Abstract
Background:
Intradialytic increases in blood pressure (BP) can complicate the management of hypertension in hemodialysis (HD) patients but the long-term consequences are uncertain. Thus, we sought to determine if BP elevations during HD were associated with higher 2-year mortality among incident HD patients.
Study Design:
Secondary analysis of a prospective dialysis cohort.
Setting and Participants:
Incident hemodialysis patients in the Dialysis Morbidity and Mortality Wave 2 Study.
Predictors:
Changes in systolic BP (SBP) during hemodialysis (ie, postdialysis SBP–predialysis SBP), averaged from 3 hemodialysis sessions prior to enrollment.
Outcome:
Time to 2-year all-cause mortality.
Measurements:
Cox regression was used to model hazard ratios for mortality associated with changes in SBP during HD, while adjusting for demographics, comorbid conditions, interdialytic weight gain, laboratory variables, and antihypertensive agents.
Results:
Of 1,748 patients, 12.2% exhibited >10mmHg increases in SBP during HD. In adjusted analyses, every 10mmHg increase in SBP during HD was independently associated with a 6% increased hazard of death (HR 1.06, CI 1.01-1.11). When also adjusted for diastolic BP and postdialysis SBP, the adjusted hazard of death associated with increasing SBP during HD remained significant (HR 1.12, CI 1.05-1.21, per 10mmHg increase in ΔSBP during HD). However, in analyses adjusted for predialysis SBP, there was a significant interaction between change in SBP and predialysis SBP. In analyses stratified by predialysis SBP, trends for an increased mortality associated with increasing SBP during dialysis were present in patients with predialysis SBP <160mmHg, however, this relationship was only significant in patients with predialysis SBP <120mmHg.
Limitations:
Secondary analysis with a limited number of baseline BP measurements and limited information on dialysis prescription.
Conclusions:
Increasing SBP >10mmHg during hemodialysis occurs in ∼10% of incident patients and while increasing systolic BP during HD was associated with decreased 2-year survival, these findings were limited to patients with predialysis systolic BP <120 mmHg.
Keywords: blood pressure, end-stage renal disease, epidemiology and outcomes, hemodialysis, hypertension, mortality
INTRODUCTION
While hemodialysis (HD) lowers blood pressure in most hypertensive patients, in some it causes intradialytic hypotension and in others it may result in intradialytic increases in blood pressure (BP). While intradialytic hypotension is a common well-recognized complication of HD associated with adverse outcomes(1), intradialytic increases in BP can occur in up to 15% of prevalent HD patients and is also associated with adverse short-term outcomes.(2, 3) However, the prevalence of intradialytic BP increases in incident patients is unknown and the clinical consequences have yet to be defined.
While the long-term consequences of intradialytic increases in blood pressure are uncertain, recent investigations into its pathophysiology have demonstrated that patients with blood pressure elevations during HD have elevated peripheral vascular resistance.(4, 5) The elevated peripheral vascular resistance associated with intradialytic increases in BP occurs independent of the renin-angiotensin system and sympathetic hormones and appears related to abnormal elevations in post-dialysis endothelin-1 (a vasoconstrictor) relative to nitric oxide (a vasodilator).(4) Considering endothelin-1 and nitric oxide are endothelial derived blood pressure mediators, abnormalities in these substances (as evidenced in patients with intradialytic increases in BP) may suggest underlying endothelial cell dysfunction, a condition that is associated with detrimental cardiovascular outcomes among dialysis patients.(6, 7) These proposed mechanisms suggest that patients with intradialytic increases in BP may have an increased risk for mortality.
In a cohort of prevalent HD patients, we recently demonstrated that systolic BP elevations of >10 mmHg with HD are associated with increased 6-month hospitalization and mortality.(3) However, the long-term consequences of intradialytic increases in BP remain to be determined. Therefore based on our previous work in prevalent HD patients, we hypothesize that a > 10 mmHg increase in blood pressure during HD is associated with increased 2-year mortality risk in a cohort of incident dialysis patients.
METHODS
Study Design
This study is a secondary analysis of a prospective cohort of incident dialysis patients enrolled in the Dialysis Morbidity and Mortality Study (DMMS) Wave 2.
Setting
All incident peritoneal dialysis (PD) and a 20% random sample of incident HD patients starting dialysis during the years 1996 and 1997 in the United States were enrolled into the DMMS cohort. Entry into the cohort began 60 days after dialysis initiation at which time demographics, comorbid conditions, clinical variables, laboratory measurements, and medications were recorded by trained health professionals through chart abstraction and patient interviews.(8)
Participants
Patients with invalid or duplicate US Renal Data System (USRDS) numbers, whose dialysis initiation date was not between 1996 and 1997, or who were undergoing PD were excluded from this analysis, leaving 1,820 incident HD patients at study entry eligible for inclusion. In addition, we excluded those with missing systolic BP (SBP; n=59) or diastolic BP (DBP; n=8) or those who died within the first 60 days of dialysis initiation (n=5). Thus, the final study cohort consisted of 1,748 (out of 1,820) incident HD patients.
Variables
Blood pressure measurements were collected as part of routine clinical care and the 3 most recent dialysis session BP measurements (sitting pre and post HD SBP and DBP) prior to the study start date were averaged. All patients included in this analysis had 12 BP parameters available (pre and postdialysis SBP and DBP for the last 3 dialysis sessions). The difference between pre- and postdialysis BP (ΔBP) was calculated as postdialysis minus predialysis BP. Mean arterial pressure (MAP) was defined as [(2 × diastolic BP) + systolic BP]/3. Pulse pressure (PP) was defined as SBP – DBP. For descriptive purposes, patients were categorized into 3 groups based on ΔBP (3 each for systolic BP, PP and MAP): 1) BP increased >10 mmHg from pre to postdialysis 2) BP unchanged, that is within the range of −10 mmHg to +10 mmHg, and 3) BP decreased > 10 mmHg from pre to postdialysis.
Interdialytic weight gain (averaged from the 3 most recent dialysis sessions) was calculated as the average change in weight between dialysis sessions. Percent interdialytic weight gain was calculated as the average interdialytic weight gain divided by the postdialysis weight(9). Tobacco use was defined as active or former tobacco use vs. nonsmoker or unknown. Coronary artery disease was defined as myocardial infarction, coronary artery bypass grafting, prior angioplasty, abnormal cardiac catheterization, or cardiac arrest. Cerebrovascular disease was defined as prior stroke or transient ischemic attack. Peripheral vascular disease (PVD) was defined as a diagnosis of PVD, an amputation due to PVD, absent foot pulses, or claudication. Hypertension was defined per the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative[ND1] (KDOQI) as predialysis SBP>140 or postdialysis SBP ≥ 130 or predialysis DBP≥ 90 or postdialysis DBP≥80)(10). Urea reduction ratio was defined as predialysis – postdialysis serum urea nitrogen divided by predialysis serum urea nitrogen. Antihypertensive medications were grouped into the following classes: angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB), beta blockers (cardio selective and nonselective), calcium channel blockers (dihydropyridine and nondihydropryidine), clonidine, hydralazine, and nitrates.
The primary outcome was time to 2-year all-cause mortality. The follow-up time began at study start date which was 60 days after dialysis initiation. Patients were censored on death, renal transplant, or end of study period. Due to the known limitations in using USRDS data to classify cause of death,(11) our primary analyses were performed assessing all-cause mortality, rather than cause-specific mortality.
Statistical Methods
The DMMS Wave 2 data was combined with the USRDS standard analytic files that contain information on mortality and cause of death.
Continuous variables are presented as means and standard deviations unless otherwise noted. Categorical variables are presented as proportions. ANOVA was used to compare normally distributed continuous variables; otherwise the nonparametric Kruskal-Wallis test was used. Chi square tests were used to compare categorical variables. For missing categorical data (<5%), the condition was assumed to be absent similar to previous investigators;(12) for missing continuous values (<5%) other than SBP or DBP, the variable was replaced with the mean. For validation, analyses were also performed without imputation of missing continuous values.
Kaplan Meier curves were used to describe unadjusted outcomes between patients stratified by 10 mmHg changes in BP with HD. For descriptive purposes, cardiovascular-related cause of death was compared among patients categorized by BP changes with HD. Cardiovascular-related cause of death was ascertained from the ESRD Death Notification Form.
Adjusted comparisons of mortality were performed using Cox proportional hazards models with ΔBP modeled as a continuous variable. Formal and graphical methods were used to test for proportional hazards for continuous variables. When the relationship was found to be nonlinear, appropriate data transformations were applied. All clinical and demographic characteristics were eligible for inclusion in the final model (Table 1). Variables known to be associated with clinical outcomes were forced in all models (age, diabetes mellitus, body mass index, interdialytic weight gain, serum albumin, serum creatinine); other variables were removed from the final model if they had a p-value of >0.1. To determine if other BP measurements modified the magnitude of the relationship between ΔBP and mortality, additional models were created which included the addition of other BP parameters and significant interactions (P<0.05) between SBP and ΔSBP in the following manner: 1) predialysis SBP, 2) postdialysis SBP, and 3) pre- and postdialysis DBP, 4) predialysis SBP and pre and postdialysis DBP, and 5) postdialysis SBP and pre and postdialysis DBP. When interactions were found to be present, a separate model was generated describing the association between ΔBP and mortality across strata of the other BP parameter.
Table 1.
Baseline Characteristics of Incident Hemodialysis Patients in the DMMS Wave 2 as categorized by Systolic Blood Pressure Changes during Hemodialysis
| Variable | SBP decreased >10 mmHg during HD (n=744) |
SBP unchanged ±10 mmHg during HD (n=791) |
SBP increased >10 mmHg during HD (n=213) |
P-value |
|---|---|---|---|---|
| Age (years, mean ±SD) | 61.0 ± 14.8 | 61.8 ± 16.0 | 62.0 ± 15.5 | 0.5 |
| Diabetes as primary cause of ESRD (%) | 45.8 | 39.7 | 45.1 | 0.2 |
| Hispanic ethnicity (%) | 10.2 | 12.9 | 8.9 | 0.1 |
| African American Race (%) | 35.4 | 32.1 | 34.3 | 0.8 |
| Male sex (%) | 54.4 | 53.1 | 47.0 | 0.2 |
| Tobacco use (%) | 42.2 | 43.1 | 44.1 | 0.9 |
| Dry weight (kg, mean ±SD) | 75.0 ± 20.0 | 70.8 ± 19.1 | 68.3 ± 17.8 | <0.001 |
| Body Mass Index (kg/m2) | 25.6 (17.9, 39.2) |
24.0 (17.3, 37.8) |
23.4 (17.0, 33.6) | <0.001* |
| IDWG (%, ±SD) | 3.33 ± 1.73 | 3.05 ± 1.81 | 2.74 ± 2.13 | <0.001 |
| Average dialysis session duration (hours) | 3.43 ± 0.55 | 3.37 ± 0.53 | 3.42 ± 0.53 | 0.2 |
| Baseline comorbidities (%) | ||||
| Diabetes mellitus | 51.6 | 47.3 | 52.6 | 0.2 |
| Coronary artery disease | 21.5 | 24.4 | 23.0 | 0.4 |
| Cerebrovascular disease | 11.7 | 15.2 | 10.8 | 0.07 |
| Congestive heart failure | 35.2 | 39.1 | 40.4 | 0.2 |
| Peripheral vascular disease | 18.8 | 19.2 | 23.5 | 0.3 |
| Blood Pressure (mmHg) | ||||
| Predialysis systolic | 159.1 ± 19.3 | 144.9 ± 20.3 | 143.2 ± 20.6 | <0.001 |
| Predialysis diastolic | 81.5 ± 11.7 | 75.9 ± 12.3 | 76.3 ± 12.6 | <0.001 |
| Postdialysis systolic | 135.3 ± 17.2 | 143.8 ± 20.4 | 161.1 ± 22.0 | <0.001 |
| Postdialysis diastolic | 73.1 ± 10.6 | 75.6 ± 12.0 | 81.4 ± 13.3 | <0.001 |
| ΔSBP | −23.8 ± 11.7 | −1.10 ± 5.5 | 17.9 ± 7.0 | <0.001 |
| Hypertension per KDOQI (%) | 85.4 | 75.2 | 93.0 | <0.001 |
| Receiving BP medications (%) | 66.1 | 70.0 | 77.9 | 0.004 |
| Laboratory Data | ||||
| Serum albumin (mg/dl) | 3.55 ± 0.55 | 3.46 ± 0.56 | 3.41 ± 0.56 | 0.001 |
| Serum creatinine (mg/dl) | 7.8 ± 3.0 | 7.4 ± 3.1 | 7.4 ± 2.6 | 0.04 |
| Serum calcium (mg/dl) | 8.7 ± 1.0 | 8.6 ± 0.96 | 8.6 ± 0.94 | 0.08 |
| Serum phosphorus (mg/dl) | 5.7 ± 1.9 | 5.5 ± 1.9 | 5.3 ± 1.8 | 0.03 |
| Calcium × phosphorus product (mg2/dl2) | 48.7 ± 16.5 | 47.0 ± 16.5 | 44.9 ± 15.2 | 0.008 |
| Hematocrit (%) | 29.9 (5.4) | 29.7 (5.7) | 29.6 (5.9) | 0.7 |
| Urea reduction ratio | 0.63 ± 0.14 | 0.62 ± 0.16 | 0.65 ± 0.09 | 0.1 |
| Baseline Medications (%) | ||||
| Aspirin | 16.1 | 20.4 | 20.7 | 0.07 |
| ACE-i/ARB | 21.8 | 21.4 | 25.8 | 0.4 |
| Beta-blocker | 14.8 | 16.1 | 23.5 | 0.01 |
| Calcium channel blocker | 45.2 | 49.6 | 55.9 | 0.02 |
| Clonidine | 11.0 | 13.2 | 22.3 | <0.001 |
| Erythropoeitin use | 92.2 | 92.3 | 93.4 | 0.8 |
| Hydralazine | 3.2 | 3.3 | 5.2 | 0.4 |
| Nitrates | 15.7 | 21.5 | 21.1 | 0.01 |
Values expressed as mean +/− SD, no. (%), or median (5th percentile, 95th percentile). Conversion factors for units: serum albumin in g/dl to g/L, ×10; creatinine in mg/dL to mmol/L, ×88.4; calcium in mg/dl to mmol/L, ×0.25; phosphorus in mg/dl to mmol/L, ×.
Nonparametric Kruskal-wallis test used
Abbreviation: DMMS, Dialysis Morbidity and Mortality; HD, hemodialysis; ESRD, end-stage renal disease; IDWG, interdialytic weight gain; IQR, interquartile range; BP, blood pressure; KDOQI, Kidney Dialysis Outcomes Quality Initiative; ACE-i, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker, SBP, systolic blood pressure.
Separate analyses were conducted to explore the relationship between 2-year mortality and ΔPP and ΔMAP. Separately, predialysis and postdialysis PP (13) and MAP were added to the final models to determine if these BP parameters modified the relationship between ΔBP and mortality. The results of these analyses are available in Item S1 (provided as supplementary material available with this article at www.ajkd.org).
Sensitivity analyses that did not censor on the date of renal transplant were performed and these results were identical to the main findings presented in this manuscript. Separate exploratory analyses were performed to investigate whether the presence or absence of certain risk factors modified the relationship between ΔSBP and 2-year mortality. For these analyses, interaction terms of interest were individually added to the final model and included the following interactions: ΔSBP × cardiac disease (defined as a history of coronary artery disease, peripheral vascular disease, cerebrovascular disease, or congestive heart disease), ΔSBP × history of congestive heart failure, ΔSBP × albumin<3.4 mg/dl, ΔSBP × body mass index <26 kg/m2, and ΔSBP × interdialytic weight gain>5%.
All analyses were performed with SAS Eguide (version 4.1, Cary, NC). This study was approved by the Duke Institutional Review Board and the UT Southwestern Institutional Review Board.
RESULTS
Participants and Descriptive Data
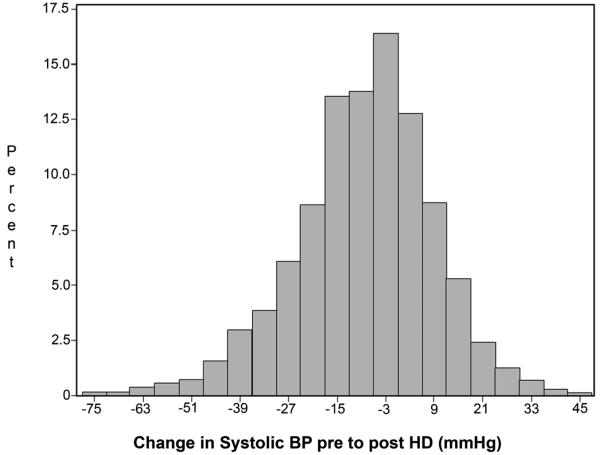
Of 1,748 incident HD patients, 213 (12.2%) exhibited >10 mmHg increases in systolic BP (SBP) during HD (Table 1). Figure 1 depicts the distribution of changes in SBP during HD across the cohort of patients. In general, patients with intradialytic increases in SBP had a lower dry weight, a smaller body mass index, and lower interdialytic weight gain compared to patients whose SBP was unchanged or fell with HD (p<0.001 for all). Patients with intradialytic increases in SBP also had lower serum albumin, lower serum creatinine, and lower serum phosphorus (p<0.05 for all). Further, patients whose SBP increased during HD were more likely to be prescribed beta-blockers, calcium-channel blockers, clonidine, and nitrates. Results were similar when values were not imputed (data not shown). Baseline characteristics of patients categorized by changes in PP and MAP during HD are available in the online supplementary material (Item S1).
Figure 1.
Distribution of change in systolic blood pressure during hemodialysis among 1,748 incident hemodialysis patients in the Dialysis Morbidity and Mortality Wave 2 Study
Outcome Data
There were a total of 570 deaths during the 2-year follow-up and the remaining patients were censored for transplantation (n=100) or study end (n=1,078). Two-year all-cause mortality was 32.6% overall and 37.0% among patients with intradialytic increases in SBP. The primary cause of mortality in this cohort was cardiovascular-related, accounting for 58.9% of deaths during the 2-year follow-up (Table 2). Among patients with >10 mmHg intradialytic increases in SBP, cardiovascular-related mortality accounted for 62.0% of deaths during the 2-year period.
Table 2.
Cause Specific 2-year Mortality among DMMS Wave 2 Patients grouped by Systolic Blood Pressure Changes during Hemodialysis
| SBP decreased >10 mmHg during HD (n=744) |
SBP unchanged ±10 mmHg during HD (n=791) |
SBP increased >10 mmHg during HD (n=213) |
|
|---|---|---|---|
| 2-year all-cause mortality, n (%) |
218 (29.3%) | 273 (34.5%) | 79 (37.1%) |
| Cardiovascular-related* | 126 (57.8%) | 161 (59.0%) | 49 (62.0%) |
includes acute myocardial infarction, pericarditis, atherosclerotic heart disease, cardiomyopathy, cardiac arrest, cardiac arrhythmia, valvular heart disease, congestive heart failure, cerebrovascular accident, and ischemic brain injury.
Abbreviation: SBP, systolic blood pressure; HD, hemodialysis; DMMS, Dialysis Morbidity and Mortality.
Unadjusted mortality associated with ΔSBP
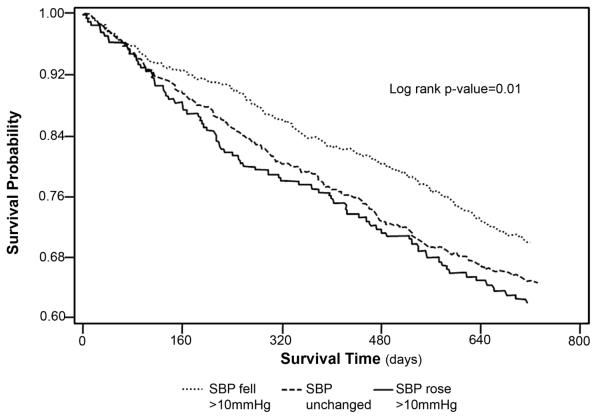
In unadjusted analyses, patients whose SBP increased >10 mmHg or was unchanged during HD had lower survival compared to patients whose SBP decreased >10 mmHg during HD (p=0.01) (Figure 2). In models with ΔSBP as a continuous variable, every 10 mmHg increase in ΔSBP during HD was associated with an unadjusted 9% increased hazard ratio of death (HR 1.09, CI 1.04-1.15, p<0.001). Results were similar when patients were stratified by intradialytic changes in PP and MAP (see Item S1).
Figure 2.
Kaplan Meier survival curves of time to death over 2-years among a national cohort of incident hemodialysis patients stratified by changes in systolic blood pressure during hemodialysis
Adjusted mortality associated with ΔSBP
Figure 3 (Model A) graphically demonstrates the association between predialysis SBP and postdialysis SBP and 2-year mortality when analyzed together in fully adjusted models. In this model, higher predialysis SBP was associated with a decreased hazard of death while postdialysis SBP was not a mortality predictor. Figure 3 (Model B) demonstrates graphically the relationship between increased SBP during HD and a higher hazard ratio of 2-year mortality in analyses adjusted for confounders but not other BP parameters.
Figure 3.
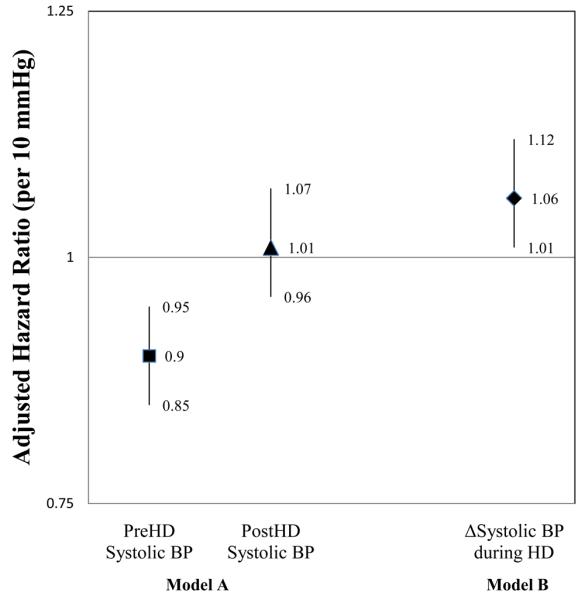
Bar plots of adjusted hazard ratios (95% confidence intervals) for death associated with predialysis and postdialysis systolic blood pressure (SBP) analyzed together and ΔSBP analyzed alone. Model A: higher predialysis SBP was associated with a decreased hazard of 2-year mortality (HR 0.90 per 10 mmHg, CI 0.85-0.95, p<0.001) while postdialysis SBP was not associated with mortality (HR 1.01 per 10 mmHg, CI 0.96-1.07, p=0.1). Model B: higher ΔSBP was associated with an increased hazard of 2-year mortality (HR 1.06 per 10 mmHg, CI 1.01-1.12, p=0.03).
*Models are also adjusted for age, body mass index<26 kg/m2, interdialytic weight gain >5%, diabetes mellitus, hypertension, peripheral vascular disease, congestive heart disease, coronary artery disease, serum albumin, creatinine, phosphorus, and the use of nitrates.
In the fully adjusted final model including predialysis SBP and ΔSBP (Table 3), increasing SBP during HD remained associated with an increased hazard of death, however a significant interaction was identified between predialysis SBP and ΔSBP during HD (p=0.02). Other variables associated with an increased hazard of death included increasing age, lower body mass index, history of peripheral vascular disease and congestive heart disease, and the use of nitrates. History of hypertension was associated with decreased mortality as was increasing serum albumin and increasing serum creatinine.
Table 3.
Adjusted Cox Proportional Hazard Model for Predictors of Time to 2-year All-cause Mortality in Incident Hemodialysis Patients
| Variable | Hazard ratio, (95% Confidence Interval) |
p-value |
|---|---|---|
| ΔSBP during dialysis, per 10 mmHg increase | 1.50 (1.08-2.07) | 0.02 |
| Predialysis SBP, per 10 mmHg increase | 0.90 (0.85-0.94) | <0.001 |
| ΔSBP during dialysis × predialysis SBP | 0.98 (0.96-0.99) | 0.02 |
| Age, per 10 year increase | 1.26 (1.18-1.35) | <0.001 |
| Body mass index <26kg/m2 vs ≥26kg/m2 | 1.34 (1.11-1.63) | 0.002 |
| Interdialytic weight gain ≥5% vs <5% | 1.12 (0.88-1.41) | 0.4 |
| Diabetes mellitus | 1.00 (0.83-1.20) | 0.9 |
| Peripheral vascular disease | 1.30 (1.07-1.57) | 0.008 |
| Congestive heart disease | 1.20 (1.00-1.43) | 0.05 |
| Coronary artery disease | 1.15 (0.95-1.40) | 0.2 |
| Hypertension | 0.83 (0.69-0.99) | 0.04 |
| Nitrates | 1.31 (1.08-1.60) | 0.008 |
| Serum albumin, per 1 mg/dl increase | 0.57 (0.49-0.67) | <0.001 |
| Serum creatinine, per 1 mg/dl increase | 0.94 (0.91-0.98) | 0.001 |
| Serum phosphorus, per 1 mg/dl increase | 1.04 (0.99-1.09) | 0.1 |
Variables tested for significance but removed with p value >0.10: race, sex, tobacco use, cerebrovascular disease, ace-inhibitor/angiotensin receptor blocker, beta-blocker, calcium channel blocker, aspirin, urea reduction ratio, hematocrit, and calcium.
Abbreviation: SBP, systolic blood pressure
Table 4 shows the relationship between increasing systolic BP during HD and 2-year mortality in adjusted models including other BP parameters. In models adjusted for ΔSBP during HD and postdialysis systolic BP (Table 4: models 3 and 6), increasing SBP during HD remained associated with an increased hazard ratio of death. There was no significant interaction between ΔSBP and postdialysis SBP (p=0.1). In models adjusted for increasing SBP during HD and DBP (Table 4: models 4, 5, and 6), diastolic BP did not persist as an independent predictor of mortality when also adjusted for SBP, but increasing SBP during HD remained as a significant predictor of 2-year mortality.
Table 4.
Adjusted* Cox Proportional Hazard Models for time to 2-year Mortality associated with ΔSBP during HD in separate models adjusted for other BP parameters
| Model | BP Variable (per 10 mmHg increase) | Hazard ratio, (95% Confidence Interval) |
p-value |
|---|---|---|---|
| Model 1 | ΔSBP during dialysis | 1.06 (1.01-1.12) | 0.03 |
| Model 2 | ΔSBP during dialysis | 1.50 (1.08-2.07) | 0.02 |
| Predialysis systolic BP | 0.90 (0.85-0.94) | <0.001 | |
| ΔSBP during dialysis × predialysis SBP | 0.98 (0.96-0.99) | 0.02 | |
| Model 3 | ΔSBP during dialysis | 1.11 (1.05-1.18) | <0.001 |
| Postdialysis SBP | 0.91 (0.87-0.95) | <0.001 | |
| Model 4 | ΔSBP during dialysis | 1.08 (1.02-1.16) | 0.01 |
| Predialysis DBP | 0.99 (0.87-1.12) | 0.81 | |
| Postdialysis DBP | 0.85 (0.74-0.97) | 0.01 | |
| Model 5 | ΔSBP during dialysis | 1.5 (1.08-2.07) | 0.01 |
| Predialysis SBP | 0.92 (0.87-0.98) | 0.01 | |
| Predialysis DBP | 1.02 (0.89-1.17) | 0.8 | |
| Postdialysis DBP | 0.90 (0.78-1.03) | 0.1 | |
| ΔSBP during dialysis × predialysis SBP | 0.98 (0.96-1.00) | 0.03 | |
| Model 6 | ΔSBP during dialysis | 1.12 (1.05-1.21) | 0.002 |
| Postdialysis SBP | 0.94 (0.88-1.00) | 0.04 | |
| Predialysis DBP | 1.03 (0.90-1.18) | 0.6 | |
| Postdialysis DBP | 0.88 (0.77-1.01) | 0.08 |
All models are also adjusted for age, body mass index<26 kg/m2, interdialytic weight gain >5%, diabetes mellitus, hypertension, peripheral vascular disease, congestive heart disease, coronary artery disease, serum albumin, creatinine, phosphorus, and the use of nitrates.
Abbreviation: DBP, diastolic blood pressure; SBP, systolic blood pressure.
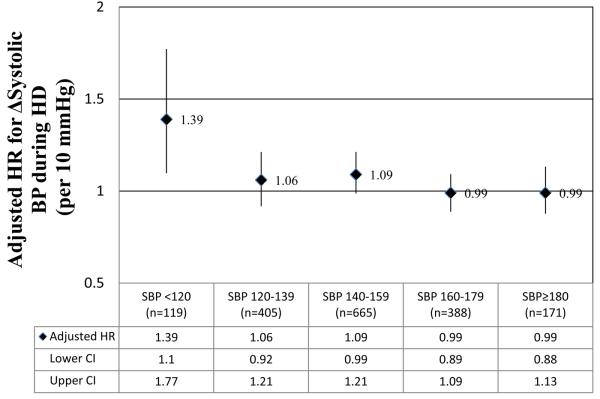
Considering we identified a significant interaction between predialysis SBP and ΔSBP during HD, the relationship between increasing SBP during HD and 2-year mortality was analyzed across strata of predialysis SBP (Figure 4). In fully adjusted stratified analyses, trends for an increased mortality associated with increasing SBP during dialysis were present in patients with predialysis SBP <160 mmHg, however, this relationship was only significant in patients with predialysis systolic BP <120 mmHg.
Figure 4.
Adjusted × hazard ratio for mortality associated with ΔSBP across strata of predialysis SBP. *model also adjusted for age, body mass index<26 kg/m2, interdialytic weight gain >5%, predialysis SBP, pre and postdialysis DBP, diabetes mellitus, hypertension, peripheral vascular disease, congestive heart disease, coronary artery disease, serum albumin, creatinine, phosphorus, and the use of nitrates. Abbreviation: SBP, systolic blood pressure; DBP, diastolic blood pressure.
Models analyzing the relationship between changes in PP and MAP during HD and 2-year mortality can be found in Item S1.
Sensitivity analyses
Considering the adverse association between increasing SBP during HD and 2-year mortality was pronounced in patients with low-normal predialysis SBP, we explored whether the presence or absence of other risk factors modified the relationship between increasing systolic BP during HD and 2-year mortality. However, we found no significant interaction between increasing SBP during HD and history of cardiovascular disease (p=0.4), history of congestive heart failure (p=0.9), hypoalbuminemia (p=0.4), body mass index <26 kg/m2 (p=0.8), or interdialytic weight gain >5% (p=0.8).
DISCUSSION
Intradialytic rise in BP is a known phenomenon of HD; however, this is the first study to identify its association with adverse 2-year clinical outcomes. While clinicians often focus on intradialytic hypotension and its complications, intradialytic increases in BP are often largely ignored. Considering our investigation identified an intradialytic increase in SBP to be associated with an increased hazard of death, particularly among patients with low predialysis SBP, further investigation into the pathophysiology measured by this phenomenon and the reason behind its association with adverse outcomes is warranted.
Previously, we demonstrated in a cohort of 438 prevalent HD patients that every 10 mmHg increase in SBP during HD is associated with a 20% increased odds of death or hospitalization at 6 months.(3) In our prior investigation, the adverse association between intradialytic increases in SBP and short-term outcomes were most pronounced in prevalent patients with KDOQI-defined hypertension. In our current investigation of incident HD patients, there was a modest association between intradialytic increases in SBP and 2-year mortality; however, these findings were limited to patients without predialysis hypertension. There are a number of potential reasons for the different findings between our two studies. In prevalent dialysis patients, those who exhibit intradialytic increases in SBP likely have an overall higher hemodynamic burden. In fact, a recent study by Agarwal et al noted the use of intradialytic BP recordings improved the diagnosis of hypertension as measured by ambulatory blood pressure (ABP).(14) In addition, a study by Mendes et al noted that in patients with intradialytic BP increases, the elevated postdialysis BP measurement was a better estimate of interdialytic BP load (measured by ABP) than the low predialysis BP parameter.(15) Considering 2 recent meta-analyses identified treatment of high BP to improve outcomes in prevalent HD patients (16, 17), the association between intradialytic increases in BP and poorer outcomes in prevalent HD patients may reflect the adverse effects of a higher hemodynamic burden over time. In incident dialysis patients, many of whom likely have not reached a target dry weight, intradialytic increases in BP may be less of a measure of hemodynamic burden and more a marker of volume overload or underlying cardiac comorbidity. In 2 small investigations of patients who exhibited intradialytic increases in BP and cardiac dilation on echocardiography, volume removal with HD improved the patient's cardiac output resulting in increased BP during HD.(18-20) Incident patients in our study with intradialytic BP elevations had a lower body mass index, lower interdialytic weight gain, lower serum albumin, and lower serum creatinine compared to patients without intradialytic BP elevations. Therefore, under recognized volume overload and/or a reluctance to aggressively remove volume (resulting in inappropriately low sodium solute removal) in patients with low albumin and low body mass may partially underlie the adverse outcomes associated with intradialytic increases in BP in incident dialysis patients. Alternatively, intradialytic increases in BP may purely be a marker of underlying cardiomyopathy as patients with low-normal predialysis SBP exhibited the highest mortality ratios associated with increasing SBP during HD; however, we did not identify a higher prevalence of cardiac disease among patients with intradialytic BP increases.
While intradialytic BP elevations have been demonstrated to be due to volume overload and dilated cardiomyopathy in select patients, more recent studies have suggested endothelial cell dysfunction may contribute to the pathogenesis of intradialytic increases in BP. In 2 small investigations, participants with intradialytic increases in BP (compared to control individuals) exhibited inappropriately elevated postdialysis peripheral vascular resistance.(4, 5) The elevated peripheral vascular resistance was not associated with an increase in catecholamines or renin but rather was associated with an imbalance of endothelin-1 (an endothelial derived vasoconstrictor) to nitric oxide (an endothelial derived vasodilator). Thus, these 2 studies raise the hypothesis that the pathophysiology of intradialytic increases in BP may be related to underlying endothelial cell dysfunction. Endothelial cell dysfunction is a precursor to vascular damage and is associated with an increased risk of future cardiovascular events.(6, 21, 22) In our present investigation of patients with intradialytic BP elevations, the primary reported cause of death was cardiovascular. Thus, underlying endothelial cell dysfunction may provide a common mechanistic explanation behind the pathophysiology of intradialytic increases in BP and its association with increased mortality risk.
While our investigation revealed unique associations between intradialytic BP elevations and mortality, these results should be interpreted in the setting of limitations. First, as a retrospective analysis, cause and effect cannot be determined and unmeasured or unknown confounders cannot be accounted for. This analysis cannot determine if intradialytic BP is merely a marker for severity of underlying disease. Second, this study is not able to identify pathophysiologic mechanisms associated with intradialytic BP increases nor was data available to determine specific dialytic techniques or intradialytic food intake which may have contributed to intradialytic BP increases. Further, we lacked information on timing of antihypertensive medication and the dose of erythropoietin which theoretically could lead to intradialytic BP changes and contribute to adverse outcomes. Third, the BP parameters used in this analysis were collected as part of routine clinical care and non-standardized BP parameters are less predictive of end-organ damage compared to standardized BP measurements or ambulatory BP.(23, 24) Finally, regression to the mean is more likely to occur in patients with extremes of systolic BP and measurement error may have contributed to our findings of intradialytic BP changes being associated with adverse outcomes. In addition, we used BP measurements from only 3 HD sessions to define intradialytic increases in BP which does not account for changes in BP over time.
In summary, among incident HD patients, we have identified increasing systolic BP during HD to be associated with poorer 2-year survival; however, these findings are limited to patients with normal predialysis systolic BP. Whether an intradialytic increase in systolic BP in incident dialysis patients is simply a marker of volume overload or comorbid illness or is an independent modifiable risk factor remains to be determined.
Supplementary Material
ACKNOWLEDGMENTS
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government. This work was presented in part at the American Society of Nephrology Annual Meeting in Philadelphia on November 6, 2008.
Support: Dr. Inrig was supported by National Institutes of Health (NIH) grant K23 HL092297. Dr. Patel was supported by NIH grant K23 DK075929. Dr. Toto was supported by NIH grant K24 DK02818.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None.
SUPPLEMENTARY MATERIAL
Item S1: Baseline characteristics of patients categorized by changes in pulse pressure and mean arterial pressure, and mortality associated with changes in pulse pressure and mean arterial pressure.
Note: The supplementary material accompanying this article (doi: ____) is available at www.ajkd.org.
REFERENCES
- 1.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Gul A, Sarnak MJ. Management of intradialytic hypertension: the ongoing challenge. Semin Dial. 2006;19:141–145. doi: 10.1111/j.1525-139X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 3.Inrig JK, Oddone EZ, Hasselblad V, et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71:454–461. doi: 10.1038/sj.ki.5002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou KJ, Lee PT, Chen CL, et al. Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int. 2006;69:1833–1838. doi: 10.1038/sj.ki.5000266. [DOI] [PubMed] [Google Scholar]
- 5.Raj DS, Vincent B, Simpson K, et al. Hemodynamic changes during hemodialysis: role of nitric oxide and endothelin. Kidney Int. 2002;61:697–704. doi: 10.1046/j.1523-1755.2002.00150.x. [DOI] [PubMed] [Google Scholar]
- 6.Koc M, Richards HB, Bihorac A, Ross EA, Schold JD, Segal MS. Circulating endothelial cells are associated with future vascular events in hemodialysis patients. Kidney Int. 2005;67:1078–1083. doi: 10.1111/j.1523-1755.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama S, Taguchi A, Iwashima S, et al. Low circulating CD34(+) cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney Int. 2008;74:1603–1609. doi: 10.1038/ki.2008.495. [DOI] [PubMed] [Google Scholar]
- 8.US Renal Data Systems . Researcher's Guide to the USRDS Database. National Institutes of Health, National Institute of Diabetes and Digestive Kidney Diseases; Bethesda, MD: 2006. [Google Scholar]
- 9.Inrig JK, Patel UD, Gillespie BS, et al. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis. 2007;50:108–118. doi: 10.1053/j.ajkd.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Kidney Foundation K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines accessed February 10, 2007. [PubMed]
- 11.Jaar BG, Plantinga LC, Fink NE, et al. Comparison of Multiple Sources of Cause of Death in a Cohort of Incident Dialysis Patients: The CHOICE Study; ASN Annual Conference SU-PO620; 2007. [Google Scholar]
- 12.Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. Beta-blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164:2465–2471. doi: 10.1001/archinte.164.22.2465. [DOI] [PubMed] [Google Scholar]
- 13.Klassen PS, Lowrie EG, Reddan DN, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. Jama. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Metiku T, Tegegne GG, et al. Diagnosing Hypertension by Intradialytic Blood Pressure Recordings. Clin J Am Soc Nephrol. 2008;3:1364–1372. doi: 10.2215/CJN.01510308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendes RB, Santos SF, Dorigo D, et al. The use of peridialysis blood pressure and intradialytic blood pressure changes in the prediction of interdialytic blood pressure in haemodialysis patients. Blood Press Monit. 2003;8:243–248. doi: 10.1097/00126097-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Heerspink HJ, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009 doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Sinha AD. Cardiovascular Protection With Antihypertensive Drugs in Dialysis Patients. Systematic Review and Meta-Analysis. Hypertension. 2009 May;53(5):860–6. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirit M, Akcicek F, Terzioglu E, et al. Paradoxical rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant. 1995;10:1417–1420. [PubMed] [Google Scholar]
- 19.Fishbane S, Natke E, Maesaka JK. Role of volume overload in dialysis-refractory hypertension. Am J Kidney Dis. 1996;28:257–261. doi: 10.1016/s0272-6386(96)90309-1. [DOI] [PubMed] [Google Scholar]
- 20.Gunal AI, Karaca I, Celiker H, Ilkay E, Duman S. Paradoxical rise in blood pressure during ultrafiltration is caused by increased cardiac output. Journal of Nephrology. 2002;15:42–47. [PubMed] [Google Scholar]
- 21.Amabile N, Guerin AP, Leroyer A, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 22.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Peixoto AJ, Santos SFF, Zoccali C. Pre- and Postdialysis Blood Pressures Are Imprecise Estimates of Interdialytic Ambulatory Blood Pressure. Clin J Am Soc Nephrol. 2006;1:389–398. doi: 10.2215/CJN.01891105. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–1180. doi: 10.1038/sj.ki.5000247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.