Abstract
Objective:
Training to perform a serial reaction time task (procedural motor learning) with one-hand results in performance improvements in the untrained as well as in the trained hand, a phenomenon referred to as intermanual transfer. The aim of this study was to investigate the neurophysiological changes associated with intermanual transfer associated with learning to perform an eminently different task involving fine force control within the primary motor cortex (M1). We hypothesized that intermanual transfer of learning such a task would reveal intracortical changes within M1.
Methods:
Speed (time to complete each sequence) and accuracy (% of accuracy errors) of motor performance were measured in both hands before and after right (dominant) hand practice. Transcranial magnetic stimulation (TMS) was used to characterize recruitment curves (RC), short intracortical inhibition (SICI), intracortical facilitation (ICF) and interhemispheric inhibition (IHI) from the left to the right M1.
Results:
Practice resulted in significant improvements in both speed and accuracy in the right trained hand and in the left untrained hand. RC increased in the left M1, SICI decreased in both M1s, and IHI from the left to the right M1 decreased. No changes were identified in ICF nor in RC in the right M1.
Conclusions:
Our results suggest that some neurophysiological mechanisms operating in the M1 controlling performance of an untrained hand may contribute to optimize the procedure for selecting and implementing correct pinch force levels.
Significance:
These results raise the hypothesis of a contribution of modulation of SICI and IHI, or an interaction between both to intermanual transfer after learning a sequential pinch force task.
Keywords: motor cortex, intermanual transfer, interhemispheric inhibition, pinch force control
Introduction
Studies in both humans and animals have demonstrated that knowledge acquired with one hand transfers to the other hand, a process called intermanual transfer (Rand et al., 1998; Grafton et al 2002; Japikse et al., 2003; Perez et al., 2007a and 2007b). This phenomenon has been characterized in different behavioral tasks including prism adaptation (Taub and Golberg, 1973), inverted and/or reversed writing (Parlow and Kinsbourne, 1989), figure drawing (Thut et al., 1996), and motor sequence learning (Grafton et al., 2002; Perez et al., 2007a and 2007b). The neural substrates underlying intermanual transfer of sequential knowledge are incompletely understood. Recent functional imaging work showed that the primary motor cortex (M1) contralateral to the untrained hand, (i.e, the hemisphere ipsilateral to the trained hand), is active during motor sequence learning tasks (Verstynen et al., 2005; Daselaar et al., 2003; Bischoff-Grethe et al., 2004). Transcranial magnetic stimulation (TMS), used to study intracortical motor physiology and interhemispheric interactions non-invasively (Ferbert et al., 1992; Shim et al., 2005) showed modulation of interhemispheric inhibition (IHI) between both M1s associated with intermanual transfer during learning to perform a serial reaction-time task ( SRTT) (Robertson, 2007; Perez et al., 2007a and 2007b).
However, the relative contribution of intracortical mechanisms within the M1 that controls performance of an untrained hand likely differs across behavioral sets (i.e., implicit vs explicit knowledge; binary correct-incorrect key presses vs fine gradations of force). Support for this view comes from previous studies demonstrating that cortical reorganization in the primate M1 is more prominent during learning of complex motor tasks relative to performance of repetitive movements (Nudo et al., 2007). In humans, intracortical mechanisms within M1 associated with intermanual transfer of procedural learning in the SRTT (Perez et al., 2007a) may differ from those present with motor sequences learning that involve precise force production. Indeed, it is well established that the neural networks activated in association with motor sequence learning such as the SRTT are different from those engaged in fine force control (Hazeltine et al., 1997; Gallea et al., 2005).
Fine force control as required to implement precise pinch force is important for carrying out activities of daily living, and loss of this ability is often present after brain lesions like stroke (Blennerhasset and al., 2007). Moreover, pinch force control relies on the activation of an extensive cortical network that engages more neuronal resources than gross whole-hand pinch control and requires the integrity of the corticospinal tract. Here, we aimed to investigate the neurophysiological mechanisms within the M1 that control performance in an untrained hand after learning a motor sequence that involves precise pinch force control with the opposite hand. We hypothesized that improved performance in the untrained hand will correlate with modifications of intracortical excitability of the M1.
Materials and methods
Subjects
19 healthy volunteers (9 females, 10 males, age range 18-40 years) participated in the study. All subjects gave written informed consent to the experimental procedure, which was approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki. All subjects were right handed according to the Edinbrugh Handness Inventory (Oldfield, 1971).
Sequential pinch force task
Subjects were seated in front of a computer screen, in a comfortable armchair with both arms in a 90° elbow flexion position. They were asked to hold a custom-made force transducer between the tips of the thumb and index fingers (fingertip precision grip). The force transducer and the two fingers rested on a support so that they were maintained in the horizontal plane. The other fingers were relaxed. At the beginning of each trial, the cursor appeared always on the left side of the computer screen. The cursor moved horizontally rightwards when the subjects increased the force. The subjects had to modulate their precision pinch force to reach sequentially 5 targets displayed on the computer screen. Subjects were instructed to reach the targets 1-4 from the home position on the left side of the screen without “undershooting” the left margin or “overshooting” the right margin, returning to the home position before proceeding to the next target. For target 5, the cursor had to be maintained static on the target for 0.5 sec in order to end each trial. Subjects were instructed to perform the sequence as fast and accurately as possible.
After baseline testing with the right (trained) and the left (untrained) hand (10 trials each), subjects performed 6 training blocks with the right trained hand. Each block was composed of 30 trials; the training lasted approximately 30 minutes including interspersed short rest periods as needed. Untrained left hand performance was retested immediately after the right hand training. Performance (speed, i.e. time to complete each trial and accuracy, i.e., percentage of errors within each trial) was analyzed offline. In a control experiment, 7 subjects were tested at baseline (10 trials for each hand) and 30 minutes later (10 trials for each hand) in the absence of right hand training; in order to characterize the extent to which testing alone in the absence of training induced any physiological modifications.
Electrophysiological measurements
Surface electrodes were positioned bilaterally on the skin overlying the first dorsal interosseous (FDI) muscles in a bipolar montage (inter-electrode distance, 2cm). EMG activity was displayed online using a Counterpoint electromyography machine (Dantec Electronics, Skovlunde, Denmark), the amplified EMG signals were filtered (band-pass, 25Hz to 1 kHz), sampled at 2 kHz, and stored on a PC for off-line analysis. TMS was delivered to the optimal scalp position for activation of the FDI muscles overlying left and right M1. Motor evoked potentials (MEPs) were elicited by magnetic stimuli delivered from a Magstim 200 stimulator (Magstim Company, Dyfed, UK) through a figure-of-eight coil (loop diameter, 8 cm). The handle of the coils pointed backwards and laterally about 45° to the mid-sagittal plane. Measures of cortical excitability included the resting motor threshold (RMT) defined as the lowest intensity of TMS output required to evoke MEPs of at least 50 μV in peak-to-peak amplitude in five out of ten consecutive trials (Rossini et al., 1994), MEPs recruitment curves (RC), short intracortical inhibition (SICI), intracortical facilitation (ICF) as tested with the paired pulse technique and interhemispheric inhibition (IHI) from the left (learning) to the right (transfer) M1 were tested immediately before and after the 30 minutes of training with the right training hand (Fig. 1a, 1b). Due to the length of the physiological determinations and to avoid excessive fatigue RMT, RC, SICI and ICF were tested bilaterally (learning and transfer hemisphere) in 9 of the 19 subjects and RMT and IHI were tested in the other 10 subjects.
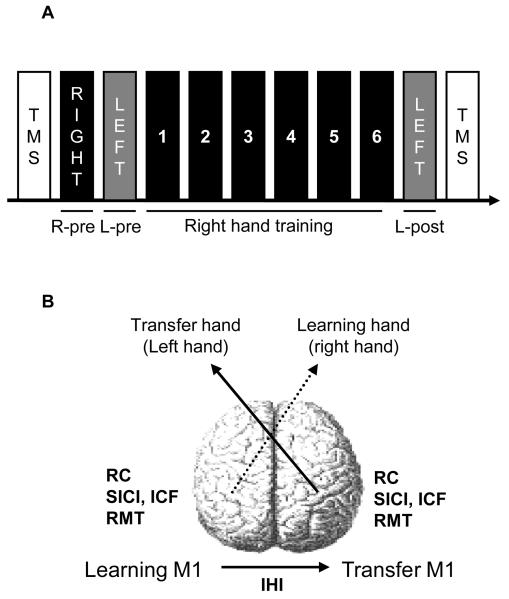
Figure 1. Experimental design.
A, Diagram showing the testing procedure. Cortical excitability (TMS) was tested at the beginning of the experiment. Measures of baseline performance for the right (R-pre) and the left (L-pre) hand were taken before the right hand training session (Right hand training, 1, 2, 3, 4, 5, and 6). After the right hand training session, left untrained hand performance was determined (L-post). Motor cortical excitability (TMS) was tested at the end of the experiment. B, Diagram showing the different measures of motor cortical excitability tested bilaterally before and after the right hand training (RC, recruitment curve; SICI, short intracortical inhibition; ICF, intra cortical facilitation; RMT, resting motor threshold and IHI, interhemispheric inhibition tested from the left to the right M1).
MEPs recruitment curves (RC)
10 TMS stimuli were applied at each stimulus intensity (from 100% RMT to 150% RMT) resulting in a total number of 60 pulses for each RC. RC determinations were done for each M1 before and after each training session with the right training hand. MEP amplitudes were measured peak-to-peak and averaged off-line. Since measurements of RMT did not unveil changes throughout the training period, the same stimulation parameters were used before and after the training session.
Short intracortical inhibition (SICI) and intracortical facilitation (ICF)
SICI and ICF were tested using the method described by Kujirai et al. (1993). A conditioning stimulus (CS) was set at 70% of the actual RMT. The RMT was retested after training to adjust accordingly the stimulus intensities to the RMT if needed. While it has been reported that this low stimulus intensity does not activate corticospinal fibers and does not change the excitability of spinal motoneurons (Di Lazzaro et al., 2001), one have to keep in mind that we do not know how these intensities would fare when expressed relative to active MT (Ilic et al 2002; Ziemann et al 2009). The test stimulus (TS) was adjusted to elicit an MEP of approximately 1mV at rest. The TS was delivered 3ms after the CS, an optimal interstimulus interval for eliciting SICI (this interval also contributes to avoid the mixture of two different phases of inhibition (Fisher et al., 2002)) or 10 ms following the CS to characterize ICF. 15 TS alone and fifteen CS-TS were presented randomly at each time interval (3 and 10 ms). Stimuli were applied every 5 seconds, and responses were recorded for off-line analysis. SICI and ICF measurements for the left and right MI were repeated (learning and transfer hemisphere) before and after the training session with the right training hand.
Interhemispheric inhibition (IHI)
IHI between left and right primary motor cortices was tested following a well described paired pulse paradigm (Ferbert et al., 1992). The two figure-of-eight coils were positioned at the optimal location for activating the learning (right) and transfer (left) FDI. The two coils were secured by straps and attached to a coil holder to ensure stability of coil positioning. A CS was given to the left M1 10 ms before a TS delivered to the right M1. The CS was always given to the left (learning) M1 and the TS to the right (transfer) M1 (Fig. 1b). The intensity for the TS was adjusted to produce an MEP of about 1.5 mV in the left FDI and the intensity of the CS was adjusted to produce 50% inhibition of the test MEP response at rest. RMT were retested after training to adjust accordingly the stimulus intensities if needed.
Data analysis
Separate repeated measures ANOVA (ANOVARM) with factor SESSION were used to evaluate the subject's performance (speed and accuracy) in the trained right and untrained left hands and RC. Paired t tests were used to evaluate (a) the percentage change in speed and accuracy in both hands and, (b) the depth (i.e. percentage) of IHI before and after training and (c) changes in SICI and ICF, the size of the CS stimulus applied during IHI measurements, and RMT.
Results
Motor performance
As shown on figure 2a ([n=19 subjects]), task performance with the right training hand improved significantly both in terms of speed and accuracy. ANOVARM revealed a significant effect of SESSION (that is of training) on performance (both on speed and on accuracy). Indeed, the time to complete the task decreased significantly over the training session (ANOVARM F(6,90) = 75.56, p < 0.0001). In addition, we found that postraining, (block 6) performed with the right training hand was faster than with the untrained hand (paired t test, p = 0.001). The overall percentage improvement in time to perform the task was significantly larger for the training hand than for the untrained hand (paired t test, p < 0.0001). The percentage of accuracy errors decreased significantly over the training session (ANOVARM with factor SESSION: F (6,90) = 28.66, p < 0.0001, training hand, see Fig. 2a). Therefore, subjects performed the task not only faster but also more accurately with the training hand after completion of the training period.
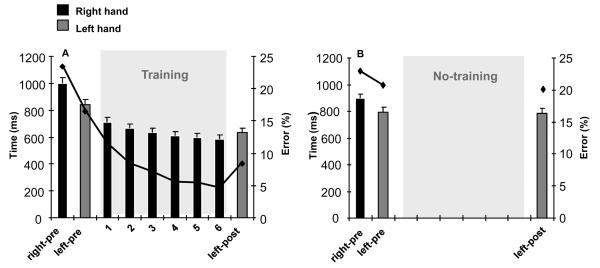
Figure 2. Behavioral results.
A, Time to perform the sequential pinch force task with the right trained and left untrained hand before (right-pre and left-pre), and after (left hand only, left-post) training with the right hand (1, 2, 3, 4, 5, and 6). The black curve indicates the percentage of errors. Note that the time required to perform the task and the percentage of errors of the left untrained hand decreased significantly after right hand training (left-post compared to left-pre). B, Time to perform the task with the right and left hand “before” (right-pre and left-pre), and “after” (left hand only, left-post) a no-training period (No-training). Note the comparable times to perform the task and percentage of errors in the absence of training (left-pre compared to left-post).
As shown on figure 2a ([n=19]), there was a significant effect of SESSION on left (untrained) hand performance (for both speed and accuracy). Indeed, the time to perform the task with the left (untrained) hand decreased significantly after the training session with the right training hand (ANOVARM: F(1,15) = 33.65, p < 0.0001). In addition, the percentage of errors in the left untrained hand decreased significantly after training (L-pre compared to L-post, paired t test, p < 0.001).
As shown on figure 2b ([n=7 subjects]), ANOVARM did not reveal any effects of repetition of testing (no-training) on performance of the left hand (speed and accuracy) (paired t test, p = 0.943). In other words, left hand testing and re-testing in the absence of right hand training did not influence performance in the left hand.
Recruitment curves
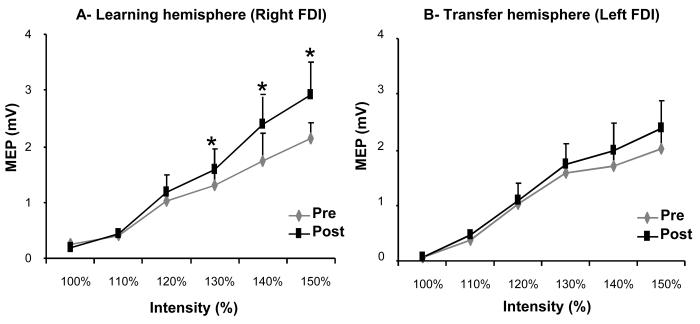
As show on figure 3a, ANOVARM revealed a significant effect of SESSION on RC in the right FDI muscle in the training hand (ANOVARM: F(5,40) = 5.54, p < 0.001) especially for larger stimulation intensities (pre/post comparison: 130% RMT, p=0.008; 140% RMT, p=0.041; 150% RMT, p=0.006). For the left FDI, ANOVARM revealed no significant effects of SESSION on RC (figure 3b).
Figure 3. MEP recruitment curves.
A, B, MEP amplitudes from FDI muscles (A, B) before (Pre) and after (Post) training. The abscissa shows the TMS stimulus intensity relative to the RMT in each subject, and the ordinate shows MEP amplitudes (mV). Note the increase in recruitment curve in the right FDI in the absence of changes in the left FDI.
Short intracortical inhibition
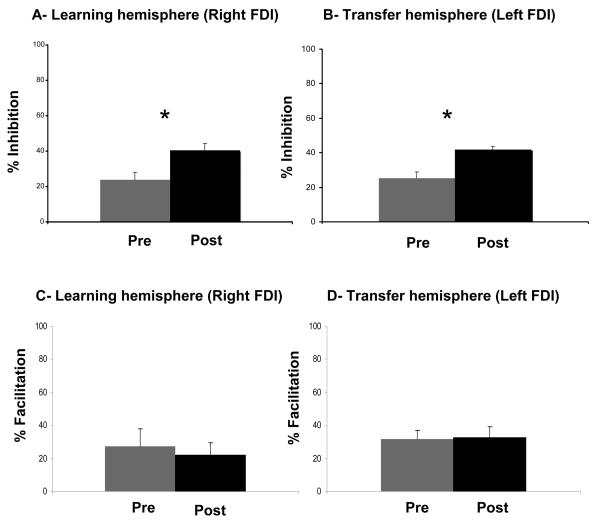
Training did not result in changes in test MEP amplitudes or RMT. However, a significant attenuation of SICI was evident in both the right training (mean pre-training, 74.67; mean post-training, 58.12; paired t test, p = 0.004, figure 4a) and the left untrained (mean pre-training, 76.18; mean post-training, 59.51; paired t test, p = 0.007, figure 4b) hands.
Figure 4. Short intracortical inhibition.
SICI (3ms) in FDI (A, B) measured before (Pre) and after (Post) training with the right hand in the left (A) and right (B) hemisphere. Note the presence of disinhibition in both left (A) and right (B) hemispheres. ICF (10ms) in FDI (C, D) measured before (Pre) and after (Post) training with the right hand in the left (C) and right (D) hemisphere.
Intracortical facilitation
Training did not result in changes in test MEP amplitudes, RMT or ICF. Test MEP amplitudes did not differ significantly with training nor did ICF in either the training (mean pre-training, 127.26; mean post-training, 121.82; paired t test, p = 0.72) or the untrained hands (mean pre-training, 131.71; mean post-training, 132.51; paired t test, p = .85.
Interhemispheric inhibition
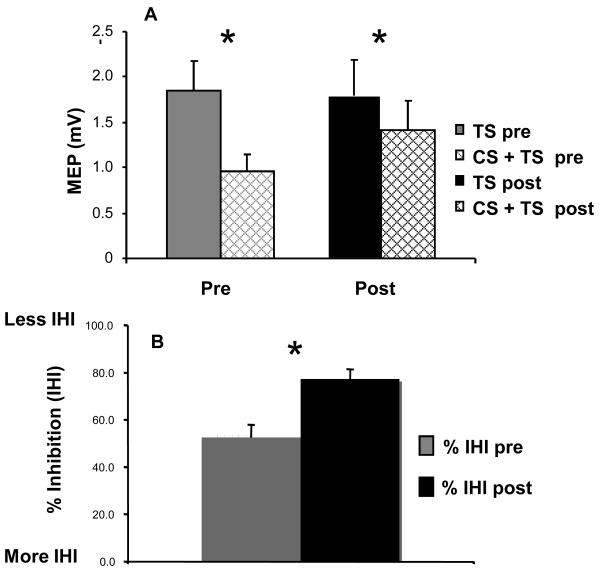
We found no significant differences in test or conditioning MEP amplitudes before and after training. More importantly, IHI from the left to the right M1 decreased with training (from 47.5% to 22.7%; paired t test, p = .0036, Figure 5).
Figure 5. Interhemispheric inhibition.
A, Amplitude of the test (TS) and conditioned (CS+TS) MEP before (pre) and after (post) training with the right hand as recorded from left FDI muscle (untrained hand). Note the comparable test MEP amplitudes before and after training (TS pre and TS post). B, Note that training led to a significant decrease in IHI.
Left hand EMG activity during right hand training
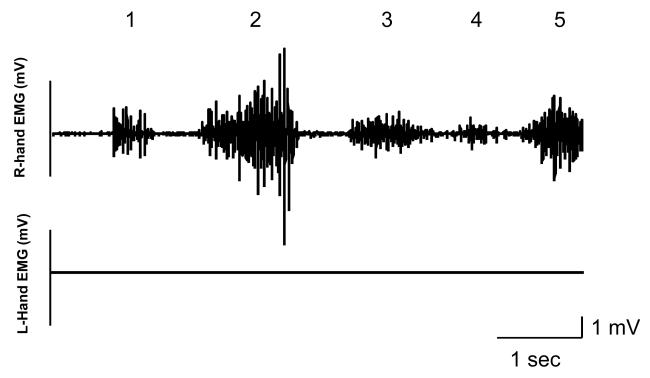
EMG activity of the resting hand (left) was monitored online during the experiment in all subjects and recorded, as a control, in 1 subject. The subjects were asked to relax completely their resting hand (left hand). Concerning the control experiment (with no right hand training) we only monitored online the EMG activity for all the subjects. As can be appreciated from figure 6, while the subject performs the training task with the right hand, the level of EMG activity for the training hand (right) is well modulated according to the level of force required to reach the different target locations while the EMG activity of the resting hand (left) remains flat.
Figure 6. Right and left hand EMG activity.
EMG activity recorded in the right (upper trace) and left (lower trace) FDI's while a subject was performing the training task with the right hand. Note that while the level of EMG activity for the training hand (right) is well modulated according to the level of force required to reach the different target locations (indicated by 1, 2, 3, 4, and 5), the level of EMG activity of the resting hand (left) remains at rest.
Discussion
The ability to transfer to an untrained hand the procedural knowledge learned with the opposite training hand represents an important property of the motor system in animals and humans (Parlow and Kinsbourne, 1989; Rand et al., 1998, 2000; Grafton et al., 2002; Japikse et al., 2003). The neural network engaged in intermanual transfer includes the supplementary motor area, primary sensorimotor cortex (M1), parietal regions, cerebellum and basal ganglia (Hazeltine et al., 1997; Grafton et al., 1998; Honda et al., 1998; Willingham et al., 2002; Daselaar et al., 2003; Bischoff-Grethe et al., 2004). M1 is active in the hemisphere contralateral to the training hand during implicit motor learning (Pascual-Leone et al., 1994; Zhuang et al., 1998; Hazeltine et al., 1997; Grafton et al., 1998; Honda et al., 1998) as well as the M1 contralateral to the untrained hand (Daselaar et al., 2003; Bischoff-Grethe et al., 2004). It is likely that interactions between the two cerebral hemispheres (Tettamanti et al., 2002; Omura et al., 2004) contribute to this intermanual transfer of procedural knowledge since split-brain and acallosal patients show clear deficits in this domain (Lassonde et al., 1995; de Guise et al., 1999). Consistent with this information, recent work demonstrated decreased GABAergic SICI but no change in RC or resting motor thresholds in the M1 contralateral to the untrained hand, and decreased IHI between M1s that correlated with non sequence-specific performance improvements in the untrained hand during acquisition of implicit procedural knowledge with the opposite hand (Perez et al., 2007a). Here, we investigated neurophysiological changes in the M1 contralateral to the untrained hand after acquisition of a qualitatively different, sequential motor learning task that involves skilled control of ballistic pinch force (Shim et al., 2005; Liang et al., 2007).
From a behavioral point of view, practice of this pinch force task resulted in clear improvements in speed as well as in accuracy in both the trained and the untrained hands. While we have not calculated in this study the speed-accuracy ratios the fact that both outcome measures improved (faster as well as more accurate performance) is strongly suggestive of a leftward movement of speed-accuracy curves rather than a simple displacement along an unchanged speed-accuracy curve (in which improved accuracy is reached at the expense of reduced speed or vice versa). These improvements were determined immediately after the end of a short 30 min training session and therefore we do not know the extent to which or if they are present at all in association with other stages of motor learning like off-line consolidation or long-term retention (Reis et al., 2009).
We tested resting motor thresholds and RC, which convey information on the excitability of motor cortical neurons, but which are also likely influenced at least partially by subcortical contributions (Siebner and Rothwell, 2003). SICI which conveys information on intracortical GABA-ergic inhibitory interneurons (Kujirai et al., 1993; Ziemann et al., 1996), and resting interhemispheric inhibition (IHI) from the left to the right M1 which provides information on interhemispheric glutamatergic connections that connect with local GABAergic interneurons in the opposite hemisphere (Ferbert et al., 1992; Di Lazzaro et al., 1999). Overall, our findings in the M1 controlling the learning training hand during this particular task are consistent with those previously reported with a different implicit procedural learning (SRTT): larger recruitment curves and decreased SICI (Perez et al., 2007a). Therefore, neurophysiological changes in the M1 controlling a training hand appear to be comparable when learning a sequential pinch force task and the SRTT task, despite the different cognitive processes involved.
More importantly, the M1 contralateral to the untrained hand in our study experienced a decrease in SICI and a decrease in IHI originating from the opposite learning M1, in the absence of changes in ICF or RC. On one hand, changes in SICI and IHI identified here are consistent with those reported in relation to intermanual transfer of implicit motor sequence learning (Perez et al 2007a). On the other hand, RC in the right M1 contralateral to the untrained hand did not show overt changes, a clear difference to what has been reported in the same cortical area when learning an implicit motor sequence (Perez et al 2007a). Overall, our findings are consistent with the interpretation that modulation of IHI between the M1s, alone or in combination with modulation of SICI (Perez and Cohen, 2008), may contribute to intermanual transfer of a sequential pinch force control task. The finding of a similar mechanism with learning of both SRRT (Perez et al 2007a) and this precise pinch force sequence may suggest the existence of common basic changes in some intracortical mechanisms across different forms of motor sequence learning. Perhaps the contribution relies in learning to optimize the procedure for selecting the correct pinch force level to execute, as well as developing the general skill necessary to implement the changes in force accurately.
Although we evaluated here M1 physiology, it is likely that activity in other areas like the basal ganglia were active in the observed functional changes in M1, as for any motor learning task (Hazeltine et al., 1997; Doyon et al., 1996; Grafton et al., 1994; Willingham and Koroshetz, 1993). Another area linked with M1 is the posterior parietal cortex (Koch et al., 2007), shown to be engaged in encoding of sequences in an abstract frame Grafton et al., (1998). Interlimb transfer has also been reported in relation to performance of other tasks (Wang & Sainburg 2003, Morton et al., 2001) and the magnitude of transfer may depend on the hand being trained since greater transfer of learning occurs when subjects first practice with the dominant hand and then subsequently perform the task with the non-dominant hand (Halsband 1992, Parlow and Kinsbourne 1989). When interpreting such results, we have to keep in mind that we actually evaluated only a range of possible interactions. It is likely, that interhemispheric interactions like those proposed (between M1s in the opposite direction), SMA-M1, PMd-M1, parietal-M1 between others might contribute as well. The problem here is that it is virtually impossible to test all those interactions within the same experiment and in the short time frame that follows a period of training. Additionally, it should be kept in mind that SICI changes described here were evaluated using CS expressed relative to resting motor thresholds (Di Lazzaro et al., 2001). We do not know how these CS intensities would fare when expressed relative to active MT (Ilic et al 2002; Ziemann et al 2009).
The finding of concomitantly decreased SICI and IHI is suggestive of a possible task-dependent interaction between these two processes that goes beyond our knowledge that in the presence of IHI, there is a reduction of SICI at rest (Daskalakis and al., 2002; Perez and Cohen, 2008). Previous work showed that IHI is mediated by glutamatergic interhemispheric fibers that link homotopic regions of both M1s which synapse with GABAergic interneurons locally, exerting a net inhibitory effect. It is conceivable that IHI as tested under our experimental design interacted with GABAergic neurons mediating SICI. Recent studies demonstrated that it is possible to evaluate the influence of IHI on SICI at both rest (Daskalakis et al., 2002) and in an activity-dependent manner (Perez et al., 2008), an important line for future research.
In summary, our results suggest that some neurophysiological mechanisms operating in the M1 controlling performance of an untrained hand may contribute to optimize the procedure for selecting and implementing correct pinch force levels, as previously shown for the implementation of key presses in the SRTT (Perez et al 2007a). These findings may have clinical implications as well, since pinch force control is crucial in many activities of the daily life, and loss of this skill is often present after brain lesions like stroke (Blennerhasset and al., 2007). Understanding the similarities and differences between mechanisms involved in controlling output from M1 to a untrained hand across different forms of motor learning could potentially contribute to plan more accurate interventions that rely on the expectation of intermanual transfer of knowledge acquired with a healthy hand to a paretic hand (mirror therapy and bilateral arm training) after stroke (Altschuler et al., 1999; Luft et al., 2004).
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–6. doi: 10.1016/s0140-6736(99)00920-4. 12. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. J Cogn Neurosci. 2004;16:127–138. doi: 10.1162/089892904322755610. [DOI] [PubMed] [Google Scholar]
- Blennerhasset JM, Matyas TA, Carey LM. Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabil Neural Repair. 2007;21(3):263–72. doi: 10.1177/1545968306295560. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Jonker C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol Aging. 2003;24:1013–1019. doi: 10.1016/s0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guise E, del Pesce M, Foschi N, Quattrini A, Papo I, Lassonde M. Callosal and cortical contribution to procedural learning. Brain. 1999;122(6):1049–62. doi: 10.1093/brain/122.6.1049. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999;129(4):494–9. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Meglio M, Cioni B, Tonali P, Rothwell JC. Descending spinal cord volleys evoked by transcranial magnetic and electrical stimulation of the motor cortex leg area in conscious humans. J Physiol. 2001;537:1047–1058. doi: 10.1111/j.1469-7793.2001.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96(4):1765–71. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci. 1996;8:637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Gallea C, de Graaf JB, Bonnard M, Pailhous J. High level of dexterity: differential contributions of frontal and parietal areas. NeuroReport. 2005;16(12):1271–4. doi: 10.1097/01.wnr.0000176514.17561.94. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Motor sequence learning with the nondominant left hand. A PET functional imaging study. Exp Brain Res. 2002;146:369–378. doi: 10.1007/s00221-002-1181-y. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Tyszka JM. Functional imaging of procedural motor learning relating cerebral blood flow with individual subject performance. Hum Brain Mapp. 1998;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Hallet M. Transcranial Magnetic Stimuation: A Primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Halsband U. Left hemisphere preponderance in trajectorial learning. Neuroreport. 1992;3(5):397–400. doi: 10.1097/00001756-199205000-00005. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry RB. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain. 1997;120:123–40. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibáñez V, Pascual-Leone A, Zhuang P, Hallett M. Honda Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain. 1998;121:2159–73. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;15(545Pt 1):153–67. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japikse KC, Negash S, Howard JH, Jr, Howard DV. Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Exp Brain Res. 2003;148:38–49. doi: 10.1007/s00221-002-1264-9. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29(1):58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassonde M, Sauerwein HC, Lepore F. Extent and limits of callosal plasticity: presence of disconnection symptoms in callosal agenesis. Neuropsychologia. 1995;33(8):989–1007. doi: 10.1016/0028-3932(95)00034-z. [DOI] [PubMed] [Google Scholar]
- Liang N, Takahashi M, Ni Z, Yahagi S, Funase K, Kato T, Kasai T. Effect of intermanual transfer induced by repetitive precision grip on input-output properties of untrained contralateral limb muscles. Exp Brain Res. 2007;182(4):459–67. doi: 10.1007/s00221-007-1004-2. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. Jama. 2004;292(15):1853–61. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Lang CE, Bastian AJ. Inter- and intra-limb generalization of adaptation during catching. Exp Brain Res. 2001;141(4):438–45. doi: 10.1007/s002210100889. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38(2 Suppl):840–5. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;1:97–113. doi: 10.1016/0028-3932(71)90067-4. 9. [DOI] [PubMed] [Google Scholar]
- Omura K, Tsukamoto T, Kotani Y, Ohgami Y, Minami M, Inoue Y. Different mechanisms involved in interhemispheric transfer of visuomotor information. Neuroreport. 2004;15(18):2707–11. 22. [PubMed] [Google Scholar]
- Pal PK, Hanajima R, Gunraj CA, Li J-Y, Wagle-Shukla A, Mogante F, Chen R. Effect of low frequency repetitive transcranial magnetic stimulation on interhemispheric inhibition. J Neurophysiol. 2005;94:1668–1675. doi: 10.1152/jn.01306.2004. [DOI] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M. Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn. 1989;11(1):98–113. doi: 10.1016/0278-2626(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–9. doi: 10.1126/science.8122113. 4. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe DT, Willingham DT, Cohen LG. Neural Substrates of intermanual transfer of a newly acquired motor skill. Current Biology. 2007b;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci. 2007a;27(5):1045–53. doi: 10.1523/JNEUROSCI.4128-06.2007. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MK, Hikosaka O, Miyachi S, Lu X, Miyashita K. Characteristics of a long-term procedural skill in the monkey. Exp Brain Res. 1998;118:293–297. doi: 10.1007/s002210050284. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci, USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586(2):325–51. doi: 10.1113/jphysiol.2007.144824. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM. The serial reaction time task: Implicit motor skill learning. J Neurosci. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Kim SW, Oh SJ, Kang N, Zatsiorsky VM, Latash ML. Plastic changes in interhemispheric inhibition with practice of a two-hand force production task: a transcranial magnetic stimulation study. Neurosci Lett. 2005;374:104–108. doi: 10.1016/j.neulet.2004.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148(1):1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Taub E, Goldberg LA. Prism adaptation: control of intermanual transfer by distribution of practice. Science. 1973;180(87):755–7. doi: 10.1126/science.180.4087.755. 18. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Paulesu E, Scifo P, Maravita A, Fazio F, Perani D, Marzi CA. Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J Neurophysiol. 2002;88(2):1051–8. doi: 10.1152/jn.2002.88.2.1051. [DOI] [PubMed] [Google Scholar]
- Thut G, Cook ND, Regard M, Leenders KL, Halsband U, Landis T. Intermanual transfer of proximal and distal motor engrams in humans. Exp Brain Res. 1996;108(2):321–7. doi: 10.1007/BF00228105. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res. 2003;149(4):520–6. doi: 10.1007/s00221-003-1392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham DB, Koroshetz WJ. Evidence for dissociable motor skills in Huntington's disease patients. Psychology. 1993;21:173–182. [Google Scholar]
- Willinghman DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophy. 2002;33:1451–60. doi: 10.1152/jn.2002.88.3.1451. 88. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Dang N, Waziri A, Gerloff C, Cohen LG, Hallett M, et al. Acta Neurol Scand. 1998;97(2):131–7. doi: 10.1111/j.1600-0404.1998.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rotwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]