Abstract
The photoluminescence in carbon dots (surface-passivated small carbon nanoparticles) could be quenched efficiently by electron acceptor or donor molecules in solution, namely that photo-excited carbon dots are both excellent electron donors and excellent electron acceptors, thus offering new opportunities for their potential uses in light energy conversion and related applications.
Quantum-sized semiconductor nanoparticles (quantum dots) have emerged as an important class of photoactive nano-materials for a variety of purposes and applications.1–4 For the utilization of semiconductor quantum dots in light energy conversion and related areas, there have been extensive investigations on their photoresponse and photoinduced charge separation and electron transfer processes.5–8 Alternative to the traditional semiconductors, other quantum-sized nanoparticles have been explored and developed for similar photophysical and photochemical properties. Of particular interest and significance is the recent finding that small carbon nanoparticles could be surface-passivated by organic molecules or polymers to become highly photoactive, exhibiting strong photoluminescence in the visible and near-infrared spectral regions.9–15 These photoluminescent carbon nano-particles, dubbed “carbon dots” (Scheme 1), were found to be physico-chemically and photochemically stable and non-blinking in their luminescent emissions.9 Here we report that the photoluminescence from carbon dots could be quenched highly efficiently by either electron acceptor or electron donor molecules in solution, namely that the photo-excited carbon dots are excellent as both electron donors and electron acceptors. These interesting photoinduced electron transfer properties may offer new opportunities in potentially using carbon dots for light energy conversion and related applications, in addition to their being valuable to the effort on mechanistic elucidation.
Scheme 1.
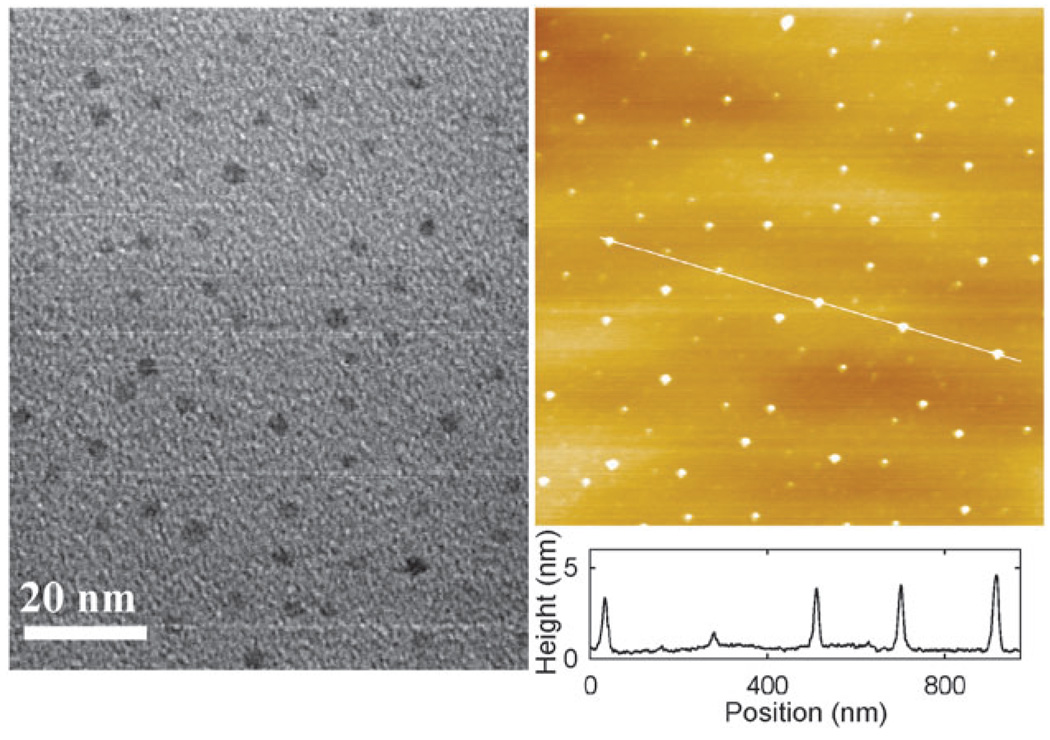
The carbon dots in this study were prepared by using the same procedures as those reported previously.9 In the preparation, the small carbon nanoparticles (separated from the laser ablation-produced powdery sample) were refluxed in aqueous nitric acid solution for the purpose of oxidizing surface carbons into carboxylic acids, followed by thionyl chloride treatment and then amidation with the oligomeric ethylene glycol diamine H2NCH2(C2H4O)35C2H4CH2NH2 (PEG1500N) to form the carbon dots with surface-attached PEGs (Scheme 1). The transmission electron microscopy (TEM) results (Fig. 1) suggested that these dots were well-dispersed, with sizes averaging about 4.2 nm (based on statistical analyses of more than 300 dots), as also supported by the atomic force microscopy (AFM) results (Fig. 1).
Fig. 1.
TEM (left) and AFM (right) images of the carbon dots used in this study. The TEM specimen was prepared by depositing a few drops of a diluted carbon dot solution onto a carbon-coated copper grid, followed by evaporation. The AFM specimen on a mica surface was similarly prepared. Hitachi HD-2000 S-TEM and Molecular Imaging PicoPlus AFM were used.
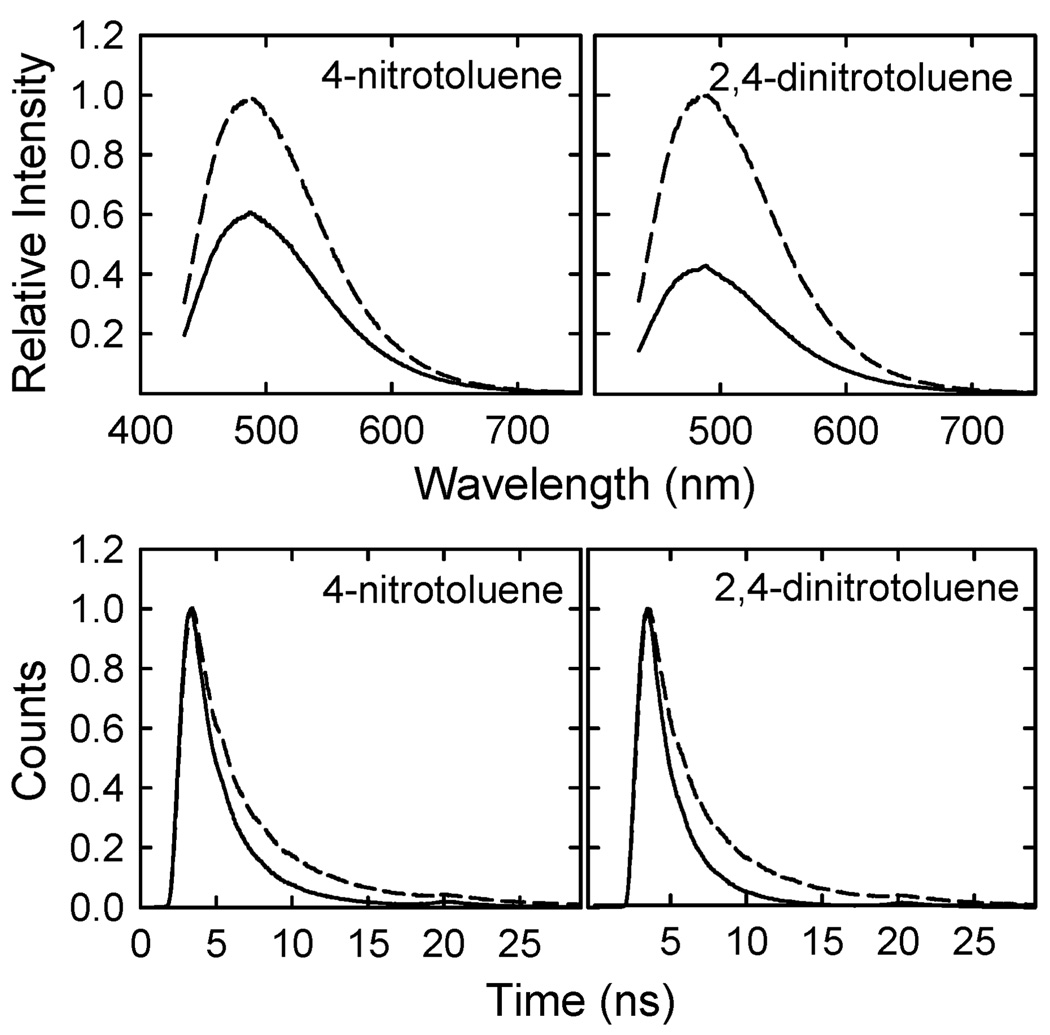
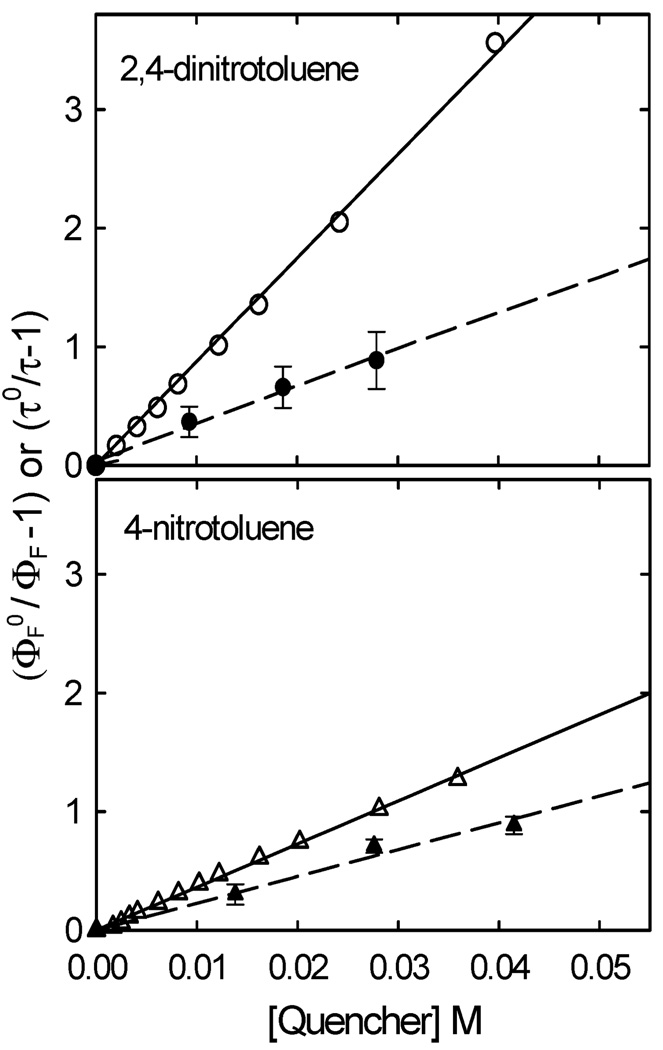
Photoluminescence spectra of the carbon dots in aqueous or organic solutions were generally broad (Fig. 2) with luminescence emission intensities (425 nm excitation) which were quenched by the known electron acceptors 4-nitrotoluene (−1.19 V vs. NHE)16 and 2,4-dinitrotoluene (−0.9 V vs. NHE)17 in toluene solution, with the observed Stern–Volmer quenching constants (KSV = τF°kq) from linear regression of 38 M−1 and 83 M−1, respectively (Fig. 3). Obviously 2,4-dinitrotoluene was a much more effective quencher than 4-nitrotoluene, consistent with its being a significantly stronger electron acceptor. The luminescence decays of the carbon dots in the absence of quenchers could not be deconvoluted with a mono-exponential function (probably due to a distribution of emissive species and/or sites),18 but could be deconvoluted with the use of a multicomponent decay function to yield an average lifetime τF° around 4 ns.9 Thus, on average the bimolecular rate constants kq for the quenching of luminescence emissions in the carbon dots by 4-nitrotoluene and 2,4-dinitrotoluene were of the order of 9.5 × 109 M−1 s−1 and 2.1 × 1010 M−1 s−1, respectively. These, especially that for 2,4-dinitrotoluene, are beyond the upper limit for any bimolecular luminescence quenching processes in solution,18 highlighting the high efficiency of the underlying electron transfer and also suggesting the presence of static quenching contributions, which were confirmed by the Stern–Volmer plots from the observed average luminescence lifetimes (Fig. 3). The corresponding quenching rate constants, kq of ~6.5 × 109 M−1 s−1 for 4-nitrotoluene and 8 × 109 M−1 s−1 for 2,4-dinitrotoluene, are still at the diffusion-controlled limit for dynamic quenching.
Fig. 2.
Top: luminescence emission spectra (425 nm excitation) of the carbon dots in toluene without (--) and with the indicated quenchers (both 0.016 M, —). Bottom: luminescence decays (407 nm excitation, monitored with 470 nm narrow bandpass filter) of the carbon dots without (--) and with the quenchers (both 0.028 M, —).
Fig. 3.
Stern–Volmer plots for the quenching of luminescence quantum yields (425 nm excitation) of the carbon dots by 2,4-dinitrotoluene (○) and 4-nitrotoluene (Δ) in toluene; and plots for the quenching of luminescence lifetimes (407 nm excitation) by 2,4-dinitrotoluene (●) and 4-nitrotoluene (▲). The lines represent the best fits (the least-square regression) of the respective data.
The electron donating capabilities of the photoexcited carbon dots were also demonstrated in the photoreduction of Ag+ to Ag. Experimentally, the reduction could be accomplished by photoirradiating (450 W xenon arc lamp coupled with a Spex 1681 monochromator) carbon dots in an aqueous solution of AgNO3 at a visible wavelength such as 450 nm, which resulted in the emergence and rapid increase of the surface plasmon absorption owing to the increasing amount of Ag produced by the photoreduction. In order to avoid the subsequent irradiation into the surface plasmon absorption band of the initially formed Ag, the same experiment was also performed with 600 nm excitation, and similar photoreduction was observed. There was no Ag formation in control experiments in the absence of carbon dots, as expected.
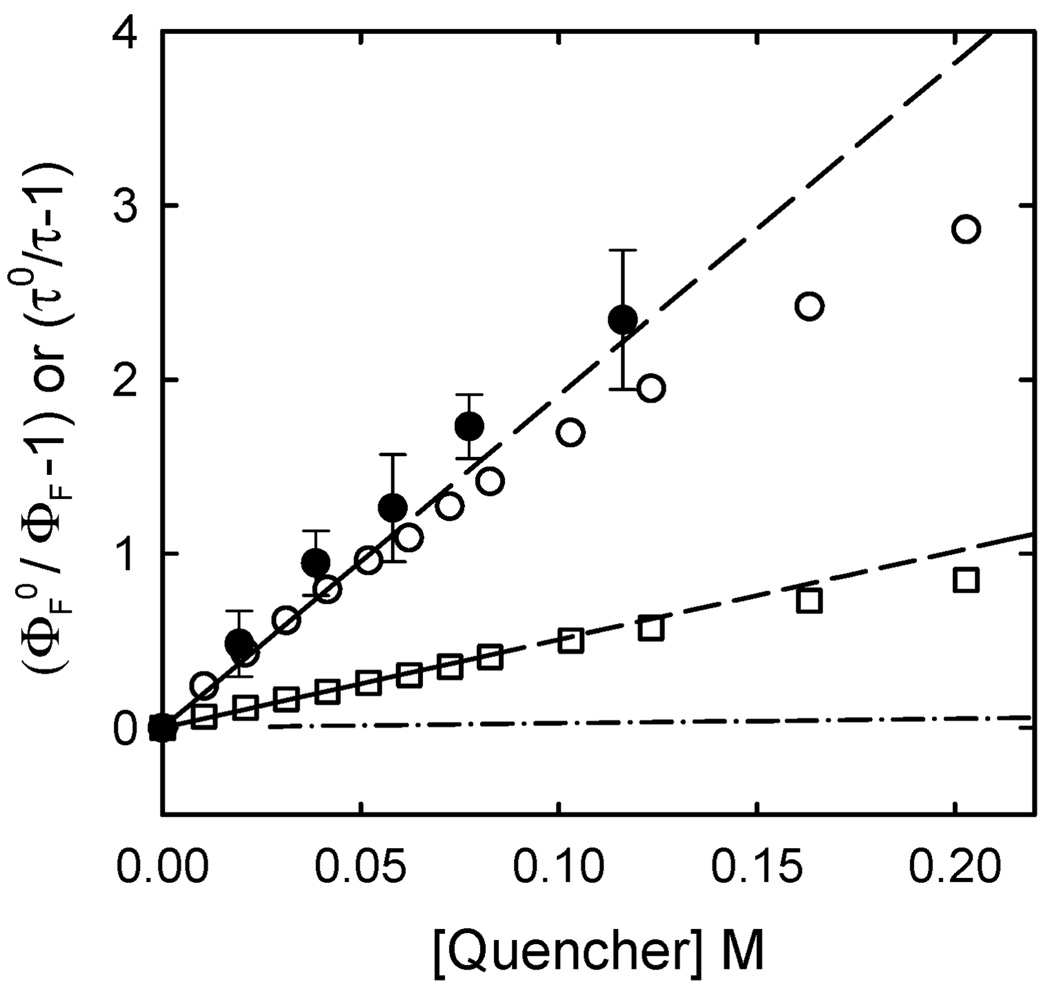
Interestingly, the carbon dots were similarly strong electron acceptors as well, allowing highly efficient luminescence quenching by known electron donors such as N,N-diethylaniline (DEA, 0.88 V vs. NHE).19,20 As shown in Fig. 4, the DEA quenching was also strongly solvent dependent, significantly more efficient in a polar solvent methanol than in chloroform. The Stern–Volmer plots for the quenching of luminescence quantum yields were curved downward at higher DEA concentrations, much more so for the quenching in methanol (Fig. 4). The linear fits for only the data points at lower DEA concentrations yielded Stern–Volmer quenching constants KSV of 19 M−1 and 5.1 M−1 in methanol and chloroform, respectively. The results from the quenching of luminescence lifetimes suggested no significant static quenching contributions. While not as extreme as those with electron acceptor quenchers discussed above, these Stern–Volmer constants are again corresponding to rate constants kq toward the upper limit for bimolecular luminescence quenching processes in solution.18
Fig. 4.
Stern–Volmer plots for the quenching of luminescence quantum yields (400 nm excitation) of the carbon dots by DEA in methanol (○, the line from fitting the data points up to 0.05 M) and chloroform (□, the line from fitting the data points up to 0.08M), and for the quenching of luminescence lifetimes (407 nm excitation) in methanol (●). The low-concentration portion of the same plot for diethylamine as the quencher in methanol (-·-) is also shown for comparison.
The strong solvent polarity dependence of the luminescence quenching by DEA is a good indication for an electron transfer quenching mechanism. As additional supporting evidence, the efficiency of the luminescence quenching was found to be strongly dependent on the electron donating ability of the quencher. For example, a weaker electron donor such as diethylamine (1.55 V vs. NHE)19 was considerably less efficient in the quenching of luminescence emissions in the carbon dots under otherwise the same experimental conditions (Stern–Volmer quenching constant KSV about 0.3 M−1, Fig. 4).
No ground-state charge transfer complexes were observed with any of the quenchers, as expected for their being used at such low concentrations.
Mechanistically, the photoluminescence in carbon dots has been attributed to energy trapping on the passivated carbon particle surface.9–11 We speculate that there could even be phenomenological similarities between the luminescence emission mechanisms in traditional semiconductor quantum dots1,2 and carbon dots (despite carbon hardly being a member of the semiconductor family), such that the emissions in carbon dots might also be a result of radiative recombination of surface-trapped electrons and holes. It is known that the carbon core in carbon dots must necessarily be very small (sub-10 nm or preferably sub-5 nm),9–11 which should create inhomogenous particle surface sites. Upon passivation via organic or polymeric functionalization, these surface sites could facilitate the trapping of photoinduced electrons and holes. As for the observed highly efficient quenching of luminescence emissions in the carbon dots by both electron acceptor and electron donor molecules,21 their disruption to the radiative recombinations on the passivated carbon surface might be responsible. Further investigations including potentially probing directly the electron–hole pairs and/or their recombination processes in the photoexcited carbon dots are desired and should be pursued. Nevertheless, the substantial photoinduced redox properties of carbon dots reported here will open up new opportunities for these newly found quantum dot-like nanomaterials in light-harvesting and related applications.
Acknowledgments
Financial support from ACS-PRF and, in part, from NIH is gratefully acknowledged. The instrumentation used in this work was acquired with funding from NSF. F. K. was a participant of the undergraduate research program jointly sponsored by NSF and Clemson University.
Notes and references
- 1.Alivisatos AP. Science. 1996;271:933–937. [Google Scholar]
- 2.Wilson WL, Szajowski PF, Brus LE. Science. 1993;262:1242–1244. doi: 10.1126/science.262.5137.1242. [DOI] [PubMed] [Google Scholar]
- 3.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW. Science. 2003;300:1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 4.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamat PV. J. Phys. Chem. C. 2008;112:18737–18753. [Google Scholar]
- 6.Landes CF, Braun M, EI-Sayed MA. J. Phys. Chem. B. 2001;105:10554–10558. [Google Scholar]
- 7.Sharma SN, Pillai ZS, Kamat PV. J. Phys. Chem. B. 2003;107:10088–10093. [Google Scholar]
- 8.Long D, Wu G, Wang W, Yao S. Res. Chem. Intermed. 2007;33:655–661. [Google Scholar]
- 9.Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang HF, Luo PJG, Yang H, Kose ME, Chen B, Veca LM, Xie SY. J. Am. Chem. Soc. 2006;128:7756–7757. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- 10.Cao L, Wang X, Meziani MJ, Lu FS, Wang HF, Luo PJG, Lin Y, Harruff BA, Veca LM, Murray D, Xie SY, Sun Y-P. J. Am. Chem. Soc. 2007;129:11318–11319. doi: 10.1021/ja073527l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y-P, Wang X, Lu FS, Cao L, Meziani MJ, Luo PJG, Gu LR, Veca LM. J. Phys. Chem. C. 2008;112:18295–18298. doi: 10.1021/jp8076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Booker C, Li R, Zhou X, Sham T-K, Sun X, Ding Z. J. Am. Chem. Soc. 2007;129:744–745. doi: 10.1021/ja0669070. [DOI] [PubMed] [Google Scholar]
- 13.Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Karakassides M, Giannelis EP. Small. 2008;4:455–458. doi: 10.1002/smll.200700578. [DOI] [PubMed] [Google Scholar]
- 14.Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Georgakilas V, Giannelis EP. Chem. Mater. 2008;20:4539–4541. [Google Scholar]
- 15.Zhao Q-L, Zhang Z-L, Huang B-H, Peng J, Zhang M, Pang D-W. Chem. Commun. 2008:5116–5118. doi: 10.1039/b812420e. [DOI] [PubMed] [Google Scholar]
- 16.Rehm JM, Mclendon GL, Fauchet PM. J. Am. Chem. Soc. 1996;118:4490–4491. [Google Scholar]
- 17.Sohn H, Calhoun RM, Sailor MJ, Trogler WC. Angew. Chem., Int. Ed. 2001;40:2104–2105. doi: 10.1002/1521-3773(20010601)40:11<2104::AID-ANIE2104>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2nd edn. New York: Kluwer Academic/Plenum Publishers; 1999. [Google Scholar]
- 19.Arbogast JW, Foote CS, Kao M. J. Am. Chem. Soc. 1992;114:2277–2279. [Google Scholar]
- 20.Sun Y-P, Bunker CE, Ma B. J. Am. Chem. Soc. 1994;116:9692–9699. [Google Scholar]
- 21.The fluorescence quenching of semiconductor QDs (CdSe and CdSe/ZnS, for example) by both electron donors and acceptors has also been reported.6–8,22,23.
- 22.Shi G, Shang Z, Wang Y, Jin W, Zhang T. Spectrochim. Acta, Part A. 2008;70:247–252. doi: 10.1016/j.saa.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 23.Breus VV, Heyes CD, Nienhaus GU. J. Phys. Chem. C. 2007;111:18589–18594. [Google Scholar]