Abstract
Although mood has a direct impact on mental and physical health, our understanding of the mechanisms underlying mood regulation is limited. I propose here that there is a direct, reciprocal relation between the cortical activation of associations and mood regulation, whereby positive mood promotes associative processing, and associative processing promotes positive mood. This relation might stem from an evolutionary pressure for learning and predicting. Along these lines, one can think of mood as a reward mechanism that guides us to use our brains in the most productive manner. The proposed framework has many implications, most notably for diagnosing and treating mood disorders such as depression, for elucidating the role of inhibition in the regulation of mood, for contextualizing adult hippocampal neurogenesis, and for a general, non-invasive improvement of well-being.
Rationale for linking mood with associative processing
Mood has direct impact on mental and physical health, ranging from depression and anxiety to cardiovascular disease, addiction, psychological resistance, cognitive performance, aging and longevity. Nevertheless, our understanding of the mechanisms underlying mood regulation is quite limited. The crux of the hypothesis presented here is that mood is directly linked to how associative or inhibited are our mental processes. There is compelling evidence in support of one direction of this link: that positive mood results in broad associative activation of related concepts. Here I propose that the reverse relation also exists, whereby broad activation of associations results in improved mood. This relation might stem from an evolutionary pressure to learn and explore many alternatives in parallel, possibly motivated by increased release of neuropeptides with increased activation of associations. One function of such simultaneous exploration could be to support humans' constant need to anticipate relevant outcomes. Therefore, the activation of associations might be beneficial for improving mood because associations afford the generation of predictions, and predictions minimize uncertainty, thus reducing anxiety and stress, both concomitants of mood disorders. The second mood-related benefit of broad associative activation is that associations prevent persistent rumination, another hallmark of mood disorders, by “distracting” the thought process away from dwelling on a narrow, negative theme. Broad associative activation helps gain a broader perspective.
I will synthesize existing evidence that collectively supports the proposed link between mood and associations and suggest testable predictions that are derived from the ideas outlined here. Finally, I will make a number of suggestions for therapeutic approaches that build on this theory to potentially alleviate symptoms of major depression and, more generally, improve well-being through better mood.
Relevant aspects of associative processing
Our world consists of patterns that typically occur together (e.g., kitchens contain refrigerators, visiting a museum requires being quiet, and showers are taken without clothes). These statistical regularities are encoded in memory as associations, linking related representations and co-activating them as necessary in subsequent encounters. The ability to associate a particular sensation (or perceptual feature, object, concept, or emotion) with another is critical for virtually every aspect of mental functioning. In addition to their fundamental and widely studied role in learning, memory encoding and retrieval, problem solving, creativity, action, and spatial navigation, associations provide the basis required for generating predictions [1]. When we encounter a novel situation, we extract from memory an analogous situation with which we are already familiar, and apply the corresponding associative knowledge from memory to anticipate relevant aspects of the present, novel but similar, situation.
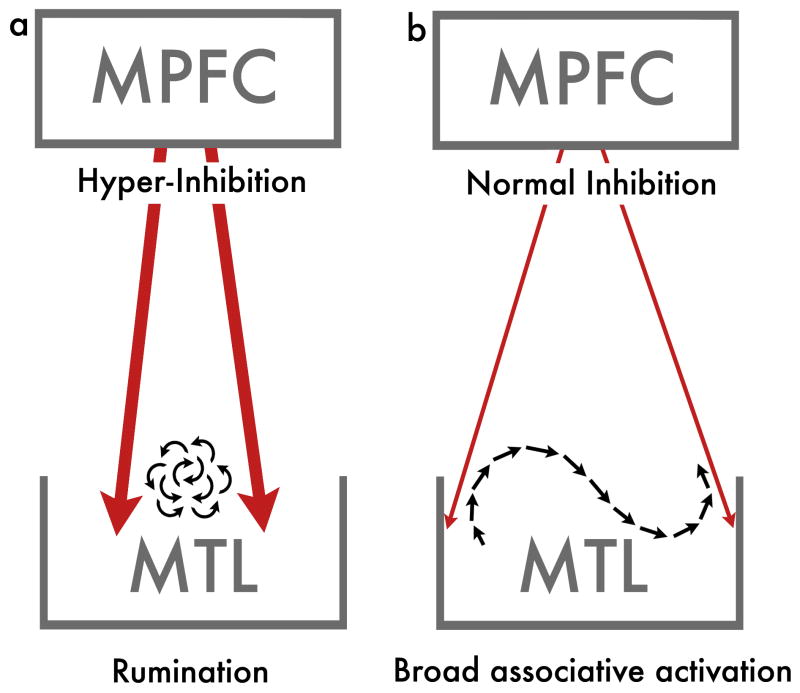
In the context of the proposal put forth here, associations provide the vehicle with which our thoughts advance from one representation to another. Central to the proposal put forward here is the distinction between narrow and broad associative activations. Narrow associative thinking, or rumination, refers to associations that surround a narrow focus (e.g., the context of my bad comment over dinner last night: what I said, what I should have said, the resulting facial expressions and verbal responses to it, possible future implications and so forth). Ruminative, narrow associative processing, as proposed below, might stem from excess prefrontal inhibition (Figure 1a). Broad associative activations, on the other hand, involve more remote associations and, critically, activate associations that make thought processes advance from one context to another smoothly (Figure 1b), thereby minimizing rumination (e.g., “in spite of that miserable comment, dinner was really tasty; never tried the combination of figs with prosciutto before; need to go grocery shopping tomorrow”).
Figure 1. – Rumination versus broadly associative thinking.
(a) Thought pattern typical of mood disorder involves rumination around a narrow focus. Even if this thought pattern is associative, it is limited in scope. Such constrained thought is proposed here to stem from hyper-inhibition from the medial prefrontal cortex (MPFC) to the medial temporal lobe (MTL). (b) The thought pattern in the brain of individuals without mood disorders is characterized by a broadly associative activation, which although is still affected by inhibition signals (for functional guidance), can seamlessly disengage from one focus and advance to another.
Using human neuroimaging, we recently studied the cortical mechanisms mediating contextual associations in particular [2-4]. The associations that tie items that share the same context consistently activate three main cortical regions: the parahippocampal cortex in the medial temporal lobe (MTL), the medial partial cortex (MPC), and the medial prefrontal cortex (MPFC) [3]. This contextual associations network shows a striking overlap with the cortical network termed the “default network” [5], as we have shown recently [4]. The default network is believed to subserve the mental processes that take place in the brain when we are not engaged in a specific goal-oriented task [6, 7]. This overlap between the default network and the network subserving associative processing of contextually related information is taken as the cortical manifestation of the idea that associative processing is a crucial element of natural thought [4]. In other words, associative activations provide the building blocks that allow our thought processes to proceed from one topic to another. Notably, other tasks, including hedonic processes, reward-related processes and affective processes, also activate parts of this associative network. We have interpreted this common activation by different processes as reflecting the fact that what unites these processes is that they all rely on associative activations at their core [4].
Contextual associations and mood are linked here directly first by noting recent reports that depressed patients tend to ignore global context [8] (e.g., “not taking things in perspective”). Such findings make sense when one considers the tendency of depressed individuals to ruminate on a single, usually negative, theme. The link to the single-track, narrow mode of thinking in depression is strengthened further by findings showing that the contextual associations network, including the MTL, MPC and MPFC, functions abnormally in depressed patients [9, 10]. Finally, the link between depression and contextual associations might further be supported by recent findings of adult neurogenesis in the MTL in the context of depression treatment.
Positive mood promotes associative processing
Human subjects in a positive mood provide more unusual associates to neutral words, compared with subjects in negative or neutral moods [11]. In addition, positive mood facilitates creative ingenuity in problem solving [12], and false memory, which is presumably a result of associative activation, is minimized in negative mood [13].
A parallel to the conclusion that positive mood promotes associative thinking is the finding that positive mood is accompanied by a wider attentional “spotlight,” whereas negative mood can be characterized by a more focused type of thinking [14]. For example, when subjects need to accomplish memory or categorization tasks, those in a happier mood utilize available global information whereas the attention of participants in a sad mood is focused on more local properties. Similarly, positive mood and optimism are directly associated with a global processing bias in visual tasks, whereas a local bias is observed for people in negative mood and depression [15]. A negative mood may signal the potential presence of threat and thus might elicit a more focused mode of processing. Indeed, dangerous environments also have the same effect on the scope of attention as that of negative affect [16]. The effects and functional merits of positive emotions, on the other hand, have been framed as expanding thought repertoire (i.e., scope) in a host of dimensions within a comprehensive related framework termed broaden-and-build [17].
Finally, associative processing has been linked with the ability to imagine future events and to predict upcoming information [1]. Therefore, the fact that foresight is severely impaired in depressed individuals [18] provides encouraging support for the idea that associative activation and mood are tightly related.
The proposal here is not only that mood broadens associations and attentional scope, as reviewed above, but most critically that associative thinking can promote positive mood. This direction of influence (associative cognition → improved mood) could have profound implications for the treatment of mood disorders.
Associative processing promotes positive mood
Rumination, the preoccupying, self-focused thinking pattern that is commonly a concomitant of mood disorders [19] could be seen as the functional opposite of broad associative thinking, given its very narrow focus. If rumination involves associative processing, such associations do not advance far beyond the main focus of rumination (Figure 1a). Inducing rumination even in healthy individuals results in a negative mood [20]. Interestingly, it has been suggested that the focus of rumination does not necessarily have to be negative in nature [21, 22], so it is possible that the process of rumination, rather than exclusively its content, is the cause of the negative mood. However, to demonstrate unequivocally that it is the rumination process, and not necessarily the content, that affects mood, one would have to show that ruminating on a positive thought also elicits negative mood. The problem is that, by definition, within the framework proposed here, ruminating on positive content for long periods is impossible because the positive content will make the individual drift and associate broadly. Nevertheless, independent of the interesting question of process versus content, it is proposed here that inducing the opposite of rumination (i.e., frequent, broad associative activation) will elicit a positive mood.
Rumination seems to be a major vulnerability factor for the negative mood associated with major depression but also with other disorders, such as post-traumatic stress disorder (PTSD) [23], anxiety, obsessive-compulsive disorder (OCD) and more. In fact, given the overlap between rumination and worry, and the prevalence of both [24], the present hypothesis may be relevant across most psychiatric disorders.
One effective therapy in treating stress disorders involves shifting attention from an upsetting stimulus to something relatively neutral [25]. However, merely shifting the focus of attention does not necessarily activate broad associations and thus might lose its efficiency of distracting the ruminating mind as a long-term remedy. Indeed, in accordance with the hypothesis presented here, distraction as a soothing technique is most effective when the identity of the distracter varies frequently [26, 27]. One can therefore consider frequent, broadly associative thinking as providing a higher rate of distraction and thus fewer opportunities to ruminate and dwell on a negative thought.
Interestingly, it has been reported that speed of thought, as manipulated by paced reading, has direct influence on mood [28]. Specifically, these studies suggest that reading faster can make one feel more positively, regardless of whether the content of the text is positive or negative. Reading causes the activation of concepts, and, presumably, faster reading activates more concepts, which could be seen as analogous to the massive activation in broad associative activation (i.e., more concepts activated per time unit). Therefore, although this intriguing finding could be attributed to the mere speed of “mental motion” [22], or perhaps to the fact that a mentally demanding reading activity could serve as a powerful means of distraction from rumination, it could also provide support to the proposal outlined here that increased associative thinking elicits increased positive mood.
The present framework goes beyond previous reports in that it describes an actual mechanism, and this mechanism integrates cognition, neuroscience and clinical findings. The result is a framework within which other proposals (e.g., [22, 28]) seem to fit naturally.
Neural elements linking mood and associative processing
Converging evidence for the proposed framework comes from research in neuroscience and psychiatry. First, that successful treatment of depression seems to be conditional upon a concomitant volume increase in the adult hippocampus [29]. Second, that electrically stimulating prefrontal regions implicated in associative processing alleviates depression symptoms [30]. These two lines of findings will be elaborated next.
Associative processing and the ability to learn associations between previously unrelated items have been attributed primarily to structures of the MTL, particularly the hippocampus [31, 32], and the parahippocampal cortex in the MTL [4, 33]. Several recent studies have demonstrated both functional disturbances and volume reduction of the hippocampal formation in depression [34, 35]. Importantly, the severity of depressive symptoms is directly increased by such hippocampal structural disturbances [36], as well as with a decrease in hippocampal activity [37]. Additionally, successful antidepressant treatment can halt the progression of hippocampal damage [35], and is associated with recovery of hippocampal volume [38]. These results demonstrate the close relationship between depression and the functional and structural integrity of the MTL.
One of the most effective medications for treating depression relies on selective serotonin reuptake inhibitors (SSRI) such as fluoxetine (e.g., Prozac). In recent years it has been observed that SSRIs have a direct influence on adult neurogenesis [39], particularly in the dentate gyrus within the hippocampus [35, 40]. Importantly serotonin has also been shown to play a central role in learning and memory formation [41], which is widely believed to be mediated by the MTL and to rely on associations.
Adult hippocampal neurogenesis is still a new and controversial topic. Nevertheless, the possibilities, especially for our understanding of mental disorder, are profound and thus warrant further attention. There is accumulating evidence that hippocampal neurogenesis is not merely an epiphenomenon of the SSRIs' chemical effect, but actually has a causal effect on the success of SSRI treatment: The newly generated neurons have direct effect on the state of depression, whereby successful antidepressant treatment is conditional upon a successful hippocampal neurogenesis. Indeed, blocking hippocampal neurogenesis substantially reduces the efficiency of antidepressants [29].
Computational modeling work [42] shows that such neurogenesis could be critical for establishing new contexts for behavior in memory, and such contexts, we have argued [4], are based on associative representations and activations. Indeed, associative long-term potentiation (LTP) can be induced in new neurons more readily than in older neurons [43], and the magnitude of LTP can be modulated by serotonin [44]. These converging findings, together, provide a potential mechanism for mediating the increase in associative processing with neurogenesis and SSRI-based therapy (see Box 1).
Box 1. Some qualifications regarding hippocampal neurogenesis and depression.
First, it is worth noting that loss of neurogenesis seems neither sufficient nor necessary for developing depression symptoms, but neurogenesis can nevertheless be critical for treatment success. Within the framework proposed here, one can imagine a thinking pattern that is broadly associative because the infrastructure that affords broad associative processing is in place, and can remain so even without neurogenesis. However, for a depressed individual such infrastructure presumably needs to be rebuilt, which can only be done with new neurons and new connections. Furthermore, the birth of new hippocampal neurons will exert no behavioral and clinical influence if these new neurons do not integrate and survive; neurogenesis without associative activity that will promote the survival of the new neurons will not be sufficient for alleviating the symptoms of mood disorders, according to this proposal. Therefore, in thinking about the reciprocal relation between neurogenesis and associative activation, neurogenesis can be seen as providing the medium, but this medium needs to be used by broadly associative activation to survive and generate the webs of associations that mediate the non-ruminative activation required for a healthy mood.
Second, neurogenesis is not always beneficial, as for example is the case in temporal lobe epilepsy [75], where new cells might migrate incorrectly. Third, depression might be accompanied by cognitive impairments that might go beyond associative processing. For example, the MPFC has been implicated in thinking about self and in reward related processing. However, these other functions rely on associative processing; for example, rumination is associated with self-reflections and reward relies on learned associations. Finally, this is not to suggest that all types of depression can be treated in the same fashion [76], or that all stem from hippocampal/prefrontal processes.
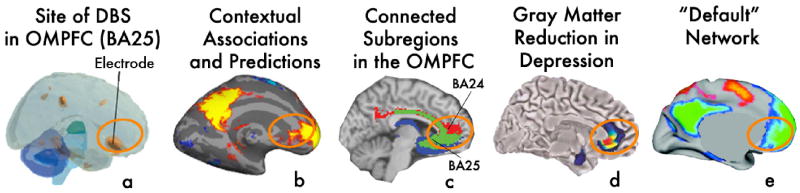
In a second line of research that is related to the proposal presented here, findings indicate that electroconvulsive therapy (ECT) and transcranial magnetic stimulation (TMS) exhibit anti-depressant effects [45]. How these treatments exert their effects remains an open question. Recently, Mayberg and colleagues [30, 46] demonstrated successfully that electrically stimulating the deep Brodmann area 25 (Figure 2a) can alleviate the symptoms of major depression patients. Area 25, the subgenual cingulate, is situated in the MPFC, which is activated in associative cognitive tasks (Figure 2b). One possible mechanism by which such deep brain stimulation (DBS) in MPFC can affect depression symptoms might be through the regulation of inhibition. Our memory consists of a web of representations that are connected with each other, within several degrees of separation. While this makes for an efficient framework for encoding and retrieval, it is crucial that the activation of one mental representation (e.g., a chair, when we see an image of a chair) would not result in the activation of representations that are irrelevant in the specific context. Inhibition is proposed to play this central role of limiting the extent of related representations that are activated at a given instance (e.g., seeing an image of a chair would activate “chair,” “table” and perhaps “legs,” but should not activate “chicken legs”), thereby giving rise to associations and predictions that are most relevant. In the context of inhibition and mood, an abnormally activated MPFC, as in depression [47], can result in over-inhibition from MPFC to MTL, which might then significantly constrain the scope of associative activation in MTL [48]. Excessive inhibition from MPFC could explain the inability of depressed patients to disengage from debilitating rumination (Figure 1a). Indeed, inhibitory dysfunction can exacerbate depression through rumination [49]. DBS might therefore operate by bringing MPFC back to its normal level of activity (Figure 1b), which brings back inhibition to a level where MTL can resume broader associative activation.
Figure 2. - Converging activations in MPFC.
The same MPFC region that is stimulated by deep brain stimulation (DBS) to treat severe depression, and where activation is most indicative of successful treatment (a), is strongly active in our studies of context-based associative predictions (b). Panel a. is modified from [71] with permission. (c) Anatomical support to the direct and extensive connection between BA25 and the MPFC region activated by contextual associations has been reported previously [72], as well as using population maps of probabilistic tractography of strongly interconnected MPFC regions [73] (reproduced with permission). (d) The same region shows reduced gray matter in depression patients [46] (reproduced with permission). (e) The default network [74], which shows a striking overlap with the same network that mediates contextual associations and predictions [4] (reproduced with permission). Recruiting this MPFC region by cognitive means, as in (b), could potentially regulate inhibition and retrain the natural tendency to engage in broad associative activation, which is compromised in mood disorders.
It is particularly interesting to note that the pattern of brain activity typically observed at “rest” (i.e., default brain activity) in healthy individuals differs in patients with depression symptoms. The MPFC and the neighboring anterior cingulate cortex in particular exhibit abnormal activity during periods of “rest” in depressed individuals compared with non-depressed individuals [50], and activation in these regions seems to predict treatment success [51]. Furthermore, the structure, function and connectivity of the same default/associative network (Fig. 2b&e) are compromised in depression [52, 53], and the integrity of this network is improved with antidepressants-related clinical improvement [53]. A therapeutic approach that promotes activation of those regions via intense associative processing can be seen as a cognitively generated analogue of DBS, and thus might elicit similar mood benefits. While cognitive, endogenous triggering of associations and regulation of inhibition is likely to be less intense than direct electrical deep stimulation, it is expected to be considerably more focused on the presumed circuitry of interest. In addition, DBS is primarily applied in cases of severe major depressive disorder (MDD), when most other methods have failed. It is possible that for less severe cases of depression, a reduced intensity of stimulation in the same area will suffice for observing a significant symptomatic improvement.
Further implications for therapy and testable predictions
Existing cognitive-behavioral therapy (CBT) techniques approach mood disorder by aiming to help patients first recognize thoughts that accompany their negative emotions, then to distance themselves from these thoughts, and then encourage the patients to question the validity of the beliefs embedded in their maladaptive thoughts [54, 55]. CBT methods have been shown to improve symptoms for certain depression patients [56], and even more so when conducted in conjunction with antidepressant medication treatment [57]. CBT has also been shown to modify brain activation patterns in depressed patients [58], with some overlap with the modifications caused by antidepressant medication [54]. The aims of CBT can be seen as teaching patients to see things in a broader perspective and to incorporate more context (and thus more associative processing) into their analysis of emotional information, an approach that could be explained with the mechanism put forth here. Nevertheless, CBT requires a high level of functionality and introspection from patients, and it is unclear whether this method is feasible and effective for the same large patient population as antidepressants. The proposal presented here gives rise to the (not necessarily conscious) acquisition of mental habits of broad associative activation and a cognitive-driven reconstruction of the underlying cortical network. Such an approach can potentially increase the efficiency of non-invasive therapy, elicit a positive response in a wider portion of patients, and demand considerably less psychotherapeutic, introspective effort from patients.
Another advantage of promoting broad associative activations as part of a therapy is that it does not require patients to actively suppress specific thoughts, but rather simply increases the shift from rumination. Explicitly attempting to suppress a specific thought has been shown to be considerably harder than anticipated, and suppression is not effective for alleviating depression, and may even be counter-productive [59]. Fostering a thinking pattern that is more associative in nature instead will divert thought to other themes more readily. Methods such as meditation require trainees to “observe thoughts and let them go.” In a healthy mind with broad associative activations, such process of “letting go” is expected to occur naturally. In this context, it is interesting to note that no claims are made here about whether the pattern of thought can be controlled voluntarily, or whether instead it is automatic. The issue of control is interesting and has already received considerable attention [60], but it is less relevant in the present proposal. The premise of the therapeutic potential of this proposal lies on the idea of restoring the cortical infrastructure that regulates inhibition and allows one to broadly associate and disengage from ruminations in a natural manner. The hypothesis is that when this infrastructure exists, thinking is more associative, independent of voluntary control.
Similar proposals can be made for other conditions that involve mood disorders. For example, patients with PTSD show abnormal activation in the same regions where microstimulation shows benefit for depression symptoms, area BA 25 (as well as BA 24) [61]. PTSD can be seen as a form of rumination, where associative memory retrieval is focused narrowly on a traumatic memory with a limited ability to shift away from this focus. One might therefore suspect that a similar approach for promoting broad associative thinking as a means of disengaging from rumination could be helpful for PTSD patients as well. Similarly, this approach is predicted to prove beneficial also in stress and anxiety disorders, phobias and possibly even in normal aging.
The present hypothesis gives rise to a counterintuitive prediction that attention deficit hyperactivity disorder (ADHD) patients, whose mental processes tend to be doing the exact opposite of ruminating, are expected to have a more positive mood than that of individuals without ADHD. There has not been much research of mood effects in ADHD, and such measurements might be influenced by multiple factors that could counteract each other. Nevertheless, the existing research indicates that, indeed, children with ADHD tend to be relatively more positive thank control subjects, as reflected, for example, by affective valance [62], and positive illusory bias [63]. Furthermore, the hippocampus of ADHD patients has been shown to be of significantly larger volume compared with that of controls [64], and, while acute depression is typically associated with an enlarged and hyperactive amygdala [65], the amygdala of ADHD patients is smaller even than this of controls [64]. With the links made here between broad associative processing, its inhibition, and mood, ADHD could be attributable, at least in part, to deficits in inhibition rather than portrayed exclusively as an attention disorder.
Previous findings show that depression patients are deficient in foresight and future-related thinking [18]. Therefore, one would predict that successful treatment of mood disorders will increase patients' foresight and ability for future-oriented mental operations, such as planning and mental simulation.
Our understanding of mood manifestation in the brain is largely lacking, but a reasonable hypothesis might be that it involves the release and binding of neuroendorphins. If activating certain neural paths results in an increased release of neuroendorphins, one can see how ruminating on the same theme for a long period exhausts the release of such rewarding opioids from the repeatedly active neurons (and why repetition is often boring), whereas activating broad associated representations and pathways that have not been activated recently increases rewarding activations (as well as encourage us to attend novelty in our environment). This aspect of the proposal---that the brain might use the release of neuroendorphins as incentive to activate associations to promote learning and minimize uncertainty---is speculative and will require further interdisciplinary investigation. Nevertheless, recent theoretical and empirical work suggests that a MTL region similar to the one we showed to be activated by contextual associations shows increased fMRI activation for pictures that participants find as pleasant [66, 67], and this area might be at the top of a hierarchy of increased concentrations of opioids. Indeed, the highest levels of opioid binding have been found both in the orbital MPFC and in the MTL [68].
Finally, new neurons that are born in the adult hippocampus must be integrated with existing networks in order to survive. In the proposed framework, increased associative processing implies recruiting more of the newly generated hippocampal neurons, which could promote the integration and survival of these new neurons. Therefore, that SSRIs help only a subset of patients but not others should be examined in parallel to what other habits these patients have. It is possible that those for which SSRIs are not effective do not engage in activities that promote the survival of new neurons, such as intense learning and other forms of associations-based mental enrichment [69, 70]. In the broad associative framework presented here, new neurons are supposedly born as a result of demand and usage, and thus their chances of integration and survival are increased.
Concluding remarks
Associations are naturally critical for learning, and therefore the healthy brain might be motivated to learn by a consequential mood reward. As we have proposed and shown in the past, associations are also critical for the generation of predictions [4]. Being able to minimize uncertainty with the generation of association-based predictions is a most effective means for improving our chances of survival. Therefore, activating associations broadly and frequently, while still being able to focus more narrowly when necessary, could provide a mechanism that promotes our survival and progress, and when using this mechanism “as intended” we are rewarded with a positive mood.
The activation of broad associations, as predicted here, makes mood more positive. Ruminating, on the other hand, makes mood negative. It might be possible that the act of rumination is by itself sufficient for eliciting a negative mood, independent of whether the focus of this rumination is negative (e.g., a bad memory). So why are we not depressed when concentrating on driving, reading, or meditating? One critical difference might be the duration of such attentive focus. Rumination that is associated with depression tends to have a much longer duration than what everyday, emotionally neutral tasks entail. Perhaps more importantly, however, this question helps sharpen the distinction between rumination and a narrow scope of attention: the narrow focus of attention while working towards a goal still advances over time (e.g., during writing), whereas the narrow attention associated with rumination remains focused on the same topic, for a long duration.
In summary, the proposal presented here outlines a cognitive neuroscience framework with which to understand mood. This framework can provide the foundations for future development of non-invasive methods for treating mood disorders, whereby the training and restructuring of the ability for broad associative thinking can elicit improvements that range from structural modifications to mood and behavior (see also Box 2).
Box 2. Questions for future research.
There are several relationships that have been alluded to here, where the directionality and causality of the relation is yet unclear: Does depression cause hippocampal and MPFC damage, or rather is it a structural disruption in MPFC and MTL that results in depression? Do more associations generate more new hippocampal neurons, or do more neurons afford more associations? The questions of directionality and causality will have to be elucidated in the future. For the purpose of developing an effective, non-invasive approach to therapy, however, we assume that addressing and improving one aspect of these facets will also bring about improvement in the other facets. In other words, it is proposed that rebuilding the brain's ability to continuously activate broad associations, and resume a normal level of prefrontal inhibition, will reduce rumination, imitate DBS internally, elicit hippocampal restructuring, and ultimately help mood disorders.
One strong prediction that stems from this framework and can be readily tested is that rumination and negative mood are associated with excessive inhibition from MPFC to MTL, as proposed in Fig. 1. Similarly, ADHD, and possibly schizophrenia (also accompanied by hyper associativity), are accompanied by insufficient inhibition from MPFC to MTL.
To what extent individuals experience associative processing as a phenomenal state, or instead are non-conscious of its operation? This question has theoretical as well as clinical implications.
Discussing neurotransmitter systems such as the dopaminergic and the serotonergic was beyond the scope of this proposal, but there is little doubt that they are both playing central roles in mood regulation. These systems have attracted a great deal of interest, which resulted in groundbreaking findings, but the question of what is their exact role in mood is yet to be elucidated. In the context of the framework proposed here, it would be especially interesting to study how the dopaminergic and serotonergic systems interact with the cognitive network responsible for associative activations. With regard to the dopaminergic system, for example, it is curious that some see dopamine as the “pleasure molecule,” providing a pleasant feeling of reward and motivating with reinforcement, whereas others see dopamine as central to anything related to predictions, reward prediction and prediction-error. But there is no satisfactory link between the two functions; how is the same system responsible both for predictions as well as for pleasurable feeling? Considering the role of associations-based predictions in positive mood, as proposed here, could provide such a bridge.
Acknowledgments
L. Barrett, L. Barsalou, J. Boshyan, D. Carney, J. Denninger, M. Fava, M. Fenske, D. Gilbert, A. Harvey, M. Mason, J. Matthews, H. Mayberg, K. Shepherd, C. Thomas, and D. Wegner for stimulating discussions, constructive comments and help with the manuscript. Supported by NIH grant R01NS050615.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 3.Bar M. Visual objects in context. Nat Rev Neurosci. 2004;5:617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- 4.Bar M, et al. The units of thought. Hippocampus. 2007;17:420–428. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- 5.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder JR, et al. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 7.Mazoyer B, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 8.Msetfi RM, et al. Depressive realism and outcome density bias in contingency judgments: The effect of the context and intertrial interval. J Exp Psychol Gen. 2005;134:10–22. doi: 10.1037/0096-3445.134.1.10. [DOI] [PubMed] [Google Scholar]
- 9.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 10.Ebert D, Ebmeier KP. The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry. 1996;39:1044–50. doi: 10.1016/0006-3223(95)00320-7. [DOI] [PubMed] [Google Scholar]
- 11.Isen AM, et al. The influence of positive affect on the unusualness of word associations. J Pers Soc Psychol. 1985;48:1413–26. doi: 10.1037//0022-3514.48.6.1413. [DOI] [PubMed] [Google Scholar]
- 12.Mednick MT, et al. Continual Association as a Function of Level of Creativity and Type of Verbal Stimulus. J Abnorm Psychol. 1964;69:511–515. doi: 10.1037/h0041086. [DOI] [PubMed] [Google Scholar]
- 13.Storbeck J, Clore GL. With sadness comes accuracy; with happiness, false memory: Mood and the false memory effect. Psychol Sci. 2005;16:785–791. doi: 10.1111/j.1467-9280.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- 14.Gasper K, Clore GL. Attending to the big picture: Mood and global versus local processing of visual information. Psychol Sci. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- 15.Basso MR, et al. Mood and global-local visual processing. J Int Neuropsychol Soc. 1996;2:249–55. doi: 10.1017/s1355617700001193. [DOI] [PubMed] [Google Scholar]
- 16.Baddeley AD. Selective attention and performance in dangerous environments. Br J Psychol. 1972;63:537–546. doi: 10.1111/j.2044-8295.1972.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 17.Fredrickson BL. The broaden-and-build theory of positive emotions. Philos Trans R Soc Lond B Biol Sci. 2004;359:1367–1378. doi: 10.1098/rstb.2004.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams JM, et al. The specificity of autobiographical memory and imageability of the future. Mem Cognit. 1996;24:116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]
- 19.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–511. [PubMed] [Google Scholar]
- 20.Segerstrom S, et al. Worry and rumination: Repetitive thought as a concomitant and predictor of negative mood. Cognit Ther Res. 2000;24:671–688. [Google Scholar]
- 21.Park RJ, et al. Effects of induced rumination and distraction on mood and overgeneral autobiographical memory in adolescent Major Depressive Disorder and controls. J Child Psychol Psychiatry. 2004;45:996–1006. doi: 10.1111/j.1469-7610.2004.t01-1-00291.x. [DOI] [PubMed] [Google Scholar]
- 22.Pronin E, Jacobs E. Thought Speed, Mood, and the Experience of Mental Motion. Perspect on Psych Science. 2008;3:461–485. doi: 10.1111/j.1745-6924.2008.00091.x. [DOI] [PubMed] [Google Scholar]
- 23.Michael T, et al. Rumination in posttraumatic stress disorder. Depress Anxiety. 2006 doi: 10.1002/da.20228. [DOI] [PubMed] [Google Scholar]
- 24.Harvey A, et al. Cognitive Behavioural Processes Across Psychological Disorders: A Transdiagnostic Approach to Research and Treatment. Oxford University Press; 2004. [Google Scholar]
- 25.Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. J Pers Soc Psychol. 1988;55:958–956. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- 26.Wegner DM, et al. Paradoxical effects of thought suppression. J Pers Soc Psychol. 1987;53:5–13. doi: 10.1037//0022-3514.53.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Harman C, Rothbart MK. Distress and attention interactions in early infancy. Motivation & Emotion. 1997;21:27–44. [Google Scholar]
- 28.Pronin E, Wegner DM. Manic thinking: independent effects of thought speed and thought content on mood. Psychol Sci. 2006;17:807–813. doi: 10.1111/j.1467-9280.2006.01786.x. [DOI] [PubMed] [Google Scholar]
- 29.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 30.Mayberg H, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 32.Squire LR, et al. The medial temporal lobe. Annu Rev Neurosci. 2004 doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 33.Stern CE, et al. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- 35.Sheline YI, et al. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 36.Benedetti F, et al. Depression and neurological disorders. Curr Opin Psychiatry. 2006;19:14–18. doi: 10.1097/01.yco.0000194147.88647.7f. [DOI] [PubMed] [Google Scholar]
- 37.Saxena S, et al. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biol Psychiatry. 2001;50:159–170. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- 38.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs BL, et al. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 40.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meneses A. A pharmacological analysis of an associative learning task: 5-HT(1) to 5-HT(7) receptor subtypes function on a pavlovian/instrumental autoshaped memory. Learn Mem. 2003;10:363–372. doi: 10.1101/lm.60503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt-Hieber C, et al. Enhanced synapticplasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 44.Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994;643:10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 45.Nobler MS, et al. Regional cerebral blood flow in mood disorders, III. Treatment and clinical response. Arch Gen Psychiatry. 1994;51:884–897. doi: 10.1001/archpsyc.1994.03950110044007. [DOI] [PubMed] [Google Scholar]
- 46.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drevets WC, et al. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bar M, editor. Philosophical Transactions of the Royal Society B: Biological Sciences. Vol. 364. 2009. Predictions: A universal principle in the operation of the human brain (Introduction) Theme issue: Predictions in the brain: using our past to generate a future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joormann J. Attentional bias in dysphoria: The role of inhibitory processes. Cognition & Emotion. 2004;18:125–147. [Google Scholar]
- 50.Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 51.Mayberg HS, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 52.Greicius MD, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 54.DeRubeis RJ, et al. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck AT, et al. Cognitive Therapy of Depression. Guilford Press; 1987. p. 425. [Google Scholar]
- 56.Deckersbach T, et al. Cognitive-behavioral therapy for depression. Applications and outcome. Psychiatr Clin North Am. 2000;23:795–809. VII. doi: 10.1016/s0193-953x(05)70198-2. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson P, Goodyer I. The effects of cognitive-behavioural therapy on mood-related ruminative response style in depressed adolescents. Child Adolesc Psychiatry Ment Health. 2008;2:3. doi: 10.1186/1753-2000-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldapple K, et al. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 59.Wenzlaff RM, Wegner DM. Thought suppression. Annu Rev Psychol. 2000;51:59–91. doi: 10.1146/annurev.psych.51.1.59. [DOI] [PubMed] [Google Scholar]
- 60.Wegner DM, Bargh JA. Control and automaticity in social life. In: Gilbert D, Fiske ST, Lindzey G, editors. Handbook of social psychology. 4. McGraw-Hill; 1998. pp. 446–496. [Google Scholar]
- 61.Bremner JD, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ornitz EM, et al. Affective valence and arousal in ADHD and normal boys during a startle habituation experiment. J Am Acad Child Adolesc Psychiatry. 1997;36:1698–705. doi: 10.1097/00004583-199712000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Owens JS, Hoza B. The role of inattention and hyperactivity/impulsivity in the positive illusory bias. J Consult Clin Psychol. 2003;71:680–91. doi: 10.1037/0022-006x.71.4.680. [DOI] [PubMed] [Google Scholar]
- 64.Plessen KJ, et al. Hippocampus and amygdala morphology in attention-deficit/Hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 66.Yue X, et al. The neural basis of scene preferences. Neuroreport. 2007;18:525–9. doi: 10.1097/WNR.0b013e328091c1f9. [DOI] [PubMed] [Google Scholar]
- 67.Biederman I, Vessel EA. Perceptual Pleasure and the Brain. Am Sci. 2006;94:247. [Google Scholar]
- 68.Wise S, Herkenham M. Opiate receptor distribution in the cerebral cortex of the Rhesus monkey. Science. 1982;218:387–389. doi: 10.1126/science.6289441. [DOI] [PubMed] [Google Scholar]
- 69.Gage FH. Mammalian Neural Stem Cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 70.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kringelbach ML, et al. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 72.Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 73.Johansen-Berg H, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2007;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox DM, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scharfman HE. Functional implications of seizure-induced neurogenesis. Adv Exp Med Biol. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]