GABA(B) receptors are evolutionarily ancient in origin, with a single human ortholog of gabbr1 (Fig. 4H).
The use of GBT-R15 facilitated two analyses in hbog not possible for the GBT-P9−based bdav allele. First, we deployed Cre recombinase to delete the core mutagenicity cassette at the hbog locus and tested the nicotine response profile of this reverted chromosome (Fig. 4G). Cre-mediated reversion could be obtained using either somatic delivery of Cre or after germline propagation followed by molecular confirmation of the excision process (germline reversion is diagrammed in Fig. 4G). The Cre-reverted chromosome results in animals with a nicotine response profile indistinguishable from wild-type, providing strong evidence of causality for this specific GBT insertion in the hbog behavioral mutation. Second, we used confocal imaging analysis to determine the specific location of the gabbr1.2/mRFP fusion protein expression and compared this with RNA distribution using whole-mount in situ hybridization (WISH) (Fig. S3). Note the strong expression of gabbr1.2 RNA in specific neural tissue; the gabbr1.2/mRFP analysis indicates enhanced expression in neuronal subsets that appear largely as a subset of the RNA-expressing cells (Fig. S3 and Movie S4). The GBT-R15 vector system thus provides options over our prior gene-breaking approach (30) by the generation of reversible genetic alleles that can also display information on expression of the trapped loci.
Discussion
The identification of a subunit of the main cytoplasmic chaperone complex with tissue-specific expression that functions in the nicotine response is intriguing, as individual subunits have been proposed to include a potential regulatory function through differential binding and folding specificity (34). In addition, altered protein kinetics has been proposed as one method of altering nicotinic receptor function as observed in recent nicotinic response work in cultured neurons (35). Evidence for a conserved role of mammalian cct8 in the nicotine response (36) includes strong expression in mouse neural tissue (37). In addition, rat cct8 mRNA is upregulated in the prefrontal cortex after exposure to nicotine (38). Determining the precise mechanism and potential regulation of cct8 in the nicotine behavioral response warrants further investigation.
GABA(B) receptors are assembled from multiple subunits (39), indicating that changes in any subunit may result in GABA(B) signaling changes. Genetic variations in the GABA(B) receptor subunit 2 are associated with nicotine dependence (40), providing evidence for a conserved role for GABA(B) signaling in the human nicotine response. GABA(B)-targeted interventions may be of therapeutic value as the addition of a GABA(B) agonist or positive modulator reduced self-administration of nicotine in rats (41, 42), although clinical testing alone or in conjunction with nicotine replacement strategies has not been conducted to date.
This study demonstrates that the zebrafish is an excellent vertebrate model for the discovery of genes in conserved behavioral paradigms. In particular, the large family size allows the detection of single gene contributions to multiloci behavioral response. The forward genetic screen represented in this study functionally demonstrates that behavioral diversity can be generated through genes that modify or alter the behavioral response. Similar genetic controls contribute to the diversity in pigmentation. For example, much of the natural variation in pigmentation lies in genetic loci such as SLC24A5 (43) that more subtly alter melanocyte function rather than the dramatic changes that occur with mutations in the core tyrosinase gene - albinism. Whether the mutations discovered using this nicotine screen are part of a shared behavioral pathway or pathways or if their functional modifications are specific to the nicotine response alone is yet to be determined and needs to be further examined. The normal response to temperature, touch, and mustard oil demonstrates that the mutants can respond normally to very strong stimuli, but we point out that more subtle defects would not be found with these assays. Moreover, additional drugs need to be tested in the future to conclusively test the nicotine specificity of the mutants. Either result will be instructive as we develop a more complete understanding of the interactions between neural signaling pathways and specific behavior.
The inclusion of loxP sites in the GBT-R15 and later GBT vectors has the potential for neural structure function mapping using tissue-specific Cre for somatic gene reversion. For example, a determination about which of the many GABA(B) receptor-expressing neurons are critical for the observed physiological response to nicotine can be made. The current limitation on this approach is the broad, genome-wide deployment of this GBT tool for the zebrafish field. The combination of this reporter-tagged insertional mutagen approach and zebrafish provides a powerful platform for a rich array of questions amenable to genetic-based scientific inquiry, including the basis of behavior, epigenetics, plasticity, stress, memory, and learning.
Experimental Procedures
Locomotion Observation.
Movement was assessed by digital snapshot capture of the fish using a “multiplex” mode of either a Nikon Coolpix 990 or Nikon Coolpix SR10 digital camera 30 s after the testing dose of nicotine. This 16-shot mode generates photographs over a period of 6 s with each photograph occurring every 0.4 s. This time window was selected to maximize the nicotine response signal compared to the basal movement while also providing a reproducible assessment of the basal movement capability of the tested animals.
Movement changes were determined by manually overlaying the initial photograph to the fourth photograph (a period of 1.6 s) in each series using Adobe Photoshop. Other frames in the sequence were used to address any ambiguity in the initial movement assessment. The percentage of fish that visibly changed position was recorded for each treatment group.
Statistical Analysis.
The behavioral data were analyzed using Microsoft Excel 2004 for Macintosh with Statistical Package (Microsoft). Percentage of fish moved was ascertained by determining the number of fish moved and dividing by the total number of fish for each treatment group. The mean of the percentages was then determined along with the standard error. All error bars in figures represent this derived standard error. Statistical significance of movement differences was assessed using two-tailed t-tests with control fish being compared to either the acute treatment group or the pretreated group. To determine statistical significance between mutant groups (Fig. 2C), we determined the mean of the mean between the pretreated mutant and wild-type siblings. Statistical significance of the difference of means was assessed using a two-tailed z-test comparing the overall mean to the individual mean tested. Statistical significance of the addition of a drug to the water of the fish was assessed using analysis of variance (ANOVA) with treatment as a grouping factor followed by a Student Newman-Keuls post hoc test using KaleidaGraph 4.1 for Macintosh (Synergy Software). A P < 0.05 was considered statistically significant.
Nicotine Administration.
A dose range up to 100 μM of nicotine was used on day 5 zebrafish for assessing the main nicotine response profile (Fig. 1B). The 10 μM dose was selected as the “standard treatment” used in the genetic screen due to the strong behavioral signal detected in both the acute and pretreated animals. Fish in a particular “Pretreatment” group were given a defined dose nicotine solution with an incubation time of 1 min. Fish were then transferred into fresh embryo water. After an incubation of 8 h, fish in both the “Pretreatment” and “Acute” groups were administered the same defined nicotine dose combined with 500 μL of embryo water. Fish in the “Control” group were given a 500 μL dose of embryo water to control for the physical action of administering nicotine. All fish were allowed a 30 s incubation period before behavioral assessment via digital imaging, described above.
GBT Transposons.
GBT-P9 was generated by subcloning the SB-P6 GBT (30) into a nearly full-length Tol2 vector (44).
GBT-R15 was generated by the addition of inverted I-Sce and direct loxP sites to miniTol2 (33), followed by insertion of the carp beta actin splice acceptor [as described in (28)]–AUG-free mRFP [derived from (45)]–zebrafish beta globin poly(A) cassette.
NMDAR Inhibitor Administration.
To test the effect of APV, an NMDAR competitive inhibitor, on the nicotine response (28, 29), fish in “Pretreatment” and “Acute” groups were each placed in a solution of 10 μM APV immediately before receiving either a nicotine pretreatment (“Pretreatment” group) or embryo water (“Acute” group) on day 5 at 9:00 AM. After a 1-min incubation, the water was replaced with fresh embryo water. “Pretreatment” and “Acute” groups were administered nicotine after an 8 h incubation as described above.
Mecamylamine Administration.
To test the affect of mecamylamine, a nicotinic receptor competitive inhibitor on the nicotine response (26), fish in the “Pretreatment” and “Acute” groups were each placed in a solution of 10 μM mecamylamine (Sigma-Aldrich) 1 h before receiving a testing dose of nicotine on day 5 at 5:00 PM. After the incubation, the water was replaced with fresh embryo water. The groups were administered nicotine as described above.
GBT Mutant Screening.
Mutant zebrafish were generated by the coinjection of plasmid DNA encoding each GBT plus Tol2-encoding synthetic mRNA as described (20). Adult fluorescent protein-expressing F1 transposon heterozygous fish were out-crossed to wild-type fish to generate a heterogeneous population of F2 larvae. The embryos were then separated into fluorescent-positive and fluorescent-negative groups and placed into similarly sized fish groupings for nicotine response assessment, as described above (Fig. 2C). The response of the pretreated GBT-positive larvae was subtracted from the pretreated GBT-negative response to determine the response differential in these lines. To ensure that GBT-mutations did not alter movement response, the basal movement rate in the control response was also monitored. Using expression linkage analysis to estimate copy number (46), we conservatively estimate at least 213 GBT-expressed loci were screened using this approach, 178 GBT-P9 and 35 GBT-R15. Candidate loci were subsequently out-crossed at least four times, recovering the noted linked nicotine response profile in each subsequent generation. The resulting alleles have been named bdavmn30 and hbogmn31, selected after celebrities who died of tobacco-related causes and to enhance communication that genetic background can and does alter the response to addictive drugs.
Genetic Analysis of bdav.
The number of single chromosome, GFP-linked fish from the initial bdav founder line that did not produce offspring with a deficiency in nicotine response was 0 out of 107 total fish, indicating tight genetic linkage. The standard linkage assessment, such as the traditional logarithm of odds (LOD) formula as described by Morton (49), is difficult to assess in this screening paradigm (and would yield a nearly infinite LOD score). Consequently, we believe the likelihood that the observed nicotine response mutation is not linked to the GFP-tagged locus is very low.
Cre Reversion.
Cre coding sequence was PCR amplified using primers BglII/Cre-F1 (5′-ACGAGATCTACAAGATGTCCAATTTAC-3′) and Spe/Cre-R1 (5′-AGCACTAGTTTAATCGCCATCTTCCA-3′) and peGFP-Cre (Halpern laboratory) as a template. PCR fragment was digested with BglII and SpeI and cloned into BglII-SpeI digested pT3TS (50). Synthetic Cre-encoding mRNA was synthesized from XbaI-linearized plasmid pT3TS-Cre and 50pg injected into homozygous hbogmn31 embryos and raised to adulthood. These animals were out-crossed, and the resulting offspring were molecularly genotyped for Cre-mediated reversion at the hbog locus using primers hbogInsertF1 (5′-TGGTCCATTCTCATTGAGCAGCG -3′) and hbogInsertR1 (5′- GCAGAAAGCGGTTCCAGGTTTGA -3′) resulting in a shifted gene product. The nicotine response profile was subsequently assessed on this allele (hbogmn32ΔGBT).
Additional Experimental Procedures.
For additional experimental procedures of zebrafish collection and preservation, response to noxious chemical, response to noxious temperature, chrna1b107 heterozygote zebrafish, Southern blot analysis for linked loci, 5′ and 3′ rapid amplification of cDNA ends (RACE), RT-PCR, in situ hybridization, isolation of full coding region for hbog/gabbr1.2, and phylogenetic analyses, see SI Experimental Procedures.
Supplementary Material
Acknowledgments.
We thank Drs. Jon Ebbert and Richard Hurt for their encouragement and insight into the tremendous impact tobacco-related issues have on our society and Dr. Keith Cheng for detailed discussion on the broad implications and challenges of genetic modifier screens. All enclosed zebrafish names have been approved by the Zebrafish Nomenclature Committee. This research was funded by National Institutes of Health Grants DA 14546 and GM 63904 (to S.C.E.), the Transdisciplinary Tobacco Use Research Center (M.J.T.), the University of Minnesota, and the Mayo Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908247106/DCSupplemental.
References
- 1.World Health Organization. Geneva, Switzerland: WHO; 2008. WHO report on the global tobacco epidemic, 2008: The MPOWER package. Available at http://www.who.int/tobacco/mpower/en. [Google Scholar]
- 2.Li MD. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Hum Genet. 2008;123:119–131. doi: 10.1007/s00439-008-0473-0. [DOI] [PubMed] [Google Scholar]
- 3.Spitz MR, Amos CI, Dong Q, Lin J, Wu X. The CHRNA5–A3 region on chromosome 15q24–25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst. 2008;100:1552–1556. doi: 10.1093/jnci/djn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss RB, et al. A candidate gene approach identifies the CHRNA5–A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorgeirsson TE, et al. A variant associated with nicotine dependence, lung cancer, and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caporaso N, et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS ONE. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–460. [PubMed] [Google Scholar]
- 8.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 9.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ. Genes and addiction. Nat Genet. 2000;26:277–281. doi: 10.1038/81570. [DOI] [PubMed] [Google Scholar]
- 11.Crabbe JC. Genetic contributions to addiction. Annu Rev Psychol. 2002;53:435–462. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- 12.Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci USA. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 14.Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacol Biochem Behav. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Ninkovic J, et al. Genetic identification of AChE as a positive modulator of addiction to the psychostimulant D-amphetamine in zebrafish. J Neurobiol. 2006;66:463–475. doi: 10.1002/neu.20231. [DOI] [PubMed] [Google Scholar]
- 16.Ninkovic J, Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39:262–274. doi: 10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Bretaud S, et al. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 18.Kily LJ, et al. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J Exp Biol. 2008;211:1623–1634. doi: 10.1242/jeb.014399. [DOI] [PubMed] [Google Scholar]
- 19.DiFranza JR, Wellman RJ. Sensitization to nicotine: How the animal literature might inform future human research. Nicotine Tob Res. 2007;9:9–20. doi: 10.1080/14622200601078277. [DOI] [PubMed] [Google Scholar]
- 20.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 22.Picciotto MR. Nicotine as a modulator of behavior: Beyond the inverted U. Trends Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- 23.Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ksir C, Hakan R, Hall DPJ, Kellar KJ. Exposure to nicotine enhances the behavioral stimulant effect of nicotine and increases binding of [3H]acetylcholine to nicotinic receptors. Neuropharmacology. 1985;24:527–531. doi: 10.1016/0028-3908(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 25.Miller DK, Wilkins LH, Bardo MT, Crooks PA, Dwoskin LP. Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacology (Berl) 2001;156:469–476. doi: 10.1007/s002130100747. [DOI] [PubMed] [Google Scholar]
- 26.Rose JE, et al. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- 27.Sepich DS, Wegner J, O'Shea S, Westerfield M. An altered intron inhibits synthesis of the acetylcholine receptor alpha-subunit in the paralyzed zebrafish mutant nic1. Genetics. 1998;148:361–372. doi: 10.1093/genetics/148.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelsey JE, Beer T, Lee E, Wagner A. Low doses of dizocilpine block the development and subsequent expression of locomotor sensitization to nicotine in rats. Psychopharmacology (Berl) 2002;161:370–378. doi: 10.1007/s00213-002-1015-4. [DOI] [PubMed] [Google Scholar]
- 29.Shoaib M, Stolerman IP. MK801 attenuates behavioural adaptation to chronic nicotine administration in rats. Br J Pharmacol. 1992;105:514–515. doi: 10.1111/j.1476-5381.1992.tb09010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivasubbu S, et al. Gene-breaking transposon mutagenesis reveals an essential role for histone H2afza in zebrafish larval development. Mech Dev. 2006;123:513–529. doi: 10.1016/j.mod.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Sivasubbu S, Balciunas D, Amsterdam A, Ekker SC. Insertional mutagenesis strategies in zebrafish. Genome Bio. 2007;(Suppl):1–S9. doi: 10.1186/gb-2007-8-s1-s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thisse B, et al. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Bio. 2004;77:505–519. doi: 10.1016/s0091-679x(04)77027-2. [DOI] [PubMed] [Google Scholar]
- 33.Balciunas D, et al. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2006;2:e169. doi: 10.1371/journal.pgen.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund PA. In: Frontiers in Molecular Biology: Molecular chaperones in the cell. Hames BD, Glover DM, editors. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 35.Nashmi R, Lester H. Cell autonomy, receptor autonomy, and thermodynamics in nicotine receptor up-regulation. Biochem Pharmacol. 2007;74:1145–1154. doi: 10.1016/j.bcp.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane JK, Konu O, Ma JZ, Li MD. Nicotine coregulates multiple pathways involved in protein modification/degradation in rat brain. Brain Res Mol Brain Res. 2004;132:181–191. doi: 10.1016/j.molbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Allen Brain Atlas. Seattle, Washington: Allen Institute for Brain Science; 2008. Available from http://www.brain-map.org. [Google Scholar]
- 38.Li MD, et al. A genome-wide scan to identify loci for smoking rate in the Framingham Heart Study population. BMC Genet. 2003;4(Suppl 1):S103. doi: 10.1186/1471-2156-4-S1-S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emson PC. GABA(B) receptors: Structure and function. Prog Brain Res. 2006;2007:43–57. doi: 10.1016/S0079-6123(06)60004-6. [DOI] [PubMed] [Google Scholar]
- 40.Beuten J, et al. Single- and multilocus allelic variants within the GABA(B) receptor subunit 2 (GABAB2) gene are significantly associated with nicotine dependence. Am J Hum Genet. 2005;76:859–864. doi: 10.1086/429839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paterson NE, Froestl W, Markou A. The GABAB receptro agonists baclofen and CGP44532 decreased nicotine self-administration in rats. Psychopharmacology (Berl) 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- 42.Paterson NE, et al. Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther. 2008;326:306–314. doi: 10.1124/jpet.108.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 44.Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify deveopmentally regulated zebrafish genes in vivo. Dev Dyn. 2004;231:449–459. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- 45.Campbell RE, et al. A monomeric red flourescent protein. Proc Natl Acad Sci USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson AE, et al. Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev Biol. 2003;263:191–202. doi: 10.1016/j.ydbio.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Prober DA, et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28:10102–10110. doi: 10.1523/JNEUROSCI.2740-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark KJ, Geurts AM, Bell JB, Hackett PB. Transposon vectors for gene-trap insertional mutagenesis in vertebrates. Genesis. 2004;39:225–233. doi: 10.1002/gene.20049. [DOI] [PubMed] [Google Scholar]
- 49.Morton NE. Sequential tests for the detection of linkage. Am J Hum Genet. 1955;7:277–318. [PMC free article] [PubMed] [Google Scholar]
- 50.Hyatt TM, Ekker SC. Vectors and techniques for ectopic gene expression in zebrafish. Methods Cell Bio. 1999;59:117–126. doi: 10.1016/s0091-679x(08)61823-3. [DOI] [PubMed] [Google Scholar]
- 51.Jowett T. Analysis of protein and gene expression. Methods Cell Bio. 1999;59:63–85. doi: 10.1016/s0091-679x(08)61821-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.