Abstract
Objective
The goal of the study was to investigate the genetic and molecular basis of a novel syndrome of marked hyperglucagonemia and pancreatic α cell hyperplasia without glucagonoma syndrome.
Methods
The glucagon receptor gene (GCGR) and glucagon gene were sequenced in a patient with hyperglucagonemia and pancreatic α cell hyperplasia without glucagonoma syndrome. Enhanced green fluorescent protein (EGFP)-conjugated WT and mutant GCGR were used to characterize the functions of the mutant GCGR.
Results
The glucagon gene sequence was normal but GCGR sequencing uncovered a homozygous missense mutation, c.256C>T, p.P86S in the extracellular domain of GCGR. When expressed in HEK293 cells, GCGR P86S localized to the plasma membrane but bound 96% less radiolabeled glucagon than WT GCGR. The EC50 of glucagon-induced cAMP production was 24 nM for GCGR P86S but 2.4 nM for WT GCGR. The patient's α cells also express glucagon-like peptide 1 and pancreatic polypeptide.
Conclusion
We hereby report the first homozygous missense mutation in the human GCGR which is associated with α cell hyperplasia and hyperglucagonemia. This mutation lowers the receptor’s affinity to glucagon and decreases cAMP production with physiological concentrations of glucagon. Thus, the P86S mutation in GCGR likely causes α cells hyperplasia and hyperglucagonemia.
Key terms: glucagon receptor, mutation, hyperglucagonemia, α cell hyperplasia
Introduction
Hyperglucagonemia is a common finding during the diagnostic workup for a pancreatic mass (1, 2). Although mild hyperglucagonemia can be encountered in a variety of clinical conditions such as hypoglycemia, pancreatitis, cirrhosis, renal failure, and acute trauma or burns, significant hyperglucagonemia is usually caused by glucagonoma. The glucagonoma syndrome may occur in patients with glucagonoma (1, 2). Rarely familial hyperglucagonemia syndrome has been reported with no significant clinical abnormalities (3). We and others have reported a few cases of a novel clinical syndrome of hyperglucagonemia and pancreatic α cell hyperplasia without glucagonoma syndrome (4–6). These patients exhibit no stigmata or family history of multiple endocrine neoplasia type I or von Hippel-Lindau disease which may cause islet cell hyperplasia (7, 8). In the patient we have described (5), glucagon levels are nearly 60,000 pg/ml but no convincing evidence of glucagonoma syndrome is found, suggesting that the glucagon receptor (GCGR) and/or glucagon may be defective in function.
GCGR is a G protein-coupled receptor that activates the stimulatory α G protein (Gs) and increases cAMP production upon glucagon binding (9). The mouse model of GCGR deficiency provides insights into the genetic and molecular basis of the patient’s disease (10–12). Mice with homozygous null mutation of the GCGR exhibit chronic hypoglycemia, extreme hyperglucagonemia, and α cell hyperplasia. The clinical features of our patient resemble the phenotype of those mice. In addition, mice with GCGR null deficiency exhibit decreased fetal survival and late-onset vision loss. We hypothesize that the index patient may harbor inactivating mutation(s) in GCGR which causes the clinical syndrome.
Subjects and Methods
Subjects
The 65-year-old female patient has been previously described (5). The current study was approved by the Cedars-Sinai Institutional Review Board, and subjects (the patient, her brother, and 28 unrelated individuals) gave informed, written consent for this study.
Sequencing
Genomic DNA was extracted from peripheral blood with a Qiagen DNA extraction kit (Valencia, CA). The coding exons of GCGR (including exons 2–13 and most of exon 14) and the corresponding intron-exon borders were amplified with 4 PCR reactions using these primers: 5'-ACGGAGTCTCGCTCTGTCACCCA-3', 5'-TTACCTCGTTACCTCACCTGCCCAC-3', 5'-AGGAAGGTGGAGGTCAGATGGGGAG-3', 5'-TTCAGCACGAAGGACGCAAACAG-3', 5'-CCAGATGGATGGCGAGGAGATTG-3', 5'-ACGATGCGGACGAAGATGAAGAAGT-3', 5'-GCACCCAGACAGGACACCAGGACA-3', and 5'-GGCACGCAGTTCACGGCACA-3'. The exons and the intron-exon borders of glucagon gene were amplified with 4 PCR reactions using these primers: 5'-CCTTCTACTTATGATATTTATCTAG-3', 5'-AAAGAGTTGGTGCGAATA-3', 5'-GCCTGCCTTACTGTTTTA-3', 5'-GTGGTTTAGAGGGGTGC-3', 5'-GAGCTTTAGCCCACCG-3', 5'-TTCTGTAATCCAGACCCATAT-3', 5'-AGATCCCAGCTCTGCTACT-3', and 5'-TGTCCATAAATCCCTCCA-3'. The PCR products were sequenced at Sequetech (Mountain View, CA).
Plasmid construction and mutagenesis
Human GCGR cDNA in a pcDNA3.1 plasmid was purchased from Missouri S&T cDNA Resource Center (Rolla, MO). GCGR cDNA was amplified with TaKaRa LA Taq DNA polymerase (Shiga, Japan) using these two primers: 5'-CGGAATTCACCATGCCCCCCTGCCAGCCACAGCG-3' and 5'-CCGCTCGAGGAAGGGGCTCTCAGCCAATCTAG-3', and subcloned into pcDNA3.1/myc-His (Invitrogen, Carlsbad, CA). To create the mutant GCGR plasmid, C256 was replaced with T by QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) using WT GCGR cDNA as template and these two primers: 5'-CCCTGGTACCTGTCTTGGCACCACAAAGTGC-3' and 5'-GCACTTTGTGGTGCCAAGACAGGTACCAGGG-3'. WT and mutant GCGR cDNA were subcloned into the pEGFP-N3 vector (Clontech, Mountain View, CA) with EcoRI/SalI restriction sites to encode GCGR-EGFP conjugate with EGFP at the C terminus of GCGR. The plasmids were designated as pGCGR-EGFP-N3 and pGCGR P86S-EGFP-N3, respectively. The plasmids were transfected into HEK293 cells by Lipofectamine 2000 (Invitrogen). Transfection conditions were adjusted so that the transfected cells express similar amount of GCGR-EGFP or GCGR P86S-EGFP.
Binding assy
HEK293 cells transfected with pGCGR-EGFP-N3 or pGCGR P86S-EGFP-N3 were split into 6-well plates 1 day after transfection. One to 2 days later, 1 ml of 0.1 nM 125I-labeled glucagon (PerkinElmer, Shelton, CT) with or without 1 µM unlabeled glucagon was added to the wells and incubated with cells at 37 C for 60 min. After washing, cells were lysed with 0.1 N NaOH and lysates counted with a γ counter.
cAMP assay
Transfected HEK 293 cells were plated and grown to near-confluence in 24-well plates, then the culture medium was aspirated and replaced with 0.25 ml of Hank’s balanced salt solution (HBSS) supplemented with 1 mM isobutyl methylxanthine (IBMX) (Sigma, St. Louis, MO), 20 mm HEPES, 0.3% BSA, and various concentrations of glucagon. After incubation at 37 C for 60 min, the plates were frozen for 1 h at −80 C and then thawed at room temperature for three times, and total cAMP was measured with the LANCE cAMP kit according to the manufacturer’s protocol (PerkinElmer).
Immunoblotting and immunofluorescent staining
Lysates from HEK293 cells transfected with pGCGR-EGFP-N3 or pGCGR P86S-EGFP-N3 were run on SDS-PAGE. The proteins were transferred to membrane and blotted with anti-EGFP (Clontech) and peroxidase-labeled secondary antibody, and the membrane developed by an enhanced chemoluminescent method. For immunofluorescent staining, pancreatic and tumor tissue sections were stained with antibodies to glucagon (1:200) (Abcam, Cambridge, MA), insulin (1:500), somatostatin (1:250), pancreatic polypeptide (1:250) (DAKO, Carpinteria, CA), ghrelin (1:50) (Santa Cruz biotechnology, Santa Cruz, CA), and Glucagon-like peptide-1 (GLP-1) (1:250) (Catalog number T-4057, not cross-reacting with glucagon, Bachem, Torrance, CA) at room temperature for 1–2 hours, followed by appropriate secondary antibodies labeled with rhodamine or FITC (1:250) at room temperature for 40 minutes.
Results
Clinical features
Details of the patient’s initial presentation have been described (5). Briefly, when she was 60 years old, she was incidentally found to harbor a pancreatic mass with elevated glucagon levels (59,284 pg/ml) but without glucagonoma syndrome or stigmata of endocrine tumor syndromes. The patient’s glucagon levels remained elevated after pancreatic mass resection. Pathological examination demonstrated that the pancreatic mass was a non-functioning islet cell tumor but the surrounding pancreatic tissue exhibited α cell hyperplasia, microglucagonoma, and non-functioning microadenoma of islet cell origin.
Periodic imaging studies during follow-up did not reveal recurrence of pancreatic mass or metastasis. She reported occasional palpitation and dizziness associated with glucose levels of 40–50 mg/dL and symptoms improved with ingestion of food or fluid juice, but fasting glucose and insulin levels were normal (92 mg/dl and 2 µIU/ml, respectively). She had no clinically apparent visual dysfunction. The patient had two live births and no miscarriages. Her Persian parents were second cousins (Fig 1). The patient’s father died of “old age” at 96 and patient’s mother is alive at 86 and has good health. The patient’s 4 siblings and 2 adult sons are healthy. The glucagon levels of two sisters were either normal or slightly above the normal reference range.
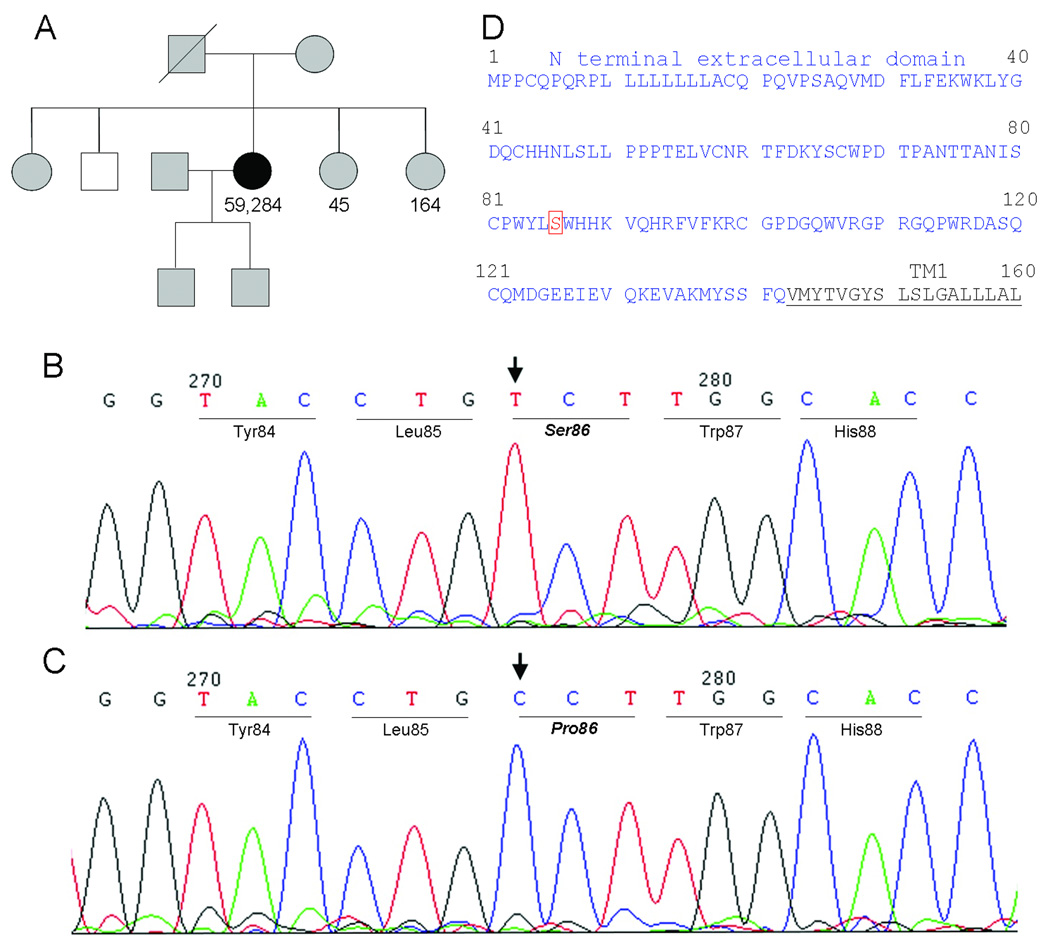
Fig. 1.
Genetic studies. A) Family tree. White, normal GCGR gene; black, mutated GCGR gene; and gray, unknown genotype. Glucagon levels (in pg/ml) were shown when available. B) DNA chromatogram from the patient showing a homozygous C to T mutation in exon 4 of GCGR gene, resulting in a P86S mutation in GCGR protein. C) DNA chromatogram from the patient’s brother who has a normal GCGR genotype. D) N terminal extracellular domain and part of transmembrane domain 1 (TM1) of GPCR P86S.
Genetic studies
As the patient’s phenotype of hyperglucagonemia and α cell hyperplasia without glucagonoma syndrome resembled those of mice with deficient GCGR (10), the patient’s and her brother’s GCGR gene were sequenced in genomic DNA. A homozygous C to T mutation was found in exon 4 of the patient’s GCGR, corresponding to nucleotide 256 in the GCGR cDNA (Fig 1). This missense mutation resulted in a proline to serine change at position 86 in the GCGR protein. The mutation was not found in the patient’s brother or in 28 unrelated United States residents. The intron-exon borders of the patient’s GCGR gene did not harbor mutations that may cause errors in mRNA splicing. The glucagon gene of the patient did not contain mutations in the exons or intron-exon borders.
Functional studies of mutant GCGR
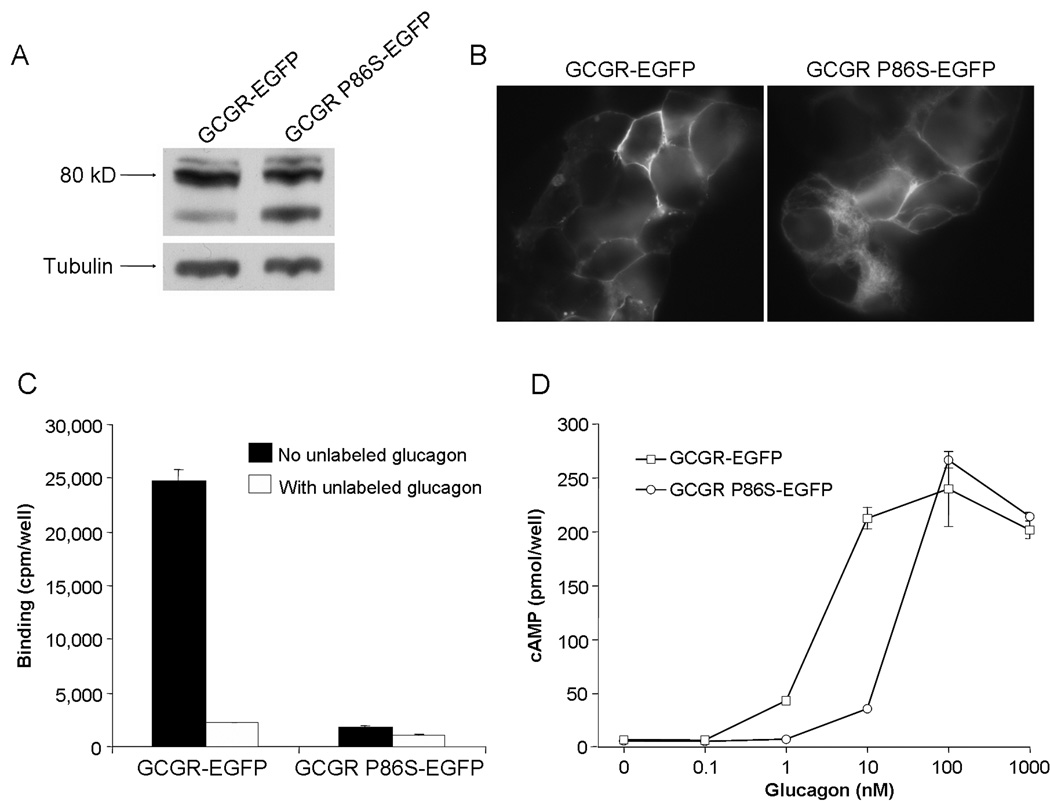
To characterize the mutant GCGR protein (GCGR P86S), the C to T mutation at position 256 in GCGR cDNA was recreated via site-directed mutagenesis, and WT GCGR and GCGR P86S were conjugated to EGFP. GCGR-EGFP and GCGR P86S-EGFP were predominantly expressed as an ~80-kD protein in HEK293 cells, as predicted by molecular weights of GCGR and EGFP (Fig 2). A minor smaller species was also expressed, which was relatively more abundant in cells expressing GCGR P86S-EGFP. Both GCGR-EGFP and GCGR P86S-EGFP were localized to the plasma membrane but the latter had significant intracellular localization in some cells (Fig 2). Both GCGR-EGFP and GCGR P86S-EGFP bound 125I-labeled glucagon and binding was inhibited by co-incubation with excess unlabeled glucagon (Fig 2). GCGR P86S-EGFP, however, bound much less 125I-labeled glucagon, only 3.5% of GCGR-EGFP. As expression levels of the two receptors were similar and both receptors were mainly localized to the plasma membrane, decreased glucagon binding by GCGR P86S-EGFP suggests a lower affinity to glucagon. Basal cAMP levels were similar in cells expressing GCGR-EGFP or GCGR P86S-EGFP (Fig 2). cAMP levels stimulated by higher concentrations of glucagon (100 nM and 1 µM) were also similar, demonstrating similar Gs-coupling efficacy of GCGR and GCGR P86S. The glucagon dose-dependent cAMP response curve of GCGR P86S, however, shifted to the right of that of GCGR, and the EC50 of glucagon stimulation was 2.4 nM for GCGR, but 23.7 nM for GCGR P86S, consistent with the lower binding affinity of GCGR P86S to glucagon.
Fig. 2.
Functional studies of GCGR P86S. A) Immunoblot of lysates from HEK293 cells expressing GCGR-EGFP or GCGR P86S-EGFP. The membrane was probed with anti-EGFP and later anti-tubulin. B) Localization of GCGR-EGFP and GCGR P86S-EGFP in HEK293 cells. C) Binding of 125I-glucagon to HEK293 cells expressing GCGR-EGFP or GCGR P86S-EGFP. D) cAMP production by HEK293 cells expressing GCGR-EGFP or GCGR P86S-EGFP after stimulation with increasing concentrations of glucagons.
Islet hormone expression pattern
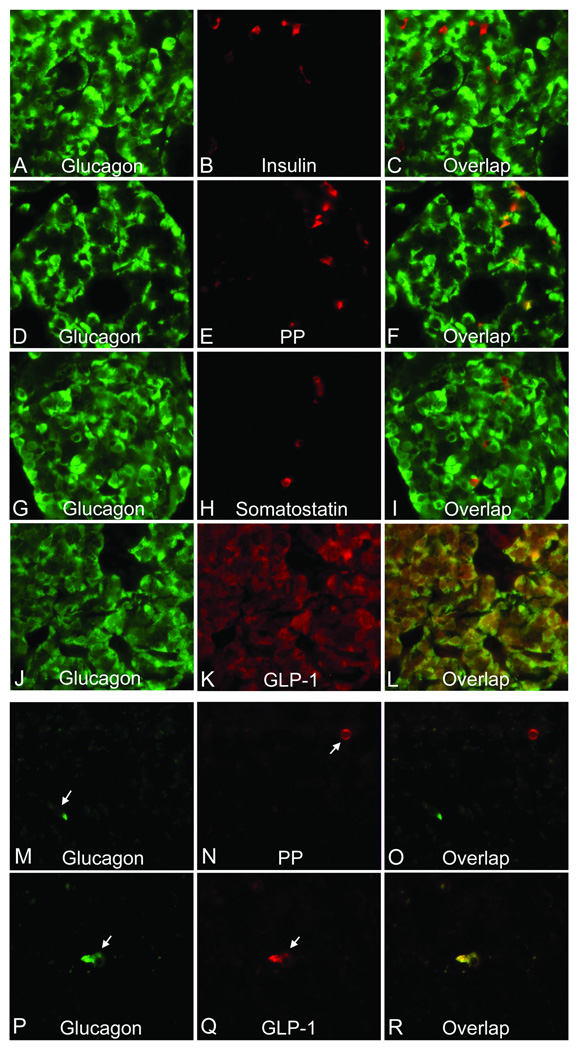
No significant hypertrophy was observed in the hyperplastic α cells of the patient. To characterize these cells further, islet hormone expression was studied by double immunoflurescent staining of glucagon and other hormones (Fig 3). Insulin was the second most abundant hormone expressed in the pancreas after glucagon, but insulin was not detected in glucagon-positive cells. Pancreatic polypeptide (PP) was the third most abundant hormone and a few aggregates of PP-positive cells were seen (data not shown). Some PP-positive cells were also glucagon-positive. Somatostatin-positive cells were scarce in the pancreas and were not positive for glucagon. We could not detect ghrelin-positive cells. GLP-1 was abundant in all glucagon-positive cells. Serum GLP-1 levels were not measured. In the non-functioning tumor, insulin, somatostatin, and ghrelin were not detected. Glucagon and PP were each found in a few cells but were not colocalized, whereas glucagon-positive cells were also positive for GLP-1.
Fig. 3.
Hormone profile of hyperplastic α cells (A–L) and the non-functioning islet cell tumor (M–R) of the patient. Tissue sections were double stained with antibodies to the indicated hormones. A, D, G, J, M, and P, glucagons; B, insulin; E and N, pancreatic polypeptide (PP); H, somatostatin; and K and Q, glucagon-like peptide 1 (GLP-1). The images on the right column (C, F, I, L, O, and R) were overlap of those in the left and middle columns. Yellow or orange indicates colocalization of hormones. Arrows, tumor cells expressing glucagon, PP, or GLP-1.
Discussion
In this study, GCGR and the glucagon gene were sequenced in a patient with hyperglucagonemia and α cell hyperplasia without the glucagonoma syndrome, and a homozygous GCGR mutation (P86S) was discovered. Effects of the P86S mutation on GCGR function were elucidated, and characteristics of the hyperplastic α cells investigated.
The GCGR P86S is processed normally and localized to the plasma membrane, as is the WT receptor. The mutation, however, is inactivating as GCGR P86S binds poorly to glucagon and requires much higher glucagon concentration to stimulate cAMP production. The inactivating mutation explains the absence of glucagonoma syndrome in this patient as it renders the GCGR much less responsive to glucagon. The P86S mutation is situated in the middle of the N terminal extracellar domain of GCGR, the deletion of which abrogates glucagon binding but not the receptor plasma membrane localization (13). GCGR is highly homologous to receptors for gastric inhibitory polypeptide (GIP) and GLP-1 (9). The crystal structures of GIP- and GLP-1-bound receptors demonstrate that P89 in GIP receptor and P90 in GLP-1 receptor, both homologous to P86 in GCGR, are critical residues in the binding surface for their respective ligands (14, 15). Thus experimental evidence in this study and the literature on homologous receptors both strongly indicate that the residue P86 in human GCGR is important for glucagon binding and replacement with a serine residue greatly reduces the receptor's ability to bind glucagon. Although the P86S mutant retains maximal cAMP production at very high glucagon concentrations (>100 nM), it is unlikely that these concentrations could be achieved in the circulation or liver. Our patient's glucagon levels of 17 nM at the time of diagnosis is the highest reported to our knowledge but even this concentration is still below the EC50 for GCGR P86S.
Regulation of α cell proliferation is complex (2). Multiple endocrine neoplasia type I and von Hippel-Lindau disease are clinically unlikely or excluded by genetic analysis in patients with α cell hyperplasia (4–6). Analogous to the hyperplasia of endocrine cells producing the cognate hormones in patients with inactivating mutations of other G protein-coupled receptors (16), α cell hyperplasia in the patient with homozygous P86S mutation may be due to a loss of negative feedback on α cell proliferation by insufficient glucagon signaling (10, 11). Specific feedback mechanisms by glucagon signaling, however, are not known. Hyperplastic α cells are not simply normal α cells in excess numbers. Our patient's α cells also express PP and GLP-1, suggesting that they have a less differentiated phenotype, similar to α cells in early embryonic stage or in mice treated with streptozotocin (17, 18). Although we did not detect cells co-expressing glucagon and insulin, such cells may exist in parts of pancreas that were not studied. α cells in mice with deficient GCGR express markers of immaturity such as GLUT2 and Pdx-1 and may produce GLP-1 as well (10, 11). It is interesting to note that the likely high GLP-1 levels in the pancreas do not result in β cell hyperplasia in this patient or in mice with deficient GCGR, suggesting a role of GCGR in mediating GLP-1 action. These results together suggest that hyperplastic α cells secondary to GCGR deficiency are of an immature and more embryonic type. Although there is only a semantic difference between hyperplastic islet and microadenoma, it is conceivable that further mutations in the hyperplastic α cells may lead to clinically significant islet cell tumorigenesis, as found in our patient. Therefore, α cell hyperplasia associated with the P86S mutation in GCGR should not be viewed as a completely benign condition and the patient should be followed by measuring islet cell tumor markers and imaging studies. The lack of fetal lethality or vision loss in our patient may be related to the partial activity of the P86S mutant; these abnormalities occur in mice with complete GCGR deficiency (11, 12).
The P86S mutation in GCGR is rare as it is not found in unrelated persons by direct sequencing or in 125 patients by single strand conformation polymorphism (SSCP) (19, 20). The rarity of the P86S mutation is also supported by the extremely low incidence of α cell hyperplasia (4–6). As the patient's parents have some consanguinity (second cousins) and other family members are apparently healthy, the patient likely has inherited the homozygous mutation from each of her parents and the inheritance of the novel disease probably is autosomal recessive (GCGR is on chromosome 17). If that is the case, heterozygous P86S mutation is rather benign because the patient’s parents should be carriers of the mutation but they are both healthy and at least the father does not die of islet cell tumors. Although homozygous deletion of GCGR results in improved glucose tolerance in mice (10), a heterozygous inactivating G40S mutation in GCGR is associated with type 2 diabetes in humans (19, 21), thus the metabolic effects of a heterozygous P86S mutation on the carriers can only be learned from observation of large numbers of patients.
If confirmed by future studies in similar patients, our study could establish the basis for a novel human disease of hyperglucagonemia and α cell hyperplasia associated with homozygous inactivating GCGR mutation. α cell hyperplasia should now be included in the differential diagnosis of hyperglucagonemia, especially in patients without glucagonoma syndrome. To further characterize this novel disease, GCGR should be sequenced in patients with suspected or confirmed α cell hyperplasia.
Acknowledgement
The authors thank Dr. Shlomo Melmed for support and critical reading of the manuscript.
Supported by NIH grant DK071870 (R.Y.) and Cedars-Sinai Medical Center
Contributor Information
Cuiqi Zhou, Division of Endocrinology, Cedars-Sinai Medical Center, Los Angeles, California.
Deepti Dhall, Department of Pathology, Cedars-Sinai Medical Center, Los Angeles, California.
Nicholas N. Nissen, Department of Surgery, Cedars-Sinai Medical Center, and Department of Surgery, David Geffen School of Medicine at UCLA, Los Angeles, California.
Chun-Rong Chen, Division of Endocrinology, Cedars-Sinai Medical Center, Los Angeles, California.
Run Yu, Division of Endocrinology, Cedars-Sinai Medical Center, Los Angeles, California.
References
- 1.Chastain MA. The glucagonoma syndrome: a review of its features and discussion of new perspectives. Am J Med Sci. 2001;321:306–320. doi: 10.1097/00000441-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Owen OE. Familial hyperglucagonemia--an autosomal dominant disorder. N Engl J Med. 1977;296:534–538. doi: 10.1056/NEJM197703102961003. [DOI] [PubMed] [Google Scholar]
- 4.Martignoni ME, Kated H, Stiegler M, et al. Nesidioblastosis with glucagon-reactive islet cell hyperplasia: a case report. Pancreas. 2003;26:402–405. doi: 10.1097/00006676-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Yu R, Nissen NN, Dhall D, et al. Nesidioblastosis and hyperplasia of alpha cells, microglucagonoma, and nonfunctioning islet cell tumor of the pancreas: review of the literature. Pancreas. 2008;36:428–431. doi: 10.1097/MPA.0b013e31815ceb23. [DOI] [PubMed] [Google Scholar]
- 6.Henopp T, Anlauf M, Schmitt A, et al. Glucagon cell adenomatosis: a newly recognized disease of the endocrine pancreas. J Clin Endocrinol Metab. 2009;94:213–217. doi: 10.1210/jc.2008-1300. [DOI] [PubMed] [Google Scholar]
- 7.Thompson NW, Lloyd RV, Nishiyama RH, et al. MEN I pancreas: a histological and immunohistochemical study. World J Surg. 1984;8:561–574. doi: 10.1007/BF01654938. [DOI] [PubMed] [Google Scholar]
- 8.Lubensky IA, Pack S, Ault D, et al. Multiple neuroendocrine tumors of the pancreas in von Hippel-Lindau disease patients: histopathological and molecular genetic analysis. Am J Pathol. 1998;153:223–231. doi: 10.1016/S0002-9440(10)65563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo KE, Miller LJ, Bataille D, et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 10.Gelling RW, Du XQ, Dichmann DS, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuguin PM, Kedees MH, Cui L, et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;147:3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umino Y, Everhart D, Solessio E, et al. Hypoglycemia leads to age-related loss of vision. Proc Natl Acad Sci U S A. 2006;103:19541–19545. doi: 10.1073/pnas.0604478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unson CG, Cypess AM, Kim HN, et al. Characterization of deletion and truncation mutants of the rat glucagon receptor. Seven transmembrane segments are necessary for receptor transport to the plasma membrane and glucagon binding. J Biol Chem. 1995;270:27720–27727. doi: 10.1074/jbc.270.46.27720. [DOI] [PubMed] [Google Scholar]
- 14.Parthier C, Kleinschmidt M, Neumann P, et al. Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc Natl Acad Sci U S A. 2007;104:13942–13947. doi: 10.1073/pnas.0706404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Runge S, Thøgersen H, Madsen K, et al. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem. 2008;283:11340–11347. doi: 10.1074/jbc.M708740200. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel AM, Weinstein LS. Inherited diseases involving G proteins and G protein-coupled receptors. Annu Rev Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- 17.Wilson ME, Kalamaras JA, German MS. Expression pattern of IAPP and prohormone convertase 1/3 reveals a distinctive set of endocrine cells in the embryonic pancreas. Mech Dev. 2002;115:171–176. doi: 10.1016/s0925-4773(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 18.Thyssen S, Arany E, Hill DJ. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology. 2006;147:2346–2356. doi: 10.1210/en.2005-0396. [DOI] [PubMed] [Google Scholar]
- 19.Hager J, Hansen L, Vaisse C, et al. A missense mutation in the glucagon receptor gene is associated with non-insulin-dependent diabetes mellitus. Nat Genet. 1995;9:299–304. doi: 10.1038/ng0395-299. [DOI] [PubMed] [Google Scholar]
- 20.Odawara M, Tachi Y, Yamashita K. Absence of association between the Gly40-->Ser mutation in the human glucagon receptor and Japanese patients with non-insulin-dependent diabetes mellitus or impaired glucose tolerance. Hum Genet. 1996;98:636–639. doi: 10.1007/s004390050274. [DOI] [PubMed] [Google Scholar]
- 21.Hansen LH, Abrahamsen N, Hager J, et al. The Gly40Ser mutation in the human glucagon receptor gene associated with NIDDM results in a receptor with reduced sensitivity to glucagon. Diabetes. 1996;45:725–730. doi: 10.2337/diab.45.6.725. [DOI] [PubMed] [Google Scholar]