Abstract
Asymmetric (NG,NG) dimethylarginine (ADMA) is present in plasma and cells. It can inhibit nitric oxide synthase (NOS) that generates nitric oxide (NO) and cationic amino acid transporters (CAT) that supply intracellular NOS with its substrate, L-arginine from the plasma. Therefore, ADMA and its transport mechanisms are strategically placed to regulate endothelial function. This could have considerable clinical impact since endothelial dysfunction has been detected at the origin of hypertension and chronic kidney disease (CKD) in human subjects and may be a harbinger of large vessel disease and cardiovascular disease (CVD). Indeed, plasma levels of ADMA are increased in many studies of patients at risk for, or with overt CKD or CVD. However, the levels of ADMA measured in plasma of about 0.5 μmol · l−1 maybe below those required to inhibit NOS whose substrate, L-arginine, is present in concentrations manifold above the Km for NOS. However, NOS activity may be partially inhibited by cellular ADMA. Therefore, the cellular production of ADMA by protein arginine methyltransferase (PRMT) and protein hydrolysis, its degradation by NG, NG-dimethylarginine dimethylaminohydrolase (DDAH) and its transmembrane transport by CAT that determines intracellular levels of ADMA may also determine the state of activation of NOS. This is the focus of the review. It is concluded that cellular levels of ADMA can be 5- to 20-fold those in plasma and in a range that could tonically inhibit NOS. The relative importance of PRMT, DDAH and CAT for determining the intracellular NOS substrate: inhibitor ratio (L-arginine:ADMA) may vary according to the pathophysiologic circumstance. An understanding of this important balance requires knowledge of at least these three processes that regulate the intracellular levels of ADMA and arginine.
Keywords: Nitric oxide synthase (NOS), protein arginine methyl transferase (PRMT), cationic amino acid (CAA), cationic amino acid transporter (CAT), cardiovascular disease, chronic kidney disease (CKD), hypertension, reactive oxygen species and oxidative stress
1. Overview of ADMA Generation, Metabolism and Transport
Asymmetric (NG,NG) dimethylarginine (ADMA) was isolated in 1987. It is shown to be metabolized by NG, NG-dimethylarginine dimethylaminohydrolase (DDAH) to citrulline and dimethylamine [1]. Certain arginine moieties on proteins are subject to asymmetric dimethylation by class 1 isoforms of protein arginine methyltransferase (PRMT). After protein hydrolysis, ADMA is released within cells where it is a potent inhibitor of constitutive [2] and inducible nitric oxide synthase (NOS) [3]. ADMA is both exported from its site of origin, and imported from the plasma at distant sites, by cationic amino acid transporters (CATs) in exchange for arginine and other cationic amino acids (CAAs) [4,5] (Fig 1).
Figure 1.
Overview of some pathways for ADMA generation, metabolism, transport and excretion.
Much interest centers on the role of ADMA in causing cardiovascular and kidney diseases or their complications. Valance et al demonstrated that ADMA accumulated substantially in the plasma of patients with chronic kidney disease (CKD) receiving dialysis [6]. During the last decade there have been a series of important epidemiologic studies that related plasma ADMA to the established risk factors for cardiovascular disease (CVD) and CKD [7,8]. Moreover, plasma concentrations of ADMA predicted mortality in patients with end-stage renal disease (ESRD) [9] and predict cardiovascular events and mortality in populations at high, intermediate or low global vascular risk [10]. Plasma levels of ADMA predicted all cause mortality, but not cardiovascular disease incidence in the Framingham Offspring Study of 3,320 normal subjects followed for 11 years [11]. ADMA is positioned strategically to be a pathophysiologic effector of cardiovascular and kidney diseases since it accumulates in the serum in those at risk and, by inhibiting NOS and cationic amino acid transport (CAT), could participate in endothelial dysfunction and the generation of microvascular oxidative stress which are at the origin of vascular and organ diseases [12,13]. However, in their initial description of the substantially increased levels of ADMA in patients with ESRD, Vallance et al also reported that the levels of ADMA measured in the plasma, even in this extreme condition, were barely sufficient to cause contraction of an isolated aortic strip that was used as a bioassay [6]. This raised the possibility that ADMA may be an excellent marker, but not a pathophysiologic mediator, of renal disease [14].
More recently, efforts have been made to look beyond circulating ADMA and to assess the factors that lead to the generation and accumulation of ADMA within cells and the exchange of ADMA between cells. Intracellular ADMA that escapes metabolism by DDAH can inhibit CAT and thereby not only block NOS activity but also limit the cellular uptake of L-arginine, thereby contributing to oxidative stress and further inhibition of NO biogenesis. These actions would be anticipated to have detrimental effects, especially in blood vessels, myocytes and the kidney. However, as has been reviewed recently, DDAH-1 and DDAH-2 are widely distributed in cells which should limit cellular accumulation of ADMA [12]. The critical step that determines the partition of ADMA between the cytosol and the extracellular fluid is its transmembrane transport via CATs. CATs are widely distributed on cell membranes either as high-affinity, low-capacity transporters exemplified by CAT-1 that transports ADMA and arginine across cell membranes in blood vessels and the distal nephron of the kidney or as higher-capacity, lower-affinity transporters such as CAT-2A that transport these CAAs across the membranes of liver cells [4] (Fig 1). A different set of renal transporters are implicated in the cellular uptake of CAAs from the proximal tubular fluid into the proximal tubule cells and the cellular export from the basolateral side of these cells into the blood.
Some recent studies of cellular arginine and ADMA are summarized in Figure 2 and Table 1. It is apparent that the cellular arginine concentrations can be comparable to, or above, those in plasma and are manifold above the Km for endothelial (e) or neuronal (n) NOS (Panel 2A and 2C) but are close to the Kt for CAT transport (Fig 2A). Estimates of intracellular ADMA concentrations in the brain (Fig 2B) and endothelial cells (Fig 2C) suggest that ADMA is 10- or 20-fold higher than in plasma (Table 1). The concentrations of ADMA in the brain are about 8-fold above the Ki for nNOS. Although the reports for the Ki for eNOS are rather variable, most place it in a similar range as for nNOS and below 1 μmol · l−1. Thus, the ADMA concentrations within neuronal and endothelial cells are manifold above the Ki for NOS. It follows that intracellular ADMA might inhibit NO generation tonically, although this would depend also on the cellular concentrations of arginine. This could provide an explanation for the arginine paradox whereby excess arginine can enhance NO generation and action in the tissues by an NOS-dependent mechanism, despite the presence of arginine at manifold higher concentration than its Km for NOS (Fig 2A). Presumably, the state of activation or inhibition of NOS will depend on the local intracellular concentrations of substrate and inhibitor, or the L-arginine:ADMA concentration ratio. The findings of remarkably high intracellular levels of ADMA imply that CAT activity normally is insufficient to equilibrate ADMA between cell cytosol and plasma. Consequently, PRMT, DDAH and CAT activities may all assume critical roles in determining cellular levels of ADMA and, hence, the state of activation or inhibition of NOS in the tissues. This is the focus of our review.
Figure 2.
Data from studies showing intracellular L-arginine in brain in relation to the Km for nNOS and the Kt for CAT-1 (Panel A), and for intracellular ADMA in brain in relation to the Ki for nNOS (Panel B) and for intracellular ADMA in endothelial cells from normal or diabetic animals in relation to the Ki for eNOS.
Panel A and B: Redrawn from data in Cardounel, AJ and Zweier, JL. Endogenous methylarginines regulate neuronal nitric-oxide synthase and prevent excitotoxic injury. J Biol Chem 277: 33995–34002, 2002.
Panel C: Redrawn from data in (1) Bogle, RG et al. Induction of NG-monomethyl-L-arginine uptake: a mechanism for differential inhibition of NO synthases? Am J Physiol 269: C750–C756, 1995. (2) Masuda, H et al. Accelerated intimal hyperplasia and increased endogenous inhibitors for NO synthesis in rabbits with alloxan-induced hyperglycaemia. Br J Pharmacol 126: 211–218, 1999. (3) MacAllister, RJ et al. Effects of guanidino and uremic compounds on nitric oxide pathways. Kidney Int 45: 737–742, 1994.
Table 1.
Some examples of plasma and intracellular levels of L-arginine and ADMA.
| Circulating levels (μmol/l) | Intracellular levels (μmol/l) | |||||
|---|---|---|---|---|---|---|
| Species | Condition | L-arginine | ADMA | L-arginine | ADMA | Reference |
| Homo | DM Type 1 | 0.40±0.06 | [109] | |||
| DM Type 1 + nephropathy | 0.46±0.08 | |||||
| Homo | Control | 78±16 | 0.391±0.067 | [108] | ||
| ADPKD | 72±19 | 0.604±0.131 | ||||
| Homo | Control | 78±12 | 0.391±0.057 | [107] | ||
| Essential hypertension | 64±17 | 0.767±0.138 | ||||
| Rat | Normoglycemia | 127.9±3.9 | 0.599±0.017 | [114] | ||
| Hyperglycemia | 79.7±6.7 | 0.581±0.078 | ||||
| Rat | Normoglycemia | 0.5±0.1 | [112] | |||
| High fat + STZ | 1.3±0.3 | |||||
| Rat | 94.0±7.81 | 5.1±0.61 | [120] | |||
| Rat | 62.7±18 | 0.33±0.10 | 151±342 | 3.6±1.02 | [157] | |
| 189±233 | 5.8±1.23 | |||||
| Rabbit | Normoglycemia | 5.0±0.34 | [119] | |||
| Hyperglycemia | 12.1±2.34 | |||||
| Mouse | Wild-type | 27.7±1.6 | 1.58±0.20 | [158] | ||
| hDDAH-1 transgenic | 31.4±3.4 | 0.70±0.15 | ||||
| Mouse | Control | 1.07±0.07 | [111] | |||
| Ang II 3 μg/kg/min for 4 weeks | 0.84±0.04 | |||||
ADPKD – Autosomal dominant polycystic kidney disease; STZ – Streptozotocin; Ang II – Angiotensin II.
Brain slice,
Endothelial cells,
Carotid,
Aortic endothelial cells.
2. Cellular Distribution of ADMA
2.1. Transport via CAT-1 and CAT-2
Although many conditions alter the metabolism and levels of amino acids, mere determination of plasma amino acid concentrations provides limited information, because the plasma pool of amino acids is very small compared with the intracellular pool [15]. In addition, amino acids undergo extensive interorgan exchange. The amino acid plasma concentration is the outcome of their rate of appearance in and disappearance from plasma which is not reflected by static measurement of plasma amino acid levels. The rapid and extensive exchange of amino acids between the intracellular and plasma pools is facilitated by specific transport systems [16]. The nomenclature to classify these systems was devised before the isolation and characterization of the individual transport proteins and is based on specificity and affinity in kinetic experiments [17,18]. Of the CAA transport systems y+, y+L, b0,+, and B0+, only system y+ is selective for these amino acids [19]. System y+ is ubiquitously expressed, whereas system y+L is more restricted, principally to erythrocytes, platelets, lymphocytes, kidney, and placenta [19–22]. The CAT molecules responsible for system y+ were among the first mammalian amino acid transporters to be identified. They form a subfamily of the solute carrier 7 (SLC7), and comprise several members of which CAT-1, CAT-2A, and CAT-2B are most relevant for transmembrane transport of arginine and ADMA in mammals. In accordance with the wide distribution of system y+, CAT-1 is ubiquitously expressed in all tissues and organs except generally for the liver. In contrast, the two splice variants of CAT-2 have a much more restricted expression. CAT-2A is expressed strongly in the liver and weakly in skeletal muscle and pancreas, whereas CAT-2B is induced by pro-inflammatory cytokines in a variety of tissues, often with inducible forms of arginase and NOS (described in more detail in section 2.3). Expression of CAT-3 in adult animals is restricted mainly to the brain, but has also been detected in the thymus and mammary gland [23,24].
CAT transport is independent of sodium. Dibasic amino acids such as arginine, lysine, and ornithine have a net positive charge at neutral pH and are transported by CAT-1 and CAT-2B. Closs et al demonstrated that monomethylarginine (MMA), ADMA, and SDMA also are good substrates for human CAT-2B [25]. In contrast, histidine is a poor substrate at physiological pH, but is transported efficiently in its protonated form at pH 5.5. CAAs have a Km of 100 to 250 μM for CAT-1 and slightly higher values for CAT-2B. In contrast, the affinity of CAT-2A for CAAs is about 10-fold lower [24]. CAT-2A is expressed principally in the liver, which does not normally express CAT-1. The low affinity of CAT-2A protects circulating arginine from extensive hepatic uptake and degradation by arginase. CAT-2A may remove surplus CAAs from the portal circulation [26,27].
The activity of CAT-1 is stimulated strongly by CAAs on the opposite side of the membrane in a process termed trans-stimulation. This exchange action may evoke an extensive net transfer of arginine between neighboring cells [24]. Cells such as activated vascular macrophages with a high arginase activity could deplete the extracellular medium of arginine by exchanging arginine for ornithine. The reverse process of exchange of ornithine for arginine may deplete neighboring cells such as vascular endothelial cells of arginine.
The membrane potential provides a driving force for the transport of CAAs into cells. Vasoactive agonists that induce membrane hyperpolarization such as acetylcholine or bradykinin increase the driving force for CAT-mediated arginine entry into the cell [16,28]. This results in a cellular uptake of arginine with acetylcholine or bradykinin that can stimulate NO production and thereby contribute to endothelium-dependent vasodilation.
The intracellular arginine concentrations normally are well above those required for maximal NOS activity in a cell-free system. However, endothelium-dependent vasodilation can be stimulated by arginine supplementation. This phenomenon has been termed the arginine paradox [29]. Inhibition of endothelial NOS by ADMA could be one explanation for this phenomenon if excess extracellular arginine was required to relieve inhibition by ADMA. This could occur by increasing the NOS substrate/inhibitor ratio or by promoting cell export of ADMA via transstimulation of CAT [30,31]. Endothelial NOS and CAT-1 are both located in caveolae in the plasma membrane [32] which ensures an efficient supply of plasma arginine to endothelial NOS. Thus, plasma arginine might sustain NOS activity because of restricted delivery of arginine to the enzyme [33]. This has been proposed as an explanation for the arginine paradox. However, it is also conceivable that the close proximity of CAT-1 and eNOS also might provide plasma ADMA with selective access to NOS. This would imply that inhibition of NOS is dependent on plasma, rather than intracellular, ADMA. A preferential channeling of plasma ADMA to eNOS could also explain the observation that modestly elevated plasma levels of ADMA can predict cardiovascular events in an increasing number of studies.
CAT activity sustains a dynamic equilibrium of basic amino acids between cells and plasma. Disturbance of this equilibrium reveals the high capacity of tissue uptake and release of amino acids by CAT. During a 30 minute infusion of a high dose of arginine or lysine, plasma levels of these amino acids increased almost 100-fold but, thereafter, the levels dropped very rapidly and were already reduced by 50% after 30 min [34]. This implies a rapid CAT-mediated clearance from the circulation. Conversely, infusion of arginase into rats reduced plasma levels of arginine to undetectable levels, but these were restored rapidly after arginase was cleared from the circulation [35]. Rapid clearance of ADMA also has been demonstrated by Kielstein and colleagues by experiments in which it was infused into humans. They calculated a mean plasma half-life of 24±7 minutes from the plasma ADMA decay curves [36].
Cell culture media, such as the commonly used medium 199 and Dulbecco’s Modified Eagle’s Medium (DMEM), contain rather high concentrations of arginine of approximately 300–400 μmol/L, which are much above the Km value of CAT-1 and CAT-2B and also higher than typical plasma concentrations of arginine that range from 50–150 μmol/L [15,37]. This may have a profound effect on cellular NOS activity and ADMA metabolism for three reasons. First, it may supply NOS with such high amounts of substrate that it works at a maximal rate even in the presence of concentrations of ADMA that would be inhibitory at lower arginine concentrations. Second, Ogawa et al reported in 1989 that arginine is a competitive inhibitor of DDAH, albeit with a relatively high inhibitory constant (Ki of 2.5 mM) [38]. However, intracellular arginine concentrations can reach millimolar levels, indicating that physiological arginine concentrations could inhibit DDAH. Indeed, Wilcken et al demonstrated that extracellular arginine, in the concentration range from 0–400 μmol/L, dose-dependently inhibited degradation of ADMA by DDAH in cultured hepatoma cells, with a concomitant increase in intracellular levels of ADMA. The authors suggested that this could regulate the metabolism of ADMA [39,40]. Third, high concentrations of arginine may saturate the CAT system and either limit (by competition) or promote (by trans-stimulation) cellular export of ADMA. Moreover, culture media also contain supraphysiological amounts of lysine, which can compete with both arginine and ADMA for transport by CAT. In contrast, the CAA ornithine, whose concentration in plasma is approximately 50 μmol/L, is not present in culture media. On a parallel note, glutamine, that is often added to culture media in supraphysiological concentrations, inhibits agonist-stimulated NO production [41,42]. Taken together, the highly unphysiological amino acid composition of culture media may influence NO production, for example by altering the kinetics of CAT and the cellular metabolism of ADMA. Therefore, it is questionable whether the results of cell culture experiments adequately reflect the in vivo situation.
2.2. Special transport mechanisms in the kidney
The kidney plays a major role in arginine metabolism. First, the kidney is responsible for approximately 60% of net arginine synthesis in adult mammals, by extracting citrulline from the circulation and converting it stoichiometrically into arginine, which is subsequently exported to the plasma [43]. Second, the kidney consumes arginine because it is involved in creatine synthesis by catalyzing the transfer of the amidino group of arginine to glycine to form guanidinoacetate and ornithine [44]. Although the kidney both produces and consumes arginine, measurement of net flux across the kidney has revealed that overall the kidney is a net producer of arginine [45]. Net production notwithstanding, uptake of arginine from the circulation in the renal cortical macula densa cells and renal medulla by CAT-1 plays an important role in local NO production [5,46].
The kidney is very sensitive to circulating levels of L-arginine or ADMA. We found that the intrarenal infusion of L-arginine in the rat leads to NOS-dependent renal vasodilation that was most prominent in salt-depleted rats [47]. Kielstein et al reported that infusion of ADMA decreased the effective renal plasma flow [36]. Moreover, plasma ADMA in elderly subjects was an independent predictor of reduced effective renal plasma flow and increased renovascular resistance [48]. It is tempting to speculate that renal release of arginine and uptake of ADMA are connected via transstimulation of the renal CAT-1 transporter, i.e. export of de novo synthesized arginine stimulating the simultaneous uptake of ADMA. Release of ADMA by proteolysis of methylated proteins in renal tissue is rapid, suggesting that endogenous ADMA production also contributes to ADMA renal tissue content [49]. The ADMA content of the rabbit kidney is much higher than the liver, heart and skeletal muscle, despite the finding that DDAH activity was highest in the kidney [50]. A high renal ADMA content, despite a high DDAH activity, seems counterintuitive but renal tissue is heterogeneous, and sites with high DDAH activity may be distinct form sites with high ADMA production or uptake. Indeed, we have observed that the two isoforms of DDAH have distinct localizations within the rat kidney with DDAH-1 mainly localized in proximal tubules and DDAH-2 in the glomerulus, macula densa and renal vasculature [12,51]. Levels of free ADMA have a heterogeneous intrarenal distribution, in contrast to protein-incorporated ADMA that has a far more homogeneous distribution [52].
Although the kidney both generates and metabolizes ADMA, overall the healthy kidney is an ADMA clearing organ. Net renal uptake of ADMA from the circulation has been demonstrated in both humans and rats by measurement of arterio-venous concentration differences [53,54]. The kidney contributes to clearance of ADMA by degradation by DDAH and by urinary excretion. It has been estimated that humans generate approximately 300 μmol of ADMA per day, of which more than 80% is metabolized by DDAH. The remainder is excreted in the urine [55]. In contrast, SDMA is not degraded by DDAH. Therefore renal excretion is the major eliminatory pathway for SDMA. Arginine, ADMA, and SDMA are freely filtered at the glomerulus but tubular reabsorption ensures that renal excretion is minimized. Less than 1% of the filtered load of arginine is lost in the urine [44]. Reabsorption of arginine occurs primary in the proximal tubule and utilizes distinct transporters in the apical and basolateral membranes [22]. Transport of arginine and other dibasic amino acids from the tubular fluid across the apical membrane is mediated by system b0,+, that also transports cystine. System b0,+ operates via an antiport mechanism whereby CAAs are transported into the cell in exchange for neutral amino acids. Dibasic amino acids are transported into the blood stream across the basolateral membrane via system y+L in exchange for sodium and a neutral amino acid. CAT-mediated transport in other tissues translocates original and methylarginine equivalently. Presently, there are no direct studies of the tubular reabsorption of methylarginines. However, indirect evidence, at least in humans, suggests that tubular reabsorption of ADMA and SDMA may be far less efficient than arginine, which is almost completely reabsorbed. As a result, the concentration of arginine in urine is much lower than its concentration in plasma, whereas urinary concentrations of ADMA and SDMA are two orders of magnitude higher than their plasma concentrations [6,56].
We studied the interaction of methylarginines with L-arginine transport in the distal nephron of the rat kidney. The loop of Henle was perfused orthogradly with artificial tubular fluid containing 10−3M methylarginines to evaluate their effects on [3H]-arginine uptake into the cells. The fractional loop [3H]-arginine absorption was reduced by 49% for ADMA, 56% for SDMA and 41% for L-NMA [57]. This suggests that methylarginines share a common transport process with L-arginine in this nephron segment.
There are striking inter-species differences in the renal handling of methylated arginines. Healthy humans excrete ADMA and SDMA in approximately equal amounts [6,56], whereas rats excrete SDMA but almost negligible amounts of ADMA, resulting in a urinary ADMA concentration approximately 25- to 100-fold below that of SDMA [52,54,56]. In line with this observation, a recent study showed that acute total nephrectomy in rats led to a dramatic increase in plasma levels of SDMA, whereas levels of ADMA were hardly influenced [58]. Low renal excretion of ADMA compared to SDMA was also observed in rabbits [59], but not in mice [52]. All in all, these data show that extrapolation of data from studies in experimental animals to humans is tricky, as renal ADMA metabolism even differs between individual species of the rodent order.
2.3. Regulation of CAT expression and activity
ADMA and SDMA are produced in most cells of the body, but also can be transported out of cells into the extracellular fluid and plasma where they can be taken up by distant cells. In contrast, most of the body’s arginine is manufactured from citrulline in the renal proximal tubules with only small amounts being synthesized in other tissues such as blood vessels. The capacity and activity of CATs determines the partition of CAAs across cells, and thereby the intracellular ratio of arginine to ADMA concentration which is believed to regulate NOS activity. Accordingly, it is important to consider the distribution and regulation of CATs to understand how intracellular arginine and methylarginine concentrations are maintained. For example, a decrease in cellular CAT activity would decrease cellular arginine and increase cellular ADMA, thereby inhibiting NOS activity without being detected as a change in the plasma levels of these CAAs. Unfortunately, there are presently rather few studies that have evaluated trans-cellular distribution of CAAs, and no studies in humans. Therefore, the concept that CAT activity is of major importance for determining NOS activity remains largely unexplored.
Baylis et al have studied L-arginine uptake into endothelial cells in uremia. She reported substantial reductions in whole body nitrate plus nitrite (NOx) generation in patients with ESRD [60]. The clinical problem was amplified by circulating methylarginines [61] and urea [62] that blocked the uptake of L-arginine into cultured endothelial cells. This interesting series of studies has demonstrated the importance of circulating ADMA and urea in limiting arginine uptake for NO generation in a clinical setting [4].
Three cationic amino acid transporters are expressed in mammals. Their respective genes have been studied extensively: CAT-1 (SLC7A1), CAT-2A (SLC7A2A) and CAT-2B (SLC7A2B). The regulation, expression and function of CATs have been reviewed [24,63,64]. CAT-1 is highly homologous between human, rat and mouse [64–67] and is regulated transcriptionaly and posttranscriptionaly [63,64]. The CAT-1 promoter lacks a TATA-box and any obvious initiator. It has a single transcriptional start site from this promoter [24,64]. Fernandez et al [68] reported that an amino acid-response element (CTGATGAAAC) within the first exon of the CAT-1 gene was required for transcription. The phosphorylated form of eukaryotic initiation factor 2α (eIF2α), which is activated by general control nonderepressible-2 kinase (GCN2), was necessary for transcriptional activation. Translation of the CAT-1 mRNA was initiated from an internal ribosomal entry sequence (IRES) within the 5′UTR (untranslated region) of the mRNA which also required the phosphorylation of eIF2α by either GCN2 or RNA-dependent protein kinase-like endoplasmic reticulum kinase (PERK) [69,70]. The translation of an upstream open reading frame (ORF) of 48 AA within the 5′UTR regulated the activity of IRES.
In addition to this classical pathway for gene regulation, Bhattacharyya et al [71] reported an independent mechanism of control of human CAT-1 translational control based on a liver-specific microRNA (miRNA) termed miR-122. MicroRNAs are single-stranded RNA molecules of 21–23 nucleotides encoded by genes from whose DNA miRNAs are transcribed, but not translated into protein. miRNAs can down-regulate gene expression by base-pairing to target mRNAs which initiates degradation of the mRNA and translational repression. Many different miRNAs are encoded in individual genomes, and approximately 30% of all human genes are predicted to be subject to miRNA regulation. Although specific functions and targets have been assigned to only a few dozen miRNAs, much experimental evidence suggests that they participate in the regulation of a spectrum of biological processes. The expression of miRNAs is tissue- or developmental-stage specific. Altered miRNA expression profiles have been linked to human diseases [71,72].
Several potential target sites for miR-122 have been found in the mRNA for human CAT-1. Chang et al [73] reported that insertion of these sites into the 3′UTR of an mRNA reduced its translation and its stability, thereby reducing its cellular RNA levels. Interestingly, amino acid starvation relieved endogenous human CAT-1 mRNA from inhibition by miR-122. This de-repression of the CAT-1 mRNA depended on its release from cytoplasmic processing bodies. These effects of miR-122 on CAT-1 gene expression and stability were independent of IRES and phosphorylation of eIF2α [24,71]. Further study is needed to define the stimuli that regulate CAT-1 gene expression via diverse transcriptional factors and specific miRNAs, and to analyze the upstream events that initiate these distinct mechanisms of RNA translation.
The activity of CAT is dependent on trans-stimulation and hyperpolarization. Thus, membrane hyperpolarization stimulates influx of L-arginine and inhibits its efflux. This accounted for changes in L-arginine transport in cells treated with adenosine [74], bradykinin and insulin [75] or glucose [76].
The gene and protein expression and activity of CAT-1 are regulated by many factors reviewed below (Fig 3). Positive regulation has been observed in the following circumstances:
Figure 3.
Diagrammatic representation of some factors described in the text that have been reported to change the expression and/or activity of CAT-1 or CAT-2 isoforms.
1) cell growth, tissue repair and regeneration
Aulak et al [66] reported that the CAT-1 gene was silent in the normal liver but was induced in response to growth stimuli, such as partial hepatectomy. CAT-1 was considered to be a member of the classic delayed early growth response genes in the regenerating liver, since its induction was blocked by cycloheximide, indicating that gene induction depended on protein synthesis. The degree of induction of CAT-1 was substantial. It was increased by 9-fold at 3 hours after partial hepatectomy. Remarkably, this increase was not accompanied by increased transcriptional activity of the gene in the regenerating liver, indicating that the regulation of expression of the CAT-1 gene was post-transcriptional [66,77–79].
2) autocoids
Helle et al [80,81] recently reported that Ang II acting on AT1-receptors increased the expression of CAT-1 and CAT-2 mRNA in preglomerular vessels isolated from the clipped and non-clipped kidneys of 2K1C Goldblatt hypertensive rats. Insulin induced the generation of CAT-1 and CAT-2B mRNA in human umbilical vein endothelial cells (HUVEC) which was accompanied by increased CAT activity. This effect was mediated by mitogen-activated protein kinases (MAPK) p42 and p44 and phosphatidylinositol 3-kinase (P13-K) [77].
3) Starvation
Starvation has been reported to increase CAT-1 mRNA via increased transcription and/or increased mRNA stability [63]. Amino acid starvation stimulated translation of CAT-1 mRNA, resulting in a 58-fold increase in its protein levels accompanied by increased arginine transport [69]. Such a remarkable increase in CAT-1 expression presumably aids the cellular uptake of plasma L-arginine at times of deficiency. The consequences for cellular levels of CAA and ADMA have not been studied.
4) inflammation, cytokines, oxidative stress, tissue damage
Tumor necrosis factor-alpha (TNF-α) added to rat hepatic stellate cells enhanced the expression of CAT-2B mRNA without changes in CAT-1 mRNA [82]. Platelet-derived growth factor added to vascular smooth muscle cells induced CAT-2B mRNA strongly with only a modest elevation of the mRNA for CAT-1 [83]. Transforming growth factor-beta (TGF-β) [84] enhanced cellular L-arginine uptake. Peroxynitrite upregulated CAT-2 mRNA in rat mesangial cells [85]. Injections of rats with Escherichia coli endotoxin [lipopolysaccharide (LPS)] resulted in increased expression of CAT-1 and CAT-2B mRNA in lung, heart, and kidney, without affecting expression of CAT-2A in the liver [86]. Injection of LPS into rats lowered plasma levels of both arginine and ADMA, whereas plasma levels of SDMA increased [54]. In contrast, acute LPS-induced endotoxemia in humans decreased the plasma level of arginine without effects on ADMA or SDMA [87]. This difference may reflect species-specific effects of LPS or differences in LPS dose and timing of blood sampling between these studies. Clinical conditions associated with severe endotoxemia including sepsis and multiple organ failure are accompanied by increased plasma levels of ADMA [88]. A decreased activity of DDAH and an endotoxin-induced alteration of the expression pattern of CAT isoforms could underlie the link between elevated ADMA levels and mortality in critically ill patients [89,90]. Ischemic acute renal failure in rats increased renal tubular CAT-2 mRNA without a change in CAT-1 mRNA [91]. It is clear that these inflammatory and ischemic stimuli provoke large, and relatively selective, increases in CAT-2 expression in many organs or tissues.
The following factors have been found to inhibit CAT expression and/or activity:
1) oxidative stress, toxins and homocysteine
Zhang et al [92] reported that a 24 hour exposure of human endothelial cells (ECs) to cigarette smoke extract reduced the expression of CAT-1 mRNA, accompanied by a reduced arginine uptake. Peroxynitrite downregulated CAT-1 mRNA in rat mesangial cells [85]. Incubation of primary cultures of bovine aortic endothelial cells (BAECs) with homocysteine downregulated CAT-1 mRNA [93]. Incubation of ECs with oxidized LDL decreased arginine uptake [94]. Thus, it appears that oxidative stress and inflammation have generally opposite effects on CAT activity, due to downregulation of CAT-1 by ROS, but induction of CAT-2 by inflammatory cytokines.
2) heart failure, hypertension, uremia and diabetes mellitus
Kaye et al reported a 76% reduction in the CAT-1 mRNA in peripheral blood mononuclear cells from patients with heart failure [95]. A substantial reduction in L-arginine uptake into vascular tissue has been reported in hypertensive patients and those with a family history of hypertension [96]. L-Arginine transport is impaired in uremic [97] and ischemic acute renal failure and in diabetic rats [98].
3) Micro-RNA
miR-122 is a mammalian-miRNA that repressed CAT-1 mRNA expression [71,73]. However, the effects in human hepatocarcinoma cells was prevented by stress conditions [71].
4) C/T genetic polymorphism
Kaye et al identified a novel C/T polymorphism in the 3′UTR of the CAT-1 gene. The T allele decreased reporter gene expression significantly. It was found in13.3% of 278 hypertensive patients compared with 7.6% of 498 normotensive subjects [99]. The same group demonstrated that the major allele contained a consensus sequence for the transcription factor SP1. The minor allele failed to bind to SP1 [100].
2.4. Relative importance of CAT versus DDAH for determining intracellular ADMA concentration
The elegant study by Achan et al concluded that DDAH activity was responsible for 80% of ADMA disposal [55] illustrating the importance of DDAH in the metabolism of ADMA, but it did not allow concluding that (changes in) DDAH activity were the main factor in determining (changes in) ADMA levels. The activity of PRMTs, proteolysis of methylated proteins, and CAT-mediated exchange between intra- and extracellular ADMA must play a role. For example, the administration of estrogens to postmenopausal women receiving hormone replacement therapy lowered circulating ADMA [101–103]. Although this may reflect increased activity of DDAH, for example by estrogen-induced protection of DDAH against oxidative stress, we have observed that the administration of estrogens reduced virtually all plasma amino acids [104]. This indicated that protein degradation and altered CAT activity are also likely involved.
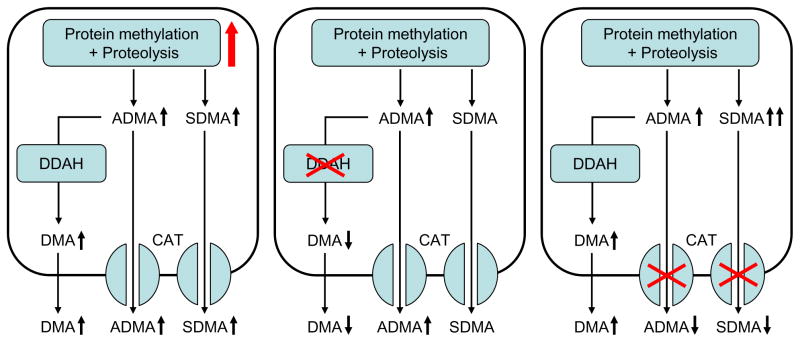
A simple scheme of the processes that determine intracellular ADMA levels, depicted in Figure 4, provides a hypothetical framework to predict what happens to both intra- and extracellular ADMA when one of these processes is perturbed. Free ADMA is formed by the sequential processes of protein methylation by PRMTs and proteolysis by intracellular proteases and/or the proteasomal system. Cellular disposal of ADMA can occur by two mechanisms: degradation by DDAH and export from the cell via CAT. Increased generation of ADMA, either caused by upregulation of PRMTs or by enhanced proteolytic activity, will increase intracellular ADMA. Assuming that the CAT system is not saturated, the increased intracellular ADMA levels will stimulate export, thereby increasing extracellular ADMA. Likewise, a lowered rate of cellular disposal of ADMA also will increase intracellular ADMA levels, but the effect on extracellular ADMA may depend on whether decreased DDAH activity or decreased CAT activity is involved. Diminished expression or inhibition of DDAH will increase both intra- and extracellular ADMA levels. Conversely, diminished CAT expression or activity will slow cellular egress of ADMA, thereby increasing intracellular, but decreasing extracellular, ADMA levels. Because enhanced ADMA generation and diminished ADMA degradation by DDAH both increase intra- and extracellular ADMA, either condition will result in a positive relation between extra- and intracellular ADMA. In contrast, inhibition of CAT may lead to an inverse association between intra- and extracellular ADMA.
Figure 4.
Simplified scheme of ADMA metabolism. Both free ADMA and SDMA are generated upon proteolysis of methylated proteins and may leave the cell via cationic amino acids transporters (CAT). In addition, ADMA is hydrolyzed by dimethylarginine dimethyaminohydrolase (DDAH) into citrulline and dimethylamine (DMA). Distinct changes in intra- and extracellular levels of ADMA, SDMA, and DMA may occur upon increased protein methylation and/or proteolysis (left panel), decreased DDAH activity (middle panel) or decreased CAT activity (right panel). See text for a detailed description.
Analysis of ADMA-related metabolites may give some clues as to what mechanism(s) are involved. Because SDMA is not a substrate for DDAH, its levels are affected by changes in protein methylation/proteolysis and CAT activity, but not by changes in DDAH activity. Reduced DDAH activity therefore will result in an increased ADMA/SDMA ratio. Changes in protein methylation or proteolytic activity may hardly affect this ratio if there are generalized changes in PRMT or proteolytic activity. However, protein arginine moieties are methylated asymmetrically by class 1 isoforms of PRMT and symmetrically by class 2 isoforms. Therefore, the ADMA/SDMA ratio may also affect relative changes in class 1 versus class 2 PRMT activity. At first sight, CAT inhibition is expected to have no influence on the ADMA/SDMA ratio. However, because the Km value of DDAH is rather high, increased intracellular ADMA levels resulting from reduced CAT activity will enhance degradation of ADMA by DDAH, thereby limiting the rise in intracellular ADMA levels. Because SDMA is not a substrate for DDAH, the relative increase of intracellular SDMA may be more prominent than that of ADMA, leading to a decreased ADMA/SDMA ratio. The endproducts of DDAH hydrolysis of ADMA are citrulline and dimethylamine. Because citrulline is involved in various metabolic pathways, its levels are not very informative with respect to ADMA metabolism. In contrast, measurements of dimethylamine may provide relevant information. Under conditions of increased ADMA generation, a parallel increase in dimethylamine production is expected, resulting in an unaltered dimethylamine/ADMA ratio. Reduced DDAH activity is associated with lower dimethylamine production resulting in a decrease of the dimethylamine/ADMA ratio. In contrast, this ratio may rise upon CAT inhibition, because, as stated above, the higher intracellular ADMA level may drive its degradation to dimethylamine by DDAH.
It should be noted that this simplified scheme may be adequate to model cell culture experiments, with ADMA in the medium equating the extracellular compartment, but it does not describe ADMA metabolism adequately at the whole body level. It is reasonable to assume in cell culture experiments that all ADMA in the culture medium has an intracellular origin. In this circumstance, the CAT system effectively causes a net export of ADMA. However, at the whole body level, the role of CAT may be more complicated, and involve cellular export as well as import of ADMA. Some organs may use CAT to export ADMA to the plasma compartment, whereas other organs, such as the liver and kidney, may serve as a sink for ADMA, by taking up ADMA from the circulation via CAT with subsequently degrading it by DDAH [88,105]. Thus CAT plays an important role in the inter-organ transport of ADMA. Whether alteration of CAT expression decrease or increase plasma ADMA levels may depend on the specific CAT system (CAT-1, CAT-2A, or CAT-2B) and the organs that are involved.
An inverse association between intracellular and plasma levels of ADMA might also provide a potential explanation for the surprising observation that elevated plasma levels of ADMA were found in a cohort of patients with chronic kidney disease to be independently associated with a decreased risk for subsequent cardiovascular events [106].
With these limitations of the model in mind, it tempting to speculate that there may be patho(physiological) conditions in which intracellular ADMA is increased whereas ADMA in the circulation is decreased or vice versa.
3. Intracellular ADMA in health and disease
3.1 Levels in normal cells versus extracellular fluid
The plasma levels of ADMA in healthy humans are in the range of 0.35–0.70 μmol/l [2,56,107–110], whereas plasma levels of up to 1.0 μmol/l have been reported in rodents [111–114] and even 1.5–3.0 μmol/l in dogs [115–118]. However, it is the intracellular concentration of ADMA that is likely to be most relevant (Table 1). Masuda et al reported that intracellular ADMA levels in harvested aortic endothelial cells were up to 10-fold higher than in plasma [119]. Cardounel and Zweier reported that the intracellular levels of ADMA, MMA and L-arginine in freshly isolated brain slices were 10.7±1.3, 5.1±0.6 and 94.0±7.8 μmol/l, respectively [120]. They showed further that the intracellular levels of L-arginine in cultured neurons was constant whereas MMA and ADMA levels decreased by more than 50% after 24h of inhibition of PRMT by S-adenosylhomocysteine. Although many disease states, including hypertension and diabetes, have been associated with increased plasma levels of ADMA, presently little is known about intracellular levels.
3.2 Studies in hypertension
There is very limited information regarding effects of hypertension on the intracellular levels of ADMA. Jacobi and colleagues infused angiotensin II into mice at either 1 or 3 μg/kg/min for four weeks [111]. It might be anticipated that the accompanying oxidative stress would increase ADMA levels. However, the authors reported a dose-dependent decrease in circulating ADMA. This highlights the complex regulation of ADMA and the need for studies of circulating and intracellular ADMA levels.
Achan et al reported that an acute infusion of ADMA into healthy human volunteers increased their blood pressure only modestly because an increased vascular resistance was offset by a decreased cardiac output and cardiac dysfunction [55].
A correlation between the levels of plasma ADMA and arterial pressure has been reported in patients without any known vascular disease [121] and with uncomplicated early hypertension [107]. Thus, elevated plasma levels of ADMA could play some role in the development of hypertension. Furthermore, Surdacki and co-workers [122] and Wang and coworkers [107] reported increased plasma levels of ADMA and reduced systemic NO production, manifested as decreased urinary NOx excretion, in newly diagnosed and untreated hypertensive subjects. Similar elevated plasma levels of ADMA have also been reported in hypertensive children [123] and elderly hypertensives [48]. Kielstein and co-workers reported elevated plasma levels of ADMA, but similar plasma levels of SDMA, in hypertensive compared to normotensive subjects. This could indicate a reduced intracellular ADMA metabolism by DDAH as outlined previously in this review. However, other studies have reached different conclusions. Böger et al [11] reported a significant inverse correlation between plasma levels of ADMA and diastolic BP in a large population of normal subjects. Delles et al did not find a correlation between plasma ADMA and blood pressure in young men with mild essential hypertension, although the study consisted of only 20 subjects [124]. Interestingly, the same authors reported that treatment of hypertension with drugs that were directed against the angiotensin system reduced the circulating levels of ADMA. A possible mechanism for these results was demonstrated later by Onozato and co-workers [51]. They reported that the addition of the angiotensin AT1-receptor blocker telmisartan to tissue slices from rat kidneys incubated with angiotensin II increased DDAH-1 protein expression. Plasma levels of ADMA and SDMA were elevated in patients with end-stage renal failure [125–127], due in part to decreased renal elimination [6].
Taken together, most available data suggest that elevated circulating ADMA levels can result in, or result from, hypertension and may increase the risk of adverse cardiovascular events.
3.3 Studies in diabetes
Several studies have reported elevated plasma levels of ADMA in patients with diabetes mellitus (DM) Type I [128,129] and II [130–132], prior gestational diabetes [133], and in rat models of insulinopenic diabetes [51,134,135] and insulin resistance [112,136–138]. Interestingly, levels of ADMA in endothelial cells from the rabbit carotid artery were 10-fold higher than plasma levels and increased further from 5.0 to 12.1 μmol/l after induction of alloxan-induced diabetes [119]. Plasma ADMA predicted progression to ESRD in diabetic patients with CKD [139] and predicted macrovascular complications in a rat model of type 1 diabetes [134]. Metformin lowered the blood glucose concentrations in diabetic patients and simultaneously reduced the circulating levels of ADMA, although there was no relationship between the decrease in ADMA concentration and the improvement of glycemic control [130]. Of note, in a later study conducted in non-obese insulin-naïve patients with type 2 diabetes, treatment with either metformin or the insulin secretagogue repaglinide did not result in lowering of plasma ADMA levels despite an improvement in glycemia [140]. Lin et al used a combination of streptozotocin and high-fat diet administration to rats. They reported elevated plasma ADMA levels [112]. However, the ADMA response to streptozotocin-induced diabetes in rats seems to be strain-dependent since diabetic Sprague-Dawley rats displayed elevated circulating ADMA [51], whereas sustained hyperglycemia in Wistar Furth rats did not elevate plasma ADMA [113,114]. This may be related to differences in basal plasma levels of arginine and ADMA between inbred rat strains [141] and effects of dosing and an underlying variability of response to streptozotocin. Moreover, ADMA was not associated with diabetes in a population-based study [11].
The mechanism of the elevated plasma levels of ADMA in diabetics has been the focus of several studies [51,112,113,134,135,142]. We reported recently that early insulinopenic diabetic rats have a 50% increase in renal angiotensin II concentrations concomitant with increased plasma levels of ADMA [51]. Interestingly, the pathways that generate and metabolize ADMA in the kidneys of these rats were altered by AT1-receptor blockade with telmisartan. Thus, the protein expression of DDAH-1 was increased whereas the protein expression of DDAH-2 was decreased by telmisartan, which also reduced the renal protein expression of the ADMA producing enzyme PRMT-1. Consequently, a reduction in circulating levels of ADMA by an angiotensin AT1-receptor blocker in this model of diabetes type 1 may be a result of both decreased ADMA synthesis by PRMT-1 and increased ADMA metabolism by DDAH-1.
Both increased [131] and decreased [143] plasma levels of ADMA have been reported in patients with type 2 diabetes. This may be related to differential effects of glucose and insulin on the metabolism of ADMA. High glucose levels may impair DDAH activity by inducing oxidative stress leading to increased intracellular ADMA levels [50]. In line with this notion, acute insulin infusion in healthy young men and type 1 diabetic patients has been shown to reduce plasma ADMA concentrations [144,145]. Moreover, intensive insulin treatment targeted at reducing blood glucose levels to near normal values reduced ADMA levels in critically ill hyperglycemic patients [146]. Although this may be ascribed to an insulin-induced reduction in glucose levels, insulin also may have glucose-independent actions. Thus insulin decreased plasma levels of most amino acids by decreasing release from muscle and activating cellular uptake [15]. Moreover, insulin may regulate CAA uptake into cells. Thus insulin upregulated the expression of CAT-1 in cardiac myocytes and CAT-1 and CAT-2B in human umbilical vein endothelial cells [77,147]. Hepatic CAT-2A expression was increased in streptozotocin-induced diabetic rats, resulting in reduced plasma levels of both arginine and ADMA [114]. The complex interplay between insulin and glucose metabolism in diabetes may thus result in either increased (upon DDAH inhibition) or decreased (upon upregulation of CAT) plasma levels of ADMA, although both situations may result in increased levels of intracellular ADMA.
3.4 Potential mechanisms of intracellular ADMA on cell function
The most clear-cut effect of elevated levels of intracellular ADMA is to reduce NO production via competitive inhibition of NOS [148,149]. However, growing evidence suggests that methylarginines also can regulate NOS-derived superoxide production [150,151]. During conditions of tetrahydrobiopterin (BH4) depletion, ADMA stimulated superoxide production by an uncoupled eNOS. Oxidative stress can oxidize BH4 to BH2, which uncouples eNOS. Since ROS may increase intracellular ADMA levels, this is a potential positive feedback mechanism to perpetuate increased oxidative stress. In a study by Chen et al, it was found that cultured endothelial cells exposed to 30 μmol/l ADMA for 24 h displayed increased ROS formation with increased production of TNF-α and interleukin (IL)-β, decreased nitrite/nitrate production and increased endothelial cell adhesion to monocytes and activation of NF-κβ, suggesting activation of the inflammatory pathway [152]. These results were similar to that of angiotensin II administration and were preventable by pretreatment with the angiotensin II AT1-receptor blocker losartan. However, the effects of ADMA on nNOS were different from eNOS. In the presence of BH4 superoxide production by nNOS was independently inhibited by both ADMA and L-arginine, whereas neither ADMA nor L-arginine altered superoxide formation by nNOS in the absence of BH4 [151].
Interestingly, ADMA can regulate its own degradation in endothelial cells [153]. NO stimulated DDAH-2 gene expression via induction of cyclic GMP. Thus, increased intracellular ADMA levels with subsequent NOS inhibition and reduced NO production could be another positive feedback loop that, in this example, may reduce ADMA degradation by reducing DDAH-2 gene expression.
Taken together, ADMA regulates cellular NO levels by inhibiting both NO production and bio-inactivation by ROS. Increasing evidence also suggests that elevated ADMA levels may induce pathways leading to inflammation.
4. Conclusions
The data discussed suggest that there can be a complicated set of interactions between ADMA regulation, metabolism, export and import that determine intracellular levels of ADMA and of the NOS substrate, L-arginine. Among these processes, the transcellular transport of arginine and methylarginine may be of particular importance in chronic illness, but presently there are few direct clinical studies to address this. An instructive example is chronic kidney disease. Studies in patients with renal disease from its origin in CKD-1 [154] to ESRD [4,155] show evidence of profound endothelial deficiency of blood vessels accompanied by impaired NOS activity. These functional defects are accompanied by reduced total body NO generation [156], increased circulating levels of ADMA and indices of ROS, and impaired endothelial uptake of L-arginine [4,60–62]. These events could represent the first steps in a process of endothelial dysfunction in small vessels that evolves though large vessel disease to end-organ dysfunction and premature cardiovascular morbidity and mortality and progression of CKD. The aim of this review has been to point to some of the steps in these processes that would benefit from further work to illuminate the pathways involved and to perhaps settle the controversy of whether intracellular levels of ADMA play a pathogenic role in disease.
Acknowledgments
We thank Ms Emily Wing Kam Chan for preparing and editing the manuscript.
Financial Support
The work described in this review was supported by research grants from the NIDDK to Christopher S. Wilcox (DK-36079; DK-49870) and to Fredrik Palm (DK-77858) and by a fellowship training grant (DK-59274) and by grants from the NHLBI (HL-68686) and by funds from the George E. Schreiner Chair of Nephrology.
Footnotes
Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ogawa T, Kimoto M, Sasaoka K. Occurrence of a new enzyme catalyzing the direct conversion of NG,NG-dimethyl-L-arginine to L-citrulline in rats. Biochem Biophys Res Commun. 1987;148:671–7. doi: 10.1016/0006-291x(87)90929-6. [DOI] [PubMed] [Google Scholar]
- 2.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20:S60–S62. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 3.Ueda S, Kato S, Matsuoka H, Kimoto M, Okuda S, Morimatsu M, et al. Regulation of cytokine induced nitric oxide synthasis by asymmetric dimethylarginine. Circ Res. 2003;92:226–33. doi: 10.1161/01.res.0000052990.68216.ef. [DOI] [PubMed] [Google Scholar]
- 4.Baylis C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol. 2006;2:209–20. doi: 10.1038/ncpneph0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch WJ, Wilcox CS. Macula densa arginine delivery and uptake in the rat regulates glomerular capillary pressure: effects of salt intake. J Clin Invest. 1997;100:2235–42. doi: 10.1172/JCI119761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 7.Zoccali C. Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int. 2006;70:26–33. doi: 10.1038/sj.ki.5000417. [DOI] [PubMed] [Google Scholar]
- 8.Zoccali C. ADMA: a critical cardio-renal link in heart failure? Eur J Clin Invest. 2003;33:361–2. doi: 10.1046/j.1365-2362.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 9.Zoccali C, Bode-Boger SM, Mallamaci F, Benedetto F, Tripepi G, Malatino LS, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–7. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 10.Boger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality-An update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009 doi: 10.1016/j.phrs.2009.07.001. In press. [DOI] [PubMed] [Google Scholar]
- 11.Boger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293:H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 14.Vallance P, Leiper J. Asymmetric dimethylarginine and kidney disease--marker or mediator? J Am Soc Nephrol. 2005;16:2254–6. doi: 10.1681/ASN.2005050539. [DOI] [PubMed] [Google Scholar]
- 15.Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002;18:761–6. doi: 10.1016/s0899-9007(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 16.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 17.Christensen HN. Organic ion transport during seven decades. The amino acids Biochim Biophys Acta. 1984;779:255–69. doi: 10.1016/0304-4157(84)90012-1. [DOI] [PubMed] [Google Scholar]
- 18.White MF. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985;822:355–74. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 19.Deves R, Boyd CAR. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 20.Brunini TM, Mendes-Ribeiro AC, Ellory JC, Mann GE. Platelet nitric oxide synthesis in uremia and malnutrition: a role for L-arginine supplementation in vascular protection? Cardiovasc Res. 2007;73:359–67. doi: 10.1016/j.cardiores.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Mendes Ribeiro AC, Brunini TM, Ellory JC, Mann GE. Abnormalities in L-arginine transport and nitric oxide biosynthesis in chronic renal and heart failure. Cardiovasc Res. 2001;49:697–712. doi: 10.1016/s0008-6363(00)00267-4. [DOI] [PubMed] [Google Scholar]
- 22.Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol. 2001;281:C1077–C1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- 23.Hosokawa H, Sawamura T, Kobayashi S, Ninomiya H, Miwa S, Masaki T. Cloning and characterization of a brain-specific cationic amino acid transporter. J Biol Chem. 1997;272:8717–22. doi: 10.1074/jbc.272.13.8717. [DOI] [PubMed] [Google Scholar]
- 24.Closs EI, Boissel JP, Habermeier A, Rotmann A. Structure and function of cationic amino acid transporters (CATs) J Membr Biol. 2006;213:67–77. doi: 10.1007/s00232-006-0875-7. [DOI] [PubMed] [Google Scholar]
- 25.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide: Biology and Chemistry. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 26.Closs EI, Albritton LM, Kim JW, Cunningham JM. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J Biol Chem. 1993;268:7538–44. [PubMed] [Google Scholar]
- 27.Kitiyakara C, Chabrashvili T, Jose P, Welch WJ, Wilcox CS. Effects of dietary salt intake on plasma arginine. Am J Physiol. 2001;280:R1069–R1075. doi: 10.1152/ajpregu.2001.280.4.R1069. [DOI] [PubMed] [Google Scholar]
- 28.Chin-Dusting JP, Willems L, Kaye DM. L-arginine transporters in cardiovascular disease: a novel therapeutic target. Pharmacol Ther. 2007;116:428–36. doi: 10.1016/j.pharmthera.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Gornik HL, Creager MA. Arginine and endothelial and vascular health. J Nutr. 2004;134:2880S–7S. doi: 10.1093/jn/134.10.2880S. [DOI] [PubMed] [Google Scholar]
- 30.Tsikas D, Boger RH, Sandmann J, Bode-Boger SM, Frolich JC. Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett. 2000;478:1–3. doi: 10.1016/s0014-5793(00)01686-0. [DOI] [PubMed] [Google Scholar]
- 31.Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134:2842S–7S. doi: 10.1093/jn/134.10.2842S. [DOI] [PubMed] [Google Scholar]
- 32.McDonald KK, Zharikov S, Block ER, Kilberg MS. A Caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem. 1997;272:31213–6. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 33.Hardy TA, May JM. Coordinate regulation of L-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med. 2002;32:122–31. doi: 10.1016/s0891-5849(01)00781-x. [DOI] [PubMed] [Google Scholar]
- 34.Smulders RA, Aarsen M, Teerlink T, de Vries PMJM, van Kamp GJ, Donker AJM, et al. Haemodynamic and biochemical responses to L-arginine and L-lysine infusions in normal subjects:L-arginine-induced vasodilation cannot be explained by non-specific effects of cationic amino acids. Clin Sci. 1997;92:367–74. doi: 10.1042/cs0920367. [DOI] [PubMed] [Google Scholar]
- 35.Prins HA, Houdijk AP, Wiezer MJ, Teerlink T, Van Lambalgen AA, Thijs LG, et al. The effect of mild endotoxemia during low arginine plasma levels on organ blood flow in rats. Crit Care Med. 2000;28:1991–7. doi: 10.1097/00003246-200006000-00051. [DOI] [PubMed] [Google Scholar]
- 36.Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, et al. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–7. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- 37.Teerlink T, Nijveldt RJ, de Jong S, Van Leeuwen PA. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem. 2002;303:131–7. doi: 10.1006/abio.2001.5575. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem. 1989;264:10205–9. [PubMed] [Google Scholar]
- 39.Wang J, Sim AS, Wang XL, Wilcken DE. L-arginine regulates asymmetric dimethylarginine metabolism by inhibiting dimethylarginine dimethylaminohydrolase activity in hepatic (HepG2) cells. Cell Mol Life Sci. 2006;63:2838–46. doi: 10.1007/s00018-006-6271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilcken DE, Sim AS, Wang J, Wang XL. Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of l-arginine on its metabolism. Mol Genet Metab. 2007;91:309–17. doi: 10.1016/j.ymgme.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Sessa WC, Hecker M, Mitchell JA, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: L-glutamine inhibits the generation of L-arginine by cultured endothelial cells. Proc Natl Acad Sci U S A. 1990;87:8607–11. doi: 10.1073/pnas.87.21.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnal JF, Munzel T, Venema RC, James NL, Bai C, Mitch WE. Interactions between L-arginine and L-glutamine change endothelial NO production. An effect independent of NO synthase substrate availability. J Clin Invest. 1995;95:2565–72. doi: 10.1172/JCI117957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr. 2004;134:2791S–5S. doi: 10.1093/jn/134.10.2791S. [DOI] [PubMed] [Google Scholar]
- 45.Tizianello A, De FG, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest. 1980;65:1162–73. doi: 10.1172/JCI109771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kakoki M, Wang W, Mattson DL. Cationic amino acid transport in the renal medulla and blood pressure regulation. Hypertens. 2002;39:287–92. doi: 10.1161/hy0202.102700. [DOI] [PubMed] [Google Scholar]
- 47.Deng X, Welch WJ, Wilcox CS. Renal vasodilation with L-arginine: Effects of dietary salt. Hypertens. 1995;26:256–62. doi: 10.1161/01.hyp.26.2.256. [DOI] [PubMed] [Google Scholar]
- 48.Kielstein JT, Bode-Böger SM, Frölich JC, Ritz E, Haller H, Fliser D. Asymmetric dimethylarginine, blood pressure and renal perfusion in elderly subjects. Circulation. 2003;107:1891–5. doi: 10.1161/01.CIR.0000060496.23144.A7. [DOI] [PubMed] [Google Scholar]
- 49.Teerlink T. ADMA metabolism and clearance. Vasc Med. 2005;10 (Suppl 1):S73–S81. doi: 10.1191/1358863x05vm597oa. [DOI] [PubMed] [Google Scholar]
- 50.Ellger B, Richir MC, Van Leeuwen PA, Debaveye Y, Langouche L, Vanhorebeek I, et al. Glycemic control modulates arginine and asymmetrical-dimethylarginine levels during critical illness by preserving dimethylarginine-dimethylaminohydrolase activity. Endocrinol. 2008;149:3148–57. doi: 10.1210/en.2007-1558. [DOI] [PubMed] [Google Scholar]
- 51.Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of DDAH and PRMT isoforms in the diabetic rat kidney; effects of angiotensin II receptor blockers. Diabetes. 2008;57:172–80. doi: 10.2337/db06-1772. [DOI] [PubMed] [Google Scholar]
- 52.Al Banchaabouchi M, Marescau B, Possemiers I, D’Hooge R, Levillain O, De Deyn PP. NG, NG-dimethylarginine and NG, NG-dimethylarginine in renal insufficiency. Pflugers Arch. 2000;439:524–31. doi: 10.1007/s004249900220. [DOI] [PubMed] [Google Scholar]
- 53.Nijveldt RJ, van Leeuwen PAM, van Guldener C, Stehouwer CDA, Rauwerda JA, Teerlink T. Net renal extraction of asymmetrical (ADMA) and symmetrical (SMDA) dimethylarginine in fasting humans. Nephrol Dial Tranplant. 2002;17:1999–2002. doi: 10.1093/ndt/17.11.1999. [DOI] [PubMed] [Google Scholar]
- 54.Nijveldt RJ, Teerlink T, van Guldener C, Prins HA, van Lambalgen AA, Stehouwer CDA, et al. Handling of asymmetrical dimethylarginine and symmetrical dimethylarginine by the rat kidney under basal conditions and during endotoxaemia. Nephrol Dial Tranplant. 2003;18:2542–50. doi: 10.1093/ndt/gfg452. [DOI] [PubMed] [Google Scholar]
- 55.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabloized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–9. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 56.Teerlink T. HPLC analysis of ADMA and other methylated L-arginine analogs in biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:21–9. doi: 10.1016/j.jchromb.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, et al. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int. 1997;52:1593–601. doi: 10.1038/ki.1997.490. [DOI] [PubMed] [Google Scholar]
- 58.Carello KA, Whitesall SE, Lloyd MC, Billecke SS, D’Alecy LG. Asymmetrical dimethylarginine plasma clearance persists after acute total nephrectomy in rats. Am J Physiol Heart Circ Physiol. 2006;290:H209–H216. doi: 10.1152/ajpheart.00208.2005. [DOI] [PubMed] [Google Scholar]
- 59.McDermott JR. Studies on the catabolism of NG-methylarginine, NG,NG-dimethylarginine and NG,NG-dimethylarginine in the rabbit. Biochem J. 1976;154:179–84. doi: 10.1042/bj1540179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294:F1–F9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- 61.Xiao S, Wagner L, Schmidt RJ, Baylis C. Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney Int. 2001;59:1466–72. doi: 10.1046/j.1523-1755.2001.0590041466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao S, wagner L, Mahaney J, Baylis C. Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am J Physiol Renal Physiol. 2001;280:F989–F995. doi: 10.1152/ajprenal.2001.280.6.F989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatzoglou M, Fernandez J, Yaman I, Closs E. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr. 2004;24:377–99. doi: 10.1146/annurev.nutr.23.011702.073120. [DOI] [PubMed] [Google Scholar]
- 64.Closs EI. Expression, regulation and function of carrier proteins for cationic amino acids. Curr Opin Nephrol Hypertens. 2002;11:99–107. doi: 10.1097/00041552-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Albritton LM, Tseng L, Scadden D, Cunningham JM. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–66. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 66.Aulak KS, Liu J, Wu J, Hyatt SL, Puppi M, Henning SJ, et al. Molecular sites of regulation of expression of the rat cationic amino acid transporter gene. J Biol Chem. 1996;271:29799–806. doi: 10.1074/jbc.271.47.29799. [DOI] [PubMed] [Google Scholar]
- 67.Yoshimoto T, Yoshimoto E, Meruelo D. Enhanced gene expression of the murine ecotropic retroviral receptor and its human homolog in proliferating cells. J Virol. 1992;66:4377–81. doi: 10.1128/jvi.66.7.4377-4381.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez J, Lopez AB, Wang C, Mishra R, Zhou L, Yaman I, et al. Transcriptional control of the arginine/lysine transporter, cat-1, by physiological stress. J Biol Chem. 2003;278:50000–9. doi: 10.1074/jbc.M305903200. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez J, Yaman I, Mishra R, Merrick WC, Snider MD, Lamers WH, et al. Internal ribosome entry site-mediated translation of a mammalian mRNA is regulated by amino acid availability. J Biol Chem. 2001;276:12285–91. doi: 10.1074/jbc.M009714200. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez J, Bode B, Koromilas A, Diehl JA, Krukovets I, Snider MD, et al. Translation mediated by the internal ribosome entry site of the cat-1 mRNA is regulated by glucose availability in a PERK kinase-dependent manner. J Biol Chem. 2002;277:11780–7. doi: 10.1074/jbc.M110778200. [DOI] [PubMed] [Google Scholar]
- 71.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–24. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 72.Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, et al. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–6. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 73.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–13. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 74.Sobrevia L, Yudilevich DL, Mann GE. Activation of A2-purinoceptors by adenosine stimulates L-arginine transport (system y+) and nitric oxide synthesis in human fetal endothelial cells. J Physiol. 1997;499:135–40. doi: 10.1113/jphysiol.1997.sp021916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bogle RG, Baydoun AR, Pearson JD, Mann GE. Regulation of L-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J Physiol. 1996;490:229–41. doi: 10.1113/jphysiol.1996.sp021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flores C, Rojas S, Aguayo C, Parodi J, Mann G, Pearson JD, et al. Rapid stimulation of L-arginine transport by D-glucose involves p42/44(mapk) and nitric oxide in human umbilical vein endothelium. Circ Res. 2003;92:64–72. doi: 10.1161/01.res.0000048197.78764.d6. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez M, Flores C, Pearson JD, Casanello P, Sobrevia L. Cell signalling-mediating insulin increase of mRNA expression for cationic amino acid transporters-1 and -2 and membrane hyperpolarization in human umbilical vein endothelial cells. Pflugers Arch. 2004;448:383–94. doi: 10.1007/s00424-004-1261-x. [DOI] [PubMed] [Google Scholar]
- 78.Liu J, Hatzoglou M. Control of expression of the gene for arginine transporter Cat-1 in rat liver cells by glucocorticoids and insulin. Amino Acids. 1998;15:321–37. doi: 10.1007/BF01320897. [DOI] [PubMed] [Google Scholar]
- 79.Wu JY, Robinson D, Kung H-J, Hatzoglou M. Hormonal regulation of the gene for the type C ectropic retrovirus receptor in rat liver cells. J Virology. 1994;68:1615–23. doi: 10.1128/jvi.68.3.1615-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Helle F, Hultstrom M, Skogstrand T, Palm F, Iversen BM. Angiotensin II-induced contraction is attenuated by nitric oxide in afferent arterioles from the nonclipped kidney in 2K1C. Am J Physiol Renal Physiol. 2009;296:F78–F86. doi: 10.1152/ajprenal.90518.2008. [DOI] [PubMed] [Google Scholar]
- 81.Hultstrom M, Helle F, Iversen BM. AT1 receptor activation regulates the mRNA expression of CAT-1, CAT-2, Arginase-1 and DDAH-2 in preglomerular vessels from angiotensin II hypertensive rats. Am J Physiol Renal Physiol. 2009;297:F163–F168. doi: 10.1152/ajprenal.00087.2009. [DOI] [PubMed] [Google Scholar]
- 82.Ookawauchi K, Saibara T, Yoshikawa T, Chun-Lin L, Hayashi Y, Hiroi M, et al. Characterization of cationic amino acid transporter and its gene expression in rat hepatic stellate cells in relation to nitric oxide production. J Hepatol. 1998;29:923–32. doi: 10.1016/s0168-8278(98)80120-7. [DOI] [PubMed] [Google Scholar]
- 83.Durante W, Liao L, Iftikhar I, Cheng K, Schafer AI. Platelet-derived growth factor regulates vascular smooth muscle cell proliferation by inducing cationic amino acid transporter gene expression. J Biol Chem. 1996;271:11838–43. doi: 10.1074/jbc.271.20.11838. [DOI] [PubMed] [Google Scholar]
- 84.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-beta(1) stimulates L-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation. 2001;103:1121–7. doi: 10.1161/01.cir.103.8.1121. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz IF, Chernichovsky T, Hagin D, Ingbir M, Reshef R, Chernin G, et al. Differential regulation of L-arginine transporters (cationic amino acid transporter-1 and -2) by peroxynitrite in rat mesangial cells. Nephrol Dial Transplant. 2006;21:3409–14. doi: 10.1093/ndt/gfl522. [DOI] [PubMed] [Google Scholar]
- 86.Hattori Y, Kasai K, Gross SS. Cationic amino acid transporter gene expression in cultured vascular smooth muscle cells and in rats. Am J Physiol. 1999;276:H2020–H2028. doi: 10.1152/ajpheart.1999.276.6.H2020. [DOI] [PubMed] [Google Scholar]
- 87.Mittermayer F, Namiranian K, Pleiner J, Schaller G, Wolzt M. Acute Escherichia coli endotoxaemia decreases the plasma l-arginine/asymmetrical dimethylarginine ratio in humans. Clin Sci (Lond) 2004;106:577–81. doi: 10.1042/CS20030363. [DOI] [PubMed] [Google Scholar]
- 88.Siroen MP, Teerlink T, Nijveldt RJ, Prins HA, Richir MC, Van Leeuwen PA. The clinical significance of asymmetric dimethylarginine. Annu Rev Nutr. 2006;26:203–28. doi: 10.1146/annurev.nutr.26.061505.111320. [DOI] [PubMed] [Google Scholar]
- 89.Nijveldt RJ, Teerlink T, van der Hoven B, Siroen MP, Kuik DJ, Rauwerda JA, et al. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22:23–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]
- 90.Nijveldt RJ, Teerlink T, van Leeuwen PAM. The asymmetrical dimethylarginine (ADMA) multiple organ failure hypothesis. Clin Nutr. 2003;22:99–104. doi: 10.1054/clnu.2002.0614. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz IF, Schwartz D, Traskonov M, Chernichovsky T, Wollman Y, Gnessin E, et al. L-Arginine transport is augmented through up-regulation of tubular CAT-2 mRNA in ischemic acute renal failure in rats. Kidney Int. 2002;62:1700–6. doi: 10.1046/j.1523-1755.2002.t01-1-00622.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertens. 2006;48:278–85. doi: 10.1161/01.HYP.0000231509.27406.42. [DOI] [PubMed] [Google Scholar]
- 93.Jin L, Caldwell RB, Li-Masters T, Caldwell RW. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J Physiol Pharmacol. 2007;58:191–206. [PubMed] [Google Scholar]
- 94.Zhang WZ, Venardos K, Finch S, Kaye DM. Detrimental effect of oxidized LDL on endothelial arginine metabolism and transportation. Int J Biochem Cell Biol. 2008;40:920–8. doi: 10.1016/j.biocel.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 95.Kaye DM, Ahlers BA, Autelitano DJ, Chin-Dusting JP. In vivo and in vitro evidence for impaired arginine transport in human heart failure. Circulation. 2000;102:2707–12. doi: 10.1161/01.cir.102.22.2707. [DOI] [PubMed] [Google Scholar]
- 96.Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, et al. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110:3680–6. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- 97.Schwartz IF, Ayalon R, Chernichovski T, Reshef R, Chernin G, Weinstein T, et al. Arginine uptake is attenuated through modulation of cationic amino-acid transporter-1, in uremic rats. Kidney Int. 2006;69:298–303. doi: 10.1038/sj.ki.5000067. [DOI] [PubMed] [Google Scholar]
- 98.Levin-Iaina N, Schwartz I, Chernichovsky T, Davidovitch A, Iaina A, Schwartz D. Tubular and glomerular L-arginine transport (uptake and transporters) and the nitric oxide synthases in ischemic acute renal failure (iARF) in streptozotocin-induced diabetic rats (STZ-DM) Ren Fail. 2007;29:1031–8. doi: 10.1080/08860220701641744. [DOI] [PubMed] [Google Scholar]
- 99.Yang Z, Venardos K, Jones E, Morris BJ, Chin-Dusting J, Kaye DM. Identification of a novel polymorphism in the 3′UTR of the L-arginine transporter gene SLC7A1: contribution to hypertension and endothelial dysfunction. Circulation. 2007;115:1269–74. doi: 10.1161/CIRCULATIONAHA.106.665836. [DOI] [PubMed] [Google Scholar]
- 100.Yang Z, Kaye DM. Mechanistic insights into the link between a polymorphism of the 3′UTR of the SLC7A1 gene and hypertension. Hum Mutat. 2009;30:328–33. doi: 10.1002/humu.20891. [DOI] [PubMed] [Google Scholar]
- 101.Teerlink T, Neele SJM, De Jong S, Netelenbos JC, Stehouwer CDA. Oestrogen replacement therapy lowers plasma levels of asymmetrical dimethylarginine in helathy postmenopausal women. Clin Sci. 2003;105:67–71. doi: 10.1042/CS20020309. [DOI] [PubMed] [Google Scholar]
- 102.Post MS, Verhoeven MO, van der Mooren MJ, Kenemans P, Stehouwer CDA, Teerlink T. Effect of hormone replacement therapy on plasma levels of the cardiovascular risk factor asymmetric dimethylarginine: a radomized placebo controlled 12 week study in healthy early postmenopausal women. J Clin Endocrinol Metab. 2003;88:4221–6. doi: 10.1210/jc.2003-030584. [DOI] [PubMed] [Google Scholar]
- 103.Holden DP, Cartwright JE, Nussey SS, Whitley G. Estrogen stimulates dimethylarginine dimethylaminohydrolase activity and the metabolism of asymmetric dimethylarginine. Circulation. 2003;108:1575–80. doi: 10.1161/01.CIR.0000091083.61609.DF. [DOI] [PubMed] [Google Scholar]
- 104.Bunck MC, Giltay EJ, Diamant M, Gooren LJ, Teerlink T. Differential effects of cross-sex hormonal treatment on plasma asymmetric dimethylarginine (ADMA) in healthy male-to-female and female-to-male transsexuals. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.01.020. In press. [DOI] [PubMed] [Google Scholar]
- 105.Richir MC, Bouwman RH, Teerlink T, Siroen MP, de Vries TP, Van Leeuwen PA. The prominent role of the liver in the elimination of asymmetric dimethylarginine (ADMA) and the consequences of impaired hepatic function. JPEN J Parenter Enteral Nutr. 2008;32:613–21. doi: 10.1177/0148607108321702. [DOI] [PubMed] [Google Scholar]
- 106.Busch M, Fleck C, Wolf G, Stein G. Asymmetrical (ADMA) and symmetrical dimethylarginine (SDMA) as potential risk factors for cardiovascular and renal outcome in chronic kidney disease - possible candidates for paradoxical epidemiology? Amino Acids. 2006;30:225–32. doi: 10.1007/s00726-005-0268-8. [DOI] [PubMed] [Google Scholar]
- 107.Wang D, Strandgaard S, Iversen J, Wilcox CS. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;296:R195–R200. doi: 10.1152/ajpregu.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang D, Strandgaard S, Borresen ML, Luo Z, Connors S, Yan Q, et al. Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kid Dis. 2008;51:184–91. doi: 10.1053/j.ajkd.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 109.Lajer M, Tarnow L, Jorsal A, Teerlink T, Parving HH, Rossing P. Plasma concentration of asymmetric dimethylarginine (ADMA) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2008;31:747–52. doi: 10.2337/dc07-1762. [DOI] [PubMed] [Google Scholar]
- 110.Horowitz JD, Heresztyn T. An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: methodological considerations. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:42–50. doi: 10.1016/j.jchromb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 111.Jacobi J, Maas R, Cordasic N, Koch K, Schmieder RE, Boger RH, et al. Role of asymmetric dimethylarginine for angiotensin II-induced target organ damage in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1058–H1066. doi: 10.1152/ajpheart.01103.2007. [DOI] [PubMed] [Google Scholar]
- 112.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–92. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 113.Palm F, Buerk DG, Carlsson PO, Hansell P, Liss P. Reduced nitric oxide concentration in the renal cortex of streptozotocin-induced diabetic rats: effects on renal oxygenation and microcirculation. Diabetes. 2005;54:3282–7. doi: 10.2337/diabetes.54.11.3282. [DOI] [PubMed] [Google Scholar]
- 114.Palm F, Friederich M, Carlsson PO, Hansell P, Teerlink T, Liss P. Reduced nitric oxide in diabetic kidneys due to increased hepatic arginine metabolism: implications for renomedullary oxygen availability. Am J Physiol Renal Physiol. 2008;294:F30–F37. doi: 10.1152/ajprenal.00166.2007. [DOI] [PubMed] [Google Scholar]
- 115.Ohnishi M, Wada A, Tsutamoto T, Fujii M, Matsumoto T, Yamamoto T, et al. Endothelin stimulates an endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine, in experimental heart failure. Clin Sci (Lond) 2002;103 (Suppl 48):241S–4S. doi: 10.1042/CS103S241S. [DOI] [PubMed] [Google Scholar]
- 116.Moesgaard SG, Pedersen LG, Teerlink T, Haggstrom J, Pedersen HD. Neurohormonal and circulatory effects of short-term treatment with enalapril and quinapril in dogs with asymptomatic mitral regurgitation. J Vet Intern Med. 2005;19:712–9. [PubMed] [Google Scholar]
- 117.Okubo K, Hayashi K, Wakino S, Matsuda H, Kubota E, Honda M, et al. Role of asymmetrical dimethylarginine in renal microvascular endothelial dysfunction in chronic renal failure with hypertension. Hypertens Res. 2005;28:181–9. doi: 10.1291/hypres.28.181. [DOI] [PubMed] [Google Scholar]
- 118.Pedersen LG, Tarnow I, Olsen LH, Teerlink T, Pedersen HD. Body size, but neither age nor asymptomatic mitral regurgitation, influences plasma concentrations of dimethylarginines in dogs. Res Vet Sci. 2006;80:336–42. doi: 10.1016/j.rvsc.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 119.Masuda H, Goto M, Tamaoki S, Azuma H. Accelerated intimal hyperplasia and increased endogenous inhibitors of NO synthesis in rabbits with alloxan-induced hyperglycaemia. Br J Pharmacol. 1999;126:211–8. doi: 10.1038/sj.bjp.0702298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cardounel AJ, Zweier JL. Endogenous methylarginines regulate neuronal nitric-oxide synthase and prevent excitotoxic injury. J Biol Chem. 2002;277:33995–4002. doi: 10.1074/jbc.M108983200. [DOI] [PubMed] [Google Scholar]
- 121.Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, et al. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation. 1999;99:1141–6. doi: 10.1161/01.cir.99.9.1141. [DOI] [PubMed] [Google Scholar]
- 122.Surdacki A, Nowicki M, Sandmann J, Tsikas D, Boeger RH, Bode-Boger SM, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol. 1999;33:652–8. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 123.Goonasekera CD, Rees DD, Woolard P, Frend A, Shah V, Dillon MJ. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J Hypertens. 1997;15:901–9. doi: 10.1097/00004872-199715080-00015. [DOI] [PubMed] [Google Scholar]
- 124.Delles C, Schneider MP, John S, Gekle M, Schmieder RE. Angiotensin converting enzyme inhibition and angiotensin II AT1receptor blockade reduce the levels of asymmetrical NG, NG-dimethylarginine in human essential hypertension. Am J Hypertens. 2002;15:590–3. doi: 10.1016/s0895-7061(02)02278-1. [DOI] [PubMed] [Google Scholar]
- 125.MacAllister R, Rambausek MH, Vallance P, Williams D, Hoffmann KH, Ritz E. Concentration of dimethyl-L-arginine in the plasma of patients with end-stage renal failure. Nephrol Dial Transplant. 1996;11:2449–52. doi: 10.1093/oxfordjournals.ndt.a027213. [DOI] [PubMed] [Google Scholar]
- 126.Anderstam B, Katzarski K, Bergström J. Serum levels of NG,NG-dimethyl-L-arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol. 1997;8:1437–42. doi: 10.1681/ASN.V891437. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt RJ, Domico J, Samsell LS, Yokota S, Tracy TS, Sorkin MI, et al. Indices of activity of the nitric oxide system in hemodialysis patients. Am J Kidney Dis. 1999;34:228–34. doi: 10.1053/AJKD03400228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tarnow L, Hovind P, Teerlink T, Stehouwer CD, Parving HH. Elevated plasma asymmetric dimethylarginine as a marker of cardiovascular morbidity in early diabetic nephropathy in type 1 diabetes. Diabetes Care. 2004;27:765–9. doi: 10.2337/diacare.27.3.765. [DOI] [PubMed] [Google Scholar]
- 129.Altinova AE, Arslan M, Sepici-Dincel A, Akturk M, Altan N, Toruner FB. Uncomplicated type 1 diabetes is associated with increased asymmetric dimethylarginine concentrations. J Clin Endocrinol Metab. 2007;92:1881–5. doi: 10.1210/jc.2006-2643. [DOI] [PubMed] [Google Scholar]
- 130.Asagami T, Abbasi F, Stuelinger M, Lamendola C, McLaughlin T, Cooke JP, et al. Metformin treatment lowers asymmeteric dimethylarginine concentrations in patients with type 2 diabetes. Metabolism. 2003;51:843–6. doi: 10.1053/meta.2002.33349. [DOI] [PubMed] [Google Scholar]
- 131.Abbasi F, Asagmi T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:1201–3. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 132.Hanai K, Babazono T, Nyumura I, Toya K, Tanaka N, Tanaka M, et al. Asymmetric dimethylarginine is closely associated with the development and progression of nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant. 2009;24:1884–8. doi: 10.1093/ndt/gfn716. [DOI] [PubMed] [Google Scholar]
- 133.Mittermayer F, Mayer BX, Meyer A, Winzer C, Pacini G, Wagner OF, et al. Circulating concentrations of asymmetrical dimethyl-L-arginine are increased in women with previous gestational diabetes. Diabetologia. 2002;45:1372–8. doi: 10.1007/s00125-002-0916-4. [DOI] [PubMed] [Google Scholar]
- 134.Xiong Y, Fu Y, Fu S, Zhou H. Elevated levels of the serum endogenous inhibitor of nitric oxide synthase and metabolic control in rats with streptozotocin-induced diabetes. J Cardiovasc Pharmacol. 2003;42:191–6. doi: 10.1097/00005344-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 135.Xiong Y, Lei M, Fu S, Fu Y. Effect of diabetic duration on serum concentrations of endogenous inhibitor of nitric oxide synthase in patients and rats with diabetes. Life Sci. 2005;77:149–59. doi: 10.1016/j.lfs.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 136.Chan NN, Chan JC. Asymmetric dimethylarginine (ADMA): a potential link between endothelial dysfunction and cardiovascular diseases in insulin resistance syndrome? Diabetologia. 2002;45:1609–16. doi: 10.1007/s00125-002-0975-6. [DOI] [PubMed] [Google Scholar]
- 137.Stuhlinger M, Abbasi F, Chu J, Lamendola C, McLaughlin T, Cooke JP, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287:1420–6. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 138.Sydow K, Mondon CE, Cooke JP. Insulin resistance: potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc Med. 2005;10 (Suppl 1):S35–S43. doi: 10.1177/1358836X0501000106. [DOI] [PubMed] [Google Scholar]
- 139.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol. 2005;16:2449–55. doi: 10.1681/ASN.2005010076. [DOI] [PubMed] [Google Scholar]
- 140.Lund SS, Tarnow L, Stehouwer CD, Schalkwijk CG, Teerlink T, Gram J, et al. Impact of metformin versus repaglinide on non-glycaemic cardiovascular risk markers related to inflammation and endothelial dysfunction in non-obese patients with type 2 diabetes. Eur J Endocrinol. 2008;158:631–41. doi: 10.1530/EJE-07-0815. [DOI] [PubMed] [Google Scholar]
- 141.Ulu N, Schoemaker RG, Henning RH, Buikema H, Teerlink T, Zijlstra FJ, et al. Proteinuria-associated endothelial dysfunction is strain dependent. Am J Nephrol. 2009;30:209–17. doi: 10.1159/000218062. [DOI] [PubMed] [Google Scholar]
- 142.Sydow K, Schwedhelm E, Arakawa N, Bode-Boger SM, Tsikas D, Hornig B, et al. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57:244–52. doi: 10.1016/s0008-6363(02)00617-x. [DOI] [PubMed] [Google Scholar]
- 143.Paiva H, Lehtimaki T, Laakso J, Ruokonen I, Rantalaiho V, Wirta O, et al. Plasma concentrations of asymmetric dimethyl arginine in type 2 diabletes assocate with glycemic control and glomerular filtration rate but not risk factors of vasculopathy. Metabolism. 2003;52:303–7. doi: 10.1053/meta.2003.50048. [DOI] [PubMed] [Google Scholar]
- 144.Eid HM, Reims H, Arnesen H, Kjeldsen SE, Lyberg T, Seljeflot I. Decreased levels of asymmetric dimethylarginine during acute hyperinsulinemia. Metabolism. 2007;56:464–9. doi: 10.1016/j.metabol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 145.Marcovecchio ML, Widmer B, Dunger DB, Dalton RN. Effect of acute variations of insulin and glucose on plasma concentrations of asymmetric dimethylarginine in young people with Type 1 diabetes. Clin Sci (Lond) 2008;115:361–9. doi: 10.1042/CS20080079. [DOI] [PubMed] [Google Scholar]
- 146.Siroen MP, Van Leeuwen PA, Nijveldt RJ, Teerlink T, Wouters PJ, Van den Berghe G. Modulation of asymmetric dimethylarginine in critically ill patients receiving intensive insulin treatment: a possible explanation of reduced morbidity and mortality? Crit Care Med. 2005;33:504–10. doi: 10.1097/01.ccm.0000155784.59297.50. [DOI] [PubMed] [Google Scholar]
- 147.Simmons WW, Closs EI, Cunningham JM, Smith TW, Kelly RA. Cytokines and insulin induce cationic amino acid transporter (CAT) expression in cardiac myocytes. J Biol Chem. 1996;271:11694–702. doi: 10.1074/jbc.271.20.11694. [DOI] [PubMed] [Google Scholar]
- 148.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide sythases. Cardiovasc Res. 1999;43:542–8. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 149.Boger RH, Vallance P, Cooke JP. Asymmetric dimethylarginine (ADMA): a key regulator of nitric oxide synthase. Atheroscler Suppl. 2003;4:1–3. doi: 10.1016/s1567-5688(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 150.Druhan LJ, Forbes SP, Pope AJ, Chen CA, Zweier JL, Cardounel AJ. Regulation of eNOS-derived superoxide by endogenous methylarginines. Biochem. 2008;47:7256–63. doi: 10.1021/bi702377a. [DOI] [PubMed] [Google Scholar]
- 151.Cardounel AJ, Xia Y, Zweier JL. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J Biol Chem. 2005;280:7540–9. doi: 10.1074/jbc.M410241200. [DOI] [PubMed] [Google Scholar]
- 152.Chen MF, Xie XM, Yang TL, Wang YJ, Zhang XH, Luo BL, et al. Role of asymmetric dimethylarginine in inflammatory reactions by angiotensin II. J Vasc Res. 2007;44:391–402. doi: 10.1159/000103284. [DOI] [PubMed] [Google Scholar]
- 153.Sakurada M, Shichiri M, Imamura M, Azuma H, Hirata Y. Nitric oxide upregulates dimethylarginine dimethylaminohydrolase-2 via cyclic GMP induction in endothelial cells. Hypertens. 2008;52:903–9. doi: 10.1161/HYPERTENSIONAHA.108.114207. [DOI] [PubMed] [Google Scholar]
- 154.Wang D, Iversen J, Wilcox CS, Strandgaard S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal polycystic kidney disease. Kidney Int. 2003;64:1381–8. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 155.Aslam S, Santha T, Leone A, Wilcox CS. Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int. 2006;70:2109–15. doi: 10.1038/sj.ki.5001983. [DOI] [PubMed] [Google Scholar]
- 156.Lyons D, Roy S, Patel M, Benjamin N, Swift CG. Impaired nitric oxide-mediated vasodilatation and total body nitric oxide production in healthy old age. Clin Sci. 1997;93:519–25. doi: 10.1042/cs0930519. [DOI] [PubMed] [Google Scholar]
- 157.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, et al. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879–87. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 158.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger M, Wang B, et al. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis. Circulation. 2003;108:3042–7. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]