Abstract
Pharmacological management of atrial fibrillation (AF) remains an important unmet medical need. Because available drugs for rhythm control of AF are often associated with a significant risk for development of ventricular arrhythmias or extracardiac toxicity, recent drug development has focused on agents that are atrial selective. Inhibition of the ultrarapid delayed rectifier potassium current (IKur), a current exclusive to atria, is an example of an atrial-selective approach. Recent studies however have shown that loss-of-function mutations in KCNA5, the gene that encodes KV1.5, the α subunit of the IKur channel, is associated with the development of AF and that inhibition of IKur can promote the induction of AF in experimental models. Another potential atrial-selective approach has recently been identified. Experimental studies have demonstrated important atrioventricular differences in the biophysical properties of the sodium channel and have identified sodium channel blockers that can exploit electrophysiological distinctions between atria and ventricles. Atrial-selective/predominant sodium channel blockers such as ranolazine effectively suppress AF in experimental models involving canine isolated right atrial preparations at concentrations that produce little to no effect on electrophysiological parameters in ventricular myocardium. Chronic administration of amiodarone was also found to exert atrial-selective depression of INa-dependent parameters and thus to prevent the induction of AF. Ranolazine and amiodarone have in common the ability to rapidly dissociate from the sodium channel and to prolong the atrial action potential duration via inhibition of IKr. Our observations suggest that atrial-selective sodium channel block may be a fruitful strategy for the management of AF.
Introduction
Safe and effective treatment of atrial fibrillation (AF) remains a major unmet medical need in our society and the problem is growing as the prevalence of AF continues to increase with the aging of the baby boom generation. AF is the most prevalent sustained clinical arrhythmia associated with increased morbidity and mortality. Prevalence of AF is 0.4–1% in the general population and greater than 8% among individuals >80 years of age. An estimated 2.5 million individuals in the North America and 4.5 million in Europe are affected by AF.1, 2 These numbers are projected to increase to 15 million in North America alone by 2050.2
Significant progress has been realized in recent years with respect to ablation therapy for AF. These advance notwithstanding, antiarrhythmic drugs (AADs) remain first-line therapy for rhythm control of AF.1, 3 Although a number of agents are available for the rhythm control of AF, the effectiveness and/or safety of these agents are not optimal. Currently available pharmacologic strategies for the rhythm control of AF include: 1) Agents that block sodium channels predominantly such as propafenone and flecainide; 2) potassium channel blockers (largely IKr) such as sotalol and dofetilide and 3) mixed ion channel blockers such as amiodarone and dronedarone.
Electrophysiological distinctions between atrial and ventricular cells
New drug development is focused on atrial-selective drugs with the goal of avoiding the ventricular proarrhythmic effects of currently available agents. In order to fully appreciate the basis for atrial-selective actions of these agents, it would be helpful to review the electrophysiological differences between atrial and ventricular cells under normal and pathophysiological conditions.
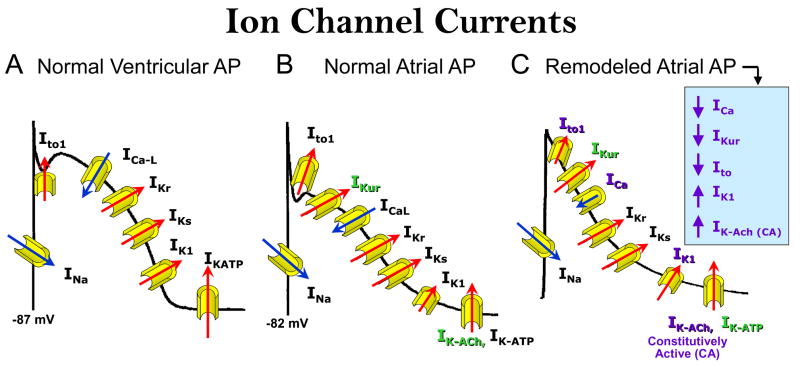
The normal action potential in atria differs from that of the ventricle with respect to ion channel currents that contribute to resting membrane potential (RMP), phase 1, and phase 3 of the action potential (Figure 1A and B).4, 5 RMP in atria is more depolarized than in the ventricle due in large part to a smaller inward rectifier potassium current, IK1. Phase 1 is more prominent in atria due to the presence of a prominent transient outward current (Ito) and a current that is exclusive to atria, known as the ultra rapid delayed rectifier potassium current, IKur. Another current that is exclusive to atria is the acetylcholine activated potassium current, IK-ACh. Phase 3 of the action potential is much slower to repolarize in atria because of weaker repolarizing currents, including the rapidly and slowly activating delayed rectifier currents (IKr and IKs) and IK1.
Figure 1. Differences in ion channel currents of action potentials from normal atria and ventricles and remodeled atria.
A and B The normal action potential in atria differs from that of the ventricle with respect to ion channel currents that contribute to resting membrane potential (RMP), phase 1, and phase 3 of the action potential. RMP in atria is more depolarized than in the atrial due a smaller IK1. Phase 1 is more prominent in atria due to the presence of a prominent Ito and IKur Both IKur and IK-ACh are exclusive to atria. Phase 3 of the action potential is much slower to repolarize in atria because of weaker repolarizing currents IKr, IKs and IK1. C: Rapid activation of the atria during AF results in a decrease in ICa, IKur and Ito, but in an increase in IK1 and constitutively active IK-ACh. The abbreviation of action potential duration is due principally to the decrease in ICa and the increase in IK1 and constitutively active -IK-ACh
The initiation of AF involves the development of both a substrate and a trigger. The electrical substrate develops as a consequence of a reduction in wavelength due largely to an abbreviation of ERP. The maintenance of AF often is facilitated by electrical and structural remodeling that is the result of the rapid activation of the atria (AF begets AF).6 The electrical remodeling further abbreviates ERP by abbreviating the atrial action potential (Figure 1C). A prolonged period of rapid activation of the atria as occurs during AF leads to a decrease in ICa, IKur and Ito, but to an increase in IK1 and constitutively active IK-ACh. The abbreviation of APD is due principally to the decrease in ICa and the increase in IK1 and constitutively active -IK-ACh.7
Atrial–selective drugs
The principal goal of rhythm control therapy is therefore to prolong the ERP and thus to eliminate the substrate for development of AF. Sodium channel blockers accomplish this by reducing excitability, thus leading to the development of post-repolarization refractoriness (PRR). Potassium channel blockers accomplish this by prolonging the atrial action potential and mixed ion channel blockers accomplish this through a combination of both actions. Because all three classes of drug have an inclination to induce ventricular arrhythmias, recent drug development for management of AF has focused on agents that selectively affect the atria but not the ventricles of the heart.
Inhibition of IKur, present in atria, but not ventricles, is an example of an atrial-selective approach.8, 9 The generation of selective IKur blockers is a great challenge because these agents often block other currents (e.g., INa by vernakalant and AZD7009 and Ito/IKACh/CA-IKACh by AVE0118) in addition to IKur.10–13 IKur density is progressively reduced with acceleration of activation rates14 and IKur density is reported to be lower in cells isolated from chronic AF atria.13, 15 Moreover, selective IKur reduction produces only minor APD90 prolongation in human remodeled atria or canine acetylcholine-treated atria (both showing a triangular action potential morphology and prone to develop AF).16, 17 These data indicate that the relative contribution of IKur to atrial repolarization in remodeled hearts is relatively low, particularly at rapid activation rates. Although IKur block may contribute to the antiarrhythmic efficacy of the IKur blockers, IKur block alone may be insufficient to effectively suppress AF and that inhibition of additional currents may be required (e.g., INa, IKr, Ito, IKACh, CA-IKAch etc).13, 17–20
The rationale for this approach has been brought into question by the recent finding that loss-of-function mutations in KCNA5, the gene that encodes the α subunit of the IKur channel is associated with the development of AF, suggesting that a reduction in IKur may promote the development of AF in humans.21 Indeed, inhibition of IKur has been shown to be capable of permitting the induction of AF in experimental models consisting of non-remodeled coronary-perfused canine right atrial preparations.17 The pro-AF action is likely related to the action of the IKur blocker to abbreviate APD90.
Atrial-selective sodium channel block
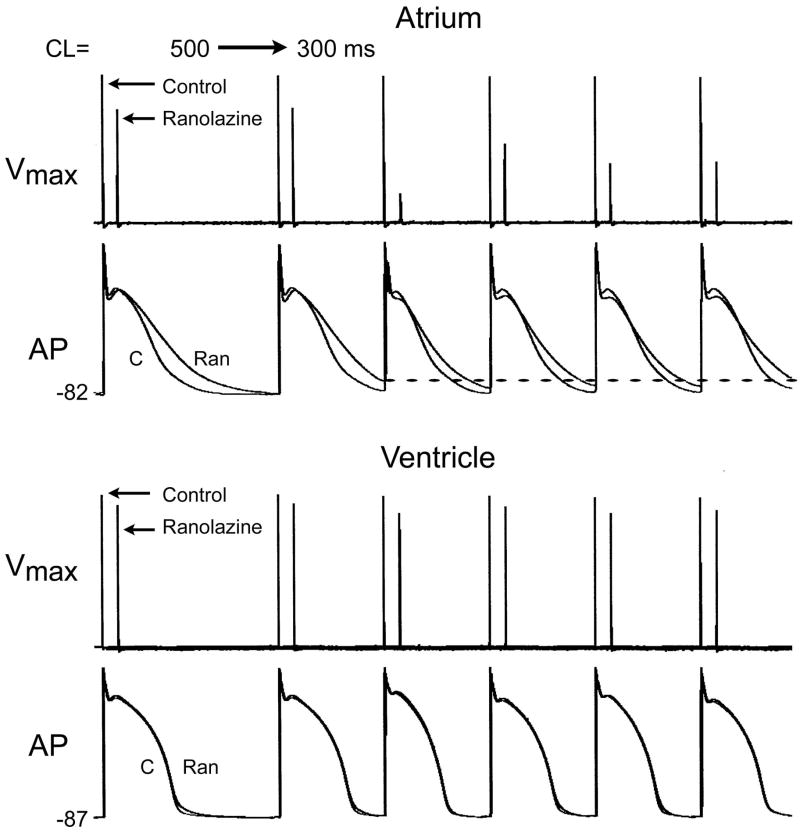
We recently introduced the concept of atrial-selective sodium channel blockers as a novel strategy for the management of AF.22–25 Two agents identified as atrial-selective sodium channel blockers are ranolazine and amiodarone. Ranolazine predominantly depresses atrial vs. ventricular sodium channel-dependent parameters and suppresses AF at concentrations that produces little to no effect in the ventricles (Figure 2).22 Chronic amiodarone likewise exerts atrial-selective depression of INa-dependent parameters, which prevent the induction of AF in experimental models.23 Ranolazine and chronic amiodarone reduce maximum rate of rise of the action potential upstroke (Vmax), prolong conduction time (CT), increase diastolic threshold of excitation (DTE), and induce PRR specifically or predominantly in the canine isolated atrial vs. ventricular coronary-perfused preparations.22, 23 Induction of PRR is a unique feature of INa blockers, occurring when the ERP is prolonged beyond the end of repolarization of the action potential. In contrast, propafenone depresses Vmax and CT, decreases DTE, and induces PRR in a chamber-independent manner at a pacing cycle length of 500 ms, but become slightly more atrial-selective at a BCL of 300 ms.26
Figure 2. Ranolazine produces a much greater rate-dependent inhibition of the maximal action potential upstroke velocity (Vmax) in atria than in ventricles.
Top: Vmax and action potentials recordings obtained from coronary-perfused canine right atrium (crista terminalis) before (C) and after ranolazine (10 μM). Bottom: Vmax and action potentials (AP) recordings obtained from coronary-perfused canine left ventricular wedge preparation (M cell) before (C) and after ranolazine (10 μM). Ranolazine prolongs late repolarization in atria, but not ventricles and acceleration of rate leads to elimination of the diastolic interval, resulting in a more positive take-off potential in atrium, which contributes to atrial selectivity of the drug. The diastolic interval remains relatively long in ventricles.
Ranolazine, first recognized as an antianginal and then as an anti-arrhythmic agent, blocks early INa, late INa, IKr, and late ICa at concentration within the therapeutic range (2–8 μM)22, 27 Amiodarone has likewise been shown to inhibit multiple cardiac ionic currents (IKr, IKs, INa, late INa, Ito, ICa-L, ICa-T, IK1, IK(ACh) IK(ATP)) as well as to block α- and β-adrenoceptors.28, 29
Sodium channel blockers generally bind more effectively to open and/or inactivated sodium channels (i.e., during the action potential) than to resting sodium channels (i.e., during the diastolic interval). Unblocking is commonly associated with the resting state of the sodium channel.30, 31 Rapid rates promote the development of sodium channel blockade by increasing the proportion of time that the sodium channels are in the open/inactivated state and reduce the time that the channels are in the resting state. There are no “pure” open or inactivated state INa blockers. As a general rule, predominantly inactivated-state blockers are INa blockers having rapid unbinding kinetics (τ ≤ 1 sec; i.e., Class IB agents) and predominantly open-state blockers are INa blockers having medium or slow unbinding kinetics (τ > 1 but < 12 sec and ≥12 sec; Class IA and IC agents, respectively).31, 32
The ability of a drug to preferentially block open vs. inactivated sodium channels does not determine the degree of atrial selectivity. While both propafenone and ranolazine are predominantly open state blockers,30, 33 (Nesterenko et al, unpublished) amiodarone and lidocaine are predominantly inactivated state blockers.34 Rate of dissociation of drug from the sodium channel on the other hand is thought to contribute to atrial selectivity. Ranolazine and amiodarone, both atrial-selective sodium channel blockers, possess relatively rapid dissociation kinetics (unbinding τ=0.2–1.6 sec)22, 35 whereas propafenone, which shows little to no atrial selectivity, displays slow dissociation kinetics (unbinding τ ≥8 sec).30
The “atrial-selective” properties of sodium channel blockers are due to atrioventricular differences in the biophysical properties of the sodium channel and differences in the morphology of atrial and ventricular action potentials (Figure 2).22, 23, 25 As previously discussed, RMP is intrinsically more depolarized in atrial vs. ventricular myocytes.36 Steady state inactivation of INa is more negative in atrial cells in dogs and guinea pigs; half inactivation voltage (V0.5) in atrial cells is 9–14 mV more negative than in ventricular myocytes.22, 37, 38 Studies conducted using human atrial and ventricular myocytes show no apparent difference in V0.5.39, 40 It is noteworthy that data for human ventricular myocytes is very limited and reported data for human atrial cells indicate wide fluctuations in V0.5 for steady-state inactivation. For example, half-inactivation for human atrial myocytes has been reported to be −88.5 mV by Lalevee et al41 and −96 mV by Sakakibara et al.39 Half-inactivation voltage measured using ruptured-patch techniques depends on a number of factors including the presence of divalents, temperature, specific voltage-clamp method and voltage protocols, as well as glycosylation.42 Thus, the absence of a difference between human atrial and ventricular cells and confirmation of this difference in other species await studies in which measurement of steady-state inactivation is made using cell-attached voltage clamp of the macroscopic sodium current in intact cells.
As a consequence of the more negative V0.5 and/or more depolarized RMP a large fraction of sodium channels are inactivated at the normal RMP in atrial cells. The fraction of resting channels is therefore smaller in atrial vs. ventricular cells at RMP. Because much of the recovery from sodium channel block commonly occurs during the resting state of the channel,31, 32 atrial cells show a greater accumulation of use-dependent sodium channel block. Atrial-selective APD prolongation (due to IKr block) may also importantly promote atrial selective depression of sodium- channel dependent parameters.
The available data suggest that atrial selectivity of sodium channel blockers at rapid activation rates is due to a number of factors working in concert: 1) The fraction of inactivated sodium channels is greater in atrial cells because of the more negative half-inactivation voltage; 2) RMP is more depolarized in atrial cells, thus further reducing the availability of sodium channel and the number of channels in the resting state; 3) The slower phase 3 in atria results in failure of the action potential to achieve maximum resting potential at rapid rates, thus leading to a depolarized take-off potential, further reducing the availability of sodium channels; 4) The slower phase 3 also leads to elimination of the diastolic interval in atria but not ventricles, thus reducing the rate of dissociation of sodium blockers from the channel; and 5) Recovery from inactivation of the sodium channel is slower in atrial cells.38
Both clinical experience and experimental evidence suggest that “dirty” drugs affecting multiple ion currents, such as amiodarone, are generally more effective than pure atrial-selective potassium or sodium channel blockers. Clinical data indicate that relatively pure INa blockers, such as lidocaine or mexiletine (Class IB agents), which have rapid binding/unbinding kinetics, are not very effective in suppressing AF.1 All clinically effective anti-AF Class I agents inhibit multiple currents (such as IKr, IKs, Ito, etc) and have relatively slow binding/unbinding kinetics from the sodium channel (e.g., flecainide or propafenone, Class IC; and quinidine, Class IA).
Agents with relatively “slow” (τ of ≥ 6 sec30, 31) sodium channel dissociation kinetics are capable of inducing VF and VT in structurally-compromised hearts (e.g., encainide, flecainide and propafenone).43 Atrial-selective INa blockers such as ranolazine and amiodarone have relatively rapid kinetics (unbinding τ=0.2–1.6 sec),22, 28 which can account for their safety in patients with acute coronary syndrome or structurally compromised hearts.29, 44, 44, 45 Despite their ability to inhibit IKr, these agents either do not (ranolazine) or very rarely (amiodarone) induce TdP. The two drugs differ with respect to extra-cardiac toxicity. Chronic amiodarone in notorious for induction of extra-cardiac toxicity,29 whereas acute and chronic use of ranolazine has been shown to be safe.44
Ranolazine, propafenone, and chronic amiodarone are effective in suppression of acetylcholine (ACh)-mediated canine isolated coronary-perfused right atria.22, 23, 26 A major difference between ranolazine and propafenone is that at clinically relevant concentrations, which effectively suppress AF (10.0 and 1.5 μM, respectively), ventricular electrophysiological parameters are strongly depressed by propafenone but not ranolazine. Ranolazine has also been shown to suppress isoproterenol-mediated AF associated with ischemia and reperfusion in canine isolated right atria.22 Chronic amiodarone (40 ms/kg/day for 6 weeks) prevents ACh-mediated AF, while causing moderate electrophysiological changes in canine isolated coronary-perfused left ventricular preparations.23 The antiarrhythmic efficacy of lidocaine (at 21 μM, also a clinically relevant concentration) in this ACh-mediated AF model is relatively poor and its electrophysiologic effects in the ventricles are much greater than those of ranolazine.22
These actions of ranolazine to suppress AF in experimental models are consistent with the results of the MERLIN-TIMI 36 clinical study in which ranolazine treatment was associated with reduced incidence of supraventricular arrhythmias and a 30% reduction in new onset AF in patients with non-ST segment elevation acute coronary syndrome.45 It is also noteworthy that in a recent single-center study, ranolazine was effective in maintaining sinus rhythm in a cohort of AF patients (most of them with structural heart diseases) in which more established AADs had failed.46
Both ranolazine and amiodarone demonstrate antiarrhythmic efficacy in the atria but have a low pro-arrhythmic potential in the ventricles likely due to their ability to significantly block late INa.47, 48 A balanced inhibition of outward IKr and inward late INa prevents the development of an exaggerated dispersion of repolarization as well as the induction of EADs, thus avoiding both the substrate and trigger for development of Torsade de Pointes arrhythmias.47, 49 Late INa inhibition plays a key role in suppression of ventricular arrhythmias in a variety of pathological conditions such a long QT syndrome, acute ischemia and heart failure.47, 49, 50
A combination of atrial-selective INa block and atrial-specific IKur block is expected to exert a more potent affect, than either approach alone, for the management of AF. Interestingly also, while the ability of IKr block alone to prolong APD and suppress AF is significantly reduced in remodeled goat atria, additional inhibition of IKur/Ito using AVE0118 was effective in suppressing AF.51
It is important to keep in mind that AF is commonly associated with electrical and structural remodeling, which can significantly modify pharmacologic response of atria to sodium and potassium channel blockers.16, 52, 53 The effects of drugs in “healthy” atria and ventricles may therefore differ from those encountered in remodeled atria.22, 23, 25 Fibrillating atria or atria susceptible to AF often display short APDs and a depolarized RMP, which reduce and promote the effectiveness of INa blockers, respectively.30 Not unexpectedly, the ability of ranolazine, chronic amiodarone, lidocaine, and propafenone to suppress sodium channel-dependent parameters is reduced in ACh-treated canine atrial preparations, possessing an abbreviated APD.22, 23, 26 Alterations in INa density or atrial conduction velocity have been reported in remodeled canine,54 but not goat6 or human55 atria. V0.5 of INa inactivation is shifted by +10 mV in cells isolated from AF vs. sinus rhythm patients,55 which may reduce atrial sensitivity to INa blockers and antiarrhythmic efficacy of INa block in persistent AF. However, the potency of Class IC agents is not altered by atrial tachypacing-induced remodeling in goats.56
Conclusion
Atrial-selective sodium channel blockers may offer a safe and effective strategy for the management of AF. Experimental studies indicate that these agents, including ranolazine and amiodarone, are effective in suppressing AF and preventing its re-induction without promoting the risk of VT/VF or TdP. The two principal factors contributing to atrial selectivity are 1) rapid dissociation of the drug from the sodium channels and 2) atrial APD prolongation secondary to inhibition of IKr, IKur and/or Ito. Additional studies specifically designed to evaluate atrial–selective sodium channel blockers for the management of AF appear to be warranted.
Acknowledgments
Supported by grant HL47678 from NHLBI (CA) and Masons of NYS and Florida
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Reiffel JA. Rate vs. rhythm control pharmacotherapy for atrial fibirillation. Journal of Atrial fibrillation. 2008;1:31–47. doi: 10.4022/jafib.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 5.Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 7.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 8.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov. 2006;5:1034–1049. doi: 10.1038/nrd2112. [DOI] [PubMed] [Google Scholar]
- 9.Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (IKur): rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52:105–120. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 10.Fedida D. Vernakalant (RSD1235): a novel, atrial-selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16:519–532. doi: 10.1517/13543784.16.4.519. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson L, Chartier D, Nattel S. Characterization of the in vivo and in vitro electrophysiological effects of the novel antiarrhythmic agent AZD7009 in atrial and ventricular tissue of the dog. J Cardiovasc Pharmacol. 2006;47:123–132. doi: 10.1097/01.fjc.0000196242.04384.c3. [DOI] [PubMed] [Google Scholar]
- 12.Blaauw Y, Gogelein H, Tieleman RG, van HA, Schotten U, Allessie MA. “Early” class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–1724. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- 13.Christ T, Wettwer E, Voigt N, Hala O, Radicke S, Matschke K, Varro A, Dobrev D, Ravens U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol. 2008;154:1619–1630. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng J, Xu D, Wang Z, Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am J Physiol. 1998;275:H1717–H1725. doi: 10.1152/ajpheart.1998.275.5.H1717. [DOI] [PubMed] [Google Scholar]
- 15.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 16.Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M, Varro A, Ravens U. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 17.Burashnikov A, Antzelevitch C. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm. 2008;5:1304–1309. doi: 10.1016/j.hrthm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burashnikov A, Antzelevitch C. How do atrial-selective drugs differ from antiarrhythmic drugs currently used in the treatment of atrial fibrillation? J Atrial Fibrillation. 2008;1:98–107. doi: 10.4022/jafib.v1i1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrlich JR, Nattel S. Atrial-selective pharmacological therapy for atrial fibrillation: hype or hope? Curr Opin Cardiol. 2009;24:50–55. doi: 10.1097/HCO.0b013e32831bc336. [DOI] [PubMed] [Google Scholar]
- 20.Burashnikov A, Antzelevitch C. New pharmacological strategies for the treatment of atrial fibrillation. Ann Noninvasive Electrocardiol. 2009;14:290–300. doi: 10.1111/j.1542-474X.2009.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson TM, Alekseev AE, Liu XK, Park SJ, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 22.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5:1735–1742. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrial-selective sodium channel block as a strategy for suppression of atrial fibrillation. Ann N Y Acad Sci. 2008;1123:105–112. doi: 10.1196/annals.1420.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel blockers: do they exist? J Cardiovasc Pharmacol. 2008;52:121–128. doi: 10.1097/FJC.0b013e31817618eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burashnikov A, Belardinelli L, Antzelevitch C. Ranolazine and propafenone both suppress atrial fibrillation but ranolazine unlike propafenone does it without prominent effects on ventricular myocardium. Heart Rhythm. 2007;4:S163. Abstract. [Google Scholar]
- 27.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;84:20R–28R. doi: 10.1016/s0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 29.Singh BN. Amiodarone as paradigm for developing new drugs for atrial fibrillation. J Cardiovasc Pharmacol. 2008;52:300–305. doi: 10.1097/FJC.0b013e31818914b6. [DOI] [PubMed] [Google Scholar]
- 30.Whalley DW, Wendt DJ, Grant AO. Basic concepts in cellular cardiac electrophysiology: Part II: Block of ion channels by antiarrhythmic drugs. PACE. 1995;18:1686–1704. doi: 10.1111/j.1540-8159.1995.tb06990.x. [DOI] [PubMed] [Google Scholar]
- 31.Carmeliet E, Mubagwa K. Antiarrhythmic drugs and cardiac ion channels: mechanisms of action. Prog Biophys Mol Biol. 1998;70:1–72. doi: 10.1016/s0079-6107(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 32.Hondeghem LM, Katzung BG. Mechanism of action of antiarrhythmic drugs. In: Sperelakis N, editor. Physiology and Pathophysiology of the Heart. 3. Kluwer Academic Publishers; 1995. pp. 589–603. [Google Scholar]
- 33.Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 noltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol. 2008;73:940–948. doi: 10.1124/mol.107.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah RR, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005;2:758–772. doi: 10.1016/j.hrthm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Kodama I, Kamiya K, Toyama J. Cellular electropharmacology of amiodarone. Cardiovasc Res. 1997;35:13–29. doi: 10.1016/s0008-6363(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 36.Golod DA, Kumar R, Joyner RW. Determinants of action potential initiation in isolated rabbit atrial and ventricular myocytes. Am J Physiol. 1998;274:H1902–H1913. doi: 10.1152/ajpheart.1998.274.6.H1902. [DOI] [PubMed] [Google Scholar]
- 37.Hiroe K, Hisatome I, Tanaka Y, Ahmmed GU, Sasaki N, Shimoyama M, Tsuboi M, Inoue Y, Manabe I, Yamamoto Y, Ohtahata A, Kinugawa T, Ogino K, Igawa O, Yoshida A, Shigemasa C, Sato R. Tonic block of the Na+ current in single atrial and ventricular guinea-pig myocytes, by a new antiarrhythmic drug, Ro 22–9194. Fundam Clin Pharmacol. 1997;11:402–407. doi: 10.1111/j.1472-8206.1997.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 38.Li GR, Lau CP, Shrier A. Heterogeneity of sodium current in atrial vs epicardial ventricular myocytes of adult guinea pig hearts. J Mol Cell Cardiol. 2002;34:1185–1194. doi: 10.1006/jmcc.2002.2053. [DOI] [PubMed] [Google Scholar]
- 39.Sakakibara Y, Wasserstrom JA, Furukawa T, Jia H, Arentzen CE, Hartz RS, Singer DH. Characterization of the sodium current in single human atrial myocytes. Circ Res. 1992;71:535–546. doi: 10.1161/01.res.71.3.535. [DOI] [PubMed] [Google Scholar]
- 40.Sakakibara Y, Furukawa T, Singer DH, Jia H, Backer CL, Arentzen CE, Wasserstrom JA. Sodium current in isolated human ventricular myocytes. Am J Physiol. 1993;265:H1301–H1309. doi: 10.1152/ajpheart.1993.265.4.H1301. [DOI] [PubMed] [Google Scholar]
- 41.Lalevee N, Nargeot J, Barrere-Lemaire S, Gautier P, Richard S. Effects of amiodarone and dronedarone on voltage-dependent sodium current in human cardiomyocytes. J Cardiovasc Electrophysiol. 2003;14:885–890. doi: 10.1046/j.1540-8167.2003.03064.x. [DOI] [PubMed] [Google Scholar]
- 42.Tyrrell L, Renganathan M, Dib-Hajj SD, Waxman SG. Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated. J Neurosci. 2001;21:9629–9637. doi: 10.1523/JNEUROSCI.21-24-09629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CAST Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 44.Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 45.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 46.Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J. 2008;8:175–181. [PMC free article] [PubMed] [Google Scholar]
- 47.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Goodrow RJ, Scornik FS, Peréz GJ. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J Cardiovasc Pharmacol Therapeut. 2004;9 (Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 48.Maltsev VA, Sabbah HN, Undrovinas AI. Late sodium current is a novel target for amiodarone: studies in failing human myocardium. J Mol Cell Cardiol. 2001;33:923–932. doi: 10.1006/jmcc.2001.1355. [DOI] [PubMed] [Google Scholar]
- 49.Antzelevitch C. Electrical heterogeneity, cardiac arrhythmias, and the sodium channel. Circ Res. 2000;87:964–965. doi: 10.1161/01.res.87.11.964. [DOI] [PubMed] [Google Scholar]
- 50.Shryock JC, Belardinelli L. Inhibition of late sodium current to reduce electrical and mechanical dysfunction of ischaemic myocardium. Br J Pharmacol. 2008;153:1128–1132. doi: 10.1038/sj.bjp.0707522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaauw Y, Schotten U, van HA, Neuberger HR, Allessie MA. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and non-specific class III drugs in the goat. Cardiovasc Res. 2007;75:89–98. doi: 10.1016/j.cardiores.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 52.Duytschaever M, Blaauw Y, Allessie M. Consequences of atrial electrical remodeling for the anti-arrhythmic action of class IC and class III drugs. Cardiovasc Res. 2005;67:69–76. doi: 10.1016/j.cardiores.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Linz DK, Afkham F, Itter G, Rutten H, Wirth KJ. Effect of atrial electrical remodeling on the efficacy of antiarrhythmic drugs: comparison of amiodarone with IKr- and Ito/IKur-blockade in vivo strial electrical remodeling and antiarrhythmic drugs. J Cardiovasc Electrophysiol. 2007;18:1313–1320. doi: 10.1111/j.1540-8167.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 54.Gaspo R, Bosch RF, Bou-Abboud E, Nattel S. Tachycardia-induced changes in Na+ current in a chronic dog model of atrial fibrillation. Circ Res. 1997;81:1045–1052. doi: 10.1161/01.res.81.6.1045. [DOI] [PubMed] [Google Scholar]
- 55.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 56.Eijsbouts S, Ausma J, Blaauw Y, Schotten U, Duytschaever M, Allessie MA. Serial Cardioversion by Class IC Drugs During 4 Months of Persistent Atrial Fibrillation in the Goat. J Cardiovasc Electrophysiol. 2006;17:648–654. doi: 10.1111/j.1540-8167.2006.00407.x. [DOI] [PubMed] [Google Scholar]