Abstract
Mice that are deficient in p53 exhibit an early onset of multiple types of tumors, especially thymic lymphoma. However, it remains unclear to what extent each of the p53-regulated pathways exerts its tumor suppressor activity. p21Cip1/Waf1, acting down stream of p53, is a major G1/S checkpoint protein that restricts cell cycle progression into S phase in the presence of DNA damage. While at old ages p21−/− mice have a higher incidence of many types of tumors than p21+/+ mice, they are more resistant to thymic lymphomagenesis. In this study, we characterized mutagenesis in vivo in T cells of p21-deficient mice, using loss of heterozygosity (LOH) at Aprt locus as an indicator. We found that the spontaneous Aprt mutant frequency in T cells of p21−/− mice is lower than that in p21+/+ mice. The mutational spectra, however, are similar, with mitotic recombination being the predominant pathway. In contrast to the remarkable induction of LOH events in T cells of p53−/− mice exposed to x-rays, LOH in T cells of p21−/− mice is not significantly induced by x-rays. Correspondingly, lymphoid cells of p21−/− mice are more sensitive to IR-induced apoptosis than those of p21+/+ mice, in contrast to the radioresistance of p53-deficient lymphocytes. Reduction in mutation load in T cell lineages may contribute to the suppression of thymic lymphomagenesis in p21−/− mice.
Keywords: p21, mutagenesis, cell cycle checkpoint, apoptosis, lymphomagenesis
1. Introduction
The tumor suppressor p53 is a master regulator of the cellular responses to DNA damage. The activation of p53 up-regulates an array of genes that initiates cell cycle checkpoints, DNA repair and apoptosis[1]. While it has been firmly established that loss or mutation of p53 can lead to a broad spectrum of human malignancy, it remains controversial which of the p53-regulated pathways, or a combination of them, functions as a tumor-suppressing mechanism [2, 3]. Whereas there is an early onset of many types of tumors, particularly thymic lymphomas, in mice that lack p53, disruption in each of the p53-regulated pathways only has limited tumor-enhancing effect [3–8]. For example, p53 mutant mice that are engineered to be deficient in induction of apoptosis, but not in other functions of p53, were found to escape the early onset of thymic lymphomas that characterize p53-null mice [3]. Moreover, the lymphomas and sarcomas that eventually developed in this particular strain of mice retained a diploid chromosome number, in sharp contrast to aneuploidy observed in tumors and cells from p53-null mice, suggesting that apoptosis is dispensable for the maintenance of chromosome euploidy in lymphoid cells. Also, knockout mice for Puma, a gene required for apoptosis, were shown to be relatively tumor free [7, 8].
Studies involving mice that lack Cdkn1a (p21), a protein that arrests cell cycle progression at G1/S checkpoint in the presence of DNA damage, showed that p21 has tumor suppressor activity in a tissue-dependent manner. While lack of p21 predisposes mice to tumor development in skin, colon, intestine, pituitary, thyroid, mammary gland, salivary gland, connective tissue and histiocystic sarcomas, p21-deficient mice are more resistant to thymic lymphomas [9–11]. For example, p21−/− mice are more protected than wild type mice from irradiation-induced lymphomas. Absence of p21 also significantly reduces the incidence of spontaneous and radiation-induced thymic lymphomas in p53−/− and p53−/− mice. Increased rate of apoptosis in thymic cells has been proposed to be responsible for the decreased lymphomagenesis [11].
Lack of p53 is associated with increased chromosome instability in vivo [12, 13]. Furthermore, chromosome instability is induced more prominently by IR in the absence of p53. For example, x-rays induce loss of heterozygosity (LOH) events more dramatically in T cells of p53−/− and p53−/− mice than in those of p53+/+ mice [14]. However, since p53 is involved in multiple cellular processes, it is unclear to what extent each of the p53-regulated pathways is responsible for the suppression of genetic instability.
In this study we addressed how a defective G1/S checkpoint contributes to mutagenesis in vivo by characterizing the somatic mutations that arise at Aprt locus in T cells of 129XC57F1 p21-null mice that are also Aprt heterozygous. In Aprt heterozygous mice, multiple pathways of LOH, including deletion, mitotic recombination and chromosome loss, can be evaluated [13, 15, 16]. The APRT-deficient mutant cells are recoverable by the virtue of their resistance to adenine analogs such as 2, 6-diaminopurine (DAP) and are designated as DAPr.
2. Materials and methods
2.1. Mice
We obtained p21 knockout mice in a mixed 129/C57 background from the Jackson Laboratory [17]. The p21 mutant mice were backcrossed to 129S2 and C57 strains, respectively, for ten consecutive generations to reach (N10) C57 and (N10)129, respectively. The (N10)129 p21−/− mice were then crossed to 129Aprt−/− mice to generate 129 p21−/−Aprt−/−. 129 p21−/−Aprt−/− mice were then crossed to C57p21−/− mice to generate 129S2 X C57 F1 hybrids that are p21(+/+, −/−, −/−) and Aprt −/−. The purpose of the backcross is to obtain strain-specific chromosome 8, so that when they are introduced in hybrids, the intervals of LOH along chromosome 8, and indirectly the pathways of LOH, in DAPr clones can be determined.
2.2. Ionizing radiation
Mice, about two months old, were subjected to whole-body x-irradiation using a Faxitron Cabinet x-ray System (Wheeling, IL) at the rate of 0.2 Gy/min using at 100 kVp. Irradiated mice were sacrificed for preparation of splenocytes two months after a single exposure. All mice, treated and untreated, were about 4 months old at the time of experiments.
2.3. Characterization of DAPr mutant T cell clones
Splenocytes were prepared and cultured as described [18]. DAPr mutant T cell clones were scored and analyzed as described [13]. The colony forming efficiency and mutant frequency were estimated for each mouse by assuming that the positive colonies are formed in the wells of a 96-well plate following a Poisson distribution.
2.4. Apoptosis assay
Apoptosis of splenic lymphocytes was estimated with the kit (Roche Applied Science, Indianapolis, IN) for detection and quantification of apoptosis based on terminal deoxyribonucleic transferase mediated dUTP nick end labeling (TUNEL) technology. Unirradiated mice and irradiated mice (6 hours following 1 Gy X-rays) were sacrificed and spleens were obtained and placed in ice-cold RPMI 1640 medium. Splenic lymphocytes were isolated as described [18] and were fixed in 2% paraformaldehyde (in 1× PBS) for 1 hour at room temperature. Cells were washed 3 times with PBS, resuspended in permeablization solution (0.1% triton X-100, 0.1% sodium citrate, freshly made), and incubated on ice for 2 minutes. Cells were washed twice with PBS, resuspended in TUNEL reaction mixture and incubated at 37°C for 1 hour. Samples were washed twice with PBS and analyzed under a fluorescence microscope.
3. Results and discussion
3.1. Spontaneous Aprt mutations in T cells of p21-deficient mice
We first compared the spontaneous frequency of DAPr T cells in vivo between p21+/+ and p21−/− mice. In 129XC57F1 p21+/+ mice, the median frequency was 17.5 × 10−6 (N=15). In contrast, the median was 8.4 × 10−6 in p21−/− mice (N=12) (Table 1). The difference between the two groups is statistically significant (P=0.03, Mann-Whitney U test). Thus, in the absence of p21, the frequency of DAPr mutant T cells, which reflect LOH at Aprt locus, is lower than when p21 is present.
Table 1.
Mutant frequency of DAPr T cell variants
| Genotype | Strain | X-rays (Gy) |
No. mice |
CFE ± SE (%) |
Median MF (× 10−6) |
|---|---|---|---|---|---|
| p21+/+ (group 1) | 129 × C57 | 0 | 15 | 8.0 ± 2.2 | 17.5 |
| P21+/+ (group 2) | 129 × C57 | 0 | 14 | 7.9 ± 1.2 | 20.3 |
| p21+/+ (group 3) | 129 × C57 | 0 | 5 | 6.7 ± 1.1 | 19.5 |
| p21−/− | 129 × C57 | 0 | 12 | 8.5 ± 0.8 | 8.4* |
| p21 wt | 129 × C57 | 1 | 2 | 5.4 ± 0.7 | 18.1 |
| p21−/− | 129 × C57 | 1 | 12 | 4.2 ± 0.4 | 13.9 |
| p21 wt | 129 × C57 | 4 | 13 | 5.1 ± 0.7 | 17.1 |
| P21+/− | 129 × C57 | 4 | 4 | 3.2 ± 0.3 | 30.8 |
| p21−/− | 129 × C57 | 4 | 11 | 5.5 ± 0.5 | 10.0 |
CFE, colony-forming efficiency; MF, mutant frequency
P = 0.03, compared to p21+/+ group 1.
To determine what types of mutation are reduced in the absence of p21, we characterized the mutational spectrum of DAPr T cell clones. We found that the spectra are similar between p21+/+ and p21−/− mice, Table 2. As we reported earlier [19], mitotic recombination is the predominant mechanism. Thus, lack of p21 renders T cells less susceptible to all types of mutations leading to functional loss of Aprt.
Table 2.
Spectrum of mutational pathways to APRT-deficiency
| Genotype | X-rays | N | No. DAPr clones |
No. PM/EI (%) |
No. MR (%) | No. del (%) |
Average ratio of MR |
|---|---|---|---|---|---|---|---|
| p21+/+ | 0 Gy (Group 1) | 13 | 60 | 29 (48) | 31 (52) | 0 (0) | 0.64 |
| 0 Gy (Group 2) | 14 | 69 | 24 (35) | 43 (62) | 2 (3) | 0.65 | |
| 0 Gy (Group 3) | 5 | 18 | 5 (28) | 13 (72) | 0 (0) | 0.72 | |
| 4 Gy | 10 | 16 | 4 (25) | 10 (63) | 2 (12) | 0.65 | |
| p21+/− | 4 Gy | 4 | 18 | 4 (22) | 14 (78) | 0 (0) | 0.74 |
| p21−/− | 0 Gy | 12 | 60 | 18 (30) | 38 (63) | 4 (7) | 0.63 |
| 1 Gy | 8 | 23 | 12 (52) | 10 (43) | 1 (5) | 0.33 | |
| 4 Gy | 11 | 29 | 11 (38) | 17 (59) | 1 (3) | 0.47 | |
PM/EI, putative point mutation/epigenetic inactivation; MR, mitotic recombination; del, deletion. Ratio of MR in each mouse was as (number of MR clones)/(number of DAPr clones analyzed). The average ratio is more representative of the distribution of MR in a group.
3.2. IR-induced Aprt mutations in T cells of p21-deficient mice
In general, the frequency of LOH at Aprt locus in T cells is only slightly induced by a single dose of ionizing radiation and the IR-induced mutations are primarily of deletion type [20]. p53−/− and p53−/− mice, on the other hand, exhibit a much more pronounced induction of multiple types of mutations [14]. To test how p21−/− mice respond to IR, we characterized the mutation frequency and spectrum of DAPr T cell clones in p21−/− mice exposed to x-rays. No significant induction in mutant frequency was detected (Table 1). In addition, the mutational spectrum of DAPr clones recovered from irradiated mice was not affected by the lack or presence of p21 (Table 2). Especially, deletions, which are usually preferentially induced by x-rays[20], were not more commonly represented in p21−/− mice than in p21+/+ mice after x-ray exposure. Thus, T cells of p21−/− mice were not more predisposed to incur mutation in response to IR than those of p21+/+ cells.
While the frequency of Aprt LOH mutants was increased about eight fold in p53−/− mice after their exposure to 4 Gy of X-rays [14], the induction of LOH in T cells of p21−/− mice is minimal, if any. Thus, the remarkable induction of mutations in T cells of p53−/− mice is not caused by a compromised G1/S checkpoint.
3.3. Lymphoid cells of p21−/− mice are more sensitive to IR-induced apoptosis
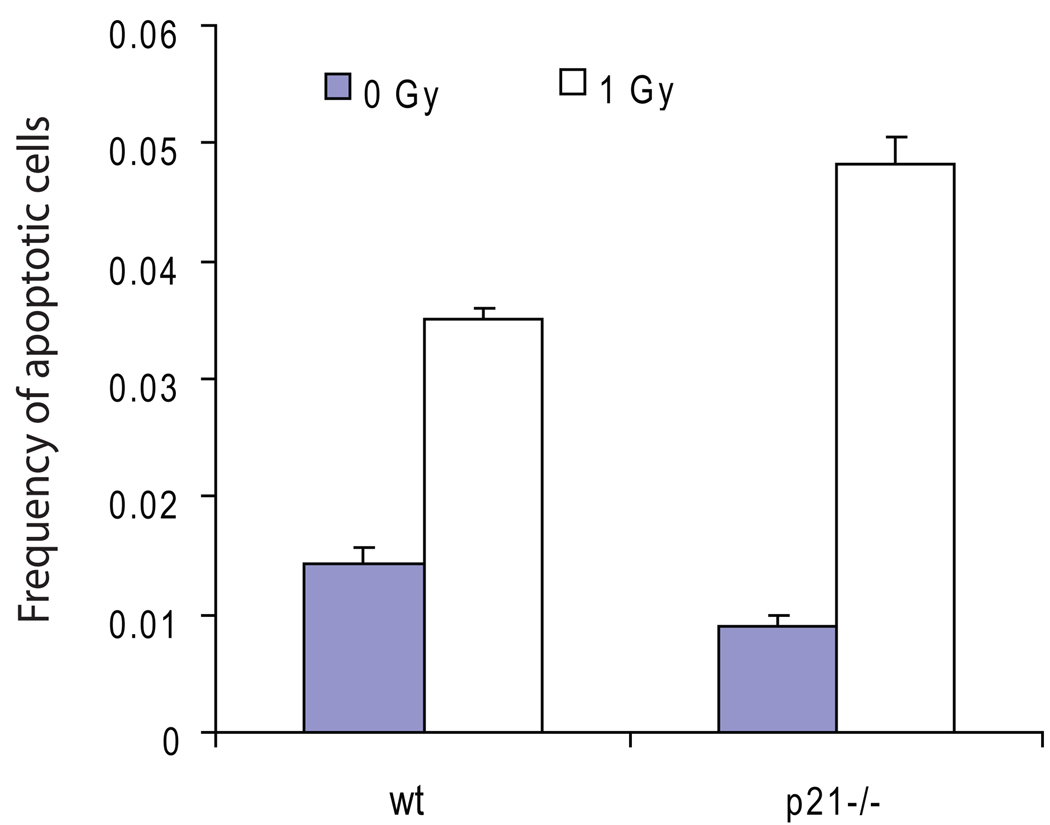
We have previously shown that reduced induction of apoptosis in mice treated with repeated exposure to x-rays is correlated to an elevation of LOH at Aprt [20]. Considering that p21 has been proposed to be an anti-apoptotic factor in some cell lines [21] and in spleens of p21−/− mice [22], and that the expression level of p21 is correlated to the resistance to apoptosis [23], we speculated that the decreased mutant frequency of LOH at Aprt in T cells of p21−/− mice might be due to their increased sensitivity to DNA damage-induced apoptosis. It is possible that DNA damage is more tolerated in T cells (or their precursor cells) with functional p21 so that p21-deficient T cells are more susceptible to DNA damage-induced apoptosis, thus less likely to harbor mutations. To gain further supporting evidence for this notion, we evaluated apoptosis in splenocytes in vivo. Mice were exposed in whole body to 1 Gy of x-rays and were sacrificed six hours later for the preparation of splenocytes. The splenic cells were then fixed and stained for scoring of apoptotic cells. As shown in Fig. 1, while apoptosis was induced two fold in splenocytes of p21+/+ mice, it was induced about five fold in those of p21−/− mice. The frequency of apoptotic splenocytes in p21−/− mice after x-ray exposure was also significantly higher than that in p21+/+ mice. This finding is consistent with previous reports [22, 23] and adds further evidence for an inverse correlation between the induction of apoptosis and the level of LOH. However, while the splenocytes of p21−/− mice were observed to have a higher rate of spontaneous apoptosis than those of p21+/+ mice in spleen sections [22], such an enhancement in spontaneous apoptosis in p21−/− mice was not detected by our assay, it is therefore unclear whether the reduced spontaneous mutagenesis in T cells of p21−/− mice was related to apoptosis in T cells per se. If spontaneous mutations leading to LOH at Aprt primarily occur at earlier stages of hematopoietic development, but not in the mature T cells, an assessment of apoptosis in the mature T cells may not necessarily reflect the true nature of the target cells in which mutations arise. Indeed, while x-rays applied to adults preferentially induce deletions and point mutations, they primarily induce mitotic recombination when applied to fetus [24], suggesting that mitotic recombination, which accounts for the majority of the LOH events at Aprt, may primarily occur before birth.
Figure 1.
p21-deficient splenocytes were more susceptible to x-ray-induced apoptosis. Mice were exposed to 1 Gy of x-rays and were sacrificed 6 hours later for the collection of splenocytes. Terminal deoxyribonucleic transferase mediated dUTP nick end labeling (TUNEL) assay was performed to determine the level of apoptosis (N= 3 mice /group).
3.4. Mutational response at Hprt is unaffected by the status of p21
While Aprt can detect a broad spectrum of mutations as a reporter, the X-linked Hprt gene as a reporter primarily detects more local genomic alterations such as point mutations and small deletions [20]. Our previous studies showed that Hprt and Aprt respond differently to x-rays [20]. In mice that were exposed to x-rays in their adulthood, there was a clear dose-dependent increase in the Hprt mutant frequency, but the increase in the Aprt mutant frequency was very modest. On the other hand, when x-rays was delivered to fetus in uteri, it had no effect on the Hprt mutant frequency, while Aprt mutant frequency was increased several fold, primarily due to an elevated mitotic recombination [24]. Interestingly, for reason(s) unknown, the mutational response to x-rays at Hprt is not affected by the status of apoptosis. We first estimated the spontaneous Hprt mutant frequency in T cells of p21−/− mice. We found that there was virtually no difference in this frequency between p21−/− and p21+/+ mice (Table 3). This result indicates that reduced spontaneous mutagenesis in the absence of p21 is locus-specific.
Table 3.
Spontaneous and IR-induced Hprt mutations in T cells of p21 null mice
| Genotype | Strain | x-rays (Gy) |
No. mice |
Median MF (× 10−6) |
|---|---|---|---|---|
| p21+/+ | 129 × C57 | 0 | 17 | 2.7 |
| p21−/− | 129 × C57 | 0 | 13 | 2.9 |
| p21+/+ | 129 × C57 | 1 | 9 | 21.5 |
| p21−/− | 129 × C57 | 1 | 6 | 37.5 |
| p21+/+ | 129 × C57 | 4 | 5 | 52.8 |
| p21+/− | 129 × C57 | 4 | 5 | 60.1 |
| p21−/− | 129 × C57 | 4 | 9 | 48.6 |
We further tested how Hprt respond to x-rays in the absence of p21 in vivo and found that p21−/− mice appeared to respond to IR similarly as p21+/+ mice. Both types of mice showed a dose dependent increase in the mutant frequency (Table 3). Thus, neither the spontaneous nor induced Hprt mutant frequency in T cells is affected by p21 status. Clearly, a defective G1/S checkpoint does not predispose the lymphoid cells of p21−/− to mutational accumulations at Hprt locus. Thus, the increased propensity to undergo apoptosis that have probably precluded the accumulation of mutation at Aprt had little effect on mutations at Hprt.
Our data showed that the reduced spontaneous mutagenesis in p21−/− mice does not apply to the X-linked Hprt. The mutagenic effect of x-rays is also locus-specific in p21−/− mice. It appears that the degree to which Aprt mutations, but not Hprt mutations, are induced is more indicative of the induction of lymphomagenesis in mice exposed to x-rays [20]. With the same total dose, fractionation at weekly intervals is more efficient than a single dose in the induction of lymphomas in mouse models. In corresponding to the more efficient induction of lymphomagenesis, x-rays fractionated at weekly intervals (4 x 1 Gy) is more potent in inducing LOH mutations at Aprt, but less so in inducing Hprt mutations. Also, a decrease in the induction of apoptosis accompanied the elevated Aprt mutant frequency.
In conclusion, lack of p21 does not cause an increased accumulation of spontaneous or induced mutations in T cells. Rather, the spontaneous mutagenesis measured by LOH at Aprt in T cells is reduced in p21−/− mice. Such a reduction in mutation load probably contributes to the reduced lymphomagenesis in those mice. The observed increase in mutagenesis in T cells of p53−/− mice in response to x-rays is therefore independent of the p21-mediated pathway.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01ES011633) and New Jersey Stem Cell Research grant (SNJ-CST-07-2042-014-90).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There is no conflict of interest.
Reference List
- 1.Levine AJ, Hu W, Feng Z. The P53 pathway: what questions remain to be explored? Cell Death. Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 2.Schmit CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El Naggar AK, Multani A, Chang S, Lozano G. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat. Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- 4.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 5.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 6.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 8.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 9.Wang YA, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14590–14595. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–6238. [PubMed] [Google Scholar]
- 11.De la CE, Garcia-Cao I, Herranz M, Lopez P, Garcia-Palencia P, Flores JM, Serrano M, Fernandez-Piqueras J, Martin-Caballero J. Tumorigenic activity of p21Waf1/Cip1 in thymic lymphoma. Oncogene. 2006;25:4128–4132. doi: 10.1038/sj.onc.1209432. [DOI] [PubMed] [Google Scholar]
- 12.Bouffler SD, Kemp CJ, Balmain A, Cox R. Spontaneous and ionizing radiation-induced chromosomal abnormalities in p53-deficient mice. Cancer Res. 1995;55:3883–3889. [PubMed] [Google Scholar]
- 13.Shao C, Deng L, Henegariu O, Liang L, Stambrook PJ, Tischfield JA. Chromosome instability contributes to loss of heterozygosity in mice lacking p53. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7405–7410. doi: 10.1073/pnas.97.13.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang L, Shao C, Deng L, Mendonca MS, Stambrook PJ, Tischfield JA. Radiation-induced genetic instability in vivo depends on p53 status. Mutat. Res. 2002;502:69–80. doi: 10.1016/s0027-5107(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 15.Shao C, Yin M, Deng L, Stambrook PJ, Doetschman T, Tischfield JA. Loss of heterozygosity and point mutation at Aprt locus in T cells and fibroblasts of Pms2−/− mice. Oncogene. 2002;21:2840–2845. doi: 10.1038/sj.onc.1205358. [DOI] [PubMed] [Google Scholar]
- 16.Shao C, Deng L, Chen Y, Kucherlapati R, Stambrook PJ, Tischfield JA. Mlh1 mediates tissue-specific regulation of mitotic recombination. Oncogene. 2004;23:9017–9024. doi: 10.1038/sj.onc.1208148. [DOI] [PubMed] [Google Scholar]
- 17.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Current Biology. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 18.Liang L, Deng L, Shao C, Stambrook PJ, Tischfield JA. In vivo loss of heterozygosity in T-cells of B6C3F1 Aprt(+/−) mice. Environmental & Molecular Mutagenesis. 2000;35:150–157. [PubMed] [Google Scholar]
- 19.Shao C, Deng L, Chen Y, Kucherlapati R, Stambrook PJ, Tischfield JA. Mlh1 mediates tissue-specific regulation of mitotic recombination. Oncogene. 2004;23:9017–9024. doi: 10.1038/sj.onc.1208148. [DOI] [PubMed] [Google Scholar]
- 20.Liang L, Mendonca MS, Deng L, Nguyen SC, Shao C, Tischfield JA. Reduced Apoptosis and Increased Deletion Mutations at Aprt Locus In vivo in Mice Exposed to Repeated Ionizing Radiation. Cancer Res. 2007;67:1910–1917. doi: 10.1158/0008-5472.CAN-06-1476. [DOI] [PubMed] [Google Scholar]
- 21.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol. Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 22.Komarova EA, Christov K, Faerman AI, Gudkov AV. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791–3798. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 23.Wallace M, Coates PJ, Wright EG, Ball KL. Differential post-translational modification of the tumour suppressor proteins Rb and p53 modulate the rates of radiation- induced apoptosis in vivo. Oncogene. 2001;20:3597–3608. doi: 10.1038/sj.onc.1204496. [DOI] [PubMed] [Google Scholar]
- 24.Liang L, Deng L, Mendonca MS, Chen Y, Zheng B, Stambrook PJ, Shao C, Tischfield JA. X-rays induce distinct patterns of somatic mutation in fetal versus adult hematopoietic cells. Repair (Amst) 2007 doi: 10.1016/j.dnarep.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]