Abstract
The pursuit of happiness is a preoccupation for many people. Yet only the pursuit can be promised, not happiness itself. Can science help? We focus on the most tractable ingredient, hedonia or positive affect. A step toward happiness might be gained by improving the pleasures and positive moods in daily life. The neuroscience of pleasure and reward provides relevant insights, and we discuss how specific hedonic mechanisms might relate to happiness or the lack thereof. Although the neuroscience of happiness is still in its infancy, further advances might be made through mapping overlap between brain networks of hedonic pleasure with others, such as the brain's default network, potentially involved in the other happiness ingredient, eudemonia or life meaning and engagement.
Introduction
Happiness is subtle and complex. It is a daunting challenge to connect happiness and pleasure to underlying neurobiology. Nevertheless, on being asked to attempt the task, after reflection we believe a few observations can be made.
Scientists have in fact made substantial progress in defining and measuring happiness in the form of subjective well-being 1-4. Since Aristotle, happiness has been thought of as consisting of two aspects: hedonia (pleasure) and eudaimonia (a life well-lived)5. More modern formulations include the corresponding psychological ingredients of pleasure and meaning but add a third distinct component of engagement related to feelings of commitment and participation in life 1. Although there is a sharp conceptual distinction between pleasure versus engagement-meaning components, hedonic and eudaimonic aspects empirically cohere together in happy people. For example, in happiness surveys over 80% of people rate their overall eudaimonic life satisfaction as “pretty to very happy”, and comparably, 80% also rate their current hedonic mood as positive (e.g., positive 6-7 on a 10 point valence scale where 5 is hedonically neutral)6. A lucky few may even live consistently around a hedonic point of 8 (although excessively higher hedonic scores may actually impede attainment of life success, as measured by riches, education, or political participation)—see Figure 1, top right 7.
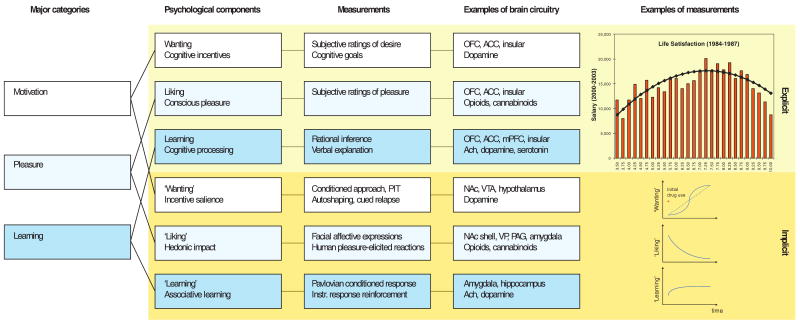
Fig 1. Measuring reward and hedonia.
Reward and pleasure are multifaceted psychological concepts. Major processes within reward (first column) consist of motivation or wanting (white), learning (blue), and – most relevant to happiness – pleasure liking or affect (light blue). Each of these contains explicit (top rows, light yellow) and implicit (bottom rows, yellow) psychological components (second column) that constantly interact and require careful scientific experimentation to tease apart. Explicit processes are consciously experienced (e.g. explicit pleasure and happiness, desire, or expectation), whereas implicit psychological processes are potentially unconscious in the sense that they can operate at a level not always directly accessible to conscious experience (implicit incentive salience, habits and ‘liking’ reactions), and must be further translated by other mechanisms into subjective feelings. Measurements or behavioral procedures that are especially sensitive markers of the each of the processes are listed (third column). Examples of some of the brain regions and neurotransmitters are listed (fourth column), as well as specific examples of measurements (fifth column), such as an example of how highest subjective life satisfaction does not lead to the highest salaries (top) 93. Another example shows the incentive-sensitization model of addiction and how ‘wanting’ to take drugs may grow over time independently of ‘liking’ and ‘learning’ drug pleasure as an individual becomes an addict (bottom)94.
Scientists have also made substantial progress in understanding the psychology and neurobiology of sensory pleasure. These advances make the hedonic side of happiness most tractable to a scientific approach to the neural underpinnings of happiness. Supporting a hedonic approach, it has been suggested that the best measure of subjective well-being may be simply to ask people how they hedonically feel right now – again and again – so as to track their hedonic accumulation across daily life3, 8, 9. These repeated self-reports of hedonic states could also be used to identify more stable neurobiological hedonic brain traits that dispose particular individuals toward happiness. Further, a hedonic approach might even offer a toehold into identifying eudaimonic brain signatures of happiness, due to the empirical convergence between the two categories, even if pleasant mood is only half the happiness story.
It is important to note that our focus on hedonic happiness should not be confused with hedonism, which is the pursuit of pleasure for pleasure's own sake, and more akin to the addiction features we describe below. Also, to focus on hedonics does not deny that some ascetics may have found bliss through painful self-sacrifice, but simply reflects that positive hedonic tone is indispensible to most people seeking happiness.
A science of pleasure
Given the potential contributions of hedonics to happiness, we now survey developments in understanding brain mechanisms of pleasure (for relevant reviews see also 10, 11). The scientific study of pleasure and affect was foreshadowed by the pioneering ideas of Charles Darwin, who examined the evolution of emotions and affective expressions, and suggested that these are adaptive responses to environmental situations. In that vein, pleasure ‘liking’ and displeasure reactions are prominent affective reactions in the behavior and brains of all mammals12, and likely had important evolutionary functions 13. Neural mechanisms for generating affective reactions are present and similar in most mammalian brains, and thus appear to have been selected for and conserved across species 14. Indeed both positive affect and negative affect are recognized today as having adaptive functions 15, and positive affect in particular has consequences in daily life for planning and building cognitive and emotional resources 16, 17.
Such functional perspectives suggest that affective reactions may have objective features beyond subjective ones 18. Progress in affective neuroscience has been made recently by identifying objective aspects of pleasure reactions and triangulating toward underlying brain substrates. This scientific strategy divides the concept of affect into two parts: the affective state, which has objective aspects in behavioral, physiological and neural reactions; and conscious affective feelings, seen as the subjective experience of emotion 18 (box 1). Note that such a definition allows conscious feelings to play a central role in hedonic experiences, but holds that the affective essence of a pleasure reaction is more than a conscious feeling.
Box 1. Interspecies pleasure research.
Pleasure has manifestations both in consciousness (subjective liking) and in brain and behavioral reactions (objective ‘liking’). While the pleasure of a reward such as sweetness can be measured by verbal reports in conscious humans, the affective core of this hedonic processing is not dependent on the presence of language. In most non-linguistic mammals, pleasure will also elicit affective ‘liking’ reactions, reflecting in a basic form the hedonic gloss to the sensation which we experience as conscious pleasure 14, 30.
Finding neural generators of pleasure such as brain hedonic hotspots has relied on employing examples of useful ‘liking’ reactions, and amplifying them with brain manipulations. One such example is the affective facial expression elicited by the hedonic impact of sweet tastes in newborn human infants. Sweet tastes elicit positive facial ‘liking’ expressions (e.g. tongue protrusions), whereas bitter tastes instead elicit facial ‘disliking’ expressions (e.g. gapes). These homologous affective expressions (sharing features such as identical allometric timing laws) seem to have developed from the same evolutionary source in humans, orangutans, chimpanzees, monkeys, and even rats and mice 12.
We suggest that ’liking’ or hedonic impact reflected by these reactions is a primary pleasure component of fundamental reward, which may be shared by higher pleasures and even happiness. Overlaps between neural circuits of fundamental pleasures and higher pleasures imply existence of a common neural currency that is shared by all positive affects. Core ‘liking’ reactions need not necessarily be conscious, but conscious experiences of pleasure, in the ordinary sense of the word, are elaborated out of core ‘liking’ reactions by cognitive brain mechanisms to form a higher level of hedonic awareness. (Beyond ‘liking’, reward also contains nonhedonic components: 1) Wanting: motivation for reward, which includes mesolimbic incentive salience ‘wanting’ processes that are not necessarily conscious, and more cortical conscious desires for incentives or cognitive goals. 2) Learning: associations, representations and predictions about future rewards based on past experiences. Learned predictions include both explicit and cognitive predictions, and implicit knowledge including simpler associative conditioning, such as basic Pavlovian and instrumental associations)
For the conscious components of pleasure, specialized but elusive brain mechanisms of conscious elaboration are likely needed to convert a ‘liking’ reaction into a subjectively-felt liking experience. It may thus be that human conscious experience of pleasure is different not only quantitatively but also qualitatively from other animals, depending on the uniqueness of human cortical mechanisms involved in the conversion into consciousness.
Cognition also vastly expands the range of events that can trigger pleasure in humans to include cognitive and cultural sources, and provides new top-down regulatory ways to amplify or dampen a pleasure or displeasure.
Evidence so far available suggests that brain mechanisms involved in fundamental pleasures (food and sexual pleasures) overlap with those for higher-order pleasures (for example, monetary, artistic, musical, altruistic and transcendent pleasures) 14, 19-25. From sensory pleasures and drugs of abuse 26 to monetary, aesthetic and musical delights, all pleasures seem to involve the same hedonic brain systems, even when linked to anticipation and memory 27, 28. Pleasures important to happiness, such as socializing with friends 1-4,24, and related traits of positive hedonic mood are thus all likely to draw upon the same neurobiological roots that evolved for sensory pleasures. The neural overlap may offer a way to generalize from fundamental pleasures that are best understood and so infer larger hedonic brain principles likely to contribute to happiness.
We note the rewarding properties for all pleasures are likely to be generated by hedonic brain circuits that are distinct from the mediation of other features of the same events (e.g., sensory, cognitive) 20. Thus pleasure is never merely a sensation or a thought 29, but is instead an additional hedonic gloss generated by the brain via dedicated systems.
The neuroanatomy of pleasure
How does positive affect arise? Affective neuroscience research on sensory pleasure has revealed many networks of brain regions and neurotransmitters activated by pleasant events and states (Figures 1 and 2). Identification of hedonic substrates has been advanced by recognizing that pleasure or ‘liking’ is but one component in the larger composite psychological process of reward, which also involves ‘wanting’ and ‘learning’ components 30. Each component also has conscious and non-conscious elements that can be studied in humans – and at least the latter can also be probed in other animals.
Fig 2. Hedonic brain circuitry.
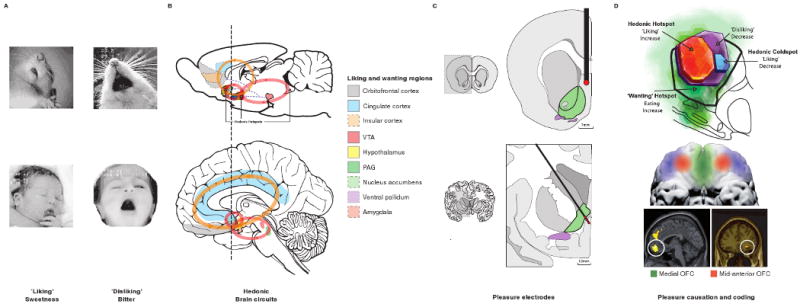
The schematic figure shows the brain regions for causing and coding fundamental pleasure in rodents and humans. (a) Facial ‘liking’ and ‘disliking’ expressions elicited by sweet and bitter taste are similar in rodents and human infants. (b, d) Pleasure causation has been identified in rodents as arising from interlinked subcortical hedonic hotspots, such as in nucleus accumbens and ventral ‘pallidum, where neural activation may increase ‘liking’ expressions to sweetness. Similar pleasure coding and incentive salience networks have also been identified in humans. (c) The so-called ‘pleasure’ electrodes in rodents and humans are unlikely to have elicited true pleasure but perhaps only incentive salience or ‘wanting’. (d) The cortical localization of pleasure coding may reach an apex in various regions of the orbitofrontal cortex, which differentiate subjective pleasantness from valence processing of aspects the same stimulus, such as a pleasant food.
Hedonic hotspots
The brain appears rather frugal in ‘liking’ mechanisms that cause pleasure reactions. As shown below, some hedonic mechanisms are found deep in the brain (nucleus accumbens, ventral pallidum, brainstem) and other candidates are in the cortex (orbitofrontal, cingulate, medial prefrontal and insular cortices) 14, 31-37. Pleasure-activated brain networks are widespread, but compelling evidence for pleasure causation (detected as increases in ‘liking’ reactions consequent to brain manipulation) has so far been found for only a few hedonic hotspots in the subcortical structures. Each hotspot is merely a cubic-millimeter or so in volume in the rodent brain (and should be a cubic-centimeter or so in humans, if proportional to whole brain volume). Hotspots are capable of generating enhancements of ‘liking’ reactions to a sensory pleasure such as sweetness, when stimulated with opioid, endocannabinoid or other neurochemical modulators 30.
Hotspots exist in nucleus accumbens shell and ventral pallidum, and possibly other forebrain and limbic cortical regions, and also in deep brainstem regions including the parabrachial nucleus in the pons (Figure 2D) 19. The pleasure-generating capacity of these hotspots has been revealed in part by studies in which microinjections of drugs stimulated neurochemical receptors on neurons within a hotspot, and caused a doubling or tripling of the number of hedonic ‘liking’ reactions normally elicited by a pleasant sucrose taste 30. Analogous to scattered islands that form a single archipelago, hedonic hotspots are anatomically distributed but interact to form a functional integrated circuit. The circuit obeys control rules that are largely hierarchical and organized into brain levels. Top levels function together as a cooperative heterarchy, so that, for example, multiple unanimous ‘votes’ in favor from simultaneously-participating hotspots in the nucleus accumbens and ventral pallidum are required for opioid stimulation in either forebrain site to enhance ‘liking’ above normal 38.
In addition, as mentioned above, pleasure is translated into motivational processes in part by activating a second component of reward termed ‘wanting’ or incentive salience, which makes stimuli attractive when attributed to them by mesolimbic brain systems 39. Incentive salience depends in particular on mesolimbic dopamine neurotransmission (though other neurotransmitters and structures also are involved).
Importantly, incentive salience is not hedonic impact or pleasure ‘liking’ 40. This is why an individual can ‘want’ a reward without necessarily ‘liking’ the same reward. Irrational ‘wanting’ without liking can occur especially in addiction via incentive-sensitization of the mesolimbic dopamine system and connected structures 26. At extreme, the addict may come to ‘want’ what is neither ‘liked’ nor expected to be liked, a dissociation possible because ‘wanting’ mechanisms are largely subcortical and separable from cortically-mediated declarative expectation and conscious planning. This is a reason why addicts may compulsively ‘want’ to take drugs even if, at a more cognitive and conscious level, they do not want to do so. That is surely a recipe for great unhappiness (Figure 2, bottom right).
Cortical pleasure
In cortex, hedonic evaluation of pleasure valence is anatomically distinguishable from precursor operations such as sensory computations, suggesting existence of a hedonic cortex proper (Figure 2) 41. Hedonic cortex involves regions such as the orbitofrontal 20, insula 42, medial prefrontal 37 and cingulate cortices 43, which a wealth of human neuroimaging studies have shown to code for hedonic evaluations (including anticipation, appraisal, experience and memory of pleasurable stimuli) and have close anatomical links to subcortical hedonic hotspots. It is important, however, to again make a distinction between brain activity coding and causing pleasure. Neural coding is inferred in practice by measuring brain activity correlated to a pleasant stimulus, using human neuroimaging 22 techniques, or electrophysiological or neurochemical activation measures in animals 44. Causation is generally inferred on the basis of a change in pleasure as a consequence of a brain manipulation such as a lesion or stimulation 30, 45. Coding and causation often go together for the same substrate, but they may diverge so that coding occurs alone.
Pleasure encoding may reach an apex of cortical localization in a mid-anterior subregion within the orbitofrontal cortex, where neuroimaging activity correlates strongly to subjective pleasantness ratings of food varieties 33 – and to other pleasures such as sexual orgasms 46, drugs 47, chocolate 21 and music 48. Most importantly, mid-anterior orbitofrontal activity tracks changes in subjective pleasure, such as a decline in palatability when the reward value of one food was reduced by eating it to satiety (while remaining high to another food) 20, 33. The mid-anterior subregion of orbitofrontal cortex is thus a prime candidate for the coding of subjective experience of pleasure 20.
Another coding site for positive hedonics in orbitofrontal cortex is along its medial edge that has activity related to the valence of positive and negative events 34, contrasted to lateral portions that have been suggested to code unpleasant events 49 (although lateral activity may reflect a signal to escape the situation, rather than displeasure per se 34, 50-52). This medial-lateral hedonic gradient interacts with an abstraction-concreteness gradient in the posterior-anterior dimension, so that more complex or abstract reinforcers (such as monetary gain and loss) 49 are represented more anteriorly in the orbitofrontal cortex than less complex sensory rewards (such as taste)21. The medial region does not, however, appear to change its activity with reinforcer devaluation, and so may not reflect the full dynamics of pleasure.
Other cortical regions implicated in coding for pleasant stimuli include parts of the mid-insular 53 and anterior cingulate cortices 43. As yet, however, it is not as clear as for the orbitofrontal cortex whether those regions specifically code pleasure or only emotion more generally 54. A related suggestion has emerged that the frontal left hemisphere plays a special lateralized role in positive affect more than the right hemisphere 55, though how to reconcile left-positive findings with many other findings of bilateral activations of orbitofrontal and related cortical regions during hedonic processing remains an ongoing puzzle 20.
It remains still unknown, however, if mid-anterior orbitofrontal cortex or medial orbitofrontal cortex or any other cortical region actually causes a positive pleasure state. Clearly, damage to orbitofrontal cortex does impair pleasure-related decisions, including choices and context-related cognitions in humans, monkeys and rats 56-64. But some caution regarding whether cortex generates positive affect states per se is indicated by the consideration that patients with lesions to the orbitofrontal cortex do still react normally to many pleasures, although sometimes showing inappropriate emotions 62-65. Hedonic capacity after prefrontal damage has not, however, yet been studied in careful enough detail (e.g. using selective satiation paradigms 33), and it would be useful to have more information on the role of orbitofrontal cortex, insular cortex, and cingulate cortex in generating and modulating hedonic states.
Pleasure causation has been so far rather difficult to assess in humans given the limits of information from lesion studies, and the correlative nature of neuroimaging studies. A promising tool, however, is deep brain stimulation (DBS) which is a versatile and reversible technique that directly alters brain activity in a brain target and where the ensuing whole-brain activity can be measured with MEG 66. Pertinent to a view of happiness as freedom from distress, at least pain relief can be obtained from DBS of periacqueductal grey in the brainstem in humans 67, where specific neural signatures of pain have been found68, and where the pain relief is associated with activity in the mid-anterior orbitofrontal cortex, perhaps involving endogenous opioid release69. Similarly, DBS may alleviate some unpleasant symptoms of depression, though without actually producing positive affect.
Famously, also, subcortical pleasure electrodes were reported decades ago in animals and humans 70, 71 (Figure 2C). However, recently we and others have questioned whether such electrodes truly caused pleasure, or instead, only a psychological process more akin to‘wanting’ without ‘liking’ 10. In our view, it still remains unknown whether DBS causes true pleasure, or if so, where in the brain electrodes produce it.
Loss of pleasure
The lack of pleasure, anhedonia, is one of the most important symptoms of many mental illnesses including depression. It is difficult to conceive of anyone reporting happiness or well-being while so deprived of pleasure. Thus anhedonia is another potential avenue of evidence for the link between pleasure and happiness72.
The brain regions necessary for pleasure – but disrupted in anhedonia – are not yet clear. Core ‘liking’ reactions to sensory pleasures appear relatively difficult to abolish absolutely in animals by a single brain lesion or drug, which may be very good in evolutionary terms. Only the ventral pallidum has emerged among brain hedonic hotspots as a site where damage fully abolishes the capacity for positive hedonic reaction in rodent studies, replacing even ‘liking’ for sweetness with ‘disliking’ gapes normally reserved for bitter or similarly noxious tastes 44, 73. Interestingly, there are extensive connections from the ventral pallidum to the medial orbitofrontal cortex 74.
On the basis of this evidence, the ventral pallidum might also be linked to human anhedonia. This brain region has not yet been directly surgically targeted by clinicians but there is anecdotal evidence that some patients with pallidotomies (of nearby globus pallidus, just above and behind the ventral pallidum) for Parkinson's patients show flattened affect 75 (Aziz, personal communication), and stimulation of globus pallidus internus may help with depression 76. A case study has also reported anhedonia following bilateral lesion to the ventral pallidum 77.
Alternatively, core ‘liking’ for fundamental pleasures might persist intact but unacknowledged in anhedonia, while instead only more cognitive construals, including retrospective or anticipatory savoring, becomes impaired. That is, fundamental pleasure may not be abolished in depression after all. Instead, what is called anhedonia might be secondary to motivational deficits and cognitive misappraisals of rewards, or to an overlay of negative affective states. This may still disrupt life enjoyment, and perhaps render higher pleasures impossible.
Other potential regions targeted by DBS to help with depression and anhedonia include the nucleus accumbens 78 and the subgenual cingulate cortex 79. In addition, lesions of the posterior part of the anterior cingulate cortex have been used for the treatment of depression with some success 80.
Bridging pleasure to meaning
It is potentially interesting to note that all these structures either have close links with frontal cortical structures in the hedonic network (e.g., nucleus accumbens and ventral pallidum) or belong to what has been termed the brain's default network which changes over early development 81, 82 (Figure 3).
Fig 3. The brain' s default network and eudaimonic – hedonic interaction.
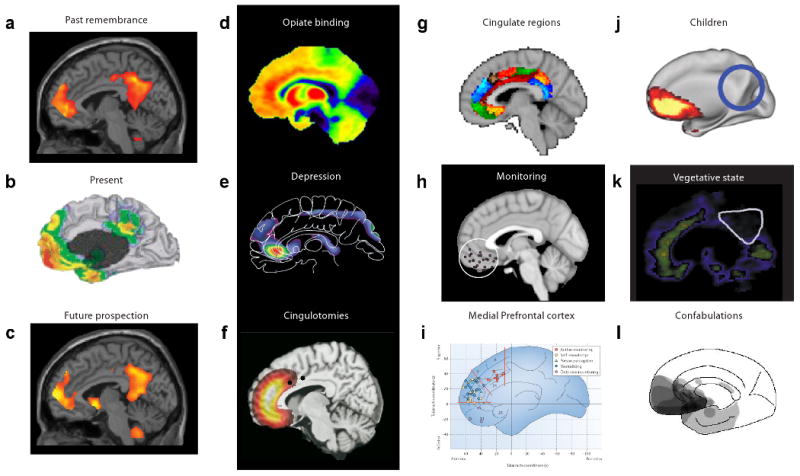
(a - c) The brain's default network 83, 90 has been linked to self awareness, remembering the past and prospecting the future 90. Some components overlap with pleasure networks, including midline structures such as the orbitofrontal, medial prefrontal and cingulate cortices. We wonder whether happiness might include a role for the default network, or for related neural circuits that contribute to computing relations between self and others, in evaluating eudaimonic meaning and interacting with hedonic circuits of positive affect. Examples show (d) key regions of the default network such as the anterior cingulate and orbitofrontal cortices that have a high density of opiate receptors 87, (e) have been linked to depression 88, and (f) its surgical treatment 80. (g) Subregional localization of function may be indicated by connectivity analyses of cingulate cortex 43 and related structures, (h) important in pleasure-related monitoring, learning and memory 34, (i) as well as self-knowledge, person perception and other cognitive functions 37. (j) The default network may change over early life in children and pre-term babies 81, 82, (k) in pathological states including depression and vegetative states86, (l) and after lesions to its medial orbitofrontal and subgenual cingulate cortices that disrupt reality monitoring and create spontaneous confabulations91.
Mention of the default network brings us back to the topic of eudaimonic happiness, and to potential interactions of hedonic brain circuits with circuits that assess meaningful relationships of self to social others. The default network is a steady state circuit of the brain which becomes perturbed during cognitive tasks 83. Most pertinent here is an emerging literature that has proposed the default network to carry representations of self 84, internal modes of cognition 85, and perhaps even states of consciousness 86. Such functions might well be important to higher pleasures as well as meaningful aspects of happiness.
Although highly speculative, we wonder whether the default network might deserve further consideration for a role in connecting eudaimonic and hedonic happiness. At least, key regions of the frontal default network overlap with the hedonic network discussed above, such as the anterior cingulate and orbitofrontal cortices 34, 37, 43, 80, and have a relatively high density of opiate receptors 87. And activity changes in the frontal default network, such as in the subgenual cingulate and orbitofrontal cortices, correlate to pathological changes in subjective hedonic experience, such as in depressed patients 88.
Pathological self-representations by the frontal default network could also provide a potential link between hedonic distortions of happiness that are accompanied by eudaimonic dissatisfaction, such as in cognitive rumination of depression 89-91. Conversely, mindfulness-based cognitive therapy for depression, which aims to disengage from dysphoria-activated depressogenic thinking might conceivably recruit default network circuitry to help mediate improvement in happiness via a linkage to hedonic circuitry92.
Concluding remarks
The most difficult questions facing pleasure and happiness research remain the nature of its subjective experience and the relation of hedonic components (pleasure or positive affect) to eudaimonic components (cognitive appraisals of meaning and life satisfaction). While some progress has been made in understanding brain hedonics, it is important not to over-interpret. In particular we have still not made substantial progress towards understanding the functional neuroanatomy of happiness.
In this review, we have, however, identified a number of brain regions that are important in the brain's hedonic networks, and speculated on potential interaction with eudaimonic networks. While it remains unclear how pleasure and happiness are exactly linked, it may be safe to say at least that the pathological lack of pleasure, in anhedonia or dysphoria, amounts to a formidable obstacle to happiness.
Further, so far as positive affect contributes to happiness, then considerable progress has been made in understanding the neurobiology of pleasure in ways that might be relevant. For example, we can imagine several possibilities to relate happiness to particular hedonic psychological processes discussed above. Thus, one way to conceive of hedonic happiness is as ‘liking’ without 'wanting. That is, a state of pleasure without disruptive desires, a state of contentment 13. Another possibility is that moderate ‘wanting’, matched to positive ‘liking’, facilitates engagement with the world. A little incentive salience may add zest to the perception of life and perhaps even promote the construction of meaning, just as in some patients DBS may help lift the veil of depression by making life events more appealing. However, too much ‘wanting’ can readily spiral into maladaptive patterns such as addiction, and is a direct route to great unhappiness. Finally, happiness might spring from higher pleasures, positive appraisals of life meaning and social connectedness, all combined and merged by interaction between the brain's default networks and pleasure networks. Achieving the right hedonic balance in such ways may be crucial to keep one not just ticking over but perhaps even happy.
Future scientific advances may provide a better sorting of psychological features of happiness and its brain bases. If so, it remains a distinct possibility that more among us may be one day shifted into a better situation to enjoy daily events, to find life meaningful and worth living – and perhaps even to achieve a degree of bliss.
Box 2. Of states and networks.
The idea that a brain hotspot or coding apex mediates pleasure or happiness can all too easily turn into phrenology if taken as a literal truth, and unconstrained chemo-phrenology poses an equal danger. Brain function is less constant than handy anatomical or chemical labels imply. Caveats, stipulations, and often even conditional (at least) retractions are sure to be needed, and if they are forgotten the effort to understand the brain will soon come to tears.
Pleasure and happiness are no exception to these cautions. The orbitofrontal cortex codes pleasure, but is embedded in many other high-level psychological functions too. The term ‘hedonic hotspot’ denotes a special capability of a site to cause pleasure under the right circumstances, but not what the site always does. For example, the nucleus accumbens hotspot causes pleasure ‘liking’ when stimulated with opioid or cannabinoid neurotransmitter signals, but the same spot only amplifies ‘wanting’ without ‘liking’ when stimulated by dopamine. Likewise opioids are no neurochemical guarantee of pleasure, except in the hotspot. If the same opioid microinjection is moved a millimeter outside the hotspot only ‘wanting’ without ‘liking’ is generated in all the rest of the nucleus accumbens. And for certain site-signal interactions, a shift in psychological context can ‘retunes’ the valence generated by a hotspot and reverse the psychological consequences from desire to fear.
If brain networks for pleasure and desire are so complicated, how much more complex must be happiness? This adds another to the many reasons for caution concerning overly simple equations between neurobiology and psychology that merge into myth (e.g., opioid = pleasure, dopamine = happiness, serotonin deficit = depression, oxytocin = love, nucleus accumbens = reward or amygdala = fear).
Box 3. Routes and diversions to pleasure and happiness.
There are many roads to pleasure, and diversions en route to happiness. Among the fundamental pleasures, the taste and smell of food is one of the most universal routes and perhaps the most experimentally accessible to affective neuroscience studies 19-23. Sex is also a potent route to pleasure, yet the neurobiological study of sexual hedonics is still in its infancy 95, 96.
In social animals like humans, social interactions with conspecifics are also fundamental and central to enhancing the other pleasures. Humans are intensely social, and data indicate that one of the most important factors for happiness is social relationships with other people. Social pleasures may still include vital sensory features such as visual faces, touch features of grooming and caress, as well as in humans more abstract and cognitive features of social reward and relationship evaluation 97. In particular, adult pair bonds and attachment bonds between parents and infants are likely to be extremely important for the survival of the species 98. The breakdown of these bonds is all too common and can lead to great unhappiness. And even bond formation can potentially disrupt happiness, such as in transient parental depression after birth of an infant (in over 10% of mothers and approximately 3% of fathers 99). Progress in understanding the hedonics of social bonds could be useful in understanding happiness.
Social neuroscience is beginning to unravel some of the complex dynamics of human social interactions. One of its major challenges is to map the developmental changes in reward processing over a lifespan. Another challenge is to understand the how brain networks underlying fundamental pleasure relate to higher pleasures such as music, dance, play and flow and to happiness.
Precious consciousness may offer the freedom of choice, pleasures, desires, and if managed correctly, perhaps even happiness. While we may be like butterflies who flutter for a day and think it is forever, we might as well enjoy the flutter.
Box: outstanding questions.
How do hedonia and eudaimonia interact to produce happiness?
In what ways are pleasure and happiness linked?
Is human pleasure similar or different to that of other animals?
Can happiness be unconscious?
Can happiness be measured by objective physiological or behavioral techniques?
How is anhedonia best measured?
Are pleasure and pain on a continuum, and can they both be present in happiness?
Does happiness have an evolutionary function?
How do genes affect happiness traits, such as hedonic bias?
What is the relation between language and happiness?
Acknowledgments
We are grateful to the editors for inviting us to assess the neural underpinnings of happiness and pleasure. We thank Christopher Peterson, Eric Jackson, Kristine Rømer Thomsen, Christine Parsons, Katie Young and several anonymous reviewers for helpful comments on an earlier version of this manuscript. Our research has been supported by grants from the TrygFonden Charitable Foundation to MLK and from the NIMH and NIDA to KCB.
Glossary of key happiness ingredients
- Eudaimonia
A life is well-lived, embedded in meaningful values, together with a sense of engagement in that life. This is the cognitive or Aristotelian ingredient of happiness. We speculate here about the possibility that cortical systems of self and cognitive appraisal, such as the default network, make a eudaimonic contribution to happiness. At a more basic brain level, the motivational component of reward, mesolimbic ‘wanting’ or incentive salience, also may contribute to eudaimonic engagement by adding attraction or zest to life; however, overly intense ‘wanting’ leads to unhappiness and becomes more akin to addiction.
- Hedonia
The conscious feelings of pleasant well-being or hedonic niceness that is the affective ingredient of happiness. We suggest here subjective hedonia occurs when orbitofrontal and related cortical brain systems elaborate core ‘liking’ reactions (which are produced by subcortical hedonic hotspots and which need not be conscious) into conscious feelings of positive affect.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seligman ME, et al. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60:410–421. doi: 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- 2.Ryan RM, Deci EL. On happiness and human potentials: a review of research on hedonic and eudaimonic well-being. Annu Rev Psychol. 2001;52:141–166. doi: 10.1146/annurev.psych.52.1.141. [DOI] [PubMed] [Google Scholar]
- 3.Diener E, et al. Personality, culture, and subjective well-being: emotional and cognitive evaluations of life. Annu Rev Psychol. 2003;54:403–425. doi: 10.1146/annurev.psych.54.101601.145056. [DOI] [PubMed] [Google Scholar]
- 4.Kahneman D. Objective happiness. In: Kahneman D, et al., editors. Foundations of hedonic psychology: Scientific perspectives on enjoyment and suffering. Sage; 1999. pp. 3–25. [Google Scholar]
- 5.Waterman AS. Two conceptions of happiness: contrasts of personal expressiveness (eudaimonia) and hedonic enjoyment. J Pers Soc Psychol. 1993;64:678–691. [Google Scholar]
- 6.Kesebir P, Diener E. In pursuit of happiness: Empirical answers to philosophical questions. Perspectives on Psychological Science. 2008;3:117–125. doi: 10.1111/j.1745-6916.2008.00069.x. [DOI] [PubMed] [Google Scholar]
- 7.Oishi S, et al. The optimal level of well-being: Can we be too happy? Perspectives on Psychological Science. 2007;2:346–360. doi: 10.1111/j.1745-6916.2007.00048.x. [DOI] [PubMed] [Google Scholar]
- 8.Kahneman D. Experienced utility and objective happiness: A moment-based approach. In: Kahneman D, Tversky A, editors. Choices, values, and frames. Cambridge University Press; 2000. pp. 673–692. [Google Scholar]
- 9.Gilbert DT, Wilson TD. Prospection: experiencing the future. Science (New York, NY. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- 10.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 12.Steiner JE, et al. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 13.Kringelbach ML. The Pleasure Center Trust your animal instincts. Oxford University Press; 2009. [Google Scholar]
- 14.Kringelbach ML. The hedonic brain: A functional neuroanatomy of human pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford University Press; 2010. pp. 202–221. [Google Scholar]
- 15.Nesse RM. Natural selection and the elusiveness of happiness. Philos Trans R Soc Lond B Biol Sci. 2004;359:1333–1347. doi: 10.1098/rstb.2004.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredrickson BL, et al. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. Journal of personality and social psychology. 2008;95:1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson A, Balleine B. Hedonics: the cognitive-motivational interface. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 74–84. [Google Scholar]
- 18.Kringelbach ML. Emotion. In: Gregory RL, editor. The Oxford Companion to the Mind. 2nd. Oxford University Press; 2004. pp. 287–290. [Google Scholar]
- 19.Peciña S, et al. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 20.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 21.Small DM, et al. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 22.Gottfried JA. Olfaction and its pleasures: human neuroimaging perspectives. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 125–145. [Google Scholar]
- 23.Veldhuizen MG, et al. The pleasure of taste, flavor and food. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford University Press; 2010. pp. 146–168. [Google Scholar]
- 24.Kahneman D, et al. A survey method for characterizing daily life experience: the day reconstruction method. Science (New York, NY. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- 25.Kringelbach ML, et al. Short Answers to Fundamental Questions about Pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 7–23. [Google Scholar]
- 26.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 27.Skov M. The pleasures of art. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 270–283. [Google Scholar]
- 28.Vuust P, Kringelbach ML. The pleasure of music. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 255–269. [Google Scholar]
- 29.Frijda N. On the nature and function of pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 99–112. [Google Scholar]
- 30.Smith KS, et al. Hedonic hotspots: generating sensory pleasure in the brain. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 27–49. [Google Scholar]
- 31.Cardinal RN, et al. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 32.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 33.Kringelbach ML, et al. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 34.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 36.Watson KK, et al. Neuroethology of pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 85–95. [Google Scholar]
- 37.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 38.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 40.Berridge K. The debate over dopamine‘s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 41.Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126:807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 42.Craig AD. Opinion: How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 43.Beckmann M, et al. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aldridge JW, Berridge KC. Neural coding of pleasure: “rose-tinted glasses” of the ventral pallidum. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 62–73. [Google Scholar]
- 45.Green AL, et al. Deep brain stimulation and pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford University Press; 2010. pp. 302–319. [Google Scholar]
- 46.Georgiadis JR, et al. Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. The European journal of neuroscience. 2006;24:3305–3316. doi: 10.1111/j.1460-9568.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- 47.Völlm BA, et al. Methamphetamine activates reward circuitry in drug naïve human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- 48.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Doherty J, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 50.Kringelbach ML, Rolls ET. Neural correlates of rapid context-dependent reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- 51.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental Brain Research. 1970:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 52.Hornak J, et al. Reward-related reversal learning after surgical excisions in orbitofrontal and dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 53.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 54.Feldman Barrett L, Wager TD. The Structure of Emotion: Evidence From Neuroimaging Studies. Current Directions in Psychological Science. 2006;15:79–83. [Google Scholar]
- 55.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 56.Nauta WJ. The problem of the frontal lobe: a reinterpretation. Journal of psychiatric research. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- 57.Baylis LL, Gaffan D. Amygdalectomy and ventromedial prefrontal ablation produce similar deficits in food choice and in simple object discrimination learning for an unseen reward. Experimental Brain Research. 1991;86:617–622. doi: 10.1007/BF00230535. [DOI] [PubMed] [Google Scholar]
- 58.Butter CM, et al. Conditioning and extinction of a food-rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- 59.Baxter MG, et al. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pickens CL, et al. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickens CL, et al. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hornak J, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003:1671–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- 63.Beer JS, et al. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of personality and social psychology. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- 64.Anderson SW, et al. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 65.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 66.Kringelbach ML, et al. Translational principles of deep brain stimulation. Nature Reviews Neuroscience. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 67.Gildenberg PL. Evolution of neuromodulation. Stereotactic and functional neurosurgery. 2005;83:71–79. doi: 10.1159/000086865. [DOI] [PubMed] [Google Scholar]
- 68.Green AL, et al. Neural signatures in patients with neuropathic pain. Neurology. 2009;72:569–571. doi: 10.1212/01.wnl.0000342122.25498.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kringelbach ML, et al. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport. 2007;18:223–228. doi: 10.1097/WNR.0b013e328010dc3d. [DOI] [PubMed] [Google Scholar]
- 70.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 71.Heath RG. Pleasure and brain activity in man. Deep and surface electroencephalograms during orgasm. Journal of Nervous and Mental Disease. 1972;154:3–18. doi: 10.1097/00005053-197201000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues in clinical neuroscience. 2008;10:291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 74.Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 75.Parkin SG, et al. Unilateral and bilateral pallidotomy for idiopathic Parkinson's disease: a case series of 115 patients. Mov Disord. 2002;17:682–692. doi: 10.1002/mds.10186. [DOI] [PubMed] [Google Scholar]
- 76.Kosel M, et al. Mood improvement after deep brain stimulation of the internal globus pallidus for tardive dyskinesia in a patient suffering from major depression. Journal of psychiatric research. 2007;41:801–803. doi: 10.1016/j.jpsychires.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Miller JM, et al. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163:786–788. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- 78.Schlaepfer TE, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 79.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Steele JD, et al. Anterior cingulotomy for major depression: clinical outcome and relationship to lesion characteristics. Biological psychiatry. 2008;63:670–677. doi: 10.1016/j.biopsych.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 81.Fransson P, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fair DA, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 84.Lou HC, et al. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Human brain mapping. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buckner RL, et al. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 86.Laureys S, et al. Brain function in coma, vegetative state, and related disorders. Lancet neurology. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 87.Willoch F, et al. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108:213–220. doi: 10.1016/j.pain.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 88.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 89.Williams JM, et al. The specificity of autobiographical memory and imageability of the future. Memory & cognition. 1996;24:116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]
- 90.Addis DR, et al. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schnider A. Spontaneous confabulation and the adaptation of thought to ongoing reality. Nat Rev Neurosci. 2003;4:662–671. doi: 10.1038/nrn1179. [DOI] [PubMed] [Google Scholar]
- 92.Teasdale JD, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of consulting and clinical psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 93.Haisken-De New JP, Frick R. Desktop companion to the German Socio-Economic Panel Study (GSOEP) German Institute for Economic Research (DIW); 2005. [Google Scholar]
- 94.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 95.Komisaruk BR, et al. Sexual pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 169–177. [Google Scholar]
- 96.Georgiadis JR, Kortekaas R. The sweetest taboo: functional neurobiology of human sexuality in relation to pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 178–201. [Google Scholar]
- 97.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 98.Kringelbach ML, et al. A specific and rapid neural signature for parental instinct. PLoS ONE. 2008;3:e1664. doi: 10.1371/journal.pone.0001664. 1610.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cooper PJ, Murray L. Postnatal depression. Bmj. 1998;316:1884–1886. doi: 10.1136/bmj.316.7148.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]