Abstract
Objective
The Energy Envelope postulates that patients with Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) will improve functioning when maintaining expended energy levels at the same level as available energy level.
Methods
Estimated weekly energy quotients were established by dividing expended energy level by perceived energy level and multiplying by 100. Two groups of patients were identified following participation in a non-pharmacologic intervention trial. Some were able to keep expended energy close to available energy and others were not successful at this task.
Results
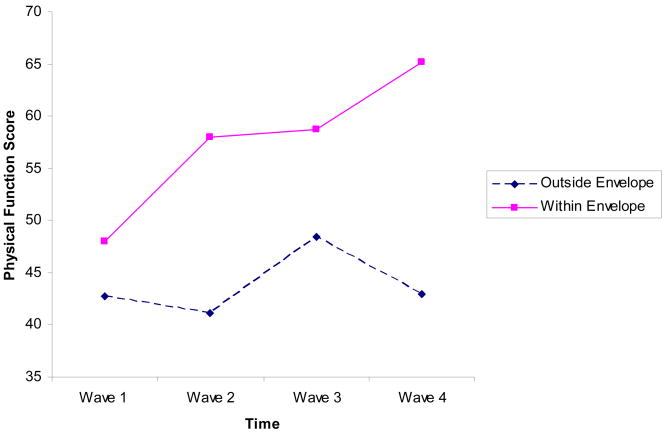
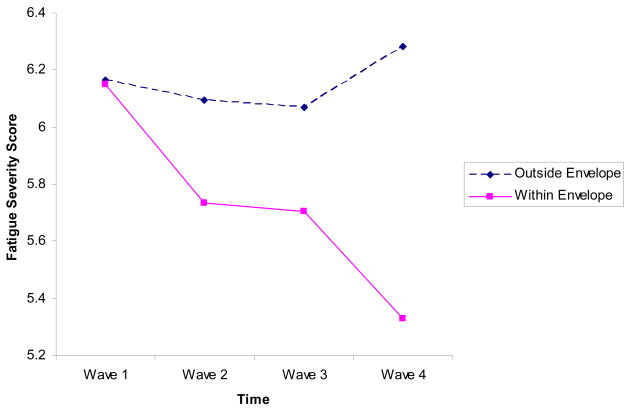
Those who were able to stay within their energy envelope had significant improvements in physical functioning and fatigue severity.
Conclusion
Findings suggest that helping patients with ME/CFS maintain appropriate energy expenditures in coordination with available energy reserves can help improve functioning over time.
Practical Implications
Health care professionals that treat patients with ME/CFS might incorporate strategies that help patients self-monitor and self-regulate energy expenditures.
Keywords: Chronic fatigue syndrome, Myalgic Encephalomyelitis, Myalgic Encephalopathy, Energy
1. Introduction
Chronic fatigue syndrome (CFS), also known as Myalgic Encephalomyelitis or Myalgic Encephalopathy (ME), is a highly incapacitating illness with an annual value of lost productivity in the US due to this illness estimated to be $9.1 billion (1). Moreover, total direct and indirect costs due to CFS ranges from $18.7 to $24 billion dollars (2). Patients with CFS are more functionally impaired than those suffering from type II diabetes mellitus, congestive heart failure, multiple sclerosis, and end-stage renal disease (3; 4). It is estimated that over 800,000 individuals have this illness (5). Given the prevalence and impact of this illness, there is a need to find ways to develop effective intervention strategies.
One of the more popular treatments for patients with ME/CFS has been cognitive behavior therapy (CBT). Price, Mitchell, Tidy, and Hunot (6) recently reviewed 15 studies of CBT with a total of 1,043 ME/CFS participants. At treatment end, 40% of people in the CBT group showing clinical improvement in contrast to only 26% in usual care, but changes were not maintained at a 1–7 month follow-up when including people who had dropped out. In addition, a survey of 3228 respondents (7) and a separate survey sponsored by the ME Association (8) found that graded exercise, which is a component of CBT, was felt to be the type of treatment that made more people with ME/CFS worse than any other. Two additional studies provide possible reasons for patient reaction to these graded exercise strategies. Jammes, Steinberg, Mambrini, Bregeon, and Delliaux (9) found that incremental exercise among individuals with CFS was associated with oxidative stress and marked alterations of muscle membrane excitability. Black, O’Connor, and McCully (10) found that 28% on average increases in daily physical activity for a four week period among a sample of people with CFS resulted in worsening overall mood, muscle pain intensity, and time spent each day with fatigue. Later after a re-analysis of this data, Black and McCully (11) concluded that CFS patients developed exercise intolerance as demonstrated by reduced total activity after 4–10 days.
Other approaches to helping patients with ME/CFS have included Envelope Theory (12) and pacing (13), and these approaches do not unilaterally increase activity for all patients. The Envelope Theory recommends that patients with ME/CFS pace their activity according to their available energy resources (12). In this approach, the phrase, “staying within the envelope,” is used to designate a comfortable range of energy expenditure, in which an individual avoids both over-exertion and under-exertion, maintaining an optimal level of activity over time. Some people with ME/CFS need to be encouraged to increase their activity, as they have the appropriate amount of perceived energy to do so. However, there are also people with ME/CFS that need to be encouraged to do less in order to decrease the discrepancy between perceived and expended energy. This theory emphasizes the need to understand the differential needs of subtypes of patients with ME/CFS. The key is to not over-expend their energy supplies or consistently go outside their “envelope” of available energy. Rather than a cure, this approach focuses on improving the ability of patients to cope with this illness.
In evaluating this energy envelope theory, Jason, Melrose et al. (12) presented evidence that when a patient kept her expended energy levels within the envelope of her perceived energy levels, her fatigue was lower and her perceived energy higher. In a second study, Jason, Tryon, et al. (14) found a positive significant relationship between current fatigue level and self-rated expended energy two-days ago. Pesek et al. (15) found that when participants with ME/CFS were provided with a buddy to reduce activities and to assist in identifying and reducing discrepancies between perceived and expended energy, overall fatigue severity as well as severity ratings for ME/CFS symptoms decreased. In a correlational study, Jason, Muldowney and Torres-Harding (16) found that the individuals with ME/CFS experienced a range of negative symptoms and disability when they extend beyond their energy envelope. Unfortunately, the studies above were either correlational or involved small samples.
There is a need to experimentally test out the Envelope theory with individuals who stay within their energy envelopes versus those who do not, and then assess whether this leads to differences on measures of physical functioning and fatigue. The present study tested out this theory with two groups of patients with ME/CFS. The hypothesis was that significant positive changes on physical functioning and fatigue severity would only occur for those patients with ME/CFS that stayed within their energy envelope.
2. Method
2.1. Participant Recruitment
Participants were recruited from a variety of sources, including physician referrals. Information about the non-pharmacologic treatment trial study was disseminated to medical colleagues through mailings, phone communication, and invited grand rounds. In addition, study announcements for new participants were placed in local newspapers and recruitment offers were made at local ME/CFS support group meetings. These efforts were continued throughout the study period until the target enrollment numbers were achieved. One hundred and fourteen individuals were recruited and enrolled in the study. The study was approved by the Institutional Review Board at DePaul University.
Of the 114 individuals, 46% were referred by physicians, 34% were recruited by media (newspapers, TV, radio, etc.), and 20% stemmed from other sources (e.g., heard about the study from a friend, family member, person in the study, etc.). There were no significant demographic differences for patients recruited from these varying sources. Twenty-four additional individuals who were screened were excluded due to a variety of reasons (i.e., lifelong fatigue, less than 4 Fukuda symptoms, BMI > 45, melancholic depression or bipolar depression, alcohol or substance abuse disorder, autoimmune thyroiditis, cancer, lupus, rheumatoid arthritis). Approaches to reduce attrition included use of letters and telephone reminders of all appointments, flexibility regarding working around vacations and medical and other crises, reimbursement for transportation costs, and participant honoraria.
2.2. Initial Screening
All participants were required to be at least 18 years of age, not pregnant, able to read and speak English, and considered to be physically capable of attending the scheduled sessions. Bedridden, housebound, and patients who used wheelchairs were excluded due to the practical difficulties of keeping therapy appointments. Referrals to local physicians who treat ME/CFS and to support groups were offered to these individuals. After a consent form was filled out, prospective participants were initially screened by the second author, using a structured questionnaire, the ME/CFS Questionnaire.
2.3. The ME/CFS Questionnaire
This screening scale was initially validated by Jason et al. (17). This scale is used to collects demographic, health status, medication usage, and symptom data, and it uses the definitional symptoms of ME/CFS (18). Hawk, Jason, and Torres-Harding (19) recently revised this ME/CFS Questionnaire, and administered the questionnaire to three groups (those with ME/CFS, Major Depressive Disorder, and healthy controls). The revised instrument, which was used in the present study, evidences good test-retest reliability and has good sensitivity and specificity.
2.3. Psychiatric Interview
Next, a semi-structured psychiatric interview called the Structured Clinical Interview for DSM-IV (SCID) (20) was administered. Axis I was used to establish psychiatric diagnoses. The professionally administered SCID allows for clinical judgment in the assignment of symptoms to psychiatric or medical categories. These measures were completed at DePaul University and took approximately two hours. After the initial interview was completed, the patients’ information was reviewed to ensure that they met all eligibility requirements. If an individual was eligible for the study, a medical appointment was set up. Conversely, if an individual was not eligible, we discussed with him or her alternate treatment options.
2.4. Medical Assessment of ME/CFS
The physician screening evaluation included an in-depth medical and neurological history, as well as general and neurological physical examinations. The evaluation also included a structured instrument, a modified version of the ME/CFS questionnaire (21). This instrument assesses the signs, symptoms, and medical history to rule out other disorders. Relevant medical information was gathered to exclude possible other medical causes of chronic fatigue, including exposure histories to tuberculosis, AIDS, and non-AIDS sexually transmitted diseases. Information on prescribed and illicit drug use was also assessed and recorded. Finally, the histories of all symptoms related to ME/CFS were gathered.
Laboratory tests in the battery were the minimum necessary to rule out other illnesses (18). Laboratory tests included a chemistry screen (which assesses liver, renal, and thyroid functioning), complete blood count with differential and platelet count, erythrocyte sedimentation rate, arthritic profile (which includes rheumatoid factor and antinuclear antibody), hepatitis B, Lyme Disease screen, HIV screen, and urinalysis. A tuberculin skin test was also performed. The project physician performed a detailed medical examination to detect evidence of diffuse adenopathy, hepatosplenomegaly, synovitis, neuropathy, myopathy, and cardiac or pulmonary dysfunction.
2.5. Fatigue Severity Scale (FS) (22)
This scale was used to measure fatigue. This scale includes nine items rated on seven-point scales and is sensitive to different aspects and gradations of fatigue severity. Most items in the Krupp fatigue scale are related to behavioral consequences of fatigue. Previous findings have shown the scale can discriminate between individuals with ME/CFS, MS, and primary depression (23). In addition, the Fatigue Severity Scale (22) was normed on a sample of individuals with MS, SLE, and healthy controls. Higher scores indicate more fatigue. Data were collected on this variable at the baseline, post-test, 6 and 12 month follow-up assessment.
2.6. Medical Outcomes Study-Short Form-36 (SF-36) (24)
This 36 item broadly-based self-report measure of functional status related to health, identifies eight health concepts as perceived by the individual. Test construction studies have shown adequate internal consistency, significant discriminate validity among subscales, and substantial differences between patient and non-patient populations in the pattern of scores (25; 26). It also has indicated sufficient psychometric properties as a measure of functional status in a ME/CFS population (4). White, Sharpe, Chalder, DeCesare and Walwyn (27) have recommended using the physical function sub-scale of the Medical Outcomes Survey-SF-36 as a primary outcome measure for ME/CFS trials. We selected this measure as one of the major dependent variables in the present study. Higher scores indicate higher levels of functioning. Data were collected on this variable at the baseline, post-test, 6 and 12 month follow-up assessment.
2.7. Perceived and Expended Energy
Participants were asked to rate perceived energy and expended energy over the past week on a 100-point scale, with 0= no energy and 100= abundant energy similar to when the person was completely well. Data were collected on this variable at the baseline and 12 month assessments. Hawk, Jason, and Torres-Harding (19) found test-retest reliability for weekly perceived energy and expended energy was .81 and .64, respectively. Perceived energy referred to the participants’ estimation of their available energy resources. Expended energy was defined as the participants’ estimation of the total amount of energy exerted. Expended energy can be greater than perceived energy, particularly when participants push themselves over their energy limits. The participants’ expended energy was divided by their perceived energy, and this number was then multiplied by 100. This represents the Energy Quotient for weekly ratings. A score of equal to or below 150 at the 12 month follow-up was considered to be remaining within one’s energy envelope. This follow-up Energy Quotient variable had a mean of 189 (SD 155, range 50–1000).
2.8. Treatment Protocols
Participants were provided 13 sessions of either Cognitive Behavior Therapy, Anaerobic Activity Alone, Cognitive Coping Skills, or Relaxation during 45 minute meetings that were held once every two weeks for 6 months (See 28 for more details). Participants attended an average of 10.0 sessions out of a possible 13 sessions, with a range from 1–13. The average drop-out rate was 25%, but it was not significantly different per condition. There were 28–29 participants randomly assigned to each of the four conditions. There were no significant socio-demographic differences among these groups at baseline.
3.0. Results
Of the sample of 81 with data collected at the 12 month follow-up on energy ratings, we classified 49 as staying within their energy envelope and 32 as going beyond their energy envelope. This decision was based on individuals at follow-up who had a score of 150 or less on weekly Energy Quotient. Therefore, 49 individuals were classified as having reduced or maintained a reasonable balance between perceived and expended energy. We also used a more severe criteria, a score of 100 or less (which included only 24 individuals who were in the category stayed within their envelope category, and similar findings as what is reported below were found).
Between the two categories of patients, there were no significant differences for race (X2 (3, N = 80) = 1.38, p = .71), gender (X2 (1, N = 81) = .03, p = .87), marital status (X2 (4, N = 80) = 6.38, p = .18), education level (X2 (3, N = 80) = 1.42, p = .70), unemployment status (X2 (1, N = 80) = .01, p = .94), and percent working full (X2 (1, N = 80) = 2.90, p = .09) or part-time(X2 (1, N = 80) = .44, p = .51). There was no significant difference among the four treatment conditions in terms of the percentage of patients who were in each of the two categories (X2 (3, N = 81) = 1.84, p = .61).
As expected, those who stayed within the energy envelope in comparison to those who did not had significantly lower Energy Quotient scores at the 12 month follow up for weekly ratings (Ms = 107 vs 315; t (31.8) = 6.38, p < .01). Those who stayed within the energy envelope also evidenced significantly lower baseline weekly Energy Quotient scores than those who were not within their energy envelopes (Ms = 171 versus 356; t (32.2) = 2.71, p = .01).
Figures 1 and 2 present the Physical Functioning and Fatigue Severity data over time for the two groups. We had predicted significant interaction effects for both variables. In these repeated measures ANOVA analyses, baseline weekly Energy Quotient was used as a covariate. For Physical Functioning, Mauchly’s Test of Sphericity was significant (W(5) = .79, p = .03), however, the tests of Within-Subjects effects were significant using different correction factors (Greenhouse-Geisser, Huynh-Feldt, Lower-bound), therefore we report results using Sphericity assumed. For Physical Functioning, we found significant time (F(3,162) = 4.20, p < .01) and interaction (F(3,162) = 6.08, p < .01) effects. Examining just those who stayed within their energy envelopes over time, for Physical Functioning, significant improvements were noted over time (F(3,105) = 18.22, p < .01), whereas for those who did not stay within the energy envelope, no significant changes emerged over time (F(3,69) = 1.05, p = .37).
Figure 1.
Physical Functioning Scores over Time
Figure 2.
Fatigue Severity Scores over Time
For Fatigue Severity, Mauchly’s Test of Sphericity was not significant (W(5) = .84, p = .12), therefore we report Sphericity assumed results. There was a significant interaction effect for Fatigue Severity (F(3,159) = 5.21, p < .01). For Fatigue Severity, significant improvements were noted over time for those in the stayed within the energy envelope (F(3,102) = 8.806, p < .01), whereas for those who did not stay within the energy envelope, no significant changes emerged for either variable (F(3,69) = 1.65, p = .19).
Because of missing data on post-test or 6 month follow-up data, sample sizes are smaller than the 81 participants with data on Energy Quotients at the Baseline and 12 month follow-up, but we completed similar analyses with just the Baseline and 12 month follow-up Physical Functioning and Fatigue Severity data, and similar findings emerged.
4.0. Discussion and Conclusion
4.1 Discussion
It is possible that when patients overexert themselves, they experience negative emotional responses due to evaluating stressful experiences as a significant threat and as exceeding available coping resources (29). Consequently, negative emotional responses can cause distressed clients to engage in behaviors (e.g., altering sleep patterns, alcohol and tobacco use, or decreasing physical activity) which conceivably modify immune responses. In addition, negative emotional states might activate the sympathetic division, whose fibers, descending from the brain to lymphoid tissues such as bone marrow, thymus, spleen, and so forth, could release substances that influence immune responses. Distress also can activate the Hypothalamus-Pituitary- Adrenal axis and hormonal products from these systems can dysregulate the immune system.
Kindling is another explanation for what might occurs when patients with ME/CFS over exert themselves and deplete energy reserves. Zalcman, Savina, and Wise (30) have found that immunogenic stimuli can alter brain circuitry, changing its sensitivity to seemingly unrelated subsequent stimuli. Exposure to stress or over exertion can induce long-term potentiation, such that the brain cells react more strongly (and releases dopamine more abundantly) in response to future exposures to the drug or stress (31). Gellhorn (32) has postulated that under prolonged stimulation of the limbic-hypothalamic-pituitary axis, a lowered threshold for activation can occur. The kindling hypothesis suggests that once this system is charged, either by high-intensity stimulation or by chronically repeated low-intensity stimulation, which might occur by going beyond energy reserves, it can sustain a high level of arousal.
Two receptors residing on the cell surface membranes of neurons are GABA (gamma aminobutyric acid), which inhibits neuronal firing and NMDA (N-methyl-D-aspartate), which excites neuronal firing. The GABA and NMDA receptors should be balanced, but after an injury or kindling, NMDA fires more than GABA. Minor and Hunter (33) have proposed that prolonged exposure to inescapable stressors will eventually deplete GABA, thus reducing an important form of inhibition on excitatory glutamate transmission. Ultimately, chronic stress sensitizes neural and behavioral fear processes, and this over-activation leads to fatigue. The limbic system plays a regulatory role pertaining to symptoms of fatigue, pain, memory, and cognition, and in part, it plays this role with the use of dopamine to control the NMDA receptors. These NMDA receptors might not function properly due to low levels of dopamine (34). Brouwer and Packer (35) have conducted research indicating that people with ME/CFS might have “unstable cortical excitability associated with sustained muscle activity resulting in varied magnitudes of descending volleys” (p. 1212). In a sense, patients with ME/CFS might have this type of cortical excitability, that might be due to kindling, and then when they go beyond their energy reserves, the kindling produces high arousal that has implications for the hypothalamus, the autonomic system, as well as the immune system.
It could be argued that the score of 150 was selected in an arbitrary way. Even when we used the tighter criteria of 100, we did find similar outcomes. Still, the criteria to decide what is within one’s energy envelope versus what is not within one’s energy envelope is subjective, and until normative data on large samples of both healthy and ill individuals are collected on this construct, there still will be some ambiguity concerning what number represents an optimal Energy Quotient.
It is also possible to argue that we should have included only those who made positive changes in their Energy Quotient into the category that was designated by staying within the envelope. In a sense, there were some individuals who at baseline remained within their energy envelope at the follow-up, and others who were outside the energy envelope at baseline and were able to make improvements by the follow-up. Certainly these types of subgroup differences are of importance, but our small sample size would make such analyses difficult to perform due to low power. In a sense, what we have in this study are people with ME/CFS who were within their energy envelopes at baseline and other people with ME/CFS who learned to stay in their energy envelopes, and the combined group were able to evidence improvements over those who were outside their energy envelopes by the follow-up assessment (whether they had been or not been in their energy envelope at the baseline). Clearly, further research is needed on this topic with larger sample sizes so that researchers can better attribute the mediators for improvements that were noted.
There are several other limitations to the present study. One potential limitation is that there were only two dependent variables in this study. Given the small sample size, the authors decided to focus on two major outcomes variables in order to reduce the probability of obtaining findings by chance. In addition, there were some initial baseline differences between the weekly Energy Quotient and this might have also influenced the findings. However, weekly baseline Energy Quotient was inserted as a covariate, and significant interaction effects were still found. Finally, due to small sample sizes, it is still unclear whether there is differential impact of the different non-pharmacologic interventions on staying within the energy envelope. Future research with larger samples within each of the four treatment conditions is needed to assess whether one or more of the treatment conditions was more effective in helping patients modulate their energy expenditures. However, there were no differences in the percentage of patients in the two Energy Quotient categories within the four treatment conditions. It is possible that a common mediator within these non-pharmacologic interventions is helping patients self-monitor and self-regulate energy expenditures, and those that are more successful in this endeavor or learned to become more skillful are the ones to make the most substantial improvements.
4.2. Conclusion
The present study found that the two groups of patients had different outcomes on measures of physical functioning and fatigue severity. In general, those patients who exerted more energy than they had available encountered less change in functioning and fatigue over time. Those patients who were able to stay within their energy boundaries made significantly more improvements over time. These findings suggest that when an individual with ME/CFS avoids both overexertion and underexertion, maintaining an optimal level of activity over time, it might be associated with improvements in physical functioning and fatigue.
4.3. Practical Implications
The present study suggests that the Energy Envelope theory has some merit, and that being overextended and going beyond energy reserves can be an impediment to improving functionality and fatigue levels. The current study supports the notion that staying within the energy envelope does result in significant improvements in physical functioning and fatigue severity for patients with ME/CFS. Clearly, additional research is warranted examining these relationships over more extended periods of time. Health care professionals that treat patients with ME/CFS might want to consider incorporating strategies that help patients self-monitor and self-regulate energy expenditures.
Acknowledgments
The authors appreciate the funding provided by NIAID (grant number AI 49720).
Footnotes
I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. 0. References
- 1.Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome [Internet] Cost effectiveness and resource allocation [cited 2005 Oct 15] doi: 10.1186/1478-7547-2-4. Available from: http://resource-allocation.com/content/2/1/4. [DOI] [PMC free article] [PubMed]
- 2.Jason LA, Benton M, Johnson A, Valentine L. The economic impact of ME/CFS: Individual and societal level costs [Internet] Dynamic Med. 2008;7(6) doi: 10.1186/1476-5918-7-6. [cited 2008 Apr 21]. Available from: http://www.dynamic-med.com/content/7/1/6. [DOI] [PMC free article] [PubMed]
- 3.Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. J Nerv Ment Dis. 185:359–367. doi: 10.1097/00005053-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. AJM. 1996;121:953–9. doi: 10.1016/S0002-9343(96)00234-3. [DOI] [PubMed] [Google Scholar]
- 5.Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR. A community-based study of chronic fatigue syndrome. Arch of Intern Med. 1999;159:2129–37. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 6.Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database of Systematic Reviews. 2 doi: 10.1002/14651858.CD001027.pub2. Art No.: CD001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preliminary Report. The severely affected. AfMe Feb 28 2001.
- 8.Cooper L. Report of survey members of local ME groups. Perspectives. 2001 [Google Scholar]
- 9.Jammes Y, Steinberg JG, Mambrini O, Bregeon F, Delliaux S. Chronic fatigue syndrome: Assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med. 2005;257:299–310. doi: 10.1111/j.1365-2796.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- 10.Black CD, O’Connor PJ, McCully KK. Increased daily physical activity and fatigue symptoms in chronic fatigue syndrome. Dynamic Med. 2005;4:3. doi: 10.1186/1476-5918-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black CD, McCully KK. Time course of exercise induced alterations in daily activity in chronic fatigue syndrome. Dynamic Med. 2005;4:10. doi: 10.1186/1476-5918-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jason L, Melrose H, Lerman A, Burroughs V, Lewis K, King C, Frankenberry E. Managing chronic fatigue syndrome: Overview and case study. AAOHN J. 1999;47:17–21. [PubMed] [Google Scholar]
- 13.Goudsmit E. Measuring the quality of trials and treatment for chronic fatigue syndrome. JAMA. 2001;286:3078–9. doi: 10.1001/jama.286.24.3078. [DOI] [PubMed] [Google Scholar]
- 14.Jason L, Tyron W, Taylor R, King K, Frankenberry E, Jordan K. Monitoring and assessing symptoms of chronic fatigue syndrome: Use of time series regression. Psych Reports. 1999;85:121–130. doi: 10.2466/pr0.1999.85.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Pesek J, Jason L, Taylor R. An empirical investigation of the envelope theory. J Hum Behav Soc Env. 2000;3:59–77. [Google Scholar]
- 16.Jason LA, Muldowney K, Torres-Harding S. The energy envelope theory of Myalgic Encephalomyelitis/chronic fatigue syndrome. AAOHN J. 2008;56:189–195. doi: 10.3928/08910162-20080501-06. [DOI] [PubMed] [Google Scholar]
- 17.Jason LA, Ropacki JA, Santoro NB, Richman JA, Heatherly W, Taylor R, et al. A screening scale for chronic fatigue syndrome: Reliability and validity. J Chronic Fatigue Syn. 1997;3:39–59. [Google Scholar]
- 18.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hawk C, Jason LA, Torres-Harding S. Reliability of a chronic fatigue syndrome questionnaire. J Chronic Fatigue Syn. 2007;13:41–66. [Google Scholar]
- 20.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for the DSM-IV—Non-Patient Edition (SCID—NP, Version 2.0) Washington DC: American Psychiatric Press; 1995. [Google Scholar]
- 21.Komaroff AL, Fagioli LR, Geiger AM, Doolittle TH, Lee J, Kornish RJ, Gleit MA, Guerriero RT. An examination of the working case definition of chronic fatigue syndrome. AJM. 1996;100:56–64. doi: 10.1016/s0002-9343(96)90012-1. [DOI] [PubMed] [Google Scholar]
- 22.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic Lupus erythematosus. Arch of Neur. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 23.Pepper CM, Krupp LB, Friedberg F, Doscher C, Coyle PK. A comparison of neuropsychiatric characteristics in chronic fatigue syndrome, multiple sclerosis, and major depression. J Neuropsychiatry Clin Neurosci. 1993;5:200–5. doi: 10.1176/jnp.5.2.200. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 25.McHorney CA, Ware JE, Raczek AE. The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 26.McHorney CA, Ware JE, Lu AW, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Test of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 27.White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R. Protocol for the PACE trial: A randomized controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise, as supplements to standardized specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy. BMC Neuro. 2007;7:6. doi: 10.1186/1471-2377-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jason LA, Torres-Harding S, Friedberg F, Corradi K, Njoku MG, Donalek J, Reynolds N, Brown M, Weitner BB, Rademaker A, Papernik M. Non-pharmacologic interventions for CFS: A randomized trial. J Clin Psych Med Settings. 2007;14:275–96. [Google Scholar]
- 29.Miller GE, Cohen S. Psychological interventions and the immune system: A meta-analytic review and critique. Health Psych. 2001;20:47–63. doi: 10.1037//0278-6133.20.1.47. [DOI] [PubMed] [Google Scholar]
- 30.Zalcman S, Savina I, Wise RA. Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res. 1999;847:276–83. doi: 10.1016/s0006-8993(99)02063-6. [DOI] [PubMed] [Google Scholar]
- 31.Saal D, Dong Y, Bonci A, Malenka R. Drugs of abuse and stress trigger a common synaptic adaption in dopamine neurons. Neuron. 2003;37(4):577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 32.Gellhorn E. The emotions and the ergotropic and trophotropic systems. Psychologische Forschung. 1970;34:48–94. doi: 10.1007/BF00422862. [DOI] [PubMed] [Google Scholar]
- 33.Minor TR, Hunter AM. Stressor controllability and learned helplessness research in the United States: Sensitization and fatigue process. Integrative Psycho Behav Sci. 2002;37:44–58. doi: 10.1007/BF02688805. [DOI] [PubMed] [Google Scholar]
- 34.Wood PB. Stress and dopamine: Implications for the pathophysiology of chronic widespread pain. Med Hypoth. 2004;62(3):420–4. doi: 10.1016/j.mehy.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Brouwer B, Packer T. Corticospinal excitability in patients diagnosed with Chronic Fatigue Syndrome. Muscle and Nerve. 1994;17:1210–12. doi: 10.1002/mus.880171012. [DOI] [PubMed] [Google Scholar]