Abstract
Human immunodeficiency virus type 1 (HIV-1) originated in chimpanzees; yet, several previous studies have shown that primary HIV-1 isolates replicate poorly in chimpanzee CD4+ T lymphocytes in vitro and in vivo. The reasons for this apparent restriction are not understood. Here, we describe a new activation protocol that led to a reproducible expansion and activation of chimpanzee CD4+ T lymphocytes in vitro. Using this protocol, we uncovered species-specific differences in the activation profiles of human and chimpanzee CD4+ T cells, including HLA-DR and CD62L. Moreover, we found that improved activation facilitated the replication of both CXCR4 and CCR5-tropic HIV-1 in CD4+ T cell cultures from over 30 different chimpanzees. Thus, the previously reported “replication block” of CCR5-tropic HIV-1 in chimpanzee lymphocytes appears to be due, at least in large part, to suboptimal T cell activation.
Keywords: Chimpanzee, CD4+ lymphocytes, Activation, HIV-1, CCR5
Introduction
Chimpanzees are 98% genetically identical to humans and were one of the first non-human primate species that were experimentally infected with HIV-1 in an attempt to develop an animal model for AIDS pathogenesis (Alter et al., 1984; Gendelman et al., 1991; Watanabe et al., 1991). Early studies showed that certain strains of HIV-1 were capable of establishing a persistent infection in chimpanzees in vivo; however, all of these were T cell line adapted viruses. In contrast, primary HIV-1 isolates generally failed to replicate in chimpanzee CD4+ T lymphocytes, and this was true in vitro as well as in vivo (Benton et al., 1999; Bogers et al., 1998; Gendelman et al., 1991; Pischinger et al., 1998; Schuitemaker et al., 1993; Shibata et al., 1995; Watanabe et al., 1991). In fact, it was subsequently determined that all HIV-1 strains capable of replicating in chimpanzee PBMCs in vitro used the CXCR4 chemokine receptor for entry, either exclusively (X4) or as dual tropic (X4R5) strains (Cho, Shibata, and Martin, 1996; Schuitemaker et al., 1993; Shibata et al., 1995). Conversely, HIV-1 primary isolates that were CCR5 tropic (R5) failed to establish a productive infection in chimpanzee PBMCs in vitro (Benton et al., 1999; Cho, Shibata, and Martin, 1996; Ondoa et al., 2002; Schuitemaker et al., 1993; Shibata et al., 1995). Collectively, these findings were taken to indicate that there was a coreceptor-dependent entry restriction for HIV-1 in chimpanzee cells.
The apparent replication block of R5 strains was difficult to reconcile with the subsequent observation that naturally occurring SIVcpz strains were all R5 tropic (Bibollet-Ruche et al., 2004; Muller-Trutwin et al., 2000; Ondoa et al., 2001; Takehisa et al., 2007). Moreover, R5 HIV-1 variants were subsequently identified that were capable of establishing a persistent infection in vivo (Conley et al., 1996; ten Haaft et al., 2001). Finally, human and chimpanzee CCR5 differ by only two amino acids, at position 13 (Asn in human, Asp in chimpanzee) and 130 (Val in human, Ile in chimpanzee) and multiple studies have shown that these two positions are not involved in HIV-1 gp120 binding (Benton, Lee, and Kennedy, 1998; Dragic et al., 1998; Martin et al., 1997; Muller-Trutwin et al., 1999; Pretet et al., 1997; Samson et al., 1996; Zacharova, Zachar, and Goustin, 1997). Survey of a large number of chimpanzees also failed to identify inactivating mutations of the CCR5 gene, such as the CCR5-Δ32 nonfunctional human allele (Martinson et al., 1997; Mummidi et al., 2000; ten Haaft et al., 1997; Voevodin, Samilchuk, and Dashti, 1998). Together, these finding argued against a coreceptor related entry block of R5 tropic HIV-1 in chimpanzees T lymphocytes.
The lack of replication of HIV-1 R5 isolates in chimpanzee T-cells could be explained by a differential CCR5 expression on in vitro activated chimpanzee compared to human lymphocytes. A minimal CCR5 threshold appears to be required for efficient viral entry and this threshold seems to be dependent on CD4 expression levels (Dejucq, Simmons, and Clapham, 1999; Lin et al., 2002; Platt et al., 1998). CCR5 cell surface expression on activated human CD4+ lymphocytes varies considerably among different human donors, possibly because of polymorphisms in the CCR5 5′ cis-regulatory region (Lee et al., 1999; Trkola et al., 1996; Wu et al., 1997). Polymorphisms in the chimpanzee CCR5 5′ cis-regulatory region have been reported, distinct from the polymorphisms found in humans (Bamshad et al., 2002; Tang et al., 1999; Wooding et al., 2005). CCR5 expression on human CD4+ T lymphocytes is known to be dependent on the degree of cellular activation, on memory versus naïve phenotypes of these cells, and on the ligand used for polyclonal T cell activation (Bleul et al., 1997; Mengozzi et al., 2001; Riley et al., 1998). Indeed phytohaemaglutinin (PHA), the mitogen used in most previous studies of chimpanzee PBMCs activation, is rather ineffective at inducing CCR5 cell surface expression in human CD4+ lymphocytes (Bleul et al., 1997). The secretion of β-chemokines, such as CCL3 (MIP-1 alpha), CCL4 (MIP-1 beta), or CCL5 (RANTES) by chimpanzee T-cells could also account for the replication block of HIV-1 R5 strains in vitro. These β-chemokines can block infection either directly via blocking gp120-CD4 interaction, or indirectly by inducing CCR5 internalization (Creson et al., 1999; Mack et al., 1998; Riley et al., 1997; Sabbe et al., 2001). Consistent with this hypothesis, the gene copy number of the β-chemokine CCL3L1, a duplicated isoform of CCL3, was reported to be higher in chimpanzees compared to humans (Gonzalez et al., 2005; Shao et al., 2007).
In this study, we asked whether the previously reported failure of R5 HIV-1 to replicate in chimpanzee CD4+ T cell cultures might reflect an experimental artifact. Specifically, we asked whether different levels of T cell activation could account for the observed replication differences, as the level of T-cell activation determines the level of HIV-1 replication. Our results show that this is indeed the case. Employing a novel lymphocyte activation protocol, we show that chimpanzee CD4+ T cells can support efficient replication of prototypic R5 HIV-1 strains.
Materials and Methods
Chimpanzee and human blood
Blood samples were collected from captive chimpanzees housed at the Yerkes Primate Research Center during their annual health survey, a procedure approved by the Emory Institutional Animal Care and Use Committee. None of the chimpanzees studied were infected with HIV-1 or SIVcpz. Human blood from healthy HIV-1-negative individuals was obtained from Research Blood Components (Boston, MA). Blood samples, collected using ACD as anticoagulant, were processed within 24 hours.
Isolation of CD4+ cells
Chimpanzee and human peripheral blood mononuclear cells (PBMCs) were isolated by density separation using Ficoll-Hypaque Plus (GE-Healthcare, Piscataway, NJ) and centrifuged at 1800 rpm for 25 min at 22°C. Interphase mononuclear cells were washed once at room temperature in Hanks Balanced Saline Solution (HBSS) + 4 mM EDTA and once at 4°C in HBSS + 1% FCS. CD4+ cells were purified from total PBMCs by positive selection using magnetic MicroBeads and an autoMACS™ (Miltenyi Biotec, Auburn, CA). Human CD4 MicroBeads were used for human and non-human primate CD4 MicroBeads for chimpanzee, according to manufacturer's protocols and recommendations. Purity of the CD4+ population was determined to be >90% by flow cytometric analysis.
Standard CD4+ T lymphocyte activation
Five to ten million CD4+ mononuclear cells (monocytes and lymphocytes) in 3 ml of RPMI media + 15% FCS were placed in one well of a 6 well plate in the presence of PHA (PHA-P, Sigma-Aldridge, St Louis, MO) (3μg/ml) or Staphylococcal Enterotoxin B (SEB, Sigma-Aldridge, St Louis, MO) (3μg/ml) for 48 to 72 hours at 37°C in a 5% CO2 incubator. Following activation, cells were cultured at 1 × 106/ml and infected in complete media (RPMI + 10% FBS with 30 units/ml of IL-2 (Roche Diagnostics)).
Improved CD4+ T lymphocyte activation
Briefly, the optimized activation procedure can be divided in three steps: (i) to enable efficient lymphocyte activation and proliferation, the ratio of monocytes to lymphocytes in the CD4+ positively selected cell fraction is changed from 1:10 to about 1:2 by successive plating, (ii) monocytes are pulsed overnight with SEB and (iii) upon monocytes differentiation in macrophages, CD4+ lymphocytes are allowed to proliferate for 6 to 7 days. For the first step, twenty to forty million CD4+ mononuclear cells (monocytes and lymphocytes) were plated in 2 ml of serum-free RPMI media in one well of a 6 well plate for 30 minutes at 37°C in a 5% CO2 incubator. Cells were allowed to adhere and non-adherent cells were subsequently placed into a new well. This procedure was repeated a total of three times to increase the ratio of adherent monocyte/macrophages (10-25% of the initial cells) to non-adherent CD4+ T lymphocytes (Koller et al., 1973). For the second step, monocytes and lymphocytes were pulsed for 12-15 hours with 3μg/ml of SEB in 1.5 ml RPMI + 15% FCS. For the third step, SEB was removed by media exchange and washing non-adherent cells with HBSS, monocytes were differentiated in 2.5 ml of DMEM with 10% Giant Cell Tumor conditioned medium (BioVeris Corp., Gaithersburg, MD) and 10% Human AB serum (Fisher Bioreagents, Fair Lawn, NJ); lymphocytes were then allowed to proliferate for 6-7 days. During this time, an additional 2 ml of the same media was added to each well to sustain lymphocyte proliferation. Combining non-adherent cells from all wells, 30-40 million activated CD4+ lymphocytes were typically harvested by day 6-7. Following proliferation, cells were cultured at a density of 1 × 106/ml in complete media (DMEM + 10% FBS with 30 units/ml of IL-2 (Roche Diagnostics)) for 24 hours prior to infection. Culturing the cells in complete media for at least 24 hours prior to infection increased the activated cells susceptibility to HIV-1 infection (data not shown). Once activated, CD4+ lymphocytes continued to proliferate in complete media in the absence of further stimuli for at least two weeks.
Immunofluorescent staining and flow cytometric analysis
Activated T cells were transferred in staining buffer (PBS + 2% FBS + 0.2% sodium azide) and the following conjugated monoclonal antibodies against human differentiation antigens were used to determine the cell surface expression of the corresponding markers (clone number indicated in parenthesis): anti-CD3 (UCHT1), anti-CD8 (RPA-T8), anti-CD4 (RPA-T4), anti-CD69 (FN50), anti-HLA-DR (G46-6), anti-CD25 (M-A251), anti-CD45RO (UCHL1), anti-CD45RA (HI100), anti-CD62L (Dreg 56), anti-CD184 (12G5), all from BD Biosciences (BD Biosciences, San Jose, CA), anti-CD195 (CTC5) from R&D Systems (R&D Systems, Inc., Minneapolis, MN). Stained cells were analyzed on a Cell Lab Quanta™ SC MPL flow cytometer (Beckman Coulter, Fullerton, CA) and a minimum of 10,000 events per 3-color stain were collected. Matching antibody isotype control stains were included as negative controls for each antibody combination in all runs. Results were analyzed using Cell Lab Quanta™ software (Beckman Coulter, Fullerton, CA) and CellQuest™ Pro (BD Biosciences, San Jose, CA).
Viruses and virus stocks
HIV-1 SG3, YU2, JRCSF and ADA virus stocks were generated by transfecting the respective full-length molecular clone into 293T cells using FuGene 6 (Roche Applied Science, Indianapolis, IN), according to manufacturer's protocol. Forty-eight to seventy-two hours post-transfection, culture supernatants were harvested, clarified by low speed centrifugation, and aliquots were stored at -70°C. The HIV-1 Bal isolate was obtained from the NIH AIDS Research and Reference Reagent Program and propagated on SEB-activated human CD4+ lymphocytes to generate virus stock. Culture supernatants were harvested, clarified by low speed centrifugation, and aliquots were stored at -70°C. Infectious titers of virus stocks were determined using the JC53BL assay as described previously (Derdeyn et al., 2000; Wei et al., 2002).
Infection and replication kinetics
Chimpanzee and human activated CD4+ T lymphocytes (0.5 106/vial) were infected overnight at a multiplicity of infection (MOI) of 0.1 (based on JC53BL infectious titer) in 300μl of complete media. Overnight incubation in this small volume was necessary for optimal infection of chimpanzee lymphocytes. After 12-15 hours, cells were washed three times with HBSS medium and plated in a well of a 24 well plate in 2 ml of complete media. Forty microliters of supernatant were collected every 2-3 days and stored at -70°C. To monitor virus replication, the p24 viral antigen was quantified in the culture supernatants using the HIV-1 p24 antigen EIA kit according to the manufacturer's protocol (Beckman Coulter, Fullerton, CA).
Bio-Plex™ cytokine assay
Activated lymphocytes culture supernatants were collected after 24 hours incubation in complete media, and stored at -70°C. Cytokines concentration for IL-4, IFN-γ, CCL3 and CCL5 were determined from 50 μl of supernatant using the corresponding Bio-Plex™ Human Cytokine Assay according to manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA). Data were collected using a Bio-Plex™ 200 suspension array system (Bio-Rad Laboratories) and statistical analyses of the results were performed using Prism software version 4.0c for Macintosh (GraphPad Software Inc.).
Statistical analyses
The nonparametric Mann-Whitney U test (two-tailed) was used to analyze the cell surface markers expression and cytokine production differences between chimpanzee and human donors. Differences in expression between the two groups were considered significant when p values were ≤0.01. Correlation analysis for the HIV-1 SG3 and YU2 replication in activated chimpanzee T cells was performed using the Spearman's rank correlation test. These statistical analyses were performed using Prism software version 4.0c for Macintosh (GraphPad Software Inc.).
Results
Limited proliferation of chimpanzee CD4+ T lymphocytes under standard activation conditions
Standard lymphocyte activation protocols make use of a variety of agents such as lectins, enterotoxins or monoclonal antibodies that induce T-cell activation and proliferation via T-cell receptor cross-linking (Kruisbeek, Shevach, and Thornton, 2004; Vicenzi and Poli, 2005). In an initial set of experiments, we thus used phytohaemaglutinin (PHA) to activate chimpanzee CD4+ T lymphocytes as previously described (Beaumont et al., 2000; Gendelman et al., 1991; Nguyen et al., 2006; Schuitemaker et al., 1993; Shibata et al., 1995; Watanabe et al., 1991). Replication of the X4-tropic HIV-1 SG3 strain in these PHA-activated T cell cultures was highly variable among chimpanzee donors (n=8, day 8 post infection median p24 = 13ng/ml, range 0-72 ng/ml), ranging from no replication (n=2) to levels 10-fold lower compared to human donors (n=6, day 8 post-infection median p24 = 193 ng/ml, range 120-430 ng/ml) (Figure 1A). Although HIV-1 SG3 was specifically selected for its ability to efficiently replicate in chimpanzee T-cells (Ghosh et al., 1993), lymphocytes from some chimpanzee donors were unable to support SG3 replication under these conditions. Moreover, the R5 strain YU2 did not replicate in any of the chimpanzee T cell cultures following this standard protocol (data not shown). Similar results were obtained when chimpanzee CD4+ lymphocytes were stimulated with concanavalin A (data not shown). Flow cytometry analysis of PHA-stimulated cells identified activated CD4+ lymphocytes in both human and chimpanzee cultures, as shown by the appearance of larger cells with increased granularity (Figure 1B). However, trypan blue staining revealed two to three-fold lower numbers of viable cells in PHA activated chimpanzee compared to human cultures. We thus explored conditions under which human and chimpanzee CD4+ lymphocytes would be activated and proliferate to the same extent and supporting HIV-1 replication.
Figure 1. HIV-1 SG3 replication in PHA-activated chimpanzee and human CD4+ lymphocytes.
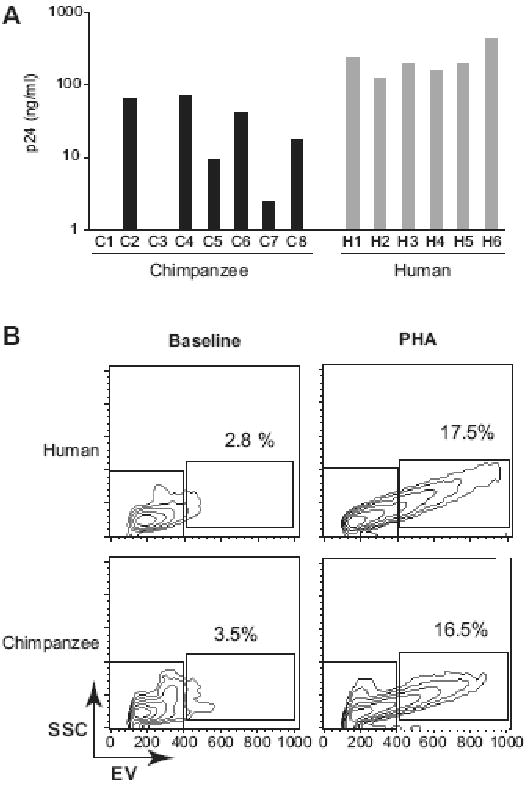
A. CD4+ lymphocytes from eight chimpanzees (C1 to C8) and six human (H1 to H6) donors were activated with PHA and tested for their ability to support HIV-1 SG3 replication. Infections were initiated at a multiplicity of infection (MOI) of 0.1. Virus replication was monitored by quantifying the p24 viral antigen in the culture supernatants on day 8 post-infection (y axis, nanograms of p24 per ml of culture supernatant). The grey box indicate the cutoff (1ng/ml) below which replication is considered negative. B. Representative flow cytometric density plots (x-axis, electronic volume; y axis, side scatter) of CD4+ lymphocytes at baseline (left panels) and 48 hours post PHA activation (right panels) for human (top panels) and chimpanzee (lower panels). The percentage of activated cells located in gate R2 are indicated.
We first evaluated a stimulation protocol using anti-CD3 or a combination of anti-CD3 + anti-CD28 monoclonal antibodies (mAbs) and the results were recently reported (Bibollet-Ruche et al., 2008). We found that not all anti-CD3 mAb isotype were equally capable of stimulating chimpanzee T cells. While immunological activation of chimpanzee lymphocytes was achieved with anti-CD3 mAbs of the IgG2a isotype, these activated cells still remained resistant to infection with the R5 tropic HIV-1 YU2 strain and less supportive of the X4-tropic HIV-1 SG3 replication compared to human lymphocytes activated under the same protocol.
We next tested the Staphylococcal Enterotoxin B (SEB) superantigen activation competence of human and chimpanzee CD4+ lymphocytes. Previous studies demonstrated that bacterial superantigens such as SEB induce a more robust proliferative response in non-human primate T-cells, including macaque and chimpanzee, compared to lectin stimulation (Bavari, Hunt, and Ulrich, 1995; Kakimoto et al., 1999; Loffredo et al., 2004). As with PHA, we found that cellular proliferation was reduced in chimpanzee compared to human CD4+ lymphocyte cultures. However, SEB stimulation increased HIV-1 SG3 replication in both chimpanzee and human CD4+ lymphocytes by 3 to 40-fold compared to PHA activated cells (Figure 2A). Although SEB stimulated chimpanzee CD4+ lymphocytes were still refractory to HIV-1 YU2, a 4-fold growth increase was observed in SEB-activated human cells (Figure 2B). We thus selected SEB as the mitogen for all further studies.
Figure 2. HIV-1 replication in PHA or SEB activated T-cells.
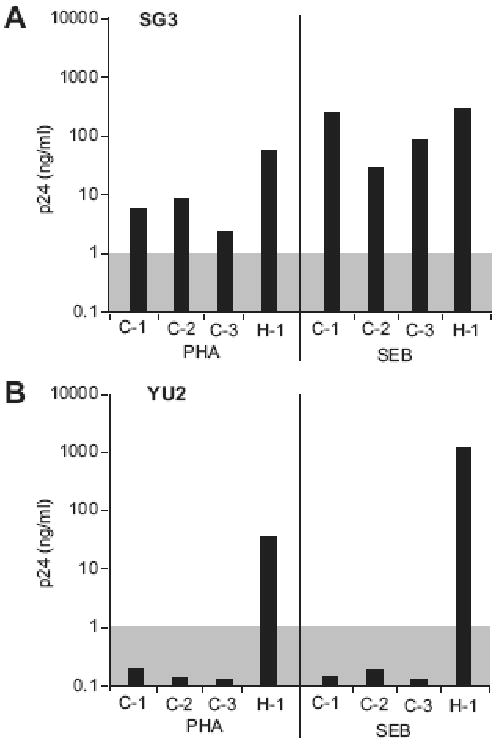
CD4+ lymphocytes from three chimpanzees (C-1 to C-3) and one human (H-1) donors were activated with PHA or SEB and tested for their ability to support HIV-1 SG3 (A) and YU2 (B) replication. Infections were initiated at a multiplicity of infection (MOI) of 0.1. Virus replication was monitored by quantifying the p24 viral antigen in the culture supernatants on day 8 post-infection (y axis, nanograms of p24 per ml of culture supernatant). Virus replication was considered positive when the p24 antigen level reaches a cutoff value of 1 ng/ml (shaded box). For the 3 chimpanzee cultures, the median p24 level for the SG3 infections the in PHA-activated cells was 5.8 ng/ml and the median p24 in SEB-activated cells was 88.3 ng/ml.
Optimization of the activation protocol for chimpanzee CD4+ T lymphocytes
Original experiments aimed at deciphering the mode of action of mitogens, such as PHA or SEB, in respect to T cell activation were performed in the presence of antigen presenting cells (APCs), including dendritic cells and macrophages (Bhardwaj et al., 1993; Boshell et al., 1996; Damaj, Mourad, and Naccache, 1992; Hewitt et al., 1992; Mills et al., 1985; Spertini, Spits, and Geha, 1991; Wakasugi et al., 1985). We thus hypothesized that APCs, specifically macrophages, may provide additional signals, via direct cell-cell contact or cytokine production, that could promote and sustain activation of chimpanzee T lymphocytes. To induce monocyte differentiation into macrophages, we supplemented the culture medium with 10% Giant Cell Tumor conditioned medium, which contains M-CSF. Similar activation schemes using SEB-pulsed dendritic cells for the expansion of human CD4+ lymphocytes have recently been described (Oswald-Richter et al., 2007; Unutmaz et al., 1999). The results showed that chimpanzee, as well as human, CD4+ lymphocytes activated by SEB and cocultured in the presence of autologous monocyte-derived macrophages exhibit a strong and reproducible proliferative response.
Phenotypic characterization of activated lymphocytes
Differences in susceptibility to HIV-1 infection have been reported for various human lymphocyte populations, including naïve and memory T-cells, subsets of memory cells, as well as cells with T helper type 1 (Th1) or type 2 (Th2) phenotypes (Annunziato et al., 2000; Bonecchi et al., 1998; Cayota et al., 1993; Chun et al., 1997; Oswald-Richter et al., 2007; Song et al., 2005b). We thus determined the phenotype of the activated lymphocytes to ensure that HIV-1 replication in human and chimpanzee cultures was not biased by species-specific differences in the expanded T cell populations.
For sixteen chimpanzee and seven human donors, activated CD4+ T cells were analyzed for the expression of lymphocyte surface markers. Flow cytometric analyses were performed using monoclonal antibodies against human cell surface antigens known to cross-react with the cognate chimpanzee antigen. The expanded cells were CD3+ CD4+ with a reproducible purity of over 90% (data not shown). The cell surface markers expression profiles for representative human and chimpanzee are shown in Figure 3. The magnitude of expression for CD4, CD69, CD25, CCR5, CD28 and CD45RO was comparable between chimpanzees and humans (Figure 4). The mean fluorescence intensity of these markers on activated lymphocytes was not statistically significant between human and chimpanzees (p > 0.1) and showed limited inter-individual differences (data not shown). Expression of the CD69 and CD25 early and late activation markers, respectively, were comparable between human and chimpanzee. On average, > 50% of the cells expressed CD25, indicative of an activated phenotype (Figure 4). The CXCR4, CD62L and CD45RA markers were consistently detected on chimpanzee T cells, while their expression was lower or not detected on human T cells (Figure 3 and 4). Significantly lower levels of HLA-DR expressing cells were detected in chimpanzee compared to human cultures (p=0.0002). Further analysis of HLA-DR expression prior to activation revealed low expression on CD4+ lymphocytes and high expression on monocytes for both human and chimpanzee cultures (Figure 5). Thus, T-cell activation induced HLA-DR expression in human but not chimpanzee CD4+ lymphocytes (Figure 5). The HIV-1 coreceptor CCR5 was expressed at high levels in both species, while CXCR4 expression was significantly lower in humans (p=0.01) (Figure 3 and 4). Activated lymphocytes also expressed the CD45RO marker, indicating that the expanded cells were derived from the memory subset (Figure 3 and 4). Analysis of the CD45RO and CD45RA markers expression on CD4+ lymphocytes prior to stimulation revealed that naïve (CD45RA+) and memory (CD45RO+) subsets were equally represented (data not shown), indicating that our activation procedure preferentially induced the proliferation of CD4+ CD45RO+ memory lymphocytes. Low levels of the CD45RA marker were consistently detected on chimpanzee cells while not detected on human cells (p = 0.0005, Figure 3 and 4). Cells from human donors expressed low to undetectable levels of the CD62L marker, while this marker was expressed at higher levels on chimpanzee T cells (p = 0.0009, Figure 3 and 4). Upon stimulation, cell surface CD62L is hydrolyzed by metalloproteases and the low expression on human cells is likely due to degradation as no metalloproteinase inhibitors were included during the activation procedure or during the 24 hours incubation in complete media (Kayagaki et al., 1995; Preece, Murphy, and Ager, 1996). The CD62L expression detected on activated chimpanzee cells suggests a differential regulation of L-selectin between human and chimpanzees.
Figure 3. Phenotype of activated human and chimpanzee lymphocytes.
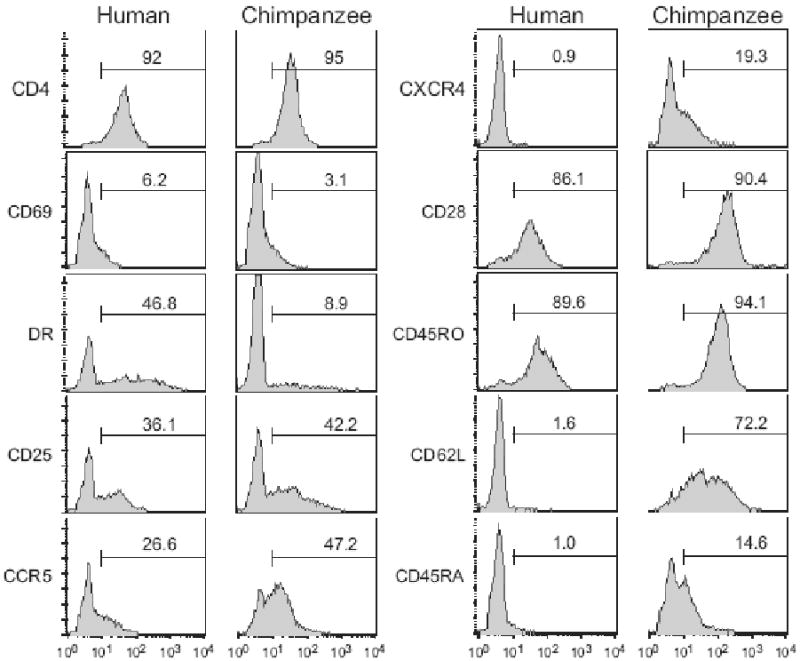
Activated CD4+ T cells were stained and analyzed for cell surface activation (CD69, HLA-DR, CD25) and phenotypic (CD28, CD45RO, CD62L and CD45RA) markers expression as described in Materials & Methods. Expression of the HIV-1 CD4 receptor as well as the CCR5 and CXCR4 coreceptors were also analyzed. Representative histogram profiles are shown for one human (left panels) and one chimpanzee (right panels) donor (x-axis: log fluorescence, y-axis: cell number). The electronic gate was set for the CD4+ lymphocyte population and the percentage of cells expressing this maker was calculated using the same gate for all stains.
Figure 4. Surface expression of select antigens on activated CD4+ lymphocytes.
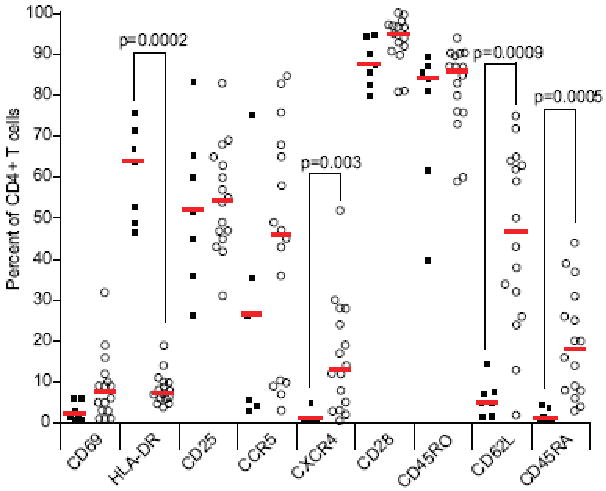
Activated CD4+ lymphocytes from 16 chimpanzee (open circles) and 7 human (filled squares) donors were stained with antibodies as described in Materials and Methods. Surface antigen expression is shown as the percentage of cells expressing the indicated antigen from the CD4+ lymphocytes population electronic gate (y axis). For each antigen, a red horizontal bar indicates the median value for human and chimpanzee donors. p values were obtained from Mann-Whitney tests between human and chimpanzee donors, and non-significant (p>0.01) values were omitted.
Figure 5. Surface expression of HLA-DR on CD4+ lymphocytes and monocytes.
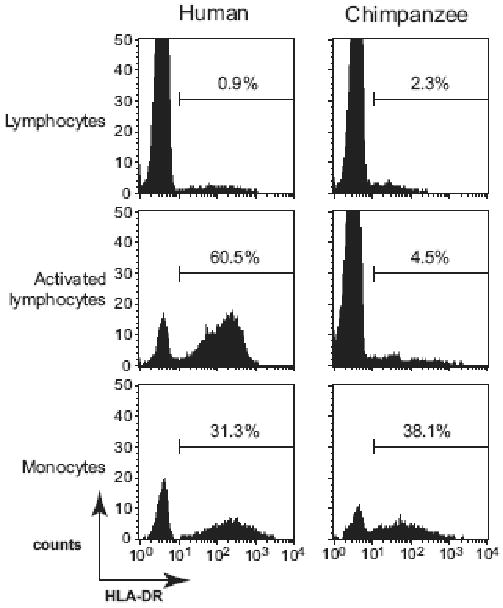
CD4-positively selected cells (lymphocytes and monocytes) and activated CD4+ lymphocytes were stained with anti-HLA-DR-FITC, anti-CD4-PECy5 and anti-CD3-PE antibodies. A representative profile for one human (left panels) and one chimpanzee (right panels) donor is shown (x-axis = log FITC fluorescence, y-axis = cell counts). Electronic gates were set for lymphocyte (CD4+ CD3+ population) or monocyte (CD4+ CD3-) populations and HLA-DR expression was analyzed. The percentage of cells expressing HLA-DR is indicated for each cell type.
We next monitored the production of interferon gamma (IFN-γ) and interleukin-4 (IL-4) by activated lymphocytes as markers for Th1 and Th2 subsets, respectively. No IL-4 production (detection limit 1 pg/ml) and high level of IFN-γ were detected in both species. The average level IFN-γ in culture supernatant was 985 pg/ml (range 77-2168, median 898 pg/ml) and 968 pg/ml (range 176-2877 pg/ml, median 780 pg/ml), in human and chimpanzee activated CD4+ lymphocytes culture supernantants, respectively. The IFN-γ levels were not statistically different between species. These results indicate that the lymphocytes expanded under our protocol are predominantly from the Th1 subset.
Beta-chemokines secretion by activated lymphocytes
Although there was no significant difference in the number of CCR5 expressing cells between activated human and chimpanzee lymphocytes (Figure 4), a higher production of CCR5-binding chemokines (CCL5, CCL3, CCL4) by chimpanzee cells could theoretically block infection (Annunziato et al., 2000; Margolis et al., 1998; Trkola et al., 1998). This possibility was of particular interest since chimpanzees were reported to have a higher copy number of the CCL3L1 gene, an isoform of the CCL3 chemokine (Gonzalez et al., 2005; Shao et al., 2007). To test this hypothesis, we quantified the production of CCL5 and CCL3 (+ CCL3L1 isoform as the Bio-Plex assay detects both chemokines) in the culture supernatants of activated lymphocytes. High levels of CCL5 were measured in both species and, surprisingly, a median 10-fold lower secretion of CCL3 was found in chimpanzee compared to human donors (Figure 6). The CCL3 expression differences were highly significant (p=0.0002). These results indicate that a higher secretion of CCR5-binding cytokines by chimpanzee cells does not explain the reported replication block observed for R5 HIV-1 in chimpanzee lymphocytes.
Figure 6. CCL3 and CCL5 secretion by activated CD4+ lymphocytes.
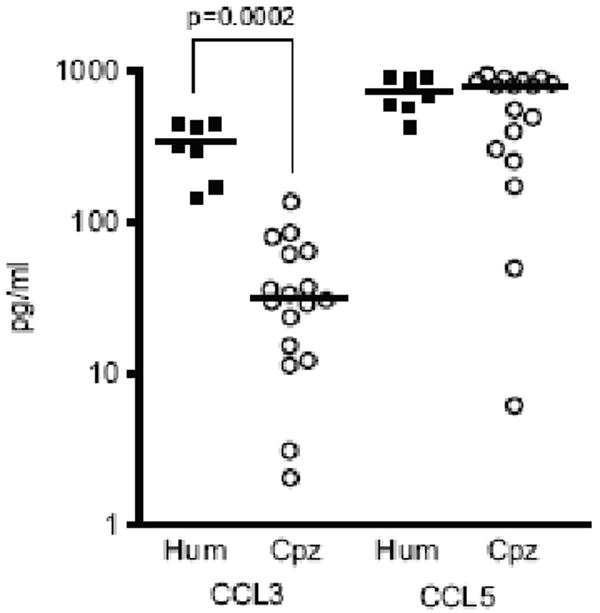
The production of the CCL3 and CCL5 beta-chemokines was quantified in the culture supernatant of activated CD4+ lymphocytes from seven human (filled squares) and seventeen chimpanzee (open circles) donors. (y axis, picograms of CCL3 or CCL5 per ml of culture supernatant)
SG3 and YU2 replication in chimpanzee CD4+ T lymphocytes
We next asked whether chimpanzee lymphocytes activated with the new protocol support HIV-1 replication, using the HIV-1 SG3 and YU2 prototypic strains. The results showed that both viruses replicated in T cells from all chimpanzee donors tested. Mean p24 levels in the supernatants of SG3- and YU2-infected CD4+ lymphocytes of 23 chimpanzees at 7 days post-infection was 375 ng/ml (range 74-2480 ng/ml, median 374 ng/ml) and 33 ng/ml (range 1-460 ng/ml, median 40 ng/ml), respectively (Figure 7A). This profile was similar to the replication levels of these two viruses in human CD4+ T lymphocytes, although relative growth differences between SG3 and YU2 were less pronounced in human lymphocytes. The chimpanzee cultures exhibited a wider range of HIV-1 replication compared to human (Figure 7B and 7C), including five individuals with reduced YU2 replication (p24 <10 ng/ml, Figure 7C). This was not due to sub-optimal activation as SG3 replicated efficiently in these same cultures (p24 values for SG3 at day 7 in these five chimpanzees: average 488 ng/ml, range 320-670ng/ml, median 550 ng/ml). These results thus showed that chimpanzee CD4+ T cell cultures differed significantly more in their ability to replicate HIV-1 than human cultures, indicating significant intrinsic inter-individual differences among chimpanzee donors in supporting the replication various HIV-1 strains.
Figure 7. HIV-1 SG3 and YU2 strains replication potential in chimpanzee and human CD4+ lymphocytes.
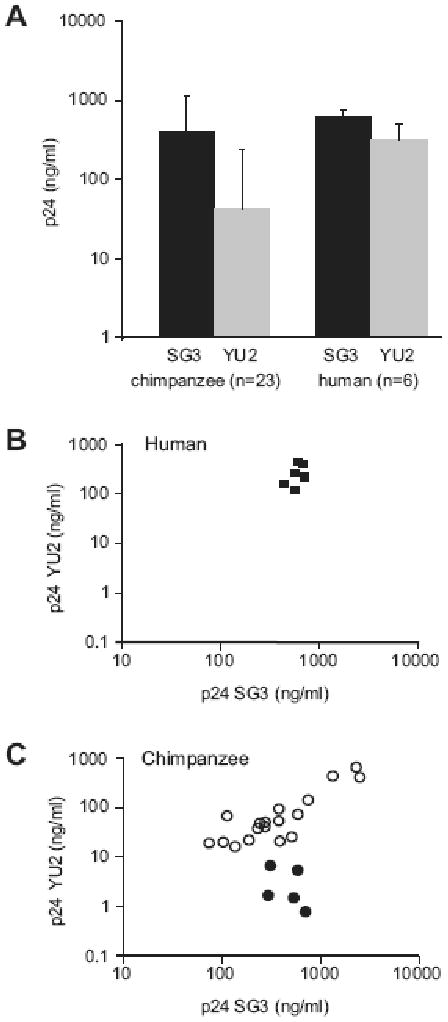
(A) Median p24 values in the culture supernatant of SG3- (black) or YU2-infected (grey) in activated CD4+ lymphocytes from chimpanzee (n=23) and human (n=4) donors. The error bars represent one standard deviation. The p24 antigen was quantified 7 days post infection (y axis, nanograms of p24 per ml of culture supernatant), initiated at day 0 at 0.1 MOI. The day 7 p.i. p24 values for SG3 (x-axis) and YU2 (y-axis) plotted for 6 human (B) and 23 chimpanzee donors (C). The filled circles represent the five chimpanzee donors that supported limited HIV-1 YU2 replication (p24 < 10 ng/ml).
Susceptibility of chimpanzee CD4+ T lymphocytes to primary HIV-1 isolates
To validate the SG3 and YU2 isolates replication results, we next analyzed the replication of the HIV-1 R5 prototypic strains ADA, BaL and JRCSF in activated CD4+ lymphocytes from four human and chimpanzee donors. All strains replicated efficiently and with comparable replication kinetics in activated human T-cells (Figure 8A). Conversely, the BaL isolate replicated efficiently in chimpanzee cells, to levels comparable to the SG3 and YU2 prototypic strains. The ADA strain typically exhibited lower replication levels and, unexpectedly, the JRCSF strain did not replicate in CD4+ lymphocytes from any of the chimpanzee donor tested (Figure 8B). Taken together these results indicate that the previously reported failure of R5 HIV-1 to replicate in chimpanzee T cells was due, at least in part, to inadequate T cell activation but also suggest that chimpanzee donors differ in their susceptibility to HIV-1 infection or that some human adapted HIV-1 strains can be restricted for replication in chimpanzee cells.
Figure 8. Primary HIV-1 strains YU2, JRCSF, ADA, and BaL replication kinetics in human and chimpanzee CD4+ lymphocytes.
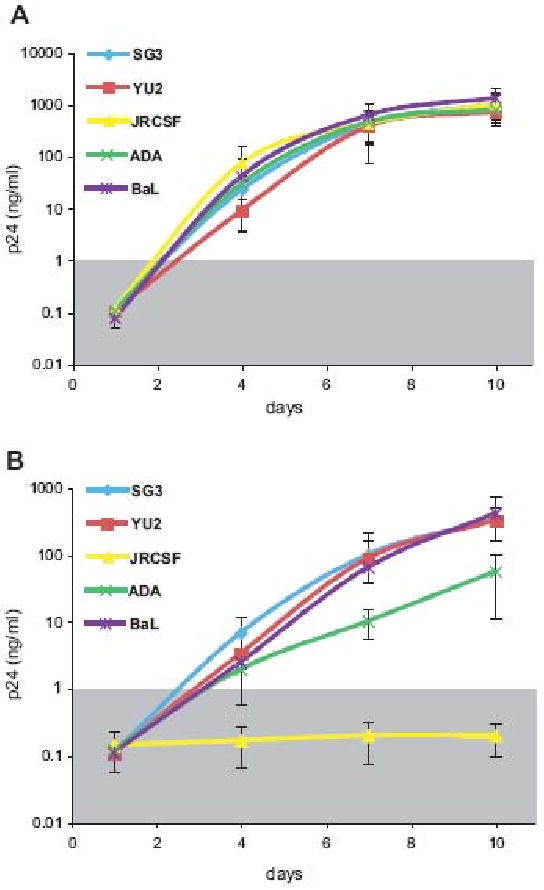
Virus replication was monitored by measuring the level of the p24 antigen in culture supernatants. Replication curves for each strain are shown as the average of independent experiments in CD4+ T lymphocytes from four different chimpanzee and human donors; the error bars represent one standard deviation calculated for each time point. The grey box indicate the cutoff (1ng/ml) below which replication is considered negative.
Discussion
In this study, we investigated the replication potential of R5 and X4 HIV-1 strains in human and chimpanzee lymphocytes. We show that efficient and reproducible activation of chimpanzee CD4+ lymphocytes can be achieved in the presence of SEB and autologous macrophages, and that this mode of activation renders chimpanzee T cells capable of supporting the replication of both prototypic X4 and R5 tropic HIV-1 strains. Characterization of activated T cell also revealed differences in surface marker between human and chimpanzee, expanding previously reported species-specific differences of cell surface protein expression.
In the past, investigators screened chimpanzees by in vitro culture to find those most susceptible to HIV-1 infection (Eichberg et al., 1987; Nguyen et al., 2006; Pischinger et al., 1998; Schuitemaker et al., 1993; Shibata et al., 1995); however, the level of T cell activation in these experiments was not determined. A more recent study noted differences in the proliferative response between human and chimpanzee T-cells (Nguyen et al., 2006). This suggested that chimpanzee lymphocytes might require different activation stimuli than human lymphocytes to support HIV-1 replication. We reasoned that autologous macrophages might provide signals that would induce a longer lasting and/or more robust activation state. We found that the presence of differentiated macrophages early in the activation process was indeed key since it facilitated sustained SEB-induced CD4+ lymphocyte proliferation, a function not provided by monocytes or B-lymphocytes in this system (FB-R, unpublished).
Chimpanzee CD4+ lymphocytes activated under the new protocol supported efficient replication of the prototypic R5 YU2 strain, revealing for the first time that the previously reported R5 replication block was likely a tissue culture artifact (Beaumont et al., 2000; Cho, Shibata, and Martin, 1996; Schuitemaker et al., 1993; Shibata et al., 1995). We did, however, find that lymphocytes from different chimpanzee donors varied in their ability to support YU2 replication, more so than comparable human cultures (Figure 7B and C). This was not due to lymphocyte activation levels, coreceptor expression, or availability of target cells, and may thus reflect as yet unidentified host factors that influence viral replication. The mechanisms underlying these differences among chimpanzee donors remain to be explored.
The phenotypic characterization of CD4+ lymphocytes activated under the new protocol also revealed species-specific differences in the expression of surface marker possibly linked to the regulation of T-cell activation. Because human and chimpanzee monocytes express comparable levels of HLA-DR, the low level of HLA-DR expression on activated chimpanzee CD4+ lymphocytes (Figure 4) suggests that these cells might be less functional as APCs in vivo compared to their human counterpart. The role of MHC class II expression on CD4+ lymphocytes remain unclear, but several reports suggest that the MHC class II-mediated interaction between T-cells could regulate adaptive immune responses (Barnaba et al., 1994; Fischer et al., 2007; LaSalle, Ota, and Hafler, 1991). In addition, MHC class II proteins differ in their ability to bind superantigens such as SEB and amino acid differences between human and chimpanzee MHC class II have been described (de Groot and Bontrop, 1999; Herman et al., 1990; Herrmann, Accolla, and MacDonald, 1989). Thus, it is possible that differences in HLA-DR cell surface expression and/or allelic polymorphism (via differences in SEB affinity) account for the limited proliferative response of chimpanzee CD4+ lymphocytes to SEB stimulation in the absence of macrophages. Activated chimpanzee CD4+ lymphocytes also expressed high levels of the CD62L homing receptor which adds to the list of adhesion molecules involved in co-stimulatory receptor/ligand interactions, including CD33-related Siglecs (Nguyen et al., 2006), ICAMs (Walter, Stebbing, and Messier, 2005) that could differentially modulate CD4+ lymphocyte activation and proliferation in vitro and in vivo. The basis of these differences between these two species, as well as their relevance to in vivo functional differences, will require further exploration.
In summary, we report here a new activation protocol that renders chimpanzee CD4 T cells capable of replicating R5 tropic HIV-1 strains. This activation protocol has already proven useful to assess the biological relevance of an HIV-1 matrix protein adaptive change selected upon cross-species transmission of SIVcpz to humans and to analyze the replication potential of novel fecal-consensus SIVcpz and SIVgor molecular clones in chimpanzee CD4+ lymphocytes (Takehisa et al., 2009; Takehisa et al., 2007; Wain et al., 2007). However, unanswered questions remain. We show here that chimpanzee donors vary significantly in the level of HIV-1 replication, much more so than human donors. The receptor and coreceptor expression levels are equivalent between human and chimpanzees (Figure 4), thus it seems improbable that, like natural SIV hosts such as sooty mangabeys and African green monkeys (Beaumier et al., 2009; Sodora et al., 2009), CD4 or CCR5 expression differences account for the susceptibility differences. Moreover, we have recently identified additional HIV-1 strains and primary isolates that, similar to JRSCF, do not replicate in chimpanzee lymphocytes, including strains that use CXCR4 as coreceptor. These results point to chimpanzee-specific host factors can may block or modulate HIV-1 replication in chimpanzee lymphocytes. These restriction factors are likely different from currently know human HIV-1 restriction factors such as TRIM5a, APOBEC3G, since the human and chimpanzee alleles seem equivalent with respect to their anti-HIV-1 function (Kratovac et al., 2008; Sawyer, Emerman, and Malik, 2004; Song et al., 2005a). Our new lymphocyte activation approach provides the basis for comparative studies of human and chimpanzee susceptibility to HIV-1 that could lead to the identification of novel host factors that control or are required for HIV-1 replication.
Acknowledgments
This work was supported by the National Institutes of Health (R21 AI080364 to F.B.-R., R01 AI50529 and R01 AI 58715 to B.H.H.), the UAB Center for AIDS Research (P30 AI 27767), and the Yerkes National Primate Research Center (RR-000165). We thank Jennifer Jones for assistance in setting up the flow cytometry assays and Cynthia Derdeyn for critical reading of the manuscript. This article is dedicated to the memory of our friend and colleague Kenneth Zammit, whose enthusiasm and meticulousness contributed greatly to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter HJ, Eichberg JW, Masur H, Saxinger WC, Gallo R, Macher AM, Lane HC, Fauci AS. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984;226(4674):549–52. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Galli G, Nappi F, Cosmi L, Manetti R, Maggi E, Ensoli B, Romagnani S. Limited expression of R5-tropic HIV-1 in CCR5-positive type 1-polarized T cells explained by their ability to produce RANTES, MIP-1alpha, and MIP-1beta. Blood. 2000;95(4):1167–74. [PubMed] [Google Scholar]
- Bamshad MJ, Mummidi S, Gonzalez E, Ahuja SS, Dunn DM, Watkins WS, Wooding S, Stone AC, Jorde LB, Weiss RB, Ahuja SK. A strong signature of balancing selection in the 5′ cis-regulatory region of CCR5. Proc Natl Acad Sci U S A. 2002;99(16):10539–44. doi: 10.1073/pnas.162046399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnaba V, Watts C, de Boer M, Lane P, Lanzavecchia A. Professional presentation of antigen by activated human T cells. Eur J Immunol. 1994;24(1):71–5. doi: 10.1002/eji.1830240112. [DOI] [PubMed] [Google Scholar]
- Bavari S, Hunt RE, Ulrich RG. Divergence of human and nonhuman primate lymphocyte responses to bacterial superantigens. Clin Immunol Immunopathol. 1995;76(3 Pt 1):248–54. doi: 10.1006/clin.1995.1123. [DOI] [PubMed] [Google Scholar]
- Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med. 2009;15(8):879–85. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont T, Broersen S, van Nuenen A, Huisman HG, de Roda Husman AM, Heeney JL, Schuitemaker H. Increased neutralization sensitivity and reduced replicative capacity of human immunodeficiency virus type 1 after short-term in vivo or in vitro passage through chimpanzees. J Virol. 2000;74(17):7699–707. doi: 10.1128/jvi.74.17.7699-7707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton PA, Lee DR, Kennedy RC. Sequence comparisons of non-human primate HIV-1 coreceptor homologues. Mol Immunol. 1998;35(2):95–101. doi: 10.1016/s0161-5890(98)00016-9. [DOI] [PubMed] [Google Scholar]
- Benton PA, Timanus DK, Shearer MH, White GL, Lee DR, Kennedy RC. Analysis of nonhuman primate peripheral blood mononuclear cells for susceptibility to HIV-1 infection and HIV coreceptor expression. Dev Comp Immunol. 1999;23(1):97–105. doi: 10.1016/s0145-305x(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Young JW, Nisanian AJ, Baggers J, Steinman RM. Small amounts of superantigen, when presented on dendritic cells, are sufficient to initiate T cell responses. J Exp Med. 1993;178(2):633–42. doi: 10.1084/jem.178.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Ruche F, Gao F, Bailes E, Saragosti S, Delaporte E, Peeters M, Shaw GM, Hahn BH, Sharp PM. Complete genome analysis of one of the earliest SIVcpzPtt strains from Gabon (SIVcpzGAB2) AIDS Res Hum Retroviruses. 2004;20(12):1377–81. doi: 10.1089/aid.2004.20.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Ruche F, McKinney BA, Duverger A, Wagner FH, Ansari AA, Kutsch O. The quality of chimpanzee T cell activation and SIV/HIV susceptibility achieved via antibody-mediated TCR/CD3 stimulation is a function of the anti-CD3 antibody isotype. J Virol. 2008 doi: 10.1128/JVI.01319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94(5):1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogers WM, Koornstra WH, Dubbes RH, ten Haaft PJ, Verstrepen BE, Jhagjhoorsingh SS, Haaksma AG, Niphuis H, Laman JD, Norley S, Schuitemaker H, Goudsmit J, Hunsmann G, Heeney JL, Wigzell H. Characteristics of primary infection of a European human immunodeficiency virus type 1 clade B isolate in chimpanzees. J Gen Virol. 1998;79(Pt 12):2895–903. doi: 10.1099/0022-1317-79-12-2895. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187(1):129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshell M, McLeod J, Walker L, Hall N, Patel Y, Sansom D. Effects of antigen presentation on superantigen-induced apoptosis mediated by Fas/Fas ligand interactions in human T cells. Immunology. 1996;87(4):586–92. doi: 10.1046/j.1365-2567.1996.509582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayota A, Vuillier F, Scott-Algara D, Feuillie V, Dighiero G. Differential requirements for HIV-1 replication in naive and memory CD4 T cells from asymptomatic HIV-1 seropositive carriers and AIDS patients. Clin Exp Immunol. 1993;91(2):241–8. doi: 10.1111/j.1365-2249.1993.tb05890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MW, Shibata R, Martin MA. Infection of chimpanzee peripheral blood mononuclear cells by human immunodeficiency virus type 1 requires cooperative interaction between multiple variable regions of gp120. J Virol. 1996;70(10):7318–21. doi: 10.1128/jvi.70.10.7318-7321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Kessler JA, II, Boots LJ, McKenna PM, Schleif WA, Emini EA, Mark GE, III, Katinger H, Cobb EK, Lunceford SM, Rouse SR, Murthy KK. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70(10):6751–8. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creson JR, Lin AA, Li Q, Broad DF, Roberts MR, Anderson SJ. The mode and duration of anti-CD28 costimulation determine resistance to infection by macrophage-tropic strains of human immunodeficiency virus type 1 in vitro. J Virol. 1999;73(11):9337–47. doi: 10.1128/jvi.73.11.9337-9347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj B, Mourad W, Naccache PH. Superantigen-mediated human monocyte-T lymphocyte interactions are associated with an MHC class II-, TCR/CD3-, and CD4-dependent mobilization of calcium in monocytes. J Immunol. 1992;149(5):1497–503. [PubMed] [Google Scholar]
- de Groot NG, Bontrop RE. The major histocompatibility complex class II region of the chimpanzee: towards a molecular map. Immunogenetics. 1999;50(34):160–7. doi: 10.1007/s002510050592. [DOI] [PubMed] [Google Scholar]
- Dejucq N, Simmons G, Clapham PR. Expanded tropism of primary human immunodeficiency virus type 1 R5 strains to CD4(+) T-cell lines determined by the capacity to exploit low concentrations of CCR5. J Virol. 1999;73(9):7842–7. doi: 10.1128/jvi.73.9.7842-7847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74(18):8358–67. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Trkola A, Lin SW, Nagashima KA, Kajumo F, Zhao L, Olson WC, Wu L, Mackay CR, Allaway GP, Sakmar TP, Moore JP, Maddon PJ. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J Virol. 1998;72(1):279–85. doi: 10.1128/jvi.72.1.279-285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg JW, Zarling JM, Alter HJ, Levy JA, Berman PW, Gregory T, Lasky LA, McClure J, Cobb KE, Moran PA, et al. T-cell responses to human immunodeficiency virus (HIV) and its recombinant antigens in HIV-infected chimpanzees. J Virol. 1987;61(12):3804–8. doi: 10.1128/jvi.61.12.3804-3808.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer UB, Jacovetty EL, Medeiros RB, Goudy BD, Zell T, Swanson JB, Lorenz E, Shimizu Y, Miller MJ, Khoruts A, Ingulli E. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo. Proc Natl Acad Sci U S A. 2007;104(17):7181–6. doi: 10.1073/pnas.0608299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Ehrlich GD, Baca LM, Conley S, Ribas J, Kalter DC, Meltzer MS, Poiesz BJ, Nara P. The inability of human immunodeficiency virus to infect chimpanzee monocytes can be overcome by serial viral passage in vivo. J Virol. 1991;65(7):3853–63. doi: 10.1128/jvi.65.7.3853-3863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SK, Fultz PN, Keddie E, Saag MS, Sharp PM, Hahn BH, Shaw GM. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology. 1993;194(2):858–64. doi: 10.1006/viro.1993.1331. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, Nibbs RJ, Freedman BI, Quinones MP, Bamshad MJ, Murthy KK, Rovin BH, Bradley W, Clark RA, Anderson SA, O'Connell R J, Agan BK, Ahuja SS, Bologna R, Sen L, Dolan MJ, Ahuja SK. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307(5714):1434–40. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- Herman A, Croteau G, Sekaly RP, Kappler J, Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990;172(3):709–17. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann T, Accolla RS, MacDonald HR. Different staphylococcal enterotoxins bind preferentially to distinct major histocompatibility complex class II isotypes. Eur J Immunol. 1989;19(11):2171–4. doi: 10.1002/eji.1830191131. [DOI] [PubMed] [Google Scholar]
- Hewitt CR, Lamb JR, Hayball J, Hill M, Owen MJ, O'Hehir RE. Major histocompatibility complex independent clonal T cell anergy by direct interaction of Staphylococcus aureus enterotoxin B with the T cell antigen receptor. J Exp Med. 1992;175(6):1493–9. doi: 10.1084/jem.175.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto WM, Gettie A, Smith S, Donahoe SM, Jin X, Marx P, Connor R, Nixon DF. Comparison of restimulation methods to elicit SIV specific cytotoxic T-lymphocytes (CTL) in vitro: Staphylococcal enterotoxin B (SEB) provides a novel method for the quantification of SIV specific CTL precursors. Immunol Lett. 1999;66(13):135–40. doi: 10.1016/s0165-2478(98)00173-4. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182(6):1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller CA, King GW, Hurtubise PE, Sagone AL, LoBuglio AF. Characterization of glass adherent human mononuclear cells. J Immunol. 1973;111(5):1610–2. [PubMed] [Google Scholar]
- Kratovac Z, Virgen CA, Bibollet-Ruche F, Hahn BH, Bieniasz PD, Hatziioannou T. Primate lentivirus capsid sensitivity to TRIM5 proteins. J Virol. 2008;82(13):6772–7. doi: 10.1128/JVI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr Protoc Immunol. 2004;Chapter 3 doi: 10.1002/0471142735.im0312s60. Unit 3 12. [DOI] [PubMed] [Google Scholar]
- LaSalle JM, Ota K, Hafler DA. Presentation of autoantigen by human T cells. J Immunol. 1991;147(3):774–80. [PubMed] [Google Scholar]
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999;96(9):5215–20. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Mettling C, Portales P, Reynes J, Clot J, Corbeau P. Cell surface CCR5 density determines the postentry efficiency of R5 HIV-1 infection. Proc Natl Acad Sci U S A. 2002;99(24):15590–5. doi: 10.1073/pnas.242134499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Sidney J, Wojewoda C, Dodds E, Reynolds MR, Napoe G, Mothe BR, O'Connor DH, Wilson NA, Watkins DI, Sette A. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J Immunol. 2004;173(8):5064–76. doi: 10.4049/jimmunol.173.8.5064. [DOI] [PubMed] [Google Scholar]
- Mack M, Luckow B, Nelson PJ, Cihak J, Simmons G, Clapham PR, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells TN, Schlondorff D, Proudfoot AE. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187(8):1215–24. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis LB, Glushakova S, Grivel JC, Murphy PM. Blockade of CC chemokine receptor 5 (CCR5)-tropic human immunodeficiency virus-1 replication in human lymphoid tissue by CC chemokines. J Clin Invest. 1998;101(9):1876–80. doi: 10.1172/JCI2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KA, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard NP. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278(5342):1470–3. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- Martinson JJ, Chapman NH, Rees DC, Liu YT, Clegg JB. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet. 1997;16(1):100–3. doi: 10.1038/ng0597-100. [DOI] [PubMed] [Google Scholar]
- Mengozzi M, Malipatlolla M, De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. Naive CD4 T cells inhibit CD28-costimulated R5 HIV replication in memory CD4 T cells. Proc Natl Acad Sci U S A. 2001;98(20):11644–9. doi: 10.1073/pnas.211205098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GB, Lee JW, Cheung RK, Gelfand EW. Characterization of the requirements for human T cell mitogenesis by using suboptimal concentrations of phytohemagglutinin. J Immunol. 1985;135(5):3087–93. [PubMed] [Google Scholar]
- Muller-Trutwin MC, Corbet S, Hansen J, Georges-Courbot MC, Diop O, Rigoulet J, Barre-Sinoussi F, Fomsgaard A. Mutations in CCR5-coding sequences are not associated with SIV carrier status in African nonhuman primates. AIDS Res Hum Retroviruses. 1999;15(10):931–9. doi: 10.1089/088922299310647. [DOI] [PubMed] [Google Scholar]
- Muller-Trutwin MC, Corbet S, Souquiere S, Roques P, Versmisse P, Ayouba A, Delarue S, Nerrienet E, Lewis J, Martin P, Simon F, Barre-Sinoussi F, Mauclere P. SIVcpz from a naturally infected Cameroonian chimpanzee: biological and genetic comparison with HIV-1 N. J Med Primatol. 2000;29(34):166–72. doi: 10.1034/j.1600-0684.2000.290310.x. [DOI] [PubMed] [Google Scholar]
- Mummidi S, Bamshad M, Ahuja SS, Gonzalez E, Feuillet PM, Begum K, Galvis MC, Kostecki V, Valente AJ, Murthy KK, Haro L, Dolan MJ, Allan JS, Ahuja SK. Evolution of human and non-human primate CC chemokine receptor 5 gene and mRNA. Potential roles for haplotype and mRNA diversity, differential haplotype-specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides in the pathogenesis of HIV-1 and simian immunodeficiency virus. J Biol Chem. 2000;275(25):18946–61. doi: 10.1074/jbc.M000169200. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A. 2006;103(20):7765–70. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondoa P, Davis D, Kestens L, Vereecken C, Garcia Ribas S, Fransen K, Heeney J, van der Groen G. In vitro susceptibility to infection with SIVcpz and HIV-1 is lower in chimpanzee than in human peripheral blood mononuclear cells. J Med Virol. 2002;67(3):301–11. doi: 10.1002/jmv.10078. [DOI] [PubMed] [Google Scholar]
- Ondoa P, Kestens L, Davis D, Vereecken C, Willems B, Fransen K, Vingerhoets J, Zissis G, ten Haaft P, Heeney J, van der Groen G. Longitudinal comparison of virus load parameters and CD8 T-cell suppressive capacity in two SIVcpz-infected chimpanzees. J Med Primatol. 2001;30(5):243–53. doi: 10.1034/j.1600-0684.2001.d01-56.x. [DOI] [PubMed] [Google Scholar]
- Oswald-Richter K, Grill SM, Leelawong M, Tseng M, Kalams SA, Hulgan T, Haas DW, Unutmaz D. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 2007;3(4):e58. doi: 10.1371/journal.ppat.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischinger K, Zimmermann K, Eibl MM, Mannhalter JW. Comparison of early events during infection of human and chimpanzee peripheral blood mononuclear cells with HIV-1. Arch Virol. 1998;143(11):2065–76. doi: 10.1007/s007050050444. [DOI] [PubMed] [Google Scholar]
- Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece G, Murphy G, Ager A. Metalloproteinase-mediated regulation of L-selectin levels on leucocytes. J Biol Chem. 1996;271(20):11634–40. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- Pretet JL, Zerbib AC, Girard M, Guillet JG, Butor C. Chimpanzee CXCR4 and CCR5 act as coreceptors for HIV type 1. AIDS Res Hum Retroviruses. 1997;13(18):1583–7. doi: 10.1089/aid.1997.13.1583. [DOI] [PubMed] [Google Scholar]
- Riley JL, Carroll RG, Levine BL, Bernstein W, St Louis DC, Weislow OS, June CH. Intrinsic resistance to T cell infection with HIV type 1 induced by CD28 costimulation. J Immunol. 1997;158(11):5545–53. [PubMed] [Google Scholar]
- Riley JL, Levine BL, Craighead N, Francomano T, Kim D, Carroll RG, June CH. Naive and memory CD4 T cells differ in their susceptibilities to human immunodeficiency virus type 1 infection following CD28 costimulation: implicatip6s for transmission and pathogenesis. J Virol. 1998;72(10):8273–80. doi: 10.1128/jvi.72.10.8273-8280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbe R, Picchio GR, Pastore C, Chaloin O, Hartley O, Offord R, Mosier DE. Donor- and ligand-dependent differences in C-C chemokine receptor 5 reexpression. J Virol. 2001;75(2):661–71. doi: 10.1128/JVI.75.2.661-671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35(11):3362–7. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- Sawyer SL, Emerman M, Malik HS. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol. 2004;2(9):E275. doi: 10.1371/journal.pbio.0020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker H, Meyaard L, Kootstra NA, Dubbes R, Otto SA, Tersmette M, Heeney JL, Miedema F. Lack of T cell dysfunction and programmed cell death in human immunodeficiency virus type 1-infected chimpanzees correlates with absence of monocytotropic variants. J Infect Dis. 1993;168(5):1140–7. doi: 10.1093/infdis/168.5.1140. [DOI] [PubMed] [Google Scholar]
- Shao W, Tang J, Song W, Wang C, Li Y, Wilson CM, Kaslow RA. CCL3L1 and CCL4L1: variable gene copy number in adolescents with and without human immunodeficiency virus type 1 (HIV-1) infection. Genes Immun. 2007;8(3):224–31. doi: 10.1038/sj.gene.6364378. [DOI] [PubMed] [Google Scholar]
- Shibata R, Hoggan MD, Broscius C, Englund G, Theodore TS, Buckler-White A, Arthur LO, Israel Z, Schultz A, Lane HC, et al. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69(7):4453–62. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, Estes JD, Hahn BH, Hirsch VM, Kaur A, Kirchhoff F, Muller-Trutwin M, Pandrea I, Schmitz JE, Silvestri G. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15(8):861–5. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Javanbakht H, Perron M, Park DH, Stremlau M, Sodroski J. Retrovirus restriction by TRIM5alpha variants from Old World and New World primates. J Virol. 2005a;79(7):3930–7. doi: 10.1128/JVI.79.7.3930-3937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Rabin RL, Hill BJ, De Rosa SC, Perfetto SP, Zhang HH, Foley JF, Reiner JS, Liu J, Mattapallil JJ, Douek DC, Roederer M, Farber JM. Characterization of subsets of CD4+ memory T cells reveals early branched pathways of T cell differentiation in humans. Proc Natl Acad Sci U S A. 2005b;102(22):7916–21. doi: 10.1073/pnas.0409720102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini F, Spits H, Geha RS. Staphylococcal exotoxins deliver activation signals to human T-cell clones via major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991;88(17):7533–7. doi: 10.1073/pnas.88.17.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehisa J, Kraus MH, Ayouba A, Bailes E, Van Heuverswyn F, Decker JM, Li Y, Rudicell RS, Learn GH, Neel C, Ngole EM, Shaw GM, Peeters M, Sharp PM, Hahn BH. Origin and biology of simian immunodeficiency virus in wild-living western gorillas. J Virol. 2009;83(4):1635–48. doi: 10.1128/JVI.02311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehisa J, Kraus MH, Decker JM, Li Y, Keele BF, Bibollet-Ruche F, Zammit KP, Weng Z, Santiago ML, Kamenya S, Wilson ML, Pusey AE, Bailes E, Sharp PM, Shaw GM, Hahn BH. Generation of infectious molecular clones of simian immunodeficiency virus from fecal consensus sequences of wild chimpanzees. J Virol. 2007;81(14):7463–75. doi: 10.1128/JVI.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Rivers C, Karita E, Costello C, Allen S, Fultz PN, Schoenbaum EE, Kaslow RA. Allelic variants of human beta-chemokine receptor 5 (CCR5) promoter: evolutionary relationships and predictable associations with HIV-1 disease progression. Genes Immun. 1999;1(1):20–7. doi: 10.1038/sj.gene.6363640. [DOI] [PubMed] [Google Scholar]
- ten Haaft P, Murthy K, Salas M, McClure H, Dubbes R, Koornstra W, Niphuis H, Davis D, van der Groen G, Heeney J. Differences in early virus loads with different phenotypic variants of HIV-1 and SIV(cpz) in chimpanzees. Aids. 2001;15(16):2085–92. doi: 10.1097/00002030-200111090-00003. [DOI] [PubMed] [Google Scholar]
- ten Haaft PJ, Murthy KK, Verstrepen BE, Eichberg JW, Heeney JL. Intact CCR-5 coreceptors in HIV-1-infected chimpanzees. Aids. 1997;11(10):1291–3. doi: 10.1097/00002030-199710001-00001. [DOI] [PubMed] [Google Scholar]
- Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384(6605):184–7. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- Trkola A, Paxton WA, Monard SP, Hoxie JA, Siani MA, Thompson DA, Wu L, Mackay CR, Horuk R, Moore JP. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72(1):396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189(11):1735–46. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicenzi E, Poli G. Infection of CD4+ primary T cells and cell lines, generation of chronically infected cell lines, and induction of HIV expression. Curr Protoc Immunol. 2005;Chapter 12 doi: 10.1002/0471142735.im1203s69. Unit 12 3. [DOI] [PubMed] [Google Scholar]
- Voevodin A, Samilchuk E, Dashti S. A survey for 32 nucleotide deletion in the CCR-5 chemokine receptor gene (deltaccr-5) conferring resistance to human immunodeficiency virus type 1 in different ethnic groups and in chimpanzees. J Med Virol. 1998;55(2):147–51. [PubMed] [Google Scholar]
- Wain LV, Bailes E, Bibollet-Ruche F, Decker JM, Keele BF, Van Heuverswyn F, Li Y, Takehisa J, Ngole EM, Shaw GM, Peeters M, Hahn BH, Sharp PM. Adaptation of HIV-1 to Its Human Host. Mol Biol Evol. 2007;24(8):1853–60. doi: 10.1093/molbev/msm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi H, Bertoglio J, Tursz T, Fradelizi D. IL 2 receptor induction on human T lymphocytes: role for IL 2 and monocytes. J Immunol. 1985;135(1):321–7. [PubMed] [Google Scholar]
- Walter NA, Stebbing J, Messier W. The potential significance of adaptive evolution and dimerization in chimpanzee intercellular cell adhesion molecules (ICAMs) J Theor Biol. 2005;232(3):339–46. doi: 10.1016/j.jtbi.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Ringler DJ, Fultz PN, MacKey JJ, Boyson JE, Levine CG, Letvin NL. A chimpanzee-passaged human immunodeficiency virus isolate is cytopathic for chimpanzee cells but does not induce disease. J Virol. 1991;65(6):3344–8. doi: 10.1128/jvi.65.6.3344-3348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S, Stone AC, Dunn DM, Mummidi S, Jorde LB, Weiss RK, Ahuja S, Bamshad MJ. Contrasting effects of natural selection on human and chimpanzee CC chemokine receptor 5. Am J Hum Genet. 2005;76(2):291–301. doi: 10.1086/427927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N, Choe H, Sodroski J, Newman W, Koup RA, Mackay CR. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185(9):1681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharova V, Zachar V, Goustin AS. Sequence of chemokine receptor gene CCR5 in chimpanzees, a natural HIV type 1 host. AIDS Res Hum Retroviruses. 1997;13(13):1159–61. doi: 10.1089/aid.1997.13.1159. [DOI] [PubMed] [Google Scholar]


