Abstract
High Mφ:T cell ratios suppress the immune response to the retroviral superantigen Mls by IFNγ-triggered production of the arg- and trp-consuming enzymes iNOS and IDO. Attempts to reverse suppression by treatment with pro-inflammatory cytokines revealed that IL-6 improved the T cell response to Mls and the pro-hematopoietic cyokines IL-3 and GM-CSF increased suppression. GM-CSF treatment increased Mφ expression of CD80, a ligand for the immune suppressive B7H1 and CTLA-4 receptors. These results illustrate potential strategies for reversing the suppression of cell-mediated immunity characteristic of the high Mφ:T cell ratios found in many tumors.
Keywords: Cytokines, Macrophages, Suppression
Introduction
The interaction between antigen presenting cells (APCs) and T cells is central to the initiation of adaptive immunity. The cellular and humoral components that dictate the subsequent immune response continue to be intensively studied. Considerable research has focused upon the elements that promote T cell activation with growing appreciation for the role that APC type and differentiation state have in this process (Steinman, et al, 2003). Interest remains in understanding how APC:T cell interaction restrains immunity, not only during normal responses but also where essential T cell effector functions have been shut down (Alaniz et al., 2004; Serafini et al., 2005). Consumption of critical amino acids (arginine, trypophan), production of particular hormones (prostaglandin E2) or cytokines (IL-10), and generation of regulatory lymphocytes are suppression mechanisms attributed to APCs (Pollard, 2004; Bronte and Zanovello, 2005; Rabinovich, et al., 2007). Understanding how APCs regulate T cell function should further the development of novel immunotherapeutic strategies for both promoting and tempering immunity.
APC presentation of superantigens (SAgs) serves as a useful model to study the regulation of T cell function. SAgs are exogenous microbial proteins or gene products of retroviruses endogenous to the murine genome (eg., the minor lymphocyte stimulatory (Mls) gene product [Mls-1a] in DBA/2J mice) (Choi, et al., 1991) that trigger CD4+ T lymphocyte activation by crosslinking the TCR Vβ chain on TH cells with the Class II MHC molecule expressed by APCs (MacDonald et al., 1988). Although macrophages (Mφs), dendritic cells (DCs), and B cells present the Mls SAg, subsets of these APCs differ with respect to their ability to trigger T cell activation, anergy or deletion (Molina et al., 1989; Webb et al., 1989; Larsson-Sciard et al., 1990; Jarvis et al., 1994; Ardavin et al., 1996; Luther and Acha-Orbea, 1997; Riggs et al., 2004). In a survey of tissues capable of SAg presentation, nonlymphoid peritoneal cavity (PerC) cells were found to suppress the T cell response to Mls (Rosini et al., 2004). Subsequent research revealed that, in PerC cell cultures, high Mφ:T cell ratios triggered the expression of indoleamine 2,3-dioxygenase (IDO) and inducible nitric oxide synthase (iNOS), enzymes responsible for tryptophan and arginine catabolism (Matlack et al., 2006). Depletion of these amino acids inhibits T cell activation (Bronte and Zanovello, 2005). High Mφ:T cell ratios and amino acid catabolism are hallmarks of the immune suppressive microenvironment found in many tumors (Pollard, 2004; Drake et al., 2006; Rabinovich, et al., 2007). Since these conditions are readily reproduced in vitro one can assess strategies to circumvent the suppressive function of myeloid cells in preventing cellular immunity in cancer.
In this report experiments to test if cytokine treatment can alter Mφ suppression of T cell activation are described. The results are discussed in the context of developing strategies to reactivate the suppressed immunity found within tumors.
Materials and methods
Mice
Two to four month old male and female C.B-17.scid (SCID), BALB.xid (XID), and DBA/2J (Mls-1a) mice, bred and maintained at Rider University, were studied. All mice were handled in accord with NIH, Animal Welfare Act, and Rider University IACUC guidelines.
Preparation of cell suspensions
Lymph node (LN), and spleen (SP) cell suspensions were obtained by gentle disruption of the organ between the frosted ends of sterile glass slides. Peritoneal cavity (PerC) cells were obtained by flushing the peritoneum with 10 mls of warm (37°C) HBSS (Life Technologies Inc., Grand Island, NY) supplemented with 3% FBS (Hyclone, Logan, UT). RBCs were depleted by treatment by hypertonic lysis. Viable cell counts were determined by Trypan blue exclusion.
Cell culture
Responder (XID) lymph node T cells (4 × 106/ml) and various dilutions (0.5 – 4.0 × 106/ml) of stimulator (DBA/2J SP and/or PerC) cells, in RPMI 1640 culture media (Life Technologies Inc.) supplemented with 10% FCS (Hyclone), 0.1 mM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 2 mM L-glutamine, antibiotics, 2 × 10−5 M 2-ME, and 10mM HEPES, were incubated in a humidified atmosphere of 5% CO2 at 37°C in ½ area 96 well microtiter plates (Costar #3696, Cambridge, MA). Recombinant IFNγ, IL-1α, IL-1β, IL-3, IL-6, and TNFα (Peprotech, Rocky Hill, NJ) were reconstituted as described by the supplier. A neutralizing MAb to IFN-γ (eBioscience, San Diego, CA), 500 μM L-NMMA (1-MA, Calbiochem, San Diego, CA) or 1-MT (Sigma Aldrich, St. Louis, MO), or 20 μM indomethacin (Indo, Sigma Aldrich) were added at the initiation of culture. After 68 hrs, 1 μCi of [3H] thymidine (Amersham, Boston, MA) was added to each well. Microtiter plates were frozen 4 hrs after labeling, then thawed for analysis using a semi-automated cell harvester (Skatron Instruments, Richmond, VA). Radioactivity was measured by scintillation spectrometry. In each experiment, 4 or 5 microtiter wells were established for each test group. Experiments were done a minimum of 3 times.
Immunofluorescence staining and flow cytometric analyses
Cell suspensions stained for Mφ phenotyping were first blocked with rat anti-mouse CD16/32 (Fc Block, eBioscience, San Diego, CA) and 2% normal rat serum (Jackson ImmunoResearch, West Grove, PA). Fc-blocked cell suspensions then had titered amounts of FITC-labeled, rat anti-mouse F4/80 added concurrent with PE-labeled rat anti-mouse Class II MHC (clone M5), CD80 (B7.1), CD86 (B7.2), or CD274 (B7H1) and CY-labeled rat anti-mouse CD11b MAb (all MAbs from eBioscience, San Diego, CA). Isotype- and fluorochrome-matched, nonspecific MAb controls were employed to establish gates. Mφs were gated based on correlating their SSC properties and F4/80 expression via multiparameter flow cytometric analyses on a FACSCalibur™ flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) using CellQuest software.
Statistical analyses
T cell proliferative responses are presented as the average CPM ± SEM or as % of control ({average CPM of test group X 100}/average CPM for DBA/2J SP cells cultured with Mls-responsive LN T cells alone). Data sets were compared using the Student’s t-Test. Unless specified otherwise, p values below 0.05 (*) were considered significant.
Results
Macrophages suppress the T cell response to Mls via amino acid catabolism
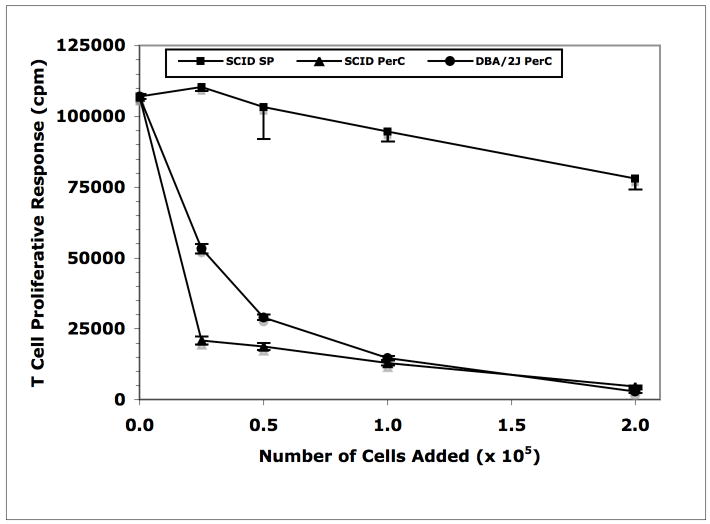
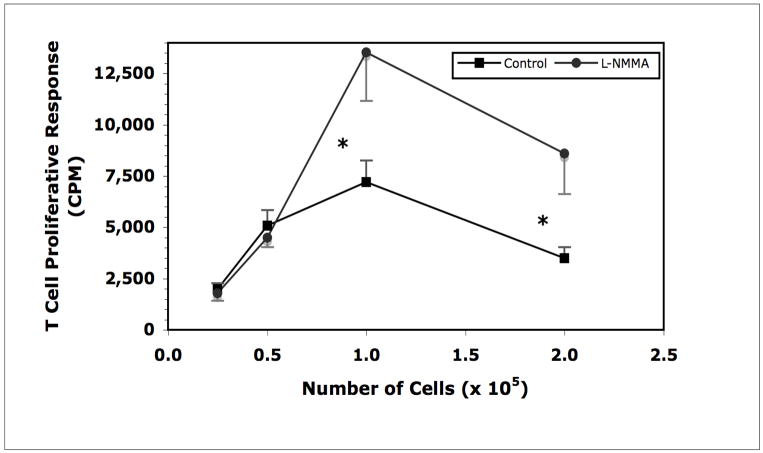
In prior studies of T cell activation triggered by Mls presentation we demonstrated that, compared with spleen (SP) cells, peritoneal cavity (PerC) cells were poor Mls APCs (Riggs et al., 2004). The PerC was found to be naturally enriched for suppressive Mφs as PerC cells from either wild-type (DBA/2J) or lymphocyte-deficient SCID mice suppressed the T cell response to Mls as presented by SP B cells (Rosini et al., 2004; Matlack et al., 2006; Figure 1). Inclusion of NG-monomethyl-L-arginine (L-NMMA), a competitive inhibitor of the arginine-consuming enzyme inducible nitric oxide synthase (iNOS), permitted Mls presentation by PerC cells (Figure 2). Thus, Mφs, via degradation of arginine, suppressed the T cell response to Mls.
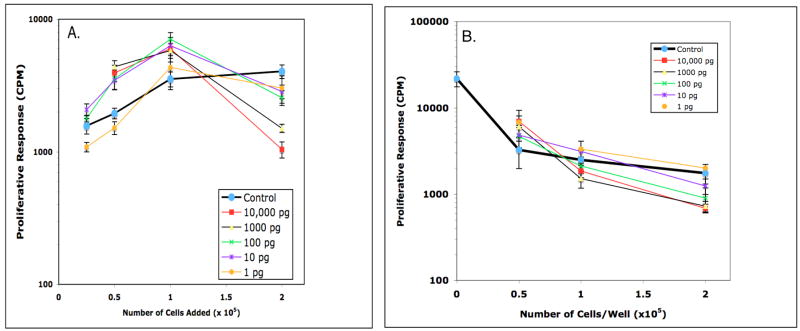
Figure 1.
Mls presentation by DBA/2J SP cells is inhibited by PerC macrophages.
Figure 2.
Mls presentation by DBA/2J PerC cells is enhanced by blocking iNOS.
* = p<.05 relative to control.
IFNγ production drives Mφ suppression of the Mls response
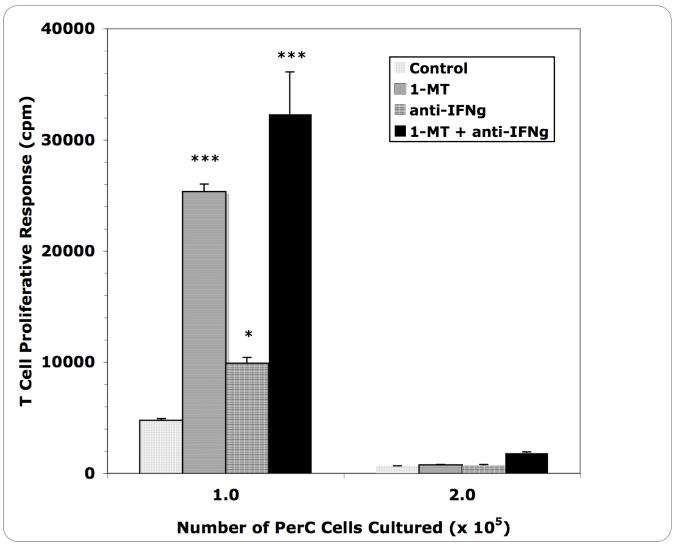
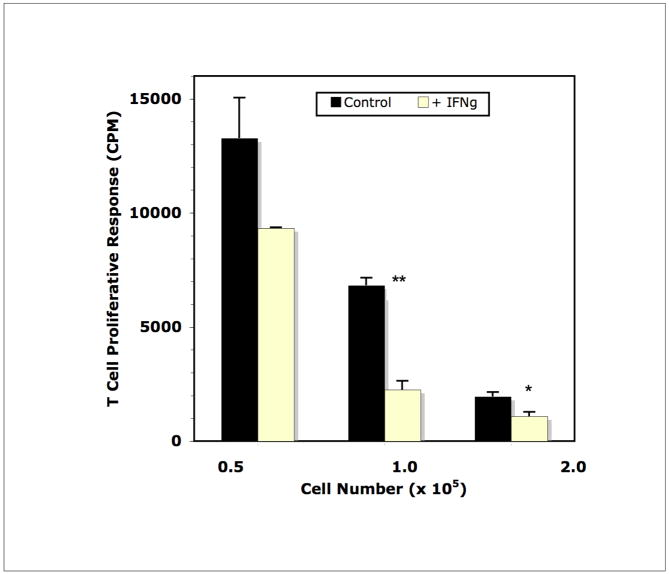
In studies of T cells activated by CD3 ligation, IFNγ drives iNOS expression leading to consumption of arginine and inhibition of T cell activation (Bronte and Zanovello, 2005; Matlack et al., 2006). The immune response to Mls is initiated by the activation of TH1 cells which are significant IFNγ producing cells (Gollob et al., 1993). The possibility that this pathway was responsible for Mφ suppression of the Mls response was tested. Addition of a neutralizing anti-IFNγ MAb at the initiation of culture led to partial recovery of the T cell response to Mls (Figure 3). This response was not as great as that seen with 1-methyl tryptophan (1-MT) treatment, a competitive inhibitor of 2,3 indoleamine dioxygenase (IDO) that is also triggered by IFNγ (Munn et al., 1999). The highest Mφ:T cell ratio did not respond to 1-MT or anti-IFNγ treatment. As anticipated from these results, the converse experiment, addition of IFNγ, increased suppression specifically in cultures with high Mφ density (Figure 4). The response triggered using SP cells, which have a normal (low) Mφ:T cell ratio, as Mls APCs was not suppressed by the addition of IFNγ (not shown). Thus, IFNγ-triggered suppression of the T cell response to Mls requires a high Mφ:T cell ratio.
Figure 3.
Blocking IFNγ and IDO permits Mls presentation by DBA/2J PerC cells.
* = p<.05; *** =p<.0005 relative to control.
Figure 4.
Addition of IFNγ, reduces Mls presentation by DBA/2J PerC cells. Addition of IFNγ to SP cell cultures had no effect on the response to Mls (control response = 46,837 ± 1599; experimental [with IFNγ] response = 53,517 ± 4269; p = .2571).
Ability of pro-inflammatory cytokines to reduce suppression of the Mls response
Aberrant Mφ:T cell ratios contribute to the immune suppression seen in many tumors (Pollard, 2004; Rabinovich, et al., 2007). Interest in advancing immunotherapy for cancer stimulated research of methods for negating Mφ-mediated suppression. Considering the apparent immaturity of PerC Mφs (Class II MHC−/lo, CD80−/lo, CD86−/lo [Lagasse and Weissman, 1996; Matlack et al., 2006]), we tested the ability of pro-inflammatory cytokines (IL-1, IL-6, TNFα) to promote PerC Mφ presentation of Mls. Treatment with IL-1α (and IL-β, not shown) and TNFα did not release T cells from suppression at high Mφ:T cell ratios. However, at more physiologic (lower) Mφ:T cell ratios IL-1α enhanced T cell responsiveness (Figs. 5 & 6, Panel A). Similar results were obtained when the cultures included SP cells as an enriched source of Mls APCs (Figs. 5 & 6, Panel B). In contrast to IL-1 and TNFα, IL-6 treatment enhanced Mls presentation by PerC cells and released the suppression seen when SP cells served as Mls APCs in cultures with high Mφ:T cell ratios (Fig. 7, Panels A & B).
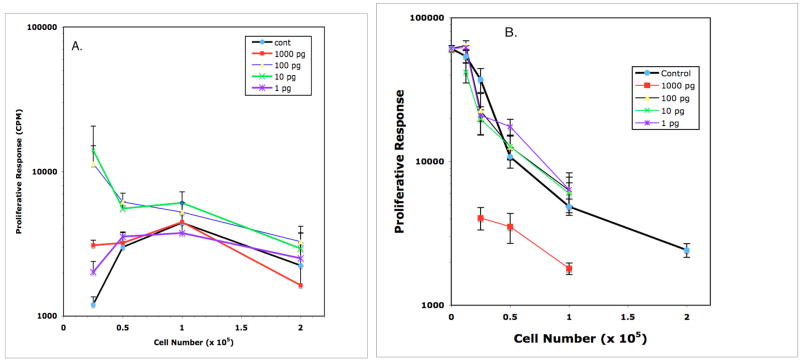
Figure 5.
Effect of IL-1α on Mls presentation by DBA/2J PerC cells (panel A) and PerC Mφ-suppressed DBA/2J SP cells (panel B). In Panel A, the T cell response to Mls was enhanced at 0.25 × 105 PerC cells/well by all concentrations of IL-1α tested and at 0.5 × 105 PerC cells/well by 10 and 100 pg/ml of IL-1α (p<.05).
Figure 6.
Effect of TNFα upon Mls presentation by DBA/2J PerC cells (panel A) and PerC Mφ-suppressed DBA/2J SP cells (panel B). In Panel A, the T cell response to Mls was enhanced at 0.25 × 105 cells/well by the addition of 100 and 1000 pg/ml of TNFα (p<.05). In Panel B, PerC Mφ suppression of SP cell Mls presentation was released by the addition of 1000 pg/ml of TNFα at 0.25 and 0.5 × 105 cells/well (p<.05).
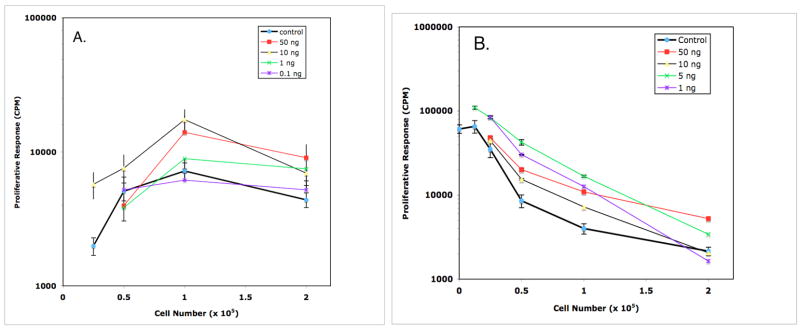
Figure 7.
IL-6 overrides macrophage suppression of Mls presentation by DBA/2J PerC (panel A) and SP (panel B) cells. In Panel A, PerC cell presentation of Mls was enhanced at: 0.25 × 105 cells/well by 10 ng/ml of IL-6; 1.0 × 105 cells/well by 10 and 50 ng/ml of IL-6; 2.0 × 105 cells/well by 1, 10, and 50 ng/ml of IL-6 (p<.05). In Panel B, PerC Mφ suppression of SP cell Mls presentation was released at: 0.25 × 105 cells/well by 1 and 5 ng/ml of IL-6; 0.5 × 105 cells/well by 1, 5, and 50 ng/ml of IL-6; 1.0 × 105 cells/well by 1, 5, 10, and 50 ng/ml of IL-6; 2.0 × 105 cells/well by 5 and 50 ng/ml of IL-6 (p<.05).
GM-CSF and IL-3 further suppress the Mls response
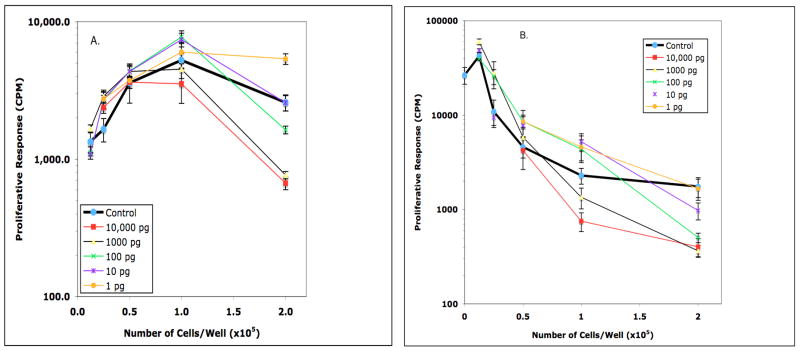
Pro-inflammatory TH1 cytokines such as IFNγ promote “classical” macrophage activation whereas TH2 cytokines such as IL-4 and IL-13 promote “alternative” Mφ activation (Mosmann and Coffman, 1989; Mosser and Edwards, 2008; Martinez, et al, 2009). However, as noted above with IFNγ, such generalizations may not be true for T cells in Mφ-dense microenvironments. The marked production of IL-3 and GM-CSF following the activation of naïve CD4+ T cells fostered interest in determining the impact of these cytokines upon Mφ suppression of the T cell response to Mls (Rochford et al., 2004). At high Mφ:T cell ratios both GM-CSF and IL-3 further suppressed T cell activation (Figs. 8 & 9, Panels A & B). GM-CSF was more suppressive and, unlike IL-3, maintained this effect at lower Mφ:T cell ratios. Neither cytokine suppressed the Mls response in cultures with a physiologically normal Mφ:T cell ratio, ie., SP cells cultured alone with Mls-responsive T cells (not shown). Thus, exposure to cytokines made early in the initiation of adaptive immunity failed to reverse the suppression of T cell activation observed at high Mφ:T cell ratios.
Figure 8.
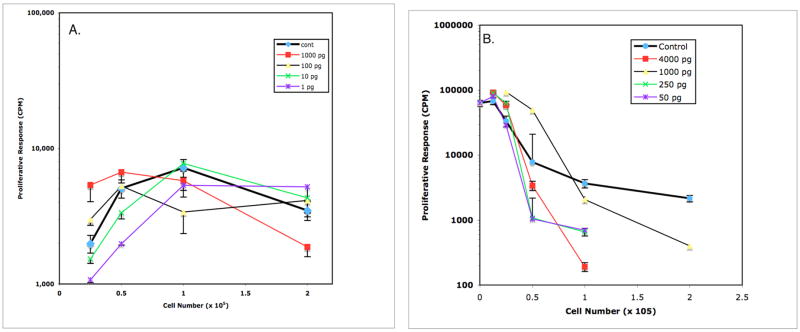
Effect of IL-3 on Mls presentation by DBA/2J PerC cells (panel A) and PerC Mφ-suppressed DBA/2J SP cells (panel B). In Panel A, the T cell response to Mls presented by PerC cells was further reduced at: 2.0 × 105 cells/well by 100, 1000, and 10,000 pg/ml of IL-3. PerC cell presentation of Mls was enhanced at: 1.0 × 105 cells/well by 10, 100, and 1000 pg/ml of IL-3; 0.5 × 105 cells/well by 10, 100, 1000, and 10,000 pg/ml of IL-3. In Panel B, the inhibition of Mls presentation by SP cells was further reduced at 2.0 × 105 cells/well by 1000 pg/ml of IL-3 (p<.05).
Figure 9.
Effect of GM-CSF on Mls presentation by DBA/2J PerC cells (panel A) and PerC Mφ-suppressed DBA/2J SP cells (panel B). In Panel A, PerC cell presentation of Mls was further suppressed at 2.0 × 105 cells/well by 100, 1000, and 10,000 pg/ml of GM-CSF. PerC cell presentation of Mls was enhanced at: 2.0 × 105 cells/well with 1 pg/ml of GM-CSF; 1.0 × 105 cells/well with 10 and 100 pg/ml of GM-CSF; 0.25 × 105 cells/well with 1000 pg/ml of GM-CSF. In Panel B, PerC Mφ suppression of the Mls response stimulated by DBA/2J SP cells was increased at: 2.0 × 105 cells/well by 100, 1000, and 10,000 pg/ml of GM-CSF. Enhancement of Mls presentation was observed at 1.0 × 105 cells/well with 10 pg/ml of GM-CSF and at 0.25 and 0.5 × 105 cells/well with 100 pg/ml of GM-CSF (p<.05).
GM-CSF and IL-6 effects not altered by inhibition of iNOS, IDO, or COX
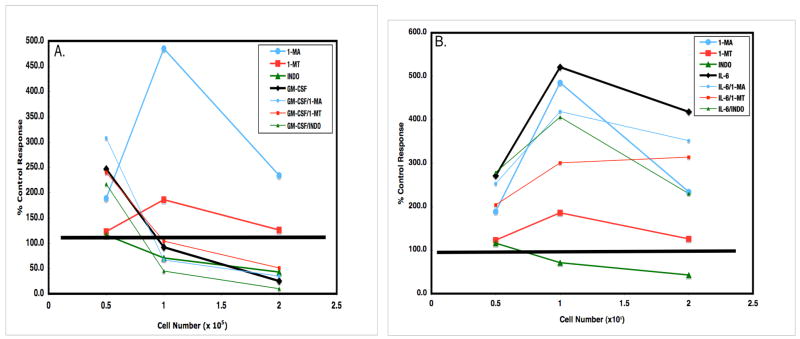
Strategies to recover T cell activation in the tumor microenvironment will likely require a multi-faceted approach (Pollard, 2004). Blocking amino acid catabolism concurrent with cytokine modulation is potentially one such option. However, in the Mls model described herein, addition of L-NMMA (1-MA), 1-MT, or the cyclooxygenase inhibitor indomethacin (Indo) failed to reduce the effects of GM-CSF (Fig 10, Panel A). Although the addition of IL-6 increased the Mls response beyond that seen with these inhibitors acting alone, none of these combinations surpassed the effect of IL-6 alone (Fig 10, Panel B). These data illustrate that these cytokine effects are not complimented by inhibition of prostaglandin production or amino acid catabolism.
Figure 10.
Inhibition of iNOS or IDO fails to negate GM-CSF-triggered suppression (panel A) and does not surpass IL-6-mediated release (panel B). In Panel A, PerC cell presentation of Mls that is further suppressed by 10 ng/ml of GM-CSF at high Mφ:T cell ratios was not recovered by blocking iNOS (1-MA), IDO (1-MT), or COX (Indo). In Panel B, addition of 50 ng/ml of IL-6 afforded the greatest recovery of the Mls response and increased the ability of 1-MA, 1-MT, and indo to recover T cell proliferation.
Increased CD80 expression by Mφs treated with GM-CSF
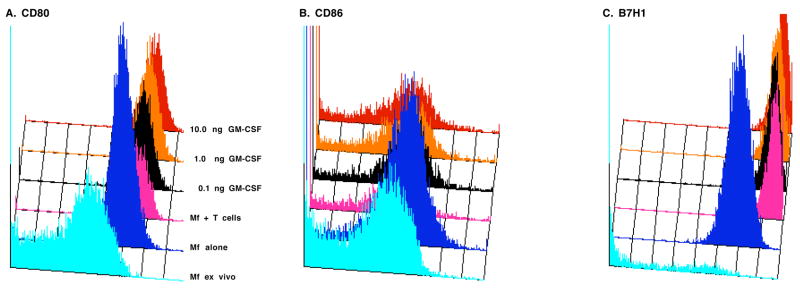
The T cell response to superantigen depends upon TCR binding of the Class II MHC/Mls complex (signal 1) and B7 binding of CD28 (signal 2) (MacDonald et al., 1988; Yeh et al., 2007). Costimulation, most often initiated by CD28-CD86 (B7.2) interaction, also triggers a counteracting, suppressive response mediated by CD152 (CTLA-4)-CD80 (B7.1) interaction (Collins et al., 2002; Pentcheva-Hoang et al., 2004). Furthermore, a new B7 family member, B7H1 (PDL1 or CD274), has been found to have negative regulatory effects (Lohr et al., 2003; Chen, 2004). To determine how high Mφ:T cell ratios and cytokine treatment impact expression of B7 family members, flow cytometric analyses of cultured Mφs was conducted. Although the majority of F4/80+ cells naturally residing in the PerC did not express these molecules ex vivo (Matlack et al., 2006), after 48 hrs of culture all Mφs cultured alone expressed CD80 and B7H1, most expressed CD86, and half expressed Class II; all of these molecules were expressed at significantly higher levels after culture (Table 1). Co-culture of Mφs with Mls-responsive T cells decreased Class II MHC expression and increased the expression of all B7 family members, particularly CD80 and B7H1. The addition of cytokines led to several notable changes. IL-6 increased, and IL-3 and GM-CSF decreased, the percentage of Class II+ Mφs. IL-3 and GM-CSF further increased Class II and CD80 expression (Table 1). Graded exposure to GM-CSF revealed a dose-dependent increase in the expression of all B7 family members, particularly CD80 (Fig 11, Table 2). CD80 expression also increased on B cells in GM-CSF-treated cultures but never surpassed the level of CD86 as routinely seen with Mφs (not shown). These results reveal that cytokine exposure leads to significant changes in the expression of molecules essential for the regulation of T cell activation.
Table 1.
Expression of Class II MHC and B7 Family Members by Macrophages
| Class II |
B7.1 |
B7.2 |
B7H1 |
|||||
|---|---|---|---|---|---|---|---|---|
| Condition |
% cellsa |
MFIb |
% cells |
MFI |
% cells |
MFI |
% cells |
MFI |
| Ex vivo | 4 | 1664 | 20 | 197 | 22 | 218 | 3 | 480 |
| Cultured Cellsc | ||||||||
| Mφs Alone | 52 | 4073 | 96 | 452 | 70 | 348 | 100 | 1353 |
| Mφs + LN T cells | 52 | 2195 | 100 | 825 | 56 | 416 | 100 | 6222 |
| “ + IL-1 | 53 | 2188 | 100 | 869 | 54 | 435 | 100 | 6596 |
| “ + IL-6 | 61 | 2272 | 100 | 807 | 62 | 410 | 100 | 6175 |
| “ + TNFα | 54 | 2254 | 99 | 749 | 64 | 438 | 100 | 5501 |
| “ + IL-3 | 39 | 2421 | 100 | 1031 | 73 | 425 | 100 | 5091 |
| “ + GM-CSF | 37 | 2471 | 100 | 1233 | 70 | 460 | 100 | 5613 |
Percentage of F4/80+/SSC-defined cells bearing the marker listed.
Mean Fluorescence Intensity.
PerC Mφs cultured at high Mφ:T cell ratio as described in Materials and Methods.
Data are representative of 3 experiments conducted.
Figure 11.
GM-CSF increases the expression of CD80 and B7H1. Resident (ex vivo) or cultured Per C cells were stained for co-expression of F4/80 and CD80 (Panel A), CD86 (Panel B), and B7H1 (Panel C) and analyzed as described in Methods.
Table 2.
B7 Family Member Expression by GM-CSF-Treated Macrophages
| B7.1 |
B7.2 |
B7H1 |
||||
|---|---|---|---|---|---|---|
| Condition | % cellsa | MFIb | % cells | MFI | % cells | MFI |
| Mφs Alone | 96 | 383 | 65 | 324 | 100 | 1168 |
| Mφs + LN T cells | 98 | 648 | 39 | 403 | 100 | 5629 |
| “ + 10 ng/ml GM-CSF | 99 | 989 | 38 | 451 | 100 | 5351 |
| “ + 1.0 ng/ml GM-CSF | 99 | 835 | 43 | 412 | 100 | 4956 |
| “ + 0.1 ng/ml GM-CSF | 99 | 747 | 51 | 385 | 100 | 4828 |
Data are representative of 3 experiments conducted.
Discussion
In this study high Mφ:T ratios were shown to inhibit the T cell response to the retroviral superantigen Mls. This form of suppression required IFNγ to trigger the arg- and trp-consuming enzymes iNOS and IDO as competitive inhibition of these enzymes with substrate analogs recovered the T cell response. In attempts to recover the T cell response, the Mφ:T cell ratio dictated the outcome of addition of exogenous cytokine. Of the three major pro-inflammatory cytokines tested only IL-6 increased T cell activation at high Mφ:T cell ratios. The pro-hematopoietic cytokines IL-3 and GM-CSF, normally produced early during the initiation of adaptive immunity, increased suppression at high Mφ:T cell ratios. Flow cytometric analysis of B7 family member expression by Mφs in cytokine-treated cultures revealed marked up-regulation of CD80, particularly after the addition of GM-CSF. Collectively these data illustrate that the Mφ:T cell ratio is critical in adaptive immunity in defining the type and quality of the ensuing response.
Mφs are a key component of immunity, widely distributed throughout the body in small numbers where they primarily function in an anti-inflammatory, housekeeping role removing apoptotic corpses and ingesting and processing antigen for delivery to regional lymphatic tissue (Gordon and Taylor, 2005; Miyake et al., 2007; Mosser and Edwards, 2008). The early phase of the adaptive immune response in organized lymphoid tissue reflects a low Mφ:T cell ratio that fosters T cell expansion (Rossner et al., 2005; Hopken et al., 2005). In contrast, the pathological aggregations of leukocytes found in tumors and certain infections, reflect how high Mφ:T cell ratios impact T cell biology and temper immunity (Goni, et al., 2002; Mencacci et al., 2002; Alaniz et al., 2004; Pollard, 2004; Shimizu, et al., 2004; Rabinovich, et al., 2007). As Mφs and TH cells are the major cytokine-producing cells stringent control mechanisms have evolved to limit their interaction and activation. Aberrant Mφ:T cell ratios likely alter normal receptor-ligand stoichiometry triggering signaling pathways that lead to immune suppression. Considering the role of this altered immunobiology in many chronic inflammatory disease processes it is imperative to seek strategies to disrupt this network and restore functional immunity (Balkwill, et al., 2005; Bunt et al., 2006).
APC maturity is an important factor in costimulating T cell activation (Steinman, et al., 2003). Based on phenotype (Lagasse and Weissman, 1996; Matlack et al., 2006), PerC Mφs appear immature and exposure to pro-inflammatory cytokines was expected to enhance their function as APCs. However, both IL-1α and TNFα failed to recover the Mls response at high Mφ:T cell ratios. In contrast, IL-6 increased T cell activation at all Mφ:T cell ratios. There are several possibilities as to how IL-6 might rescue this response. IL-6 has been shown to override the immunosuppressive effects of IFNγ-triggered IDO production by dendritic cells (DCs) (Grohman et al., 2001; Orabona et al., 2004). IL-6 has also been shown to negate the apoptosis of T cells triggered by mesenchymal stem cells (Grohman et al., 2001; Xu et al., 2007). These observations are particularly relevant considering the shared physical and functional phenotypic characteristics of mesenchymal stem cells, tolerogenic DCs, myeloid suppressor cells (MSCs) and the PerC Mφs described in this report and prior studies (Bronte and Zanovello, 2005; Matlack et al., 2006). Also, the generation of immunosuppressive regulatory T cells (Tregs) under Mφ-dense conditions is reversed by IL-6, which instead fosters development of pro-inflammatory TH17 cells (Fallarino et al., 2003; Pasare et al., 2003; Wan et al., 2007). These hypotheses are not mutually exclusive as Tregs trigger increased IDO expression by APCs (Fallarino et al., 2003). The variety of myeloid cell types that exhibit these regulatory properties led to the study of cytokines responsible for myeloid differentiation.
The pro-myelopoietic cytokines lL-3 and GM-CSF are among the major immunoregulatory proteins produced by TH1 cells shortly after TCR ligation (Rochford, et al., 2004; Mossman and Coffman, 1989). Both of these molecules increased suppression at high Mφ:T cell ratios consistent with evidence that responses skewed towards a TH1 phenotype alter Mφ maturation and promote suppression (Nonaka et al., 2008). GM-CSF, routinely employed in vitro to generate APCs from bone marrow precursors, has both pro-inflammatory and immunosuppressive properties and appears essential for generating MSCs (Fu et al., 1991; Apolloni et al., 2000; Serafini et al., 2004; Rossner et al., 2005). IL-3 has been shown to enhance Mφ–mediated suppression by increasing production of PGE2 (Kuroda et al., 2007). Although IL-3 and GM-CSF bind receptors with an identical signaling subunit they differ in function and exhibited differences in this study, notably the ability of IL-3 to increase the Mls response at lower Mφ:T cell ratios. Excepting IL-6, all of the cytokines tested in this system only enhance the T cell response at low Mφ:T cell ratios (Rosini et al., 2004). This suggests that a cytoreduction strategy focused upon reducing Mφ number would be essential for cytokine therapy to reverse the immune suppression found in tumor microenvironments.
An alternative, or perhaps complementary, strategy to cytokine treatment might include modulation of the receptor-ligand combinations that promote and maintain suppression (Korman, et al., 2006; Rabinovich, et al., 2007). This concept becomes particularly attractive considering how the convergence of Mφ density and receptor-ligand expression levels could alter both the polarity of immunological synapse formation and the avidity of such molecular interactions. In this regard, the increased expression of CD80 and B7H1 by Mφs reported herein is significant considering that both molecules are also expressed by T cells, that they have very high affinity for each other, and that their binding promotes T cell suppression (Yamazaki et al., 2002; Butte et al., 2007). Considering that CD80 also binds with high affinity to CTLA-4 to suppress T cells, focus upon this molecule might be prudent (Collins et al., 2002; Pentchova-Hoang et al., 2004). There is evidence of a role for CD80 in the generation of Tregs and in cancer (Yang et al., 2006; Perez et al., 2008). The demonstrated efficacy of modulating B7 family members and their ligands bode well for this therapeutic approach (Curiel et al., 2003; Waldmann, 2006; Zou and Chen, 2008).
The immune suppression witnessed in tumors may result from a microenvironment in which APCs encounter antigen in a non- or “smoldering” inflammatory context (Balkwill, et al., 2005; Rabinovich, et al., 2007). The “full-blown” inflammation that leads to adaptive immunity is the outcome of a specific spatiotemporal sequence of events. TLR ligation, antigen processing and presentation, and cognate interaction with T cells in organized lymphoid tissue with physiologic APC:T cell ratios, are essential elements of APC initiation of immunity. The tumor microenvironment subverts this highly evolved organization. Culture systems that model the high Mφ:T cell ratios found in tumors will serve in the development of immunotherapies to activate the dormant immunity characteristic of cancer.
Acknowledgments
This work was supported by a grant to J. Riggs from the NIH AREA program (R15 AI 060356-01). D. Silberman was supported by fellowships from the New Jersey Commission for Cancer Research and the Rider University Marvin Talmadge Memorial Research Fund. We are grateful to A. Sepulveda, S. Wisniewski, and M. Orlowski for mouse husbandry.
Abbreviations
- APC
antigen presenting cell
- IDO
indoleamine 2,3-dioxygenase
- iNOS
inducible nitric oxide synthase
- LN
lymph node
- Mφ
Macrophage
- Mls
minor lymphocyte stimulatory
- MSC
myeloid suppressor cell
- PerC
peritoneal cavity
- SAg
superantigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaniz R, Sandall S, Thomas E, Wilson C. Increased dendritic cell numbers impair protective immunity to intracellular bacteria despite augmenting antigen-specific CD8+ T lymphocyte responses. J Immunol. 2004;172:3725–3735. doi: 10.4049/jimmunol.172.6.3725. [DOI] [PubMed] [Google Scholar]
- Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, Segal D, Young H, Zanovello P. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J Immunol. 2000;165:6723–6730. doi: 10.4049/jimmunol.165.12.6723. [DOI] [PubMed] [Google Scholar]
- Ardavin C, Waanders G, Ferrero I, Anjuere F, Acha-Orbea H, MacDonald H. Expression and presentation of endogenous mouse mammary tumor virus superantigens by thymic and splenic dendritic cells and B cells. J Immunol. 1996;157:2789–2794. [PubMed] [Google Scholar]
- Balkwill F, Charles K, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Bunt S, Sinha P, Clements V, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- Butte M, Keir M, Phamduy T, Sharpe A, Freeman G. Programmed death-ligand 1 interacts specifically with B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–350. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Choi Y, Kappler J, Marrack P. A superantigen encoded in the open reading frame of the 3′ long terminal repeat of mouse mammary tumor virus superantigen. Nature. 1991;350:203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Collins A, Brodie D, Gilbert R, Laboni A, Manso-Sancho R, Walse B, Stuart D, Anton van der Merwe P, Davis S. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- Curiel T, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson K, Daniel B, Zimmerman M, David O, Burrow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez R, Emilie D, Curiel D, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;5:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- Drake C, Jaffee E, Pardoll D. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang K, Orabona C, Vacca C, Bianchi R, Belladonna M, Fioretti M, Alegre M, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Fu Y-X, Watson G, Kasahara M, Lopez D. The role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. I Induction of regulatory macrophages in normal mice by the in vivo administration of rGM-CSF. J Immunol. 1991;146:783–789. [PubMed] [Google Scholar]
- Gollob K, Nagelkerken L, Coffman R. Endogenous retroviral superantigen presentation by B cells induces the development of type 1 CD4+ T helper lymphocytes. Eur J Immunol. 1993;23:2365–2371. doi: 10.1002/eji.1830231028. [DOI] [PubMed] [Google Scholar]
- Goni O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+)CD11b(+)) immature myeloid suppressor cells. Int Immunol. 2002;14:1125–1134. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor P. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Fallarino F, Bianchi R, Belladonna M, Vacca C, Orabona C, Uyttenhove C, Fioretti M, Puccetti P. IL-6 inhibits the tolerogenic function of CD8α dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- Hopken U, Lehmann I, Droese J, Lipp M, Schuler T, Rehm A. The ratio between dendritic cells and T cells determines the outcome of their encounter: Proliferation versus deletion. Eur J Immunol. 2005;35:2851–2863. doi: 10.1002/eji.200526298. [DOI] [PubMed] [Google Scholar]
- Jarvis C, Germain R, Hager G, Damschroder M, Matis L. Tissue-specific expression of messenger RNAs encoding endogenous viral superantigens. J Immunol. 1994;152:1032–1038. [PubMed] [Google Scholar]
- Korman A, Peggs K, Alllison J. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda E, Noguchi J, Doi T, Uematsu S, Akira S, Yamashita U. IL-3 is an important differentiation factor for the development of prostaglandin E2-producing macrophages between C57BL/6 and BALB/c mice. Eur J Immunol. 2007;37:2185–2195. doi: 10.1002/eji.200737041. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Weissman I. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Meth. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Larsson-Sciard F, Spetz-Hagberg A, Casrouge A, Kourilsky P. Analysis of T cell receptor Vβ useage in primary mixed lymphocyte reactions: Evidence for directive useage by different antigen-presenting cells and Mls-like determinants on T cell blasts. Eur J Immunol. 1990;20:1223–1229. doi: 10.1002/eji.1830200605. [DOI] [PubMed] [Google Scholar]
- Lohr J, Knoechel B, Jiang S, Sharpe A, Abbas A. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol. 2003;4:664–669. doi: 10.1038/ni939. [DOI] [PubMed] [Google Scholar]
- Luther S, Acha-Orbea H. Mouse mammary tumor virus: immunological interplays between virus and host. Adv Immunol. 1997;65:139–243. [PubMed] [Google Scholar]
- MacDonald H, Schneider R, Lees R, Howe R, Acha-Orbea H, Festenstein H, Zinkernagel R, Hengartner H. T-cell receptor Vβ use predicts reactivity and tolerance to Mls-1a encoded antigens. Nature. 1988;332:40–54. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunological perspective. Ann Rev Immunol. 2008 Dec 23; doi: 10.1146/annurev.immunol.021908.132532. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Matlack R, Yeh K, Rosini L, Gonzalez D, Taylor J, Silberman D, Pennello A, Riggs J. Peritoneal macrophages suppress T cell activation by amino acid catabolism. Immunology. 2006;117:386–395. doi: 10.1111/j.1365-2567.2005.02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencacci A, Montagnoli C, Bacci A, Cenci E, Pitzurra L, Spreca A, Kopf A, Sharp A, Romani L. CD80+ Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J Immunol. 2002;169:3180–3190. doi: 10.4049/jimmunol.169.6.3180. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina I, Cannon N, Hyman R, Huber B. Macrophages and T cells do not express Mlsa determinants. J Immunol. 1989;143:39–43. [PubMed] [Google Scholar]
- Mosmann T, Coffman R. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosser D, Edwards J. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D, Shafizadeh E, Attwood J, Bondarev I, Pashine A, Mellor A. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka K, Saoi M, Suwa T, Frey A, Umemura N, Imai H, Ouyang G, Osada S, Balazs M, Adany R, Kawaguchi Y, Yoshida K, Takami T. Skewing the Th cell phenotype toward Th1 alters the maturation of tumor-infiltrating mononuclear phagocytes. J Leuk Biol. 2008;84:679–688. doi: 10.1189/jlb.1107729. [DOI] [PubMed] [Google Scholar]
- Orabona C, Grohmann U, Belladonna M, Fallarin F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon B, Fioretti M, Romani L, Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pentcheva-Hoang T, Egen J, Wojnoonski K, Allison J. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Perez N, Karumuthil-Melethil S, Li R, Prabhakar B, Holterman M, Vasu C. Preferential costimulation by CD80 results in IL-10-dependent TGF-β1+-adaptive regulatory T cell generation. J Immunol. 2008;180:6566–6576. doi: 10.4049/jimmunol.180.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. Tumour-educated macrophages promote tumour progression and metastasis. Nat Revs Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Rabinovich G, Gabrilovich D, Sotomayor E. Immunosuppressive strategies that are mediated by tumor cells. Ann Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs J, Howell K, Taylor J, Mahjied T, Prokopenko N, Alvarez J, Coleman C. Mls presentation by peritoneal cavity B cells. Immunobiol. 2004;209:255–264. doi: 10.1016/j.imbio.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Rochford R, Riggs J, Clavo A, Ernst D, Hobbs M. Differential effects of CD28 costimulation upon cytokine production by CD4+ and CD8+ T cells. Immunobiol. 2004;209:513–522. doi: 10.1016/j.imbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Rosini L, Matlack R, Taylor J, Howell K, Yeh K, Pennello A, Riggs J. Nonlymphoid peritoneal cells suppress the T cell response to Mls. Immunobiol. 2004;209:575–584. doi: 10.1016/j.imbio.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rossner S, Voigtlander C, Wiethe C, Hanig J, Seifarth C, Lutz M. Myeloid dendritic cell precursors generated from bone marrow suppresss T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533–3544. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Sem Cancer Biol. 2005;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan K, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Sano C, Tomioka H. The role of B7 molecules in the cell contact-mediated suppression of T cell mitogenesis in immunosuppressive macrophages induced with mycobacterial infection. Clin Exp Immunol. 2004;35:373–379. doi: 10.1111/j.1365-2249.2004.02403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R, Hawiger D, Nussenzweig M. Tolerogenic dendritic cells. Ann Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Waldmann T. Effective cancer therapy through immunodulation. Ann Rev Med. 2006;57:65–81. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- Wan W, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- Webb S, Okamoto A, Ron Y, Sprent J. Restricted tissue distribution of Mls-a determinants. Stimulation of Mls-a-reactive T cells by B cells but not by dendritic cells or macrophages. J Exp Med. 1989;169:1–12. doi: 10.1084/jem.169.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Comm. 2007;361:745–750. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll D, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- Yang R, Cai Z, Zhang Y, Yutzy W, Roby K, Roden R. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- Yeh K, Silberman D, Gonzalez D, Riggs J. Complementary suppression of T cell activation by peritoneal macrophages and CTLA-4-Ig. Immunobiol. 2007;212:1–10. doi: 10.1016/j.imbio.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]