Abstract
The serine/threonine kinase Akt (cellular homolog of murine thymoma virus akt8 oncogene), also known as PKB (protein kinase B), is activated by lipid products of phosphatidylinositol 3-kinase (PI3K). Akt phosphorylates numerous protein targets that control cell survival, proliferation and motility. Previous studies suggest that Akt regulates transcriptional activity of the nuclear factor-κB (NFκB) by inducing phosphorylation and subsequent degradation of inhibitor of κB (IκB). We show here that NFκB-driven transcription increases in chicken embryonic fibroblasts (CEF) transformed by myristylated Akt (myrAkt). Accordingly, both a dominant negative mutant of Akt and Akt inhibitors repress NFκB-dependent transcription. The degradation of the IκB protein is strongly enhanced in Akt-transformed cells, and the loss of NFκB activity by introduction of a super-repressor of NFκB, IκBSR, interferes with PI3K- and Akt-induced oncogenic transformation of CEF. The phosphorylation of the p65 subunit of NFκB at serine 534 is also upregulated in Akt-transformed cells. Our data suggest that the stimulation of NFκB by Akt is dependent on the phosphorylation of p65 at S534, mediated by IKK (IκB kinase) α and β. Akt phosphorylates IKKα on T23, and this phosphorylation event is a prerequisite for the phosphorylation of p65 at S534 by IKKα and β. Our results demonstrate two separate functions of the IKK complex in NFκB activation in cells with constitutive Akt activity: the phosphorylation and consequent degradation of IκB and the phosphorylation of p65. The data further support the conclusion that NFκB activity is essential for PI3K- and Akt-induced oncogenic transformation.
Keywords: NF-κB, PI-3 kinase, Akt, IKK, IκB, oncogenic transformation
Introduction
NFκB, nuclear factor-κB, is a family of transcription factors that regulates diverse cellular activities related to inflammation and innate and adaptive immune responses.1 Deregulation of NFκB activity is implicated in the development of autoimmune diseases and cancer.2 The common form of NFκB is p65/RelA-p50. In most cell types, the p65/RelA-p50 heterodimer is sequestered in the cytoplasm by the inhibitor of κB (IκB), because IκB binding masks the nuclear localization sequence of p65. Upon stimulation, IκB is phosphorylated at critical serine residues, resulting in polyubiquitination and degradation by the 26 S proteasome. The p65/RelA-p50 dimer then translocates into the nucleus, binds to specific NFκB-sites in the enhancer regions of target genes and regulates transcriptional activity.3
In addition to shuttling in and out of the nucleus, NFκB proteins are post-translationally modified by phosphorylation, acetylation or ubiquitination. These modifications fine-tune the activity of NFκB and can alter its binding specificity to different promoters.4
The NFκB signaling cascade interacts with several parallel pathways including the signaling cascades initiated by phosphatidylinositol 3-kinase (PI3K) and Akt.5-7 Two PI3K inhibitors, LY294002 and Wortmannin, block the interleukin (IL)-1- induced increase in the DNA binding activity of NFκB.8 Akt, functioning as a key downstream target of PI3K, has been suggested to function as an IKK (IκB kinase) kinase.9 The IKK complex is mainly comprised of the catalytic subunits IKKα and IKKβ and the regulatory subunit IKKγ/NEMO (NFκB essential modulator).
Here we show that constitutively active Akt stimulates IKK activity by phosphorylation on T23 in the IKKα subunit. The IKK complex then phosphorylates both the IκB protein and the p65/RelA subunit, inducing enhanced activation of the NFκB transcription factor. Inhibition of NFκB by overexpression of non-degradable IκB strongly interferes with oncogenic transformation induced by Akt or PI3K.
Materials and methods
Plasmids and mutagenesis
The plasmids encoding mouse p65, p50 and non-degradable super-repressor of NFκB (IκBSR)12 were kind gifts from Dr. Inder M. Verma. The NFκB promoter reporter from Stratagene (La Jolla, CA) contains 5 individual binding sites for the NFκB dimer, (TGGGGACTTTCCGC)5. The myristylated Akt and the dominant negative Akt-T308A/S473A expression vectors have been described.13 The FLAG-IKKα and FLAG-IKKβ vectors were kind gifts from Dr. Thomas D. Gilmore. Mutations from S to A or to D at positions 276, 527, and 534 of p65 and at position 335 of p50 were introduced using the QuikChange® II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA).
Antibodies and chemicals
Polyclonal antibodies directed against IκBα, p65 and p50 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-FLAG antibody M2 was obtained from Sigma Chemical Co. (St. Louis, MO). Anti-phospho-p65 (S534), IKKα, IKKβ and GST were obtained from Cell Signaling (Beverly, CA). The monoclonal anti-AU1 tag antibody was from Covance Research Products Inc. (Denver, PA). The Akt inhibitors IV and V were purchased from Calbiochem (La Jolla, CA).
Cell culture, transfection and infection using the avian retroviral vector RCAS
Fertilized chicken eggs (white Leghorn) were obtained from Charles River Breeding Laboratories (Wilmington, MA). Preparation and cultivation of primary chicken embryonic fibroblasts (CEF) have been described previously.14 Briefly, CEF were maintained in Ham's F-10 medium (Sigma, St Louis, MO) supplemented with 10% FBS (Omega Scientific Inc., Tarzana, CA), 5% chicken serum (Sigma, St Louis), 1 × MEM vitamin solution (Sigma, St Louis, MO), 8 mg/L folic acid, and 1% L-glutamine-penicillin-streptomycin solution (Sigma, St Louis, MO) at 37°C in 5% CO2. For interference assays, stable transfection using 2 μg of the subgroup B RCAS (Replication Competent ALV LTR with a Splice acceptor) avian retroviral vector15 was carried out by using the dimethylsulfoxide/Polybrene method (DMSO shock).16 After 2 to 3 passages, cells were seeded onto 6-well plates and infected with the following viruses and vectors: PR-A (Prague strain of Rous sarcoma virus), carrying the v-src oncogene; ASV17, expressing the v-jun oncogene;17, 18 subgroup A RCAS vector expressing myristylated Akt (myrakt) and subgroup A RCAS vector expressing the myristylated catalytic subunit of PI3K (myrP3K).13, 19 The cells were fed with nutrient agar every 3 days and stained with 2% crystal violet in 20% methanol after 10 to 14 days.20 The human embryonic kidney cells HEK293 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 50 units of penicillin and streptomycin per ml and 10% fetal bovine serum (FBS). The human breast cancer cell line, BT-20 (American Type Culture Collection, Manassas, VA) was maintained in DMEM supplemented with 10% fetal bovine serum, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate and L-glutamine at 37°C. BT-20 cells were transfected with PolyFect transfection reagent (Qiagen, Valencia, CA) according to the manufacture's instructions.
Luciferase assay
Cells were transfected by using the PolyFect transfection reagent. CEF or BT-20 cells were seeded into MP-24 tissue culture plates at 4×104 or 6×104 cells per well, respectively. Forty-eight h after transfection, the cultures were washed with PBS and then lysed in 200 μl of 1 × Passive Lysis Buffer (Promega, Madison, WI). Firefly luciferase activities and Renilla luciferase activities were measured by using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) with a Berthold Biolumat LB 9501 Luminometer. Firefly luciferase activities were normalized against Renilla luciferase activities. Each set of experiments was repeated at least three times with consistent results.
Metabolic labeling and Immunoprecipitation
Vector control or CEF transformed with myrakt were washed twice with phosphate-free F-10 and subsequently incubated with medium containing 1 mCi/ml [32P]orthophosphate (Perkin Elmer Life Sciences, Boston, MA) for 3 h. Cells were lysed in 1 × Passive Lysis Buffer and immunoprecipitated with anti-p65 antibody. The precipitated proteins were washed three times with cold cell lysis buffer and then analyzed by Western blotting and autoradiography.
Western blots
Cells were lysed in Nonidet P-40 lysis buffer (20 mM Tris·HCl, pH 7.5/150 mM NaCl/10% glycerol/1% Nonidet P-40/10 mM NaF/1 mM sodium pyrophosphate/1 mM sodium orthovanadate) containing a protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN). After incubation on ice for 15 min, cellular debris was removed by centrifugation at 13,000 rpm for 15 min. For immunoblotting, lysates containing 60 μg of protein were separated by SDS-PAGE and transferred to Immobilon-P membranes (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat dry milk, Tris-buffered saline, and 0.05% Tween 20 for 1 h at room temperature and then probed overnight with a primary antibody. After incubation with horseradish peroxide-coupled antibody, reactive bands were visualized by chemiluminescence (Pierce, Rockford, IL). For immunoprecipitation, cell extracts were incubated with 1 μl of primary antibody for 4 h followed by incubation for 1 h with 30 μl of protein A-agarose beads (Pierce, Rockford, IL). The beads were washed three times with lysis buffer, and samples were analyzed by SDS-PAGE and chemiluminescence.
In vitro kinase assays
Cells were lysed in Passive Lysis Buffer containing a protease inhibitor mixture (Roche, Indianapolis, IN) and 1 mM PMSF/50 mM NaF/1 mM Na3VO4. 200 μl of the supernatant (cell lysate) was incubated with anti-HA tag agarose at 4°C. The resulting immunoprecipitate was mixed with 1 μl [γ-32P]ATP (1.0 μCi/μl in dH2O, Perkin Elmer Life Sciences, Boston, MA) and substrate in kinase buffer (25 mM HEPES pH7.5, 25 mM β-glycerophosphate, 25 mM MgCl2, 2 mM dithiothreitol, 0.1 mM Na3VO4, 20 μM ATP) and incubated at 30°C for 30 min. Phosphorylated proteins were washed twice with cold kinase buffer and then separated by SDS-PAGE and detected by autoradiography. 1 μg of bacterially expressed GST-fusion proteins IKKα(1-101), IKKβ (1-101), or p65 (335-550) was used as substrate.
Results
Constitutively active Akt increases transcription from NFκB-binding sites
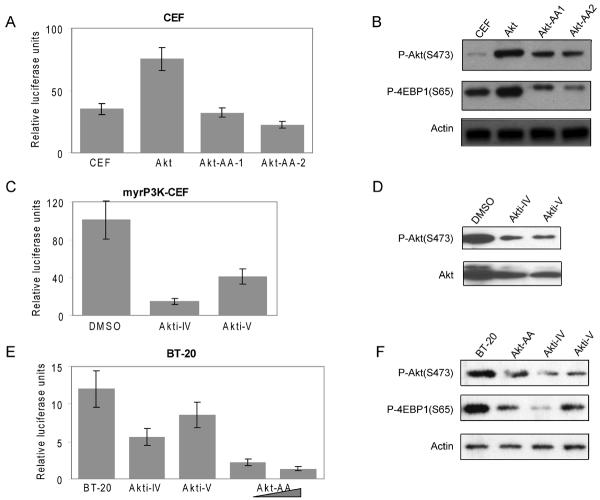
Previous studies have shown that constitutively active Akt or a constitutively active catalytic subunit of PI3K can transform CEF in culture and induce tumors in young chickens.13, 21 NFκB is reported to be important in tumorigenesis and to be involved in PI3K/Akt pathway.5-7, 9, 22 To better understand and clarify the functional importance of NFκB in PI3K- or Akt-induced oncogenic transformation, we transfected a myristylated form of Akt, myrAkt, which is constitutively active and membrane-bound,13 into CEF. Stable expression of myrAkt resulted in cellular transformation accompanied by phosphorylation of Akt at S473 and of the downstream target 4EBP1 at S65 (Fig. 1B). These cells showed increased activity of endogenous NFκB compared to control cells transfected with the empty RCAS vector (Fig. 1A). Transiently transfecting the Akt-transformed cells with the dominant negative Akt mutant T308A/S473A (Akt-AA) that cannot be activated by phosphorylation and lacks kinase activity13 decreased NFκB-mediated transcription in a dose-dependent manner. We conclude that Akt-mediated enhancement in NFκB activity depends on Akt kinase activity.
Fig. 1.
Akt promotes NFκB-driven transcription. (A) CEF were transfected with vector control or myrAkt by using the DMSO method.16 After 2 to 3 passages, cells were transfected with 100 ng of p5×κB firefly reporter plasmid and 5 ng of pRL-CMV Renilla luciferase construct. The Akt mutant (T308A/S473A, Akt-AA) was transiently transfected into Akt-transformed CEF at 0.5 μg (Akt-AA-1) and 1 μg (Akt-AA-2). After 48 h, luciferase activities were determined with the Dual-Luciferase Reporter Assay. (B) Western analysis of phosphorylated Akt (S473), phosphorylated 4EBP1 (S65) and actin in cell lysates from (A). (C) NFκB-driven luciferase transcription assays were performed in P3K-transformed cells pretreated 4 h with DMSO, Akti-IV (62.5nM) or Akti-V (2 μM). Total and phosphorylated Akt at S473 were detected by Western blotting (D). (E) The NFκB-driven reporter assay was carried in human BT-20 breast cancer cells. Cells were transfected with 100 ng of p5×κB firefly reporter plasmid and 5 ng of pRL-CMV Renilla luciferase construct. Akt inhibitors, Akti-IV (1 μM) and Akti-V (2 μM) were added 2 h before collecting the cell lysates. The Akt-AA mutant was co-transfected with reporter plasmids at 0.5 and 1 μg, respectively. (F) Western blot analysis of phosphorylated Akt (S473), phosphorylated 4EBP1 (S65) and actin in cell lysates from (E). Data shown are the average of three experiments ± standard deviation (SD). Luciferase activities are expressed in relative units (Firefly luciferase activity over Renilla luciferase activity).
Inhibitors of Akt reduce NFκB-dependent transcription
Akt is a critical component of signal transduction following PI3K activation. Two Akt inhibitors, Akti-IV and Akti-V, can efficiently block phosphorylation of Akt at T308 and S473 and inhibit Akt kinase activity.23, 24 We introduced an expression vector bearing the myristylated catalytic subunit p110α of PI3K (myrP3K) into CEF, which results in oncogenic transformation and activation of Akt.21 The inhibitors reduce the activating phosphorylation of Akt on S473 (Figs. 1D, F). Treatment of these cells with the Akt inhibitors Akti-IV and Akti-V for 2 h also reduced the NFκB transcriptional activity as revealed in reporter assays (Fig. 1C). In order to demonstrate that the regulation of NFκB activity by Akt extends to human cancer cells, we repeated the NFκB-mediated luciferase reporter assay in BT-20 breast cancer cells. BT-20 cells contain two gain-of-function mutations in the PI3K catalytic subunit p110α (P539R and H1047) and as a result, show increased phosphorylation of Akt.25 Consistent with the experiments carried out in CEF, both the dominant negative mutant of Akt and the Akt inhibitors, Akti-IV and Akti-V, led to a decrease of NFαB activity (Fig. 1E, F).
The IκB protein is degraded in Akt-transformed cells, and this degradation is a prerequisite for PI3K- and Akt-induced oncogenic transformation
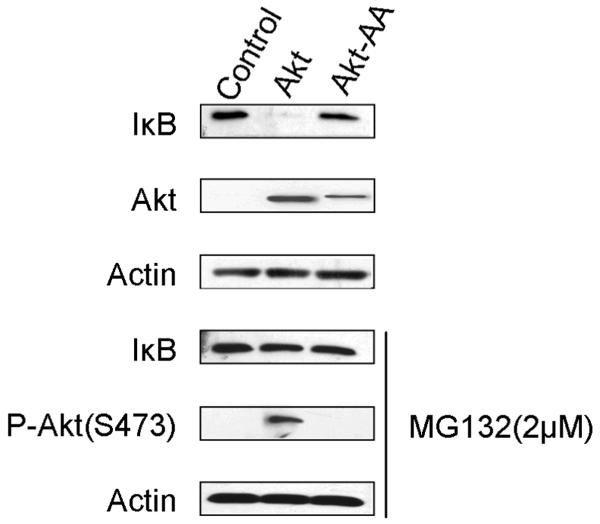
NFκB activity is tightly controlled by binding to the IκB inhibitor protein, which prevents NFκB from entering the nucleus. Once phosphorylated by the IKK complex at several NH2-terminal serines, IκB dissociates from the NFκB subunits, is ubiquitinated and rapidly degraded by the proteasome.3 We checked the endogenous level of the IκB protein in Akt-transformed cells and found that the total amount of IκB protein was dramatically decreased (Fig. 2, top). Treatment of the cells with a proteasome inhibitor, MG132,26 for 2 h restored normal levels of IκB (Fig. 2, lower half). This result suggests that the decreased IκB protein level in Akt-transformed cells is caused by proteolytic degradation and not by reduced IκB expression.
Fig. 2.
IκB is degraded in Akt-transformed cells. CEF transfected with vector control, HA-tagged myrAkt (Akt) or HA-tagged Akt-T308A/S473A (Akt-AA) were treated 2 h with either DMSO or the proteasome inhibitor MG132 (2 μM). Whole cell lysates were collected, electrophoresed and immunoblotted with antibodies to IκBα, phospho-S473-Akt, HA or actin. Data presented are representative of three independent experiments.
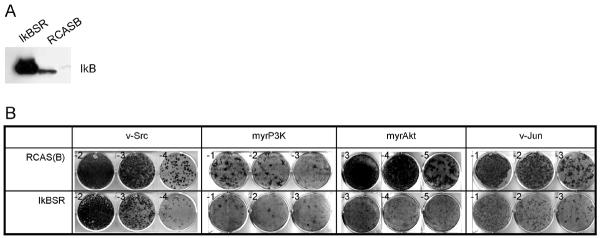
To test the functional significance of the IκB protein in Akt-induced cellular transformation, we expressed the super-repressor of NFκB (IκBSR) in CEF by using an RCAS vector with the subgroup B envelope protein followed by challenge infection with transforming RCAS constructs carrying the subgroup A envelope protein and expressing the v-src, myrp3k, myrakt or v-jun oncogenes. Overexpression of IκBSR was nontoxic to CEF and induced strong resistance to transformation by myrp3k and myrakt. IκBSR was much less effective in reducing transformation by v-jun and v-src (Fig. 3 A, B, Table 1).
Fig. 3.
IκBSR interferes preferentially with oncogenic transformation induced by P3K or Akt. CEF were transfected with RCAS(B)-IκBSR or empty vector and cultured for 10 days. (A) The expression of IκBSR was confirmed by Western blotting using anti-IκBα antibody. (B) The cells were then infected with oncogenic viruses using the subgroup A envelope protein and carrying the oncogenes v-src, myrp3k (PI3K), myrakt and v-jun. Virus dilutions (exponents of 10) are shown in the corner of each well. IκBSR induced strong resistance to transformation by myrp3k or myrakt (cf. Table 1). The experiments were repeated at least three times. The data presented here were from a representative experiment.
Table 1.
Effects of IκBSR on transforming activities of various oncoproteins in CEF
| RCAS vector control |
IκBSR |
|||
|---|---|---|---|---|
| Oncoprotein | FFU/ml | EOT | FFU/ml | EOT |
| v-Src | 9×105 | 1.0 | 3×105 | 0.3 |
| myrP3K | 3×103 | 1.0 | 2×102 | 0.07 |
| myrAkt | 2.8×106 | 1.0 | 1.2×105 | 0.04 |
| v-Jun | 1.8×105 | 1.0 | 6×104 | 0.3 |
FFU, focus-forming units; EOT, efficiency of transformation (FFU/ml on IκBSR- expressing cells divided by FFU/ml on RCAS-transfected cells).
The p65 subunit of NFκB is phosphorylated in Akt-transformed cells
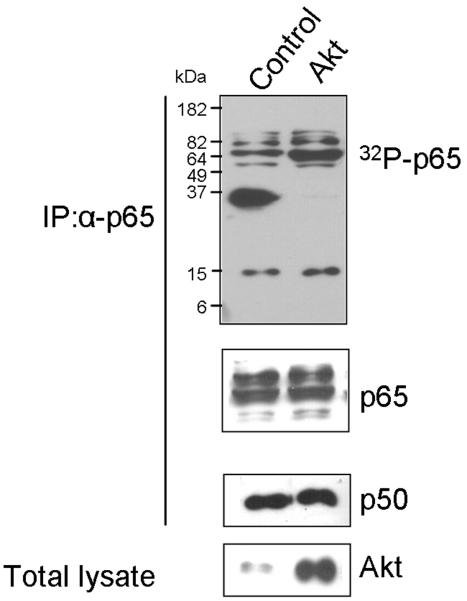
Recent investigations have focused on posttranslational modification of NFκB and elucidated the pivotal importance of phosphorylation for the transcriptional activity of NFκB.27 Phosphorylation of the p65 NFκB subunit increases transcriptional activity.28 We therefore analyzed the in vivo phosphorylation status of p65 in Akt-transformed cells and detected increased levels of 32P-labelled p65. The total amounts of p65 in control and transformed cells as determined in Western blots were comparable (Fig. 4).
Fig. 4.
Phosphorylation of p65 is induced in Akt-transformed cells. CEF transfected with vector control or myrAkt were incubated with [32P]orthophosphate for 3 h and lysed with Passive Lysis Buffer. Endogenous p65 was immunoprecipitated with p65 antibody. Phosphorylated p65 was detected by radioautography (top panel). Equal amounts of p65 loaded and the Co-IP of p50 were confirmed by Western blots (middle panels). Expression of Akt is shown using anti-HA antibody (bottom panel).
Phosphorylation of S534 in p65 is mediated by Akt
Several studies have demonstrated the phosphorylation of NFκB in response to various stimuli.1 Individual phosphorylation sites are targeted by a single or by several kinases.29 Phosphorylation on S335 of p50 increases the DNA binding capacity of this subunit.30, 31 S276 of p65 is phosphorylated by protein kinase A and mitogen- and stress-activated protein kinase and is necessary for the recruitment of cAMP response element-binding protein/p300 to p65.32 The phosphorylation of S527 by casein kinase II33 and of S53434 following TNF and LPS stimulation increase transcriptional activity. S276 is within NH2-terminal Rel homology35 domain which mediates dimerization and DNA-binding. S527 and 534 of p65 are in the COOH-terminal transactivation domain and are conserved in the human and mouse proteins (the residue numbers in this paper refer to the mouse p65 which was used in all experiments).1
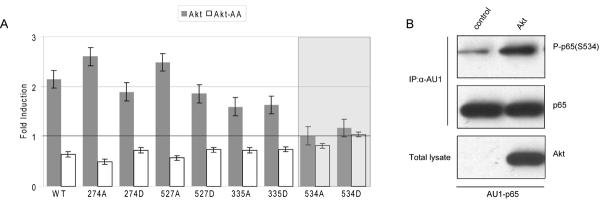
In order to identify phosphorylation events that are critical in the Akt-dependent regulation of NFκB, we mutated S335 in p50 and S276, 527 and 534 separately in p65. We generated phospho-deficient mutants (S to A) and phosphomimetic mutants (S to D). We then tested these mutants in reporter assays for their ability to respond with increased transcriptional activity to constitutively active Akt and with decreased activity to dominant negative Akt. Mutations affecting phosphorylation sites that are nonessential for the Akt-mediated regulation of NFκB would still show the positive effect of Akt and the negative effect of dominant negative Akt. Mutations of phosphorylation sites that are essential for the Akt-NFκB connection would no longer transmit the Akt-induced modulations of NFκB activity. The results are summarized in Fig. 5A. By this measure, only one phosphorylation site was identified as essential for Akt to NFκB signaling: S534 of p65. This observation suggests that S534 is the critical residue that links p65 to Akt. p65 phosphorylated at S534 was indeed detected at increased levels in cells transformed by Akt (Fig. 5B), thus Akt induces phosphorylation of p65 on S534 also in vivo.
Fig. 5.
Akt mediates p65 phosphorylation at S534. (A) CEF infected with vector control, myrAkt or dominant negative Akt were transiently transfected with either 150 ng of different p65 mutants and 150 ng of p50 wild type or 150 ng of p65 wild type and 150 ng of the p50 mutant, together with 100 ng of p5×κB and 5 ng of pRL-CMV reporter plasmids. After 48 h, the cell lysates were assayed for luciferase activity. All p65 or p50 mutants remained responsive to Akt, showing increased activity in myrAkt-CEF and decreased activity in Akt-AA-CEF, except the S534 mutant of p65 in which the effect of Akt was abolished. (B) CEF were co-infected with vector (control) or HA-Akt and AU1-p65. After 2 to 3 passages, whole cell lysates were immunoprecipitated with anti-AU1 agarose, electrophoresed and immunoblotted with antibodies to phospho-S534-p65 and p65. Expression of Akt is shown using anti-HA antibody.
IKKs bridges Akt and NFκB
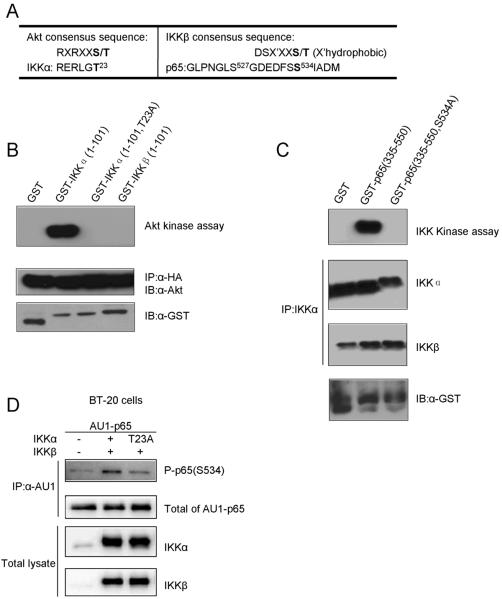
The flanking sequence around S534 of p65 does not conform to the consensus for phosphorylation by Akt (Fig. 6A). Akt is therefore unlikely to phosphorylate p65 directly. However, the site matches the IKKβ phosphorylation consensus sequence,36 and IKKα in turn has a perfect Akt phosphorylation site at T23.9 This information suggests that the IKK complex may link Akt to p65.
Fig. 6.
Phosphorylation of p65 and IKK in vitro and in vivo. (A) Alignment of the amino acid sequences around the Akt and the IKK consensus sequences compared to potential substrates. Predicted phosphorylation sites are denoted by bold letters. (B) CEF were transfected with HA-myrAkt. After 2 to 3 passages, Akt was immunoprecipitated with anti-HA agarose. Radioactive kinase assays, as detailed in Materials and Methods, were performed using GST, GST-IKKα (1-101 or 1-101/T23A) and GST-IKKβ (1-101) as substrates (upper panel). Levels of HA-Akt and total GST or GST-IKKs are shown as loading controls. (C) Endogenous IKKα was immunoprecipitated from lysates of HEK239 cells transfected with myrAkt. Radioactive kinase assays were performed using GST and GST-p65 (335-550 or 335-550/S534A) as the substrates (upper panel). The immunoprecipitated proteins were immunoblotted with antibodies to IKKα, IKKβ or GST for loading controls. (D) Phosphorylation of p65 by IKKs in vivo. BT-20 cells were cotransfected with 2 μg of AU1-p65 together with 2 μg IKKβ and IKKα or IKKα-T23A. Cotransfection of AU1-p65 and pcDNA was carried as control. The α-AU1-immunoprecipitated proteins were immunoblotted with antibodies to phospho-S534-p65 or p65. Whole cell lysates were subjected to Western blot using antibodies to IKKα and IKKβ.
Phosphorylation of IKKα at T23 by Akt in vitro was reported before9, and we confirmed this result in our system (Fig. 6B). In an in vitro kinase assay, immunoprecipitated HA-myrAkt was incubated with GST, GST-tagged NH2-terminal fragment (1-101) of IKKα or IKKβ. Only IKKα was phosphorylated by Akt but not IKKβ which has no phosphorylation site that corresponds to T23 of IKKα. The IKKα T23A mutant was not phosphorylated. The Western blot using antibodies to Akt or GST from immunoprecipitates demonstrated that equal amounts of Akt and of the substrates were present in each reaction (Fig 6B). These results suggest that Akt phosphorylates IKKα at T23 in vitro.
We then examined whether IKKs could phosphorylate p65 at S534. Sakurai et al. have reported that the endogenous IKK complex, overexpresssed IKKs, and recombinant IKKβ can phosphorylate S534 of p65 in vitro. We did not observe p65 phosphorylation by overexpressing either IKKα or IKKβ alone in Akt-transformed cells (data not shown) and therefore suggest that in vivo both IKKα and IKKβ contribute to the phosphorylation of S534 in p65. We immunoprecipitated endogenous IKKs from myrAkt-expressing HEK 293 cells using anti-IKKα agarose (Fig. 6C). The COOH-terminal (335-550) fragment of p65 was phosphorylated by this IKK complex, whereas the S534A mutant of p65 was not. A Western blot showed the presence of both IKKα and IKKβ in the IKK kinase assay.
We examined the functional significance of T23 phosphorylation in IKKα by co-transfecting BT20 human breast cancer cells with AU1-tagged p65 and Flag-tagged IKKs. The IKK complex increased p65 phosphorylation at S534 compared to the basal level in the absence of IKKs. However, co-transfection of an IKK complex with the T23A mutation in IKKα failed to stimulate the p65 S534 phosphorylation (Fig. 6D). We conclude that T23 is important for IKK kinase activity on S534 in p65.
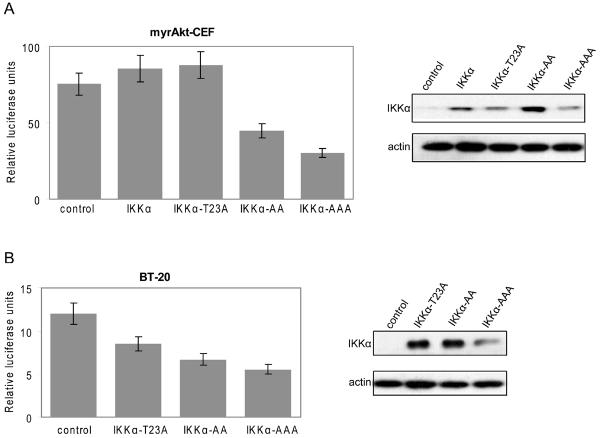
We then examined the effects of IKKα mutants on NFκB reporter activity. When empty vector (control) was co-transfected into BT20 cells or Akt-transformed CEF with the NFκB luciferase reporter construct (control), high levels of NFκB transcriptional activity were recorded (Fig. 7A, B). In myrAkt-CEF, wild type IKKα failed to further enhance the reporter activity (Fig. 7A), probably because the activity level of NFκB had reached a state of saturation. Expression of the inactive IKKα-T23A mutant also showed no significant effect in Akt-transformed CEF, probably for the same reason (Fig. 7A). In BT20 cells, the T23A mutant reduced NFκB activity. Importantly, the dominant negative IKKα-S176A/S180A mutant (AA) inhibited the potentiation of NFκB reporter activity strongly in both Akt-transformed CEF and in BT-20 cells, confirming dependence of NFκB activity on IKKα kinase activity. Expression of the triple mutant IKKα-T23A/S176A/S180A (AAA) showed a further decrease in activity compared with IKKα-S176A/S180A (Fig. 7A, B).
Fig. 7.
Akt activates NFκB through IKK. (A) CEF transformed by myrAkt were transfected with 300 ng of pcDNA vector (control) or constructs encoding IKKα wild type (IKKα) or the mutants T23A, S176A/S180A (AA), or T23A/S176A/S180A (AAA), together with 100 ng of p5×κB and 5 ng of pRL-CMV reporter plasmids. After 48 h, luciferase activities were determined. (B) BT-20 cells were transfected with 300 ng of pcDNA vector (control) or the IKKα mutantsT23A, AA and AAA as in (A). Total cell lysates were immunoblotted with antibodies to FLAG or actin. The error bars represent standard deviations of triplicate transfection experiments.
Discussion
This study contributes to our understanding of the link between Akt and NFκB. The Akt pathway is actively involved in the regulation of NFκB, and NFκB activity is essential for oncogenic transformation by PI3K and Akt.5-7, 9, 22 In cells with a gain of function in Akt, the transcriptional activity of NFκB is upregulated, and inhibition of Akt interferes with this upregulation of NFκB. In CEF transformed by myristylated Akt, there is greatly enhanced degradation of the IκB protein and increased phosphorylation of the p65 NFκB subunit. Our data rule out direct phosphorylation of the p65 or p50 NFκB subunits by Akt. Neither protein contains an Akt phosphorylation consensus sequence, and Akt fails to phosphorylate p65 in an in vitro kinase assay. Rather, our observations show that Akt phosphorylates IKKα, in accordance with published studies.9 The IKK complex then not only targets the IκB inhibitor protein, but, in agreement with previous observations, also phosphorylates the p65 NFκB subunit.22, 34, 37, 38 Thus the IKK complex targets both the IκB and NFκB p65 proteins and functions as the intermediary between Akt and NFκB. NFκB p65 and IκB are located in the same cellular compartment and are phosphorylated in a similar time-dependent pattern.34 The putative IKK phosphorylation site of p65, S534, matches the IKKβ consensus sequence, is located in the COOH-terminal transactivation domain 1, and is conserved in human, mouse, chicken and Xenopus. Phosphorylation of this residue eliminates a hydrogen bond to the nearby D531 residue in p65, and results in an efficient association of p65 with TAFII31, a component of the basal transcriptional machinery. At the same time phosphorylation reduces the affinity of p65 for the transcriptional corepressor AES (Amino-terminal Enhancer of Split). This change of affinities suggests a possible mechanism for the activation of NFκB by phosphorylation.39 In more general terms, a phosphate group, providing additional negative charge is likely to enhance the transcriptional activity of the acidic activator domain of NFκB.40 Our data document the functional significance of the phosphorylation cascade that originates with Akt and progresses through T23 of IKKα to S534 of p65.
Signaling between Akt and NFκB is complex, and the published data contain several seemingly contradictory observations. At least some of these discrepancies may reflect the use of different cell types and signaling conditions. For instance, Sizemore et al. showed that PI3K/Akt was necessary for the phosphorylation and activation of p65 in response to TNF and IL-1, and that Akt-mediated NFκB activation required IKK activity.6, 41 In contrast, Yang et al. reported that in mouse macrophages, LPS-induced p65 phosphorylation at S534 was unaffected by LY294002, an inhibitor of PI3K.42 Ozes and associates9 found that Akt was an essential mediator of the TNFα-induced activation of NFκB, operating through the phosphorylation of IKKα at T23, but this finding has been challenged by Delhase and coworkers.43
Studies on cells with genetic inactivation of components of Akt signaling have produced some surprising results. Deletion of glycogen synthase kinase 3β (GSK3β) interferes with the TNFα-induced activation of NFκB, and, surprisingly, even with the TNFα-triggered activation of Akt.44 Cells that are deficient in tuberous sclerosis complex 1/2 proteins (TSC1/TSC2) are also defective in the TNFα-induced activation of NFκB, and this defect can be eliminated by rapamycin, suggesting that in this constellation, mTOR is a negative regulator of the TNFα- NFκB signal.45 In contrast, PTEN -negative cells depend on the mTOR-Rictor complex for the TNFα-induced activation of NFκB.46, 47 These divergent observations suggest that the activation of NFκB by extracellular stimuli can be affected by numerous signaling components and that these effects are dependent on the physiological conditions and genetic make-up of the cells. NFκB is an important factor in cancer development and progression, in addition to being a central coordinator of immune responses.27, 48, 49 The viral protein v-rel (homolog of c-rel) was originally identified as retroviral oncogene.50, 51 Many tumors show constitutively elevated levels of NFκB activity caused by genetic changes, including loss-of-function mutations in the IκB gene or activation of upstream regulators such as IKKs.27 Blocking NFκB activity decreases tumorigenicity.52-54 Our results show that suppression of NFκB activity by IκBSR induces a strong and selective resistance to P3K- or Akt-induced oncogenic transformation, suggesting an essential role for NFκB in the transforming mechanisms induced by these oncoproteins. The relative importance of IκB degradation versus p65 phosphorylation in mediating transformation cannot be determined from the available data. However, the essentialness of NFκB activity for PI3K /Akt oncogenicity is particularly significant in view of the fact that the PI3K pathway is dysregulated in many human cancers. PI3K and Akt are considered promising cancer targets, and the dependence of these oncoproteins on NFκB needs to be considered in the search for therapeutically effective inhibitors.
In Akt-transformed CEF as well as in BT20 human breast cancer cells, signaling through Akt is upregulated, and the downstream targets GSK-3β, ribosomal protein S6 kinase, and eukaryotic translation initiation factor 4E-binding protein are all phosphorylated.16, 25 The constitutive activity of Akt in these cells defines a signaling landscape that is shared with other cells harboring a gain of function in the PI3K-Akt-TOR pathway. These types of cells will probably show the direct signal from activated Akt via the IKK complex to p65 that is suggested by the data presented here. Whether this pathway also applies to all external stimulus-induced activations of NFκB will have to be decided by future investigations.
Acknowledgments
We thank Dr. Sohye Kang and Dr. Jonathan Hart for helpful discussions. We also thank Anja Zembrzycki and Dr. Chao Yang for critical reading of the manuscript. This work was supported by grants from the National Cancer Institute. This is manuscript number 19902 of The Scripps Research Institute.
Abbreviations
- Akt
cellular homolog of murine thymoma virus akt8 oncogene
- myrAkt
myristylated Akt
- PKB
protein kinase B
- PI3K
phosphatidylinositol 3-kinase
- myrP3K
myristylated catalytic subunit of PI3K
- NFκB
nuclear factor-κB
- IκB
inhibitor of κB
- CEF
chicken embryonic fibroblasts
- IKK
IκB kinase
- IκBSR
super-repressor of NFκB
- IL
interleukin
- NEMO
NFκB essential modulator
- DMSO
dimethylsulfoxide
- PR-A
Prague strain of Rous sarcoma virus
- HEK293
human embryonic kidney cells
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- TNFα
tumor necrosis factor α
- LPS
lipopolysaccharide
- GSK3β
glycogen synthase kinase 3β
- TSC1/TSC2
tuberous sclerosis 1/2
- mTOR
mammalian target of rapamycin
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- TAFII31
transcription factor II31
- AES
amino-terminal enhancer of Split
References
- 1.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto T, Sanda T, Asamitsu K. NF-kappa B signaling and carcinogenesis. Curr Pharm Des. 2007;13:447–62. doi: 10.2174/138161207780162944. [DOI] [PubMed] [Google Scholar]
- 3.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 4.Ito K. Impact of post-translational modifications of proteins on the inflammatory process. Biochem Soc Trans. 2007;35:281–3. doi: 10.1042/BST0350281. [DOI] [PubMed] [Google Scholar]
- 5.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–4. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 6.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3- kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 8.Gustin JA, Ozes ON, Akca H, Pincheira R, Mayo LD, Li Q, Guzman JR, Korgaonkar CK, Donner DB. Cell type-specific expression of the IkappaB kinases determines the significance of phosphatidylinositol 3-kinase/Akt signaling to NF-kappa B activation. J Biol Chem. 2004;279:1615–20. doi: 10.1074/jbc.M306976200. [DOI] [PubMed] [Google Scholar]
- 9.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 10.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li Jw, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: Cytokine-Activated I{kappa}B Kinases Essential for NF-B Activation. Science. 1997;278:860–6. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 11.Ling L, Cao Z, Goeddel DV. NF-{kappa}B-inducing kinase activates IKK-{alpha} by phosphorylation of Ser-176. Proceedings of the National Academy of Sciences of the United States of America; 1998. pp. 3792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tergaonkar V, Bottero V, Ikawa M, Li Q, Verma IM. IkappaB kinase-independent IkappaBalpha degradation pathway: functional NF-kappaB activity and implications for cancer therapy. Mol Cell Biol. 2003;23:8070–83. doi: 10.1128/MCB.23.22.8070-8083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The Akt kinase: Molecular determinants of oncogenicity. Proc.Natl.Acad.Sci.USA. 1998;95:14950–5. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt PK. Focus assay of Rous sarcoma virus. In: Habel K, Salzman NP, editors. Fundamental Techniques in Virology. First ed. Academic Press; New York: 1969. pp. 198–211. [Google Scholar]
- 15.Federspiel MJ, Hughes SH. Retroviral gene delivery. Methods Cell Biol. 1997;52:179–214. [PubMed] [Google Scholar]
- 16.Bader AG, Felts KA, Jiang N, Chang HW, Vogt PK. Y box-binding protein 1 induces resistance to oncogenic transformation by the phosphatidylinositol 3-kinase pathway. Proc Natl Acad Sci U S A. 2003;100:12384–9. doi: 10.1073/pnas.2135336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maki Y, Bos TJ, Davis C, Starbuck M, Vogt PK. Avian sarcoma virus 17 carries the jun oncogene. Proc Natl Acad Sci U S A. 1987;84:2848–52. doi: 10.1073/pnas.84.9.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duff RG, Vogt PK. Characteristics of two new avian tumor virus subgroups. Virology. 1969;39:18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- 19.Aoki M, Schetter C, Himly M, Batista O, Chang HW, Vogt PK. The catalytic subunit of phosphoinositide 3-kinase: requirements for oncogenicity. J Biol Chem. 2000;275:6267–75. doi: 10.1074/jbc.275.9.6267. [DOI] [PubMed] [Google Scholar]
- 20.Bos TJ, Monteclaro FS, Mitsunobu F, Ball AR, Chang CH, Nishimura T, Vogt PK. Efficient transformation of chicken embryo fibroblasts by c-Jun requires structural modification in coding and noncoding sequences. Genes & Development. 1990;4:1677–87. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- 21.Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of Chicken Cells by the Gene Encoding the Catalytic Subunit of PI 3-Kinase. Science. 1997;276:1848–50. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 22.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr., Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–76. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 25.Wu G, Xing M, Mambo E, Huang X, Liu J, Guo Z, Chatterjee A, Goldenberg D, Gollin S, Sukumar S, Trink B, Sidransky D. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Research. 2005;7:R609–R16. doi: 10.1186/bcr1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinsey TA, Brockman JA, Scherer DC, Al-Murrani SW, Green PL, Ballard DW. Inactivation of IkappaBbeta by the tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-kappaB. Mol Cell Biol. 1996;16:2083–90. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 28.Campbell KJ, Perkins ND. Post-translational modification of RelA(p65) NF-kappaB. Biochem Soc Trans. 2004;32:1087–9. doi: 10.1042/BST0321087. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 30.Hou S, Guan H, Ricciardi RP. Phosphorylation of serine 337 of NF-kappaB p50 is critical for DNA binding. J Biol Chem. 2003;278:45994–8. doi: 10.1074/jbc.M307971200. [DOI] [PubMed] [Google Scholar]
- 31.Guan H, Hou S, Ricciardi RP. DNA binding of repressor nuclear factor-kappaB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. J Biol Chem. 2005;280:9957–62. doi: 10.1074/jbc.M412180200. [DOI] [PubMed] [Google Scholar]
- 32.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–71. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–7. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 34.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–6. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 35.Mayo MW, Madrid LV, Westerheide SD, Jones DR, Yuan XJ, Baldwin AS, Jr., Whang YE. PTEN blocks tumor necrosis factor-induced NF-kappa B-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J Biol Chem. 2002;277:11116–25. doi: 10.1074/jbc.M108670200. [DOI] [PubMed] [Google Scholar]
- 36.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 37.Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279:55633–43. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–43. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 39.Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and Interleukin-1-inducible Phosphorylation of p65 NF-{kappa}B at Serine 536 Is Mediated by Multiple Protein Kinases Including I{kappa}B Kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF Family Member-associated (TANK)-binding Kinase 1 (TBK1), and an Unknown Kinase and Couples p65 to TATA-binding Protein-associated Factor II31-mediated Interleukin-8 Transcription. J. Biol. Chem. 2004;279:55633–43. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz M, dos Santos Silva M, Altmann H, Czisch M, Holak T, Baeuerle P. Structural and functional analysis of the NF-kappa B p65 C terminus. An acidic and modular transactivation domain with the potential to adopt an alpha-helical conformation. J. Biol. Chem. 1994;269:25613–20. [PubMed] [Google Scholar]
- 41.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem. 2002;277:3863–9. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- 42.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 43.Delhase M, Li N, Karin M. Kinase regulation in inflammatory response. Nature. 2000;406:367–8. doi: 10.1038/35019154. [DOI] [PubMed] [Google Scholar]
- 44.Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–54. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh S, Tergaonkar V, Rothlin CV, Correa RG, Bottero V, Bist P, Verma IM, Hunter T. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and cell survival. Cancer Cell. 2006;10:215–26. doi: 10.1016/j.ccr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Dan HC, Baldwin AS. Differential involvement of IkappaB kinases alpha and beta in cytokine- and insulin-induced mammalian target of rapamycin activation determined by Akt. J Immunol. 2008;180:7582–9. doi: 10.4049/jimmunol.180.11.7582. [DOI] [PubMed] [Google Scholar]
- 47.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–6. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilhelmsen KC, Eggleton K, Temin HM. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984;52:172–82. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–37. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- 52.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 53.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]