Abstract
The ability to elicit cross-neutralizing antibodies makes human papillomavirus (HPV) L2 capsid protein a possible HPV vaccine. We examined and compared the humoral response of mice immunised with a HPV-16 L2 DNA vaccine or with HPV-16 L2 protein. The L2 DNA vaccine elicited a non-neutralising antibody response unlike the L2 protein. L2 DNA vaccination suppressed the growth of L2-expressing C3 tumor cells, which is a T cell mediated effect, demonstrating that the lack of non-neutralizing antibody induction by L2 DNA was not caused by lack of T cell immunogenicity of the construct.
INTRODUCTION
Cancer of the cervix is the second most prevalent cancer in women worldwide, with HPV-16 being the most prevalent high-risk HPV type associated with cervical cancer [1] Gardasil, the quadrivalent and Cervarix the bivalent prophylactic HPV vaccines are based on L1, the major capsid protein, and protect against four or two HPV genotypes, but will not provide complete protection against all HPV types as the protection is primarily type specific. Therefore a single antigen vaccine that protects against multiple HPV types would be a good alternative to develop.
L2, the minor capsid protein, has been shown to have a cross-type neutralising epitope [2] and a L2 DNA vaccine may be an acceptable approach for a candidate vaccine. Many factors including safety, stability and low cost make the choice of a DNA vaccine attractive for use in developing countries. A number of HPV-specific DNA vaccines have been designed, with the focus mainly on therapeutic vaccines using the early genes E6, E7 and E2 [3], [4].
In this study we constructed a HPV-16 L2 DNA vaccine (L2 DNA) using the pTH vector [5], immunised mice and investigated the induction of L2 antibody titres and also T cell responses by analyzing protection against tumor formation by C3 tumor cells, a cell line containing the entire HPV-16 genome and growth of which is controlled by T cells [6].
METHODS AND RESULTS
Vaccines
HPV-16 L1 VLP prepared in insect cells were isolated as described [7]. The HPV-16 L2 gene [8] was cloned into the expression vector pProEx ™ and L2 protein was expressed and purified from E. coli. The human codon-optimised HPV-16 L2h gene (EMBL accession AJ313180) was cloned into the mammalian expression vector pTH [5]. Endotoxin-free L2 DNA was prepared with Qiagen Endofree-Giga-kits. Western blotting showed L2 expression in L2 DNA transfected HEK-293 cells (Fig 1). The presence of the L2 gene in the C3 tumor cell line was confirmed by PCR using internal primers and primers that amplify the whole gene (Fig 2A). Western blotting of C3 cell lysates confirmed L2 protein content at low levels. (Fig 2B). This result is a new observation.
Figure 1.

Expression of L2 protein in L2 DNA transfected HEK 293 cells. Transfected cells were lysed and L2 protein detected by Western blot using a rabbit anti-HPV16 L2 antibody. Lane 1: L2 protein; Lane 2: pTH transfected cells; Lane 3,4,5: 24, 48 and 72h L2 DNA transfected cells respectively
Figure 2.
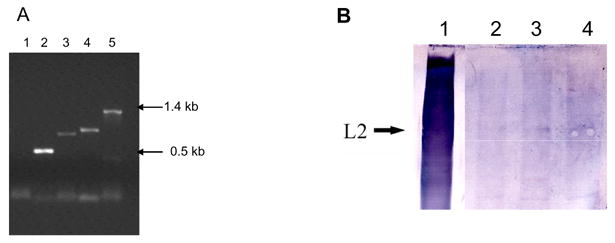
HPV-16 L2 in C3 cells: (A) DNA was isolated from C3 tumor cells and the L2 gene was amplified using different primer pairs. Lane 1 negative control, Lane 2, 3, and 4, amplified L2 fragments of 509 bp, 802 and 885 bp, respectively, Lane 5 amplification of the L2 gene (1.4kb). (B) C3 cells were lysed and run on a PAGE gel, blotted, and probed with L2 antibody. Lane 1 L2 expressed in E. coli, Lanes 2–4 increasing amounts of 5, 10 and 15 μl of cell lysate was run.
Immunisation and tumor challenge of mice
Eight week-old female C57BL/6 mice (6 per group) were inoculated on day 0 and day 28 with HPV-16 VLP (10 μg/mouse, sc), L2 protein (50 μg/mouse, sc), L2 DNA (im), 1 μg/mouse, 10 μg/mouse and 100 μg/mouse and the DNA vector pTH (100 μg/mouse). All mice were bled before immunisation and at the end of the experiment (6 weeks post tumor challenge). The animals were challenged by sc injection of 0.5 × 106 C3 tumor cells 2 weeks after the second vaccination. Tumor size was measured every week until tumor size exceeded 1.5 cm3 or 6 weeks post challenge and volume calculated as: (length × width2)/2. All animal procedures were passed by the University of Cape Town Faculty of Health Sciences Animal Ethics Committee.
Antibodies in response to HPV-16 L2 DNA vaccination
L2-specific antibodies were assessed using sera from immunized mice to probe L2 protein by western blotting. Sera collected at the end of the experiment from mice immunised with the L2 protein reacted positively with an L2 band at dilutions of 1:5000 or higher. In comparison, sera from mice immunised with 100 μg L2 DNA reacted with the L2 band only at 1:50 dilution, suggesting the L2-specific antibody titres elicited by the L2 DNA vaccine were 100-fold lower than those elicited by L2 protein immunisation (data not shown).
HPV-16-specific antibody neutralisation of HPV-16 Pseudovirions
Serum from mice immunised with the L2 DNA vaccine did not neutralise HPV-16 pseudovirions in a HPV-16 neutralisation assay [9] in contrast to sera (1:200 dilution) from mice immunised with the L2 protein which inhibited entry of pseudovirions in 293TT cells (data not shown).
L2 DNA vaccine is immunogenic
Immunisation with L2 DNA vaccine (irrespective of dose) reduced average volumes of L2 expressing tumor cells 2.5-fold compared with those receiving the empty vector at day 34 post tumor challenge (Fig 3). Tumor volume in HPV-16 VLP vaccinated mice, the positive control for T cell mediated responses[6] was 13 fold less than that induced in mice vaccinated with the pTH vector (p=0.02; Mann-Whitney U Test). This indicates that the L2 DNA vaccine is immunogenic as it protects against a tumor cell line whose growth is controlled by T cells [6].
Figure 3.
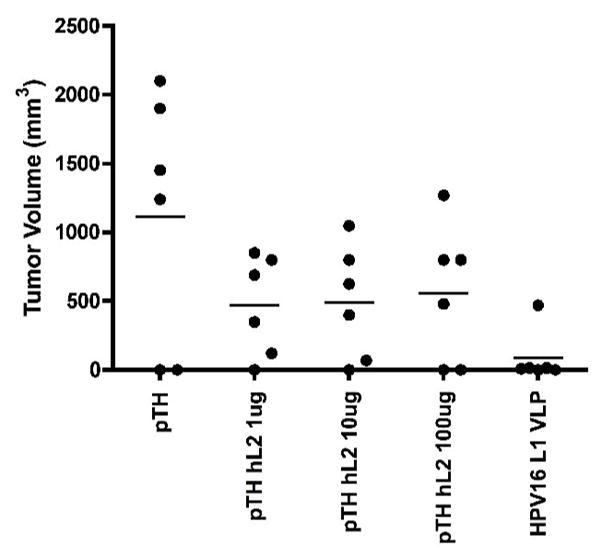
Tumor volume at 34 days post challenge with C3 tumor cells two weeks after vaccination on day 0 and day 28 with the indicated vaccines.
DISCUSSION
In the current study we investigated whether antibodies raised against L2 after immunisation with DNA vaccines were able to neutralise virus, and whether DNA vaccines based on L2 genes have the capacity to induce T cell responses by challenging mice with tumor-forming cells containing the whole HPV-16 genome.
Our preliminary work showed that HPV-16 L2 expressed in E. coli resulted in an insoluble product (Hitzeroth, et al, unpublished) affecting folding and/or exposure of correct L2 epitopes to the immune system. We hypothesized that expressing the protein in animal cells by means of a DNA vaccine might mimic in vivo L2 exposure to the immune system, and achieve authentic folding and presentation of the cross-neutralising L2 antibody epitope(s).
We generated the DNA vaccine L2 DNA using the pTH vector [5] already used in human trials of HIV DNA vaccines, and investigated its immunogenic potential. Although L2 DNA was expressed in mammalian cells when injected into mice only weak antibody responses were detected, and these antibodies did not have neutralising activity. In animals vaccinated with L2 DNA, the tumor volume was reduced to about 50% at all three doses of DNA compared to animals vaccinated with empty vector alone. Although this was only a trend, it indicates the immunogenic potential of this construct. L1 VLP gave the best protection against tumor challenge in our experiments. These results corroborate previous results where a single dose of L1/L2 VLP was enough for tumor protection. [6]. These results suggest that in vivo expression of L2 protein alone favours a cellular immune response over a humoral immune response. A reason for a lack of neutralizing antibodies could be that the L2 protein was not released from host antigen presenting cells for activation of B cells in the right quantity or frequency.
There have been a number of reports on prophylactic HPV vaccine development utilising L2. Peptides derived from L2 protein or L2 fused to E7 and E6 have been investigated as possible vaccine candidates, with some measure of success [10]. Vaccination with the N-terminal 88 residues of the L2 protein alone protects against appropriate PV challenge in rabbits [11]; [12] with BPV-1 L2 being effective in inducing antisera which neutralised a wide range of cutaneous and mucosal HPVs. More recently a DNA vaccine consisting of a human calreticulin gene linked to L2, E6 and E7 was shown to elicit neutralising antibody responses to L2 DNA after DNA vaccination in addition to tumor regression of E6/E7 expressing tumors [4]. In that study relatively low doses of 2μg – 50μg of DNA were effective, but these mice received DNA vaccine boosts at 1 week intervals for 3 weeks which might be a reason why we did not obtain the same titre of neutralising antibodies against L2. In our study we gave a single DNA vaccine booster dose 28 days apart.
In conclusion, we have shown that an L2 DNA vaccine provides some protection from tumor challenge in a mouse model indirectly indicating it can induce T cell responses that mediate this protection. We showed also that an L2 DNA vaccine alone was not a successful prophylactic vaccine. It remains to be tested whether a L2 DNA vaccine could serve as a good priming agent in a DNA prime, protein boost regime.
Acknowledgments
This work was supported by grants from the Poliomyelitis Research Foundation of South Africa and NCI grant RO1 CA 74397. W. Martin Kast holds the Walter A. Richter Cancer Research Chair. We thank the animal technicians Marleze Rheeder, Rodney Lucas and Erika Janse van Rensburg, John Schiller for providing plasmids and cell line for the pseudovirion assay, Thomas Hanke for providing pTH, Neil Christensen for MAbs and Shreya Kanodia and Diane Da Silva for critically reading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maclean J, Rybicki EP, Williamson AL. Vaccination strategies for the prevention of cervical cancer. Expert Reviews Anticancer Therapy. 2005;5:97–107. doi: 10.1586/14737140.5.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Kawana K, Yasugi T, Kanda T, et al. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine. 2003;21:4256–60. doi: 10.1016/s0264-410x(03)00454-7. [DOI] [PubMed] [Google Scholar]
- 3.Hung Chien-Fu, Monie A, Alvarez RD, Wu TC. DNA vaccines for cervical cancer: from bench to bedside. Experimental and Molecular Medicine. 2007;39:679–89. doi: 10.1038/emm.2007.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D, Gambhira R, Karanam B, et al. Generation and characterization of a preventative and therapeutic HPV DNA vaccine. Vaccine. 2008;26:351–60. doi: 10.1016/j.vaccine.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanke T, Schneider J, Gilbert SC, Hill AV, McMichael A. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum: immunogenicity in mice. Vaccine. 1998;16:426–35. doi: 10.1016/s0264-410x(97)00296-x. [DOI] [PubMed] [Google Scholar]
- 6.De Bruijn ML, Greenstone HL, Vermeulen H, et al. L1-specific protection from tumor challenge elicited by HPV16 virus-like particles. Virology. 1998;250:371–6. doi: 10.1006/viro.1998.9372. [DOI] [PubMed] [Google Scholar]
- 7.Varsani A, Williamson AL, de Villiers D, Becker I, Christensen ND, Rybicki EP. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. Journal of Virology. 2003;77:8386–93. doi: 10.1128/JVI.77.15.8386-8393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leder C, Kleinschmidt JA, Wiethe C, Muller M. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. Journal of Virology. 2001;75:9201–9. doi: 10.1128/JVI.75.19.9201-9209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastrana DV, Buck CB, Pang YY, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden R. Vaccination of Healthy Volunteers with human papillomavirus type 16 L2E7E6 Fusion Protein induces serum antibody that neutralizes across papillomavirus species. Cancer Research. 2006;66:11120–4. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 11.Pastrana DV, Gambhira R, Buck CB, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Gambhira R, Jagu S, Karanam B, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. Journal of Virology. 2007;81:11585–92. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


