Introduction
Osteoarthritis (OA) is a common and painful disorder characterized by the degeneration of the articular surfaces of diarthrodial joints. One of the most promising approaches to treat OA is the design of engineered tissue that reproduces the functional properties of healthy cartilage. This tissue engineering approach typically entails the use of living cells embedded in a three-dimensional scaffold that is cultured over time in a growth medium supplemented with various physical or biological stimulants to encourage development.
Recently our laboratory has been able to cultivate engineered cartilage with an equilibrium modulus (EY) and an glycosaminoglycan (GAG) content that match the native tissue using the temporal application of growth factors over a six-week culture period1. While these findings are encouraging, the dynamic modulus (G*) and the collagen content for these constructs amount to less than a quarter of those of native articular cartilage. Of the two mechanical measurements, G*, which is correlated with the collagen content, is considered to be the more physiologically relevant as it measures the behavior of the tissue during the application of cyclic loads and better captures fluid pressurization within the biphasic tissue. No group has currently been able to reproduce native values of collagen regardless of the tissue engineering strategy employed. Obtaining native values of G* and collagen therefore remains an important but elusive challenge.
Cross-linking agents such as glutaraldehyde, formaldehyde, or epoxy are typically used to chemically-treat (or fix) devitalized xenografts and allografts to reduce the immune rejection or the enzymatic degradation that is typical of transplanted bioprostheses2,3. Typically, however, cross-linking agents are lethal to cells. The use of genipin, a naturally occurring crosslinker with toxicity levels ten thousand fold less than glutaraldehyde4 as a biocompatible and stable cross-linker has been established by other groups5,6 and provides a promising new avenue to explore for tissue-engineering.
Genipin cross-linking works by forming intra- and intermolecular cross-links of the amino residues on tropocollagen or proteoglycan molecules. A modified cyclic form of genipin can reside stably within the extracellular network, adding bridges across adjacent fibers. As such, previous groups have explored the use of genipin as a onetime treatment to fix tissue prior to implantation7, for the assembly of tissue-engineering scaffolds prior to cell seeding8,9, or in order to modulate the release of growth factors from degradable scaffolds or beads5. Genipin has also been studied for its anti-inflammatory effects, either administered directly to different cell lines10,11, or administered orally to animals12,13. Moreover, genipin cross-linking has been shown to affect the mechanical properties of biological tissues, increasing the tensile strength of bovine pericardium14, porcine tendon15, and type I collagen gels16.
Our laboratory uses agarose hydrogel as a scaffold system for cartilage tissue engineering. This gel has been used extensively in chondrocyte biology studies and has shown some promise for tissue engineering applications. Agarose is a neutrally charged polysaccharide and as such is unaffected by genipin. However the chondrocytes embedded in the gel elaborate an extracellular matrix over time in culture that exhibits amine groups, which are subject to genipin cross-linking. In this set of studies we propose a novel use for genipin: not as a scaffold cross-linker, but as a medium supplement to promote cross-linking of de novo cell products as they are produced. We hypothesize that the application of genipin will stabilize the extracellular matrix components and increase the mechanical properties of developing cartilaginous tissue in our agarose hydrogels. We hypothesize two mechanisms through which the physical enhancement of tissue properties is fostered: (1) by reorganization and enhanced retention of cell synthesized extracellular matrix components, and (2) through reduction of the loss of extracellular matrix components by increasing their resilience to catabolic degradation.
Materials and Methods
Both the concentration and duration of genipin were modulated as variables in the current set of studies. In a preliminary study, three concentrations of genipin were examined to establish cell toxicity. Based on these results the two most promising concentrations were selected for use in long-term culture. In Study 1 genipin was applied to the culture medium continuously throughout the culture duration. In Study 2 genipin was applied to the culture medium briefly (24 hours) on selected days only. Finally, we examined one of our hypotheses for the mechanisms behind the accumulated benefits in Study 3 by perturbing the system with the inflammatory cytokine interleukin-1α (IL-1α).
Cell Isolation
Articular cartilage was harvested from bovine carpo-metacarpal (CMC) joints of freshly slaughtered 1–3 month old calves. Three to five joints were used for each study and cells were pooled from all joints. Each study was repeated at least once with different cell isolations and the results combined and averaged. The sample size stated before each study represents the combined number of samples for all repeated studies. The cartilage tissue was combined, weighed, and digested with type IV collagenase (Sigma Chemicals, St. Louis, MO) for 11 hours at 37°C with stirring in high-glucose Dulbecco’s Modified Eagle’s Medium (hgDMEM). Collagenase supplemented medium mixture was added at 7.5 mL per gram of tissue with the collagenase concentration normalized to 390 activity units/ml. The resulting cell suspension was filtered through a 70 μm pore size mesh and sedimented in a benchtop centrifuge for 10 minutes at 1000 g. Viable cells were counted using a hemacytometer and trypan blue. One volume of chondrocyte suspension (at 60 × 106 cells/ml) was then mixed with an equal volume of 4% low-melt agarose (Type VII, Sigma) at 37°C to yield a final cell concentration of 30 × 106 in 2% agarose. The chondrocyte/agarose mixture was cast into slabs and cored using a sterile disposable punch (Miltex, York, PA) to final dimensions of 0.4 cm diameter and 0.23 cm thickness (0.029 cm3). Some bovine explants (used in the preliminary study) were also cored from the original undigested tissue and cut to the same dimensions (resulting in midzone cartilage only).
Chemically-defined cell culture
Constructs were maintained in serum-free growth medium for up to 52 days (with variable genipin concentrations and time courses as illustrated in Figure 1. The serum-free growth medium for Studies 1 and 2 consisted of hgDMEM supplemented with 1× PSF (100 units/ml Penicillin, 100 μg/ml Streptomycin, 0.25 μg/ml Fungizone), 0.1 μM dexamethasone, 50 μg/mL ascorbate 2-phosphate, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, and 1× ITS+ premix (insulin, human transferrin, and selenous acid, Becton Dickinson, Franklin Lakes, NJ). The growth medium for Study 3 was identical to the previous two studies with the exception that beginning on day 42 dexamethasone supplementation was discontinued (it is a known anti-inflammatory agent that interferes with IL-1α). For all studies growth medium was changed every three days and supplemented additionally with 10 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN) for the first two weeks of culture.
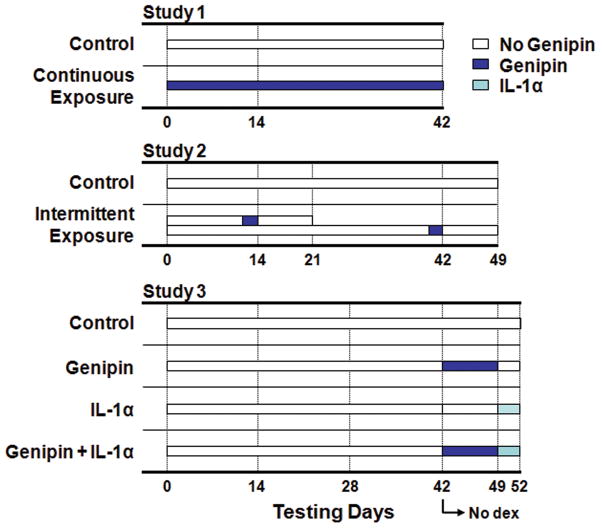
Figure 1.
Schematic of genipin exposure over culture period. Control groups were not exposed to genipin. Study 1: Constructs were exposed to genipin continuously (Continuous Exposure) in the culture medium. Study 2: Constructs were exposed to genipin for 24 hours only (Brief Exposure) and tested 1 and 7 days later. Study 3: On day 42 dexamethasone was removed from the culture medium and constructs were exposed to genipin continuously for 1 week prior to a 3 day interleukin-1α (IL-1α) challenge.
Genipin Supplementation
In the preliminary study, cell-seeded agarose constructs, cell-free controls, and freshly harvested bovine explant cartilage were incubated in culture media supplemented with 0, 22, 220, 2200 μMolar of sterile genipin (Sigma-Aldrich) for 7 days. Mechanical testing and cell-viability (using the Molecular Probes Live/Dead Viability/Cytotoxicity kit) was carried out on day 0, day 3, and day 7. Based on these results three additional studies were performed. In Study 1 constructs were exposed to 0 μM, 22 μM, or 220 μM genipin continuously throughout the 42 day culture period and tested on day 0, day 14, and day 42. In Study 2, on days 13 and 41 a subset of the genipin-free controls were isolated and exposed to genipin for 24 hours at the same concentrations as Study 1. These constructs were tested immediately after 24 hour exposure (day 14 and day 42) and also 7 days after exposure (day 21 and day 49). In Study 3, constructs were exposed to 22 μM of genipin (the low range reported in the literature14) from day 42 to day 49, after which genipin supplementation was discontinued and the cross-linked constructs were challenged with IL-1α (10 ng/mL) for three days (until day 52). For concentrations and time-courses see Figure 1.
Material Testing
Cylindrical constructs were tested for both their equilibrium Young’s modulus (EY) and their dynamic modulus (G*) in unconfined compression using a custom computer-controlled testing system 17. To measure EY an initial 0.02N tare load was applied, followed by a compression to 10% strain, at a strain rate of 0.05%/sec. EY was calculated from the equilibrium stress at 10% strain. Previous studies have shown EY to remain invariant across strain magnitudes ranging from 0% to 20% for cartilage explants. G* was measured after achieving stress-relaxation equilibrium by superimposing a 2% peak to peak sinusoidal strain at 0.1 Hz.
Biochemical Content
The biochemical content of each sample was assessed by first measuring the sample wet weight, lyophilizing overnight, and measuring dry weight. Gross water content was then determined from the difference. Once dry, the samples were digested in protenase-K overnight at 56°C, as described previously18. Aliquots of digest were analyzed for glycosaminoglycan (GAG) content using the 1,9-dimethylmethylene blue (DMMB) dye-binding assay19,20. Additional aliquots were analyzed for DNA content using the PicoGreen assay. A further aliquot was acid hydrolyzed in 12 N HCl at 110°C for 16 hours, dried over NaOH, and resuspended in assay buffer (24 mM citric acid monohydrate, 0.012 v/v glacial acetic acid, 85 mM sodium acetate trihydrate, 85 mM sodium hydroxide, pH 6.0). Ortho-hydroxyproline (OHP) content was then determined via a colorimetric assay by reaction with chloramine T and dimethylaminobenzaldehyde21, scaled for microplates. OHP content was converted to total collagen content using the conversion of 1:10 ratio of OHP:Collagen. Each biochemical constituent (DNA, GAG, and collagen) was then normalized to the tissue wet weight to correct for differences in construct size.
Statistical Analysis
Statistics were performed with the Statistica (Statsoft, Tulsa, OK) software package. Each data point represents the mean and standard deviation. Groups were examined for significant differences by analysis of variance (α = 0.05), with EY, G*, GAG, or OHP as the dependent variable. Tukey’s Honest Significant Difference Test (HSD) for unequal n post-hoc tests were carried out with a statistical significance set at p = 0.05.
Results
For the preliminary study: Live/dead assays established that after 7 days of continuous supplementation there were no differences in cell mortality from the genipin-free control for both the 22 μM and 220 μM genipin groups (>98% cells vital), however there was complete cell death in the same period for the 2200 μM group (Figure 2). There were no differences in the mechanical properties (EY, G*) of these tissue-engineered constructs at any concentration tested (EY=11 ± 1 kPa, G* at 0.1Hz = 0.07 ± 0.01 MPa). Likewise there were no changes in the mechanical properties or visual appearance of the cell-free agarose scaffolds.
Figure 2.
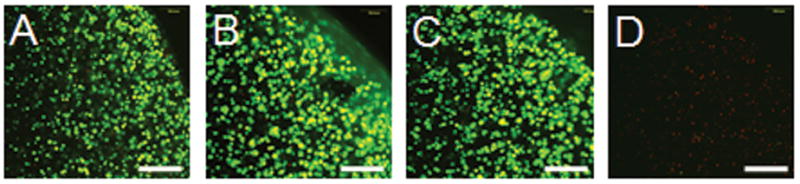
Cell viability staining after 7 day continuous incubation in genipin-supplemented media at 4 genipin concentrations: A) 0 μM B) 22 μM C) 220 μM D) 2200 μM. Bright green indicates living cells. Dim red indicates dead cells. Scale bar = 250 μm.
Explant cartilage treated with genipin became dark purple over a 24-hour period, but just as in the case of tissue-engineered constructs, the mechanical properties of genipin-treated explants were found to be identical to untreated controls (EY=1011 ± 97 kPa, G* at 0.1Hz = 13 ± 1.3 MPa). From these results, only the 22 μM and the 220 μM concentrations were deemed suitable for long term culture; the highest concentration (2200 μM) was eliminated.
In Study 1: Live/dead assays carried out after 42 days of continuous incubation in genipin confirmed no change in cell viability compared to untreated controls using these concentrations (data not shown). The mechanical properties and the physical appearance of the constructs treated with genipin were significantly different from controls. Engineered constructs turned purple with the addition of genipin with higher concentrations of genipin yielding greater intensities of purple (Figure 3). This change in color is characteristic of genipin as it spontaneously reacts with the amine groups and indicates cross-linking. In Study 2: Brief exposure groups (constructs were removed from genipin 24 hours after exposure and cultured in genipin-free growth medium) visually maintained the intensity of color for the 7 days tested (data not shown). Genipin-supplemented culture medium also turned purple, even when there were no constructs present, suggesting that constituents within the aqueous formulation were being cross-linked.
Figure 3.
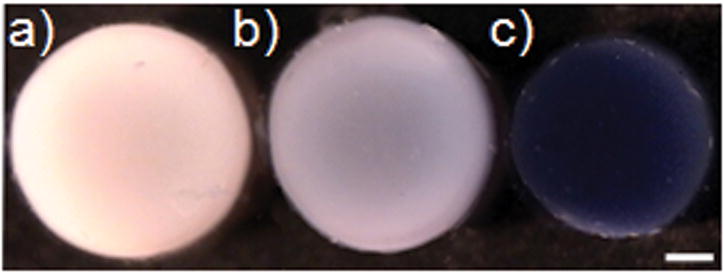
Gross morphology of tissue-engineered cylindrical constructs on day 42 after continuous incubation in genipin-supplemented media at three concentrations: a) 0 μM, b) 22 μM, and c) 220 μM. The tissue volume at the lower concentrations has increased by 20–30% compared to the 220μM concentration which remained at the original volume. Scale Bar = 1mm
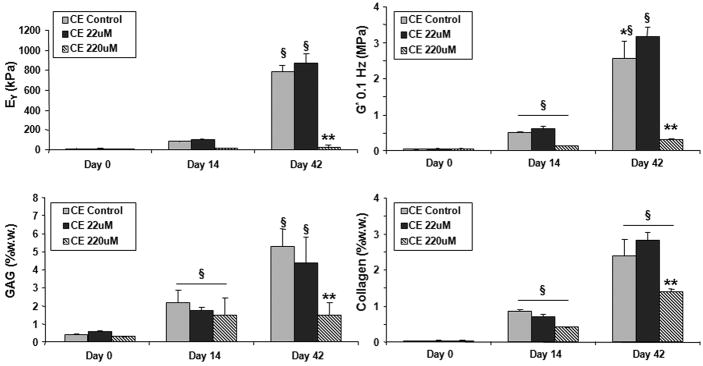
By day 42 in culture the mechanical properties of the continuous exposure (CE) groups were as follows: both CE controls (EY=788 ± 63 kPa, G*= 2.5 ± .5 MPa) and CE 22 μM (EY=873 ± 92 kPa, G*=3.2 ± .2 MPa) developed significantly higher mechanical properties over time in culture (Study 1, Figure 4). The G* in particular was significantly higher for the CE 22μM group than the control group. The CE 220μM group, by contrast, remained at day 0 mechanical properties (EY=23 ± 22 kPa, G*= 0.3 ± .03 MPa). Cell viability assays of this group indicated the cells remained alive while PicoGreen assays indicated no differences in cell proliferation over other groups. GAG and collagen levels increased significantly over time in culture for all groups however total GAG values for the CE 220μM group was significantly less than the CE 22μM and control groups.
Figure 4.
Equilibrium Young’s modulus (EY), dynamic modulus at 0.1 Hz (G*), GAG (%ww), and collagen (%ww) of constructs incubated in genipin continuously throughout the culture period. CE stands for continuous exposure. *p<0.05 for control vs. genipin groups. §p<0.05 against all groups in the previous time points. **p<0.05 for 220uM vs. other day 42 groups.
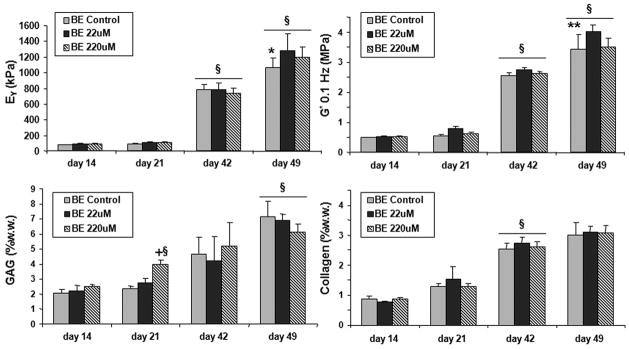
For brief exposure (BE) groups (24 hour duration, Study 2), there were no significant differences in mechanical properties from controls immediately after 24hr incubation (day 42: BE 22μM: EY=790 ± 60kPa, G*=2.7 ± 0.2MPa; BE 220μM: EY=739 ± 69 kPa, G*=2.6 ± 0.3MPa), however after 7 days of culture without genipin these groups developed significantly higher mechanical properties compared to controls (day 49: BE Control: EY=1068 ± 123kPa, G*= 3.4 ± 0.43MPa; BE 22 μM: EY=1279 ± 214kPa, G*=4.02 ± .9MPa; BE 220 μM: EY=1200 ± 128kPa, G*=3.5 ± 0.5MPa). There were no differences in GAG and collagen between the day 42 or day 49 groups (Figure 5).
Figure 5.
Equilibrium Young’s modulus (EY), dynamic modulus at 0.1 Hz (G*), GAG (% ww), and collagen (%ww) of constructs incubated for 24 hrs in genipin (on days 13 and 41). A subset of untreated control were used at each treatment point. BE stands for brief exposure. §p<0.05 against all groups in the previous time points. *p<0.05 for control vs. genipin groups. **p<0.05 for control vs. 22μM group. +p<0.05 for BE 220uM vs. other day 21 groups.
Since the culture medium itself changed colors when exposed to genipin it is possible that genipin cross-links constituents within the culture medium and diluted the dose that was delivered to the constructs. To account for this possibility, a separate study (not shown) was carried out in which constructs were bathed briefly (1–3 hours) in genipin supplemented phosphate buffered saline (PBS) and subsequently returned to genipin-free culture medium. The results using this protocol were consistent with what was observed in Study 2 with direct medium supplementation for 24 hours.
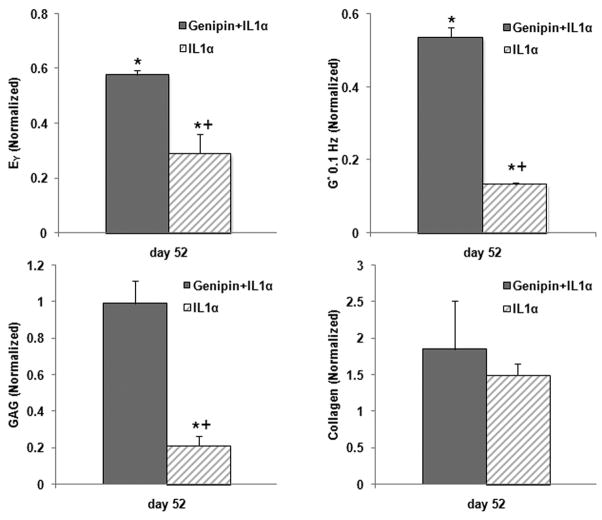
In Study 3: Constructs treated with IL-1alpha (and not genipin) dropped significantly in EY (-69%), G* (-86%), and GAG (-81%), but not collagen (Figure 6). The genipin + IL-1α group also developed significantly lower EY (-33%), G* (-44%), but interestingly GAG values did not measurably change compared to controls. Collagen values, similarly, did not change significantly. When compared to the IL-1α group, the genipin + IL-1α group maintained significantly higher EY (+218%), G* (+390%) and GAG (+477%). Figure 6 presents these values normalized to controls for comparison, the actual values obtained on day 52 were as follows: Control (EY=640±88kPa, G*=2.90±0.35MPa, GAG=6.6±0.2 %ww, Col =2.33±0.2%ww), Genipin treated control (EY=731±134kPa, G*=2.74±0.41MPa, GAG=6.8±0.3%ww, Col =2.3± 0.3%ww), IL-1α treated (EY=197±10kPa, G*=0.34±0.01MPa, GAG=1.3±.5%ww, Col =3.1±0.5%ww), and genipin + IL-1α treated (EY= 431±47 kPa, G*= 1.34±0.07 MPa, GAG= 6.1±0.8 %ww, Col =3.8±0.4%ww).
Figure 6.
Equilibrium Young’s modulus (EY), dynamic modulus at 0.1 Hz (G*), GAG (% ww), and collagen (%ww) of constructs challenged with IL-1α for 3 days. Values are normalized to day 49 controls. *p<0.05 against either control group. +p<0.05 for Genipin+IL-1α vs. IL-1α group.
Discussion
The hypothesis governing this set of studies was that genipin would cross-link the extracellular matrix within the developing engineered constructs as the extra cellular matrix proteins are produced and would thereby increase the mechanical properties of the constructs over time. The fundamental premise of the hypothesis was supported: there were increases in mechanical properties without significant changes in cell viability or biochemical quantities of GAG or collagen, suggesting a genipin-induced enhancement of tissue stiffness.
There is no established concentration for the use of genipin; therefore in the current set of studies we used values that span both the low (22μM14) and high (2000 μM16) range reported in the literature. Additionally, we adopted two durations for medium supplementation – continuous and intermittent supplementation of genipin. The toxicity of genipin when supplemented directly to the culture medium was found to be dependent both on dose and duration of exposure. Long-term continuous exposure (Study 1) was lethal at 2200μM, detrimental (but not lethal) at 220μM, and beneficial at 22μM. Short term exposure (Study 2) at 220μM and 22μM on the other hand was sufficient to obtain the benefits on the tissue-level without the detrimental effects on the cellular level.
In both long-term and short term exposure protocols we observed similar increases in mechanical properties over time in culture, but in both cases these benefits did not occur until later time points. This suggests that the tissue must reach some degree of extracellular maturity prior to genipin treatment. It also suggests that prolonged medium supplementation is probably unnecessary, but that brief exposure, in growth medium or in PBS, followed by continuing cultivation may be the best method to realize the benefits of biocompatible cross-linking.
It is notable that genipin-induced changes did not occur immediately, but took several days to manifest (Figure 5). Initially we believed this was due to the nature of the agarose hydrogel scaffold. However, we found that there were similarly no immediate changes to native cartilage explant mechanical properties when exposed to the same concentrations of genipin. Together these observations indicate that the increased stiffness of the constructs is not due directly to physical strengthening of the extracellular network via genipin cross-links, but attributable to other mechanisms that lead to a cumulative increase of tissue properties over time.
One possibility is that genipin directly affected cell metabolism in addition to cross-linking the extracellular matrix. There have been no studies reported on the effect of genipin on chondrocytes specifically, but there is evidence that genipin suppresses cell proliferation and alters RNA activity in subconjunctival fibroblast10 and some epithelial cell lines11. There was no decrease in cell proliferation in the current set of studies as confirmed by PicoGreen DNA quantification (not shown). Likewise there were no significant differences in matrix accumulation of GAG or collagen over time. Additionally, we examined the effect of genipin on chondrocytes cultured on tissue culture plates, but found no significant differences on cell proliferation and cell morphology after 14 days of continuous exposure at 22 μM (results not shown); therefore we do not believe that alterations in cell metabolism were the main cause for the increased mechanical properties observed.
Instead, we speculate that the presence of cross-links may have modulated the nature of subsequent matrix deposition by the chondrocytes, either by leading to a more consistent fiber alignment for genipin-treated constructs relative to untreated controls, or by reducing the catabolic loss of matrix components as suggested by Study 3.
Chondrocytes in culture balance catabolic and anabolic activities to remodel their surrounding extracellular matrix. By challenging the chondrocytes with IL-1α (Study 3) we artificially amplified the catabolic process. Cross-linking has been shown to increase resistance to enzymatic degradation through collagenase digestion2,4,22,23. We have previously observed that in our culture system IL-1α triggers a rapid loss in GAG content, along with higher matrix metalloproteinase-3 (MMP-3) levels, but with no significant changes in collagen content within the first 14 days of treatment 24,25. Here we observed that indeed the extracellular matrix degraded less after pretreatment with genipin, demonstrating an increased resilience to degradation through cell-mediated processes. Genipin cross-linking may have led to mechanical benefits over time by increasing the chemical resistance of a variety of extracellular matrix components, such as GAG, to catabolism associated with normal matrix remodeling. Interestingly, although the mechanical properties of these genipin treated tissues dropped with exposure to IL-1α, their GAG values remained unchanged. This suggests that some other, as of yet unmeasured matrix constituent was affected by the cytokine and responsible for the decreased construct stiffness. Alternatively, as we have not analyzed the size of GAGs that are retained in genipin-treated constructs subjected to interleukin (and compared to control) we could speculate that GAGs were indeed broken down and their interrelationship with the collagen network disrupted, leading to the observed significant decrease in functional material properties. In this scenario GAG and collagen content may not have changed because the genipin cross-links entrapped the degradative products within the construct rather than permit them to diffuse into the culture medium.
As a corollary to Study 3, genipin treatments, as described in this manuscript, may be useful to reduce the inflammatory response of the engineered tissue upon implantation. Implanting allogenic tissue in the body generally invokes an immune reaction except for certain immunologically privileged area26 such as the eye and the brain. Allogenic chondrocytes are considered to be to some degree immunoprivileged27,28 because they reside within a dense extracellular matrix that is not vascularized and, therefore, has less interaction with blood born leukocytes. Even so, the presence of extracellular matrix constituents, such as collagen, can themselves invoke an immune reaction29 and can lead to the rejection and degradation of the allograft within the joint30,31. The immunologic reactivity and susceptibility of grafts appear to be inversely related to the amount and maturity of cartilage-specific extracellular matrix present32. Reducing the number of free amino residues in the collagen through various cross-linking methods, such as genipin, has been shown to reduce the antigenicity of the resulting tissue33,34.
Inflammatory cytokines, such as interleukin-1α, are often present in the joint, either as preexisting chronic condition, or as a result of the surgical intervention itself35–38. Previously we have shown that engineered cartilage can be more susceptible to degradation by these cytokines than mature endogenous cartilage24. In those studies, engineered tissue that was exposed temporarily to IL-1α stopped growing, catabolically degraded the surrounding extracellular matrix, and did not recover. In Study 3 genipin modification of the tissue resulted in increased resistance to IL-1α degradation. This result both suggests a mechanism by which the tissue developed higher mechanical properties over time and suggests that it might provide a means to protect the tissue from transient insults of inflammatory cytokines during or after surgery.
In summary, genipin supplementation of the culture medium as described here led to significant increases over control in both the dynamic (+28 %) and Young’s (+20 %) modulus. This demonstrates the potential for long term gains in mechanical properties using biocompatible cross-linking agents as direct medium supplements. However, considering that the magnitude of the findings were relatively incremental in the context of the dynamic modulus value for native articular cartilage (4 MPa vs. 20–40 MPa for explants25) the most clinically relevant finding may be the potential reduction in antigenecity and the increased resistance to enzymatic degradation with genipin treatment. This approach warrants further investigation towards optimizing effects of genipin on engineered constructs used in vivo.
Acknowledgments
This work was supported in part by NIH grant AR46568 and a grant from the Musculoskeletal Transplant Foundation (CU07-194).
References
- 1.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15(9):1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung HW, Huang RN, Huang LL, Tsai CC, Chiu CT. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42(4):560–7. doi: 10.1002/(sici)1097-4636(19981215)42:4<560::aid-jbm12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials. 2004;25(17):3541–52. doi: 10.1016/j.biomaterials.2003.09.109. [DOI] [PubMed] [Google Scholar]
- 4.Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res A. 2003;64(3):427–38. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 5.Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006;12(9):2657–63. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 6.Huang LL, Sung HW, Tsai CC, Huang DM. Biocompatibility study of a biological tissue fixed with a naturally occurring crosslinking reagent. J Biomed Mater Res. 1998;42(4):568–76. doi: 10.1002/(sici)1097-4636(19981215)42:4<568::aid-jbm13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Englert C, Blunk T, Muller R, von Glasser SS, Baumer J, Fierlbeck J, Heid IM, Nerlich M, Hammer J. Bonding of articular cartilage using a combination of biochemical degradation and surface cross-linking. Arthritis Res Ther. 2007;9(3):R47. doi: 10.1186/ar2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo YC, Ku IN. Effects of gel concentration, human fibronectin, and cation supplement on the tissue-engineered cartilage. Biotechnol Prog. 2007;23(1):238–45. doi: 10.1021/bp060253h. [DOI] [PubMed] [Google Scholar]
- 9.Moffat KL, Marra KG. Biodegradable poly(ethylene glycol) hydrogels crosslinked with genipin for tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2004;71(1):181–7. doi: 10.1002/jbm.b.30070. [DOI] [PubMed] [Google Scholar]
- 10.Kitano A, Saika S, Yamanaka O, Ikeda K, Reinach PS, Nakajima Y, Okada Y, Shirai K, Ohnishi Y. Genipin suppresses subconjunctival fibroblast migration, proliferation and myofibroblast transdifferentiation. Ophthalmic Res. 2006;38(6):355–60. doi: 10.1159/000096231. [DOI] [PubMed] [Google Scholar]
- 11.Kitano A, Saika S, Yamanaka O, Reinach PS, Ikeda K, Okada Y, Shirai K, Ohnishi Y. Genipin suppression of fibrogenic behaviors of the alpha-TN4 lens epithelial cell line. J Cataract Refract Surg. 2006;32(10):1727–35. doi: 10.1016/j.jcrs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM, Kim SJ, Kim BC, Jin C, Lim CJ, Park EH. Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol. 2004;495(2–3):201–8. doi: 10.1016/j.ejphar.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 2006;103(3):496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J Biomed Mater Res. 1999;47(2):116–26. doi: 10.1002/(sici)1097-4636(199911)47:2<116::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Sung HW, Hsu CS, Lee YS, Lin DS. Crosslinking characteristics of an epoxy-fixed porcine tendon: effects of pH, temperature, and fixative concentration. J Biomed Mater Res. 1996;31(4):511–8. doi: 10.1002/(SICI)1097-4636(199608)31:4<511::AID-JBM11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31715. [DOI] [PubMed] [Google Scholar]
- 17.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31(10):927–34. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 18.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2005 doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 20.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 21.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen CN, Sung HW, Liang HF, Chang WH. Feasibility study using a natural compound (reuterin) produced by Lactobacillus reuteri in sterilizing and crosslinking biological tissues. J Biomed Mater Res. 2002;61(3):360–9. doi: 10.1002/jbm.10153. [DOI] [PubMed] [Google Scholar]
- 23.Sung HW, Chen CN, Chang Y, Liang HF. Biocompatibility study of biological tissues fixed by a natural compound (reuterin) produced by Lactobacillus reuteri. Biomaterials. 2002;23(15):3203–14. doi: 10.1016/s0142-9612(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 24.Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, Cook JL, Hung CT. Differences in Interleukin-1 Response between Engineered and Native Cartilage. Tissue Eng. 2008 doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- 25.Bian L, Lima EG, Angione SL, Ng KW, Williams DY, Xu D, Stoker AM, Cook JL, Ateshian GA, Hung CT. Mechanical and biochemical characterization of cartilage explants in serum-free culture. Journal of Biomechanics in press. doi: 10.1016/j.jbiomech.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streilein JW, Stein-Streilein J. Does innate immune privilege exist? J Leukoc Biol. 2000;67(4):479–87. doi: 10.1002/jlb.67.4.479. [DOI] [PubMed] [Google Scholar]
- 27.Garrett JC, Steensen RN. Meniscal transplantation in the human knee: a preliminary report. Arthroscopy. 1991;7(1):57–62. doi: 10.1016/0749-8063(91)90079-d. [DOI] [PubMed] [Google Scholar]
- 28.Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am. 2000;82-A(8):1071–82. doi: 10.2106/00004623-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71(2):343–54. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 30.Galili U, LaTemple DC, Walgenbach AW, Stone KR. Porcine and bovine cartilage transplants in cynomolgus monkey: II Changes in anti-Gal response during chronic rejection. Transplantation. 1997;63(5):646–51. doi: 10.1097/00007890-199703150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Stone KR, Walgenbach AW, Abrams JT, Nelson J, Gillett N, Galili U. Porcine and bovine cartilage transplants in cynomolgus monkey: I A model for chronic xenograft rejection. Transplantation. 1997;63(5):640–5. doi: 10.1097/00007890-199703150-00005. [DOI] [PubMed] [Google Scholar]
- 32.Arnoczky SP. The biology of allograft incorporation. J Knee Surg. 2006;19(3):207–14. doi: 10.1055/s-0030-1248109. [DOI] [PubMed] [Google Scholar]
- 33.Murayama Y, Satoh S, Oka T, Imanishi J, Noishiki Y. Reduction of the antigenicity and immunogenicity of xenografts by a new cross-linking reagent. ASAIO Trans. 1988;34(3):546–9. [PubMed] [Google Scholar]
- 34.Paul RG, Bailey AJ. Chemical stabilisation of collagen as a biomimetic. ScientificWorldJournal. 2003;3:138–55. doi: 10.1100/tsw.2003.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391(Suppl):S108–15. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 36.Schiff MH. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann Rheum Dis. 2000;59(Suppl 1):i103–8. doi: 10.1136/ard.59.suppl_1.i103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smeets TJ, Barg EC, Kraan MC, Smith MD, Breedveld FC, Tak PP. Analysis of the cell infiltrate and expression of proinflammatory cytokines and matrix metalloproteinases in arthroscopic synovial biopsies: comparison with synovial samples from patients with end stage, destructive rheumatoid arthritis. Ann Rheum Dis. 2003;62(7):635–8. doi: 10.1136/ard.62.7.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Berg WB, Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis: evidence of a dominant role for interleukin-I. Baillieres Best Pract Res Clin Rheumatol. 1999;13(4):577–97. doi: 10.1053/berh.1999.0047. [DOI] [PubMed] [Google Scholar]