Abstract
Ionizing irradiation significantly affects hippocampal neurogenesis and is associated with cognitive impairments; these effects may be influenced by an altered microenvironment. Oxidative stress is a factor that has been shown to affect neurogenesis, and one of the protective pathways to deal with such stress involves the antioxidant enzyme superoxide dismutase (SOD). This study addressed how the deficiency of cytoplasmic (SOD1) or mitochondrial (SOD2) SOD impacts radiation effects on hippocampal neurogenesis. Wild type (WT), SOD 1 and SOD2 knock out (KO) mice received a single x-ray dose of 5 Gy, and quantification of the survival and phenotypic fate of newly generated cells in the dentate subgranular zone was performed 2 months later. Radiation exposure reduced neurogenesis in WT mice but had no apparent effect in KO mice, although baseline levels of neurogenesis were reduced in both SOD KO strains prior to irradiation. Additionally, there were marked and significant differences between WT and both KO strains in how irradiation affected newly generated astrocytes and activated microglia. The mechanism(s) responsible for these effects are not yet known, but a pilot in vitro study suggests a ‘protective’ effect of elevated levels of superoxide. Overall, these data suggest that under conditions of SOD deficiency, there is a common pathway dictating how neurogenesis is affected by ionizing irradiation.
Keywords: Radiation, brain, neurogenesis, oxidative stress, inflammation, SOD
Introduction
Radiation-related brain injury is a limiting factor during therapeutic irradiation of the brain [1], and while overt tissue injury generally occurs only after relatively high doses [2], there is a strong likelihood of developing adverse reactions in terms of cognitive decline after relatively lower doses [3, 4]. Such impairment has a diverse character, but in humans it often includes hippocampal dependent functions involving learning, memory and spatial information processing [5–8]. Similar findings have been reported in experimental settings, confirming the importance of hippocampus-related effects in the evolution of radiation-induced cognitive injury [9–13]. The underlying mechanisms responsible for radiation-induced cognitive impairment have remained elusive, but important possibilities include alterations in the neurogenic cell populations in the dentate gyrus [9, 10, 12–17], loss of mature neurons in the dentate gyrus [10, 18], alterations in NMDA subunits [19], genetic risk factors [20] and reductions in the immediate early gene Arc [21].
Neurogenesis occurs in the subgranular zone (SGZ) of the dentate gyrus [22], and recent data show that newly born neurons from the SGZ are functionally integrated into the hippocampal circuitry [23, 24]. The importance of this is highlighted by considerable data showing a positive correlation between dentate neurogenesis and behavioral performance [25, 26]. Data from us and others, show that the neurogenic cells are very radiosensitive, and that the process of neurogenesis is reduced after doses that are below the threshold for overt tissue injury [9, 12, 14, 27]. Recent data from human patients similarly show that neurogenesis is severely depressed after treatment of malignant brain tumors with irradiation [28].
Radiation-induced reductions in neurogenesis and, presumably, cognitive performance, are strongly associated with the neurogenic microenvironment [12, 14, 27, 29–32]. One of the microenvironmental factors that significantly impacts neurogenesis is oxidative stress, a biochemical mechanism involving multiple reactive species that have been shown to regulate the function and fate of neural precursor cells [33]. The central nervous system (CNS) is inherently susceptible to oxidative injury, and because there are relatively low levels of endogenous antioxidants in the CNS [34, 35], that sensitivity has been implicated as playing a causative or contributory role in a number of pathologic conditions [36–39]. It has been suggested that radiation-induced increases in the production of reactive oxygen species (ROS) can contribute to the spread and ultimate expression of tissue injury [1, 40], and in irradiated cultured hippocampal neural precursor cells, early apoptosis, specific cell cycle blocks, and activation of functional cell cycle checkpoints have been shown to be coupled with elevated levels of ROS [41]. Indications of persistent oxidative stress after irradiation have also been demonstrated in the brains of mice [42] and rats [43]. Taken together, these data suggest that ROS may constitute critical environmental cues to control precursor cell survival and differentiation, and may play an important role in altered neurogenesis and perhaps cognitive impairment after irradiation [41].
There are several pathways that mitigate the physiological and pathological effects of ROS in mammalian cells [44]. One of the pathways involves the antioxidant enzyme, superoxide dismutase (SOD), which exists as three genetically and geographically distinct isoenzymes [45]. The SODs convert superoxide anions to hydrogen peroxide, which is then enzymatically removed by catalase and peroxidases. Superoxide can come from mitochondrial metabolism, or can be derived from monoamine oxidase, cylco-oxygenase, nitric oxide synthase or NADPH oxidase, and has been shown to have both positive and negative effects in the CNS [46–48]. Regardless of the source and relevant targets of superoxide signaling (e.g. kinases, phosphatases), it is clear that ROS are important molecules underlying learning and memory [46]. One way to characterize the extent to which ROS affects processes relevant to cognition, and if they are affected by ionizing irradiation, is to use mutant mice that lack specific antioxidant molecules like SOD. We recently assessed how irradiation affected dentate neurogenesis in mice deficient in extracellular isoform of SOD (EC-SOD, SOD3) [32]. That study showed that a persistent level of oxidative stress in EC-SOD knock out (KO) mice was associated with a lower baseline level of neurogenesis relative to wild type (WT) mice. However, when those same mice were subjected to a modest dose of x-rays (5 Gy), there was no effect on neurogenesis in KO mice but a highly significant reduction in WT animals [32]. Thus, we saw both negative (baseline neurogenesis) and positive (no apparent effect of irradiation) effects in the EC-SOD KO mice, presumably as a result of mechanisms associated with redox balance. These paradoxical effects highlight the importance of better understanding the delicate balance in redox homeostasis [48], and how that may ultimately affect cell/tissue function. Given this idea, we were interested in determining if the site of SOD deficiency influenced how irradiation impacted neurogenesis. In the present study we assessed neurogenesis after irradiation in mice deficient in the other SOD isoforms [45], CuZn SOD (SOD1) which is generally localized in the cytoplasm, and MnSOD (SOD2) which is localized in the mitochondria. Our results suggest that regardless of SOD isoform, when there is a deficiency in SOD, there is a common mechanism with respect to how the process of neurogenesis is affected by ionizing irradiation.
Methods
Animals
Heterozygous CuZnSOD (Sod1−/+) and MnSOD (Sod2−/+) mutant mice were generated as described previously [49, 50]. Both strains were congenic on the C57BL/6J background with Sod1−/+ and Sod2−/+ mice generated from 21st generation (N21) and 29th generation (N29) of backcross to C57BL/6J, respectively. Sod1−/+ and Sod2−/+ will be referred to as SOD1 KO and SOD2 KO, respectively throughout this manuscript. All animal handling procedures were done according to institutional IACUCs. Mice were kept in a temperature and light-controlled environment with a 12 hr light/dark cycle, and provided food and water ad libitum.
Because the SOD mutant mice reported here (SOD1, SOD2) and previously (SOD3 KO; [32]) were congenic on C57BL/6J and were raised in the same room with the same routine of animal care within the same animal facility (VMU, VA Palo Alto Health Care System), a common set of wild type (WT) controls were used for neurogenesis studies. Data from WT controls were previously reported with SOD3 KO [32]. However, to ensure consistency in cell counting across groups, all WT control sections were recounted by the same person who counted all the sections from SOD1 KO and SOD2 KO mice.
Irradiations
For irradiation, animals were anesthetized with a mixture of ketamine hydrochloride (60 mg/kg, Abbott Laboratories, North Chicago, IL) and medetomidine hydrochloride (0.25 mg/kg, Orion Corp., Espoo, Finland), administered by intraperitoneal (i.p.) injection. Irradiations were done using a Phillips orthovoltage X-ray system as previously described [32]. Briefly, animals were exposed to a single dose of 5 Gy using a special positioning jig so 4 animals could be irradiated simultaneously; the heads were centered in a 5 × 6 cm treatment field. The beam was directed down onto the head and the body was shielded with lead. Dosimetry was done using a Keithley electrometer ionization chamber calibrated using lithium fluoride thermal luminescent dosimeters. The corrected dose rate was approximately 175 cGy/min at a source to skin distance of 21 cm. Mice were irradiated at 8 weeks of age and were sacrificed, along with age-matched non-irradiated controls, 2 months later.
Quantification of antioxidant enzymes
To determine if the major antioxidant enzymes were altered before or after irradiation, cortices and hippocampi were collected from WT and KO mice one month after irradiation, which is the time when 5-bromo-2’deoxyuridine (BrdU, Sigma, St. Louis, MO) was administered for determination of neurogenesis (see below). For tissue collection, mice were anesthetized with i.p. injection of a mixture of ketamine hydrochloride (120 mg/kg) and medetomidine hydrochloride (0.5 mg/kg) and decapitated. Brains were removed and dissected on ice, and the hippocampus and cortex were isolated, frozen in dry ice and stored at −80°C. Cortex and hippocampal tissues from each animal were homogenized separately in four volumes (weight to volume ratio 1:4) of PBS, pH 7.4, containing Complete® protease inhibitor cocktail (Roche, Switzerland), followed by 3 short pulses of sonication (5 sec each). To ensure complete disruption of mitochondrial membranes, 3 rounds of freeze-thaw cycles between liquid nitrogen and room-temperature water were carried out. The samples were centrifuged at 20,800 g at 4°C for 5 minutes, and the supernatants were stored in 20 µl aliquots at −80°C. Protein concentration of each sample was measured in triplicates using the BCA Protein Assay Reagent (Pierce, Rockford, IL).
Non-denaturing isoelectric focusing gel analysis
CuZnSOD (SOD1) and MnSOD (SOD2) activities were determined by Ampholine PAGplate (Amersham Pharmacia Biotech, Inc. Piscataway, NJ), pH 3.5 – 9.5, as described previously [51]. Two-fold serial dilution of tissue lysates from 60 to 7.5 µg total protein were analyzed on the gel for CuZnSOD and MnSOD activities. The optimal range was between 15 and 7.5 µg total protein for measuring CuZnSOD and 60 and 30 µg for MnSOD. The activity stain creates clear bands on a dark purple background and identifies the location of CuZnSOD and MnSOD in the gel. The band intensity, which is proportional to the enzyme activity, was quantified by Image J 1.36b (NIH, USA) and normalized to total protein loading.
Western blot analysis
The protein levels of CuZnSOD, MnSOD, EC-SOD, catalase, peroxiredoxin (Prx1), and thioredoxin 2 (Trx2), were determined by western blot analysis. Equal amounts of protein (cortex, 30 µg; hippocampus, 50 µg) from each sample were separated by NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Primary and secondary antibodies used in this study are listed in Table 1. Protein bands were visualized using DuoLuX Chemiluminescent/Fluorescent Substrate Kit (Lumigen, Southfield, MI) after incubation with HRP-conjugated secondary antibody. All blots were stripped with stripping buffer (Tris-HCl, 62.5 mM; β-mercaptoethanol, 90 mM; 1% SDS) and reprobed with an antibody against β-actin (A 3854, 1:50,000, Sigma) as a loading control. Quantification of Western blot results was done by normalizing the signal intensity of each sample to that of β-actin.
Table 1.
Primary and secondary antibodies used in this study
| Target | Origin | Company | Catalog no. | Dilution |
|---|---|---|---|---|
| CuZnSOD | Rabbit | LabFrontier | LF-PA0013 | 1:2,000 |
| MnSOD | Rabbit | Stressgen | SOD-110 | 1:4,000 |
| EC-SOD | Rabbit | Custom-made | 1 µg/mL | |
| Catalase | Mouse | Sigma | C0979 (clone CAT- 505) |
1:4,000 |
| Peroxiredoxin 1 (Prx1) |
Rabbit | LabFrontier | LF-PA0001 | 1:2,000 |
| Thioredoxin 2 (Trx2) |
Rabbit | LabFrontier | LF-PA0012 | 1:2,000 |
| β-actin* | Mouse | Sigma | A3854 (clone AC-15) | 1:50,000 |
| Mouse IgG* | Goat | Bio-Rad | 172–1011 | 1:10,000 |
| Rabbit IgG* | Goat | Bio-Rad | 170–6516 | 1:40,000 |
| Neurons (NeuN) | Mouse | Millipore | MAB377 | 1:200 |
| Mouse IgG | Goat | Invitrogen | A31553 | 1:200 |
| Astrocytes (GFAP) |
Rabbit | Dakocytomation | Z0334 | 1:500 |
| Rabbit IgG | Goat | Vector | BA-1000 | 1:200 |
| S-phase cells (BrdU) |
Rat | Accurate | OBT0030S | 1:10 |
| Rat IgG | Donkey | Jackson | 712-295-153 | 1:200 |
| Microglia (CD68) |
Rat | Serotec | MCA1957S | 1:20 |
| Rat IgG | Mouse | Vector | BA-4001 | 1:200 |
tagged with Horseradish peroxidase
Neurogenesis
To determine the effects of irradiation on the survival and fate of newly generated cells in the SGZ as a function of genotype, groups (n = 4–5) of sham irradiated and irradiated SOD1 and SOD2 KO mice received a single i.p. injection (50 mg/kg) of BrdU daily for 7 days starting 30 days after irradiation. Three weeks after the last BrdU injection, mice were anesthetized as described and perfused with ice-cold saline followed by freshly prepared, ice-cold 4% paraformaldehyde. The brains were removed, processed, and sectioned using a sliding microtome [32]. Fifty micrometer sections were stored at 4°C in cryoprotectant solution until needed. Free floating sections were immunostained as described [14, 32] using antibodies listed in Table 1.
To calculate the numbers of BrdU-positive (BrdU+) cells in the dentate SGZ, at least 12 sections of a one-in-six series were scored per animal. All counts were limited to the dentate granule cell layer and a 50 µm border along the hilar margin that included the SGZ. Total numbers were obtained by multiplying the measured value by 6; overestimation was corrected using the Abercrombie method [52]. Total numbers of BrdU+ cells displaying neuron-specific (NeuN) or astrocytic-specific (GFAP) markers were determined using confocal microscopy to score the colocalization of BrdU and phenotypic indicators in representative sections from each animal [14, 32]. Confocal microscopy was performed using a Nikon C-1 confocal microscope (Melville, New York), using techniques previously described [14, 32]. Appropriate gain and black-level settings were obtained on control tissues stained with secondary antibodies alone. Upper and lower thresholds were always set using a range indicator function to minimize data loss due to saturation. Each cell was manually examined in its full ‘z’ dimension with use of split panel analysis, and only those cells for which the BrdU+ nucleus was unambiguously associated with the lineage-specific marker were scored as positive. For each lineage-specific marker, the percentage of BrdU+ cells expressing that marker was determined. Total numbers of lineage-specific BrdU+ cells were then calculated by multiplying this percentage by the total number of BrdU+ cells in the dentate gyrus.
Activated microglia
The impact of irradiation on the numbers of newly generated (BrdU+) activated microglia in the SGZ was determined as previously described [14, 32], using an antibody against CD68 (Table 1). Total numbers of newly generated activated microglia were quantified similarly as described above.
Xanthine/xanthine oxidase treatments of neural precursor cells
Neural precursor cells were isolated from postnatal C57BL6 mice and grown as neurospheres in culture under serum free conditions [40]. For chronic exposure to superoxide, cells were subjected to xanthine and/or xanthine oxidase, a superoxide generating system, using 1 mM xanthine (Sigma, St. Louis, MO) and 0.5mU/ml of xanthine oxidase (Sigma) for 6 consecutive days. Cultures undergoing treatments were given complete media changes every other day and stocks of xanthine and xanthine oxidase were prepared fresh, in PBS, the day of use. On the last day of treatment cells were γ-irradiated using a 137Cs irradiator (J.L. Shepard and Associates Mark I) at a dose rate of 1.17 Gy/min over a dose range of 0 to 5 Gy. Following irradiation, cells were counted and seeded into multi-well plates for the assessment of survival 5 days later.
Statistics
For each endpoint, values for all animals of a given treatment group were averaged and standard errors of mean (SEM) were calculated. Analysis of variance (ANOVA) was performed using the statistical package SAS. Differences between WT and KO strains at baseline and post irradiation were tested using contrast statements without adjustment for multiple comparisons. For consideration of the effect of dose, initially an interaction term was included to determine if the dose response appeared to be different among the animal strains. If there was no indication of an interaction, then the simplified model without interaction was fit and the main effects were tested for statistical significance. At the point that overall statistical significance was determined, subgroup analyses were conducted to determine the nature of the differences. For the analysis of the in vitro study, each plate of cells was considered an independent sample. Means and SEM were calculated and an ANOVA was used to analyze the data. For the analysis of protein levels and enzyme activities determined by western blotting and IEF gel, normalized signal intensities from each genotype/treatment group were analyzed by two-tailed Student’s t test.
Results
No differences in the level of major antioxidant enzymes between WT and SOD 1 or SOD 2 KO mice
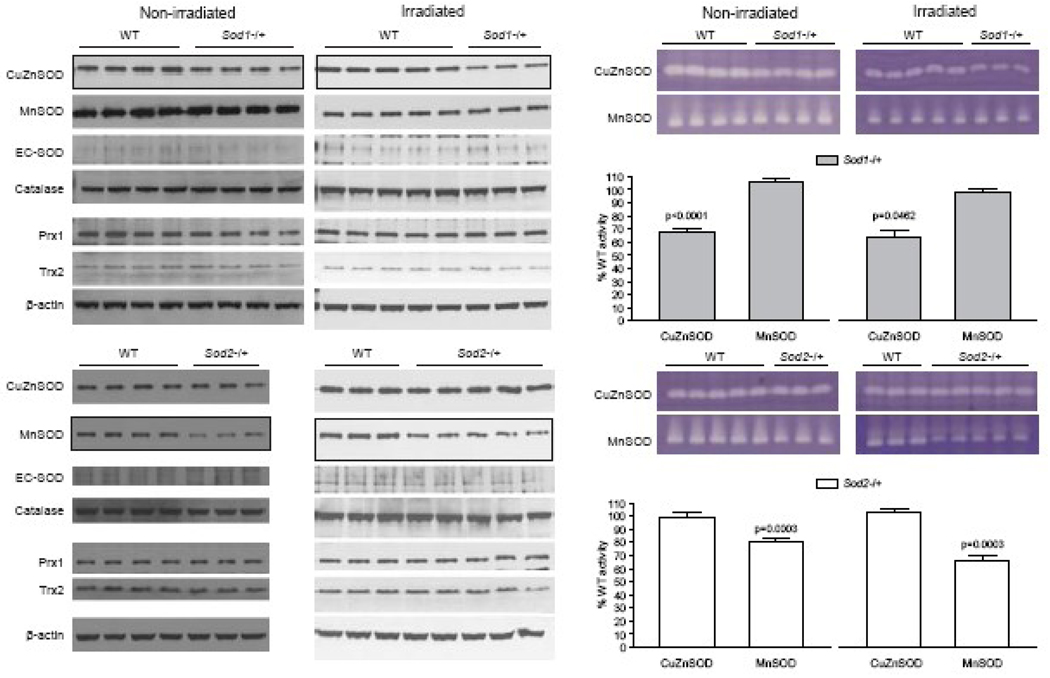
The KO mouse strains used here were heterozygous for SOD KO, so detectable protein levels (Fig. 1, left panel) and enzyme activities (Fig. 1, right panel) of the specific SOD isoforms in each strain were reduced as expected, albeit not reaching the theoretical 50% reduction based on gene dosage effect. Further, within each KO strain, there were no apparent compensatory changes in the protein levels and activities for the other SOD isoforms (Fig. 1). In addition to the three SOD isoforms, we investigated levels of several major peroxidases in this study, including catalase, peroxiredoxin 1 (Prx1), and thioredoxin 2 (Trx2). Catalase is the major enzyme for H2O2 metabolism, while Prx1 is a cytosolic thiol-based peroxidase [53] and is estimated to be present in 10 times the concentration of glutathione peroxidase [54]. Peroxiredoxins can react with lipid peroxide, peroxinitrite, and H2O. Trx2 is a mitochondrial protein that is important in protection against oxidative stress [55]; it complements the glutathione system and controls mitochondrial thiol redox state. There were no apparent changes in the protein levels of catalase, Prxl or Trx2 either prior to or after irradiation (Fig. 1, left panel).
Figure 1.
Antioxidant profiles in SOD1 and SOD2 KO mice. Hippocampi from 3-month-old male SOD1, SOD2, and WT mice were analyzed by western blotting (left panel) for the levels of major antioxidant enzymes and by non-denaturing IEF gels (right panel) for CuZnSOD and MnSOD enzymatic activities. SOD1 and SOD2 KO mice showed the expected reductions in CuZnSOD and MnSOD protein and activity, respectively when compared to WT controls. The western blot images of CuZnSOD and MnSOD from SOD1 and SOD2 KO, respectively, are outlined with dark border to bring attention to the differences between WT and the corresponding KO. There were no apparent compensatory changes in extracellular SOD (EC-SOD) or in catalase, peroxiredoxin 1 (Prx1), or thioredoxin 2 (Trx2). SOD enzyme activities in the non-denaturing IEF gels were quantified by Image J as pixel intensities and the value of percent WT controls calculated. Three – 5 mice/genotype were analyzed/experimental group.
Newly generated cells and SOD deficiency in un-irradiated mice
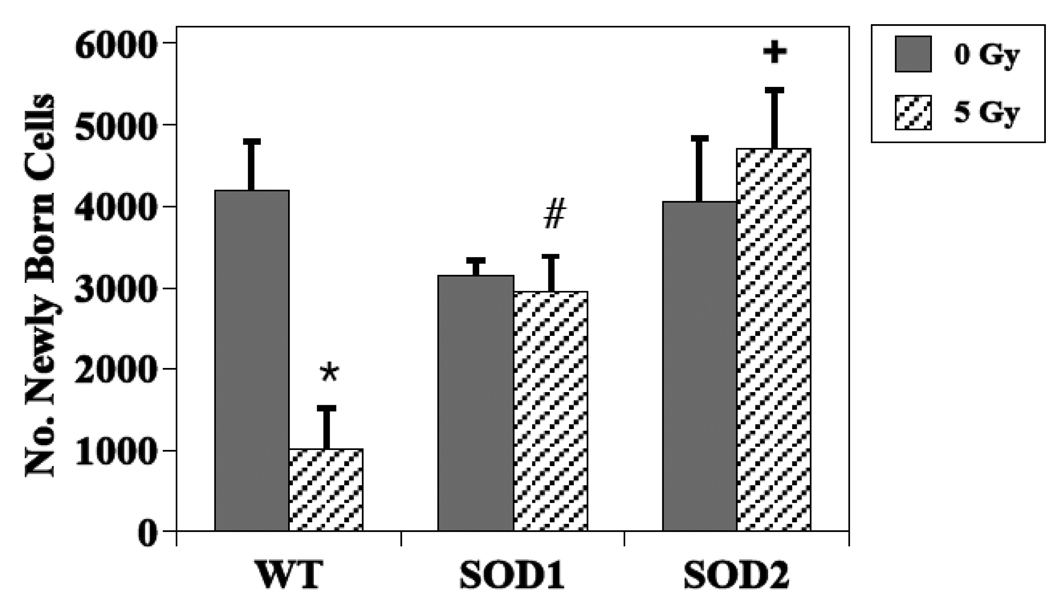
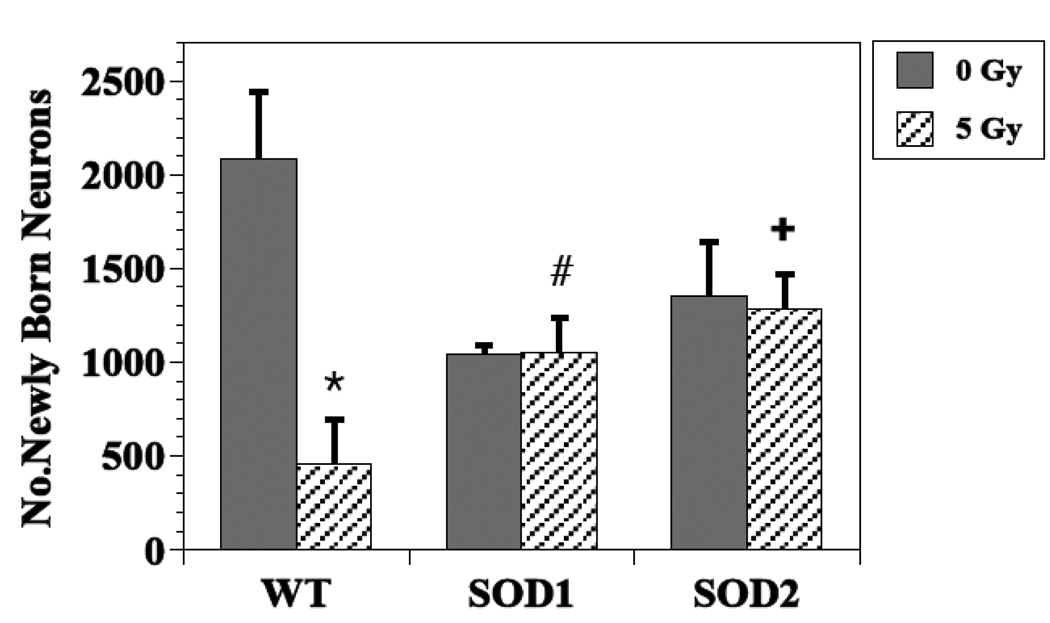
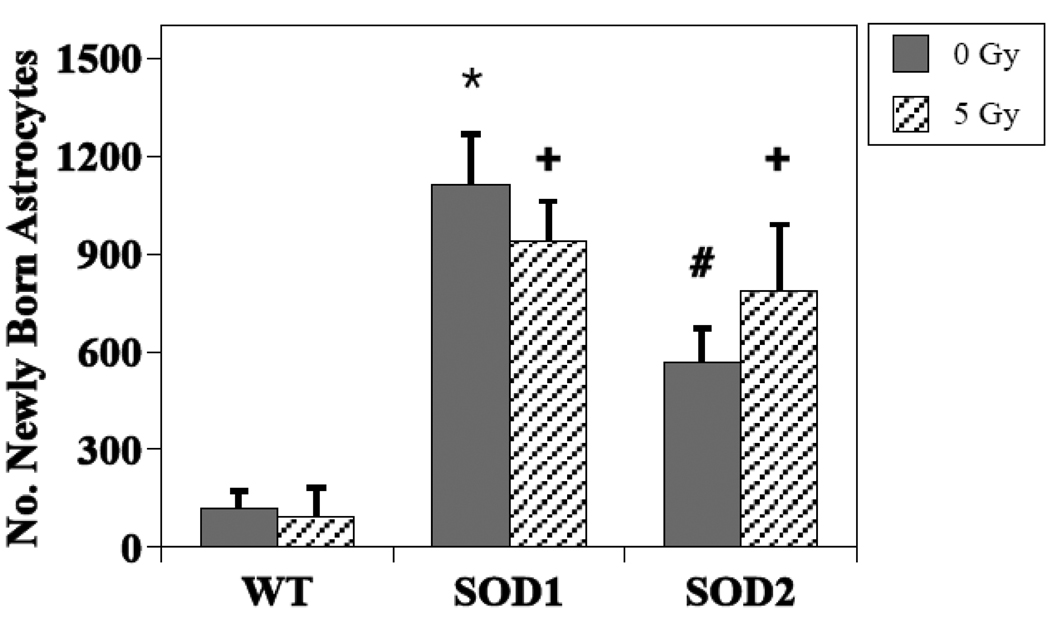
The presence of BrdU+ cells 3–4 weeks following the administration of BrdU, represents the long-term survival of newly generated cells, independent of phenotype. In non-irradiated WT mice, the total number of BrdU+ cells in the dentate SGZ averaged 4186 ± 614, while the average values were 3145 ± 188 and 4062 ± 766, in SOD 1 and SOD 2 KO mice, respectively (Fig. 2). These differences were not statistically significant (SOD1, P =0.23 and SOD2, P =0.88). In WT mice, an average of 2086 ± 354 newly generated cells had differentiated into neurons (BrdU+/NeuN+; Fig. 3), while in non-irradiated SOD1 and SOD2 KO mice, the numbers were lower, averaging 1048 ± 41 (49% reduction, P = 0.02) and 1354 ± 290 (35% reduction, P =0.08), respectively (Fig. 3). With respect to newly generated cells that differentiated into astrocytes (Brdu+/GFAP+), there was a significant difference across the groups (p<0.001). In WT mice there was an average of 121 ± 50 newly generated astrocytes, while in SOD1 and SOD2 KO mice the average numbers were 1112 ± 157 (P < .001 compared to WT) and 568 ± 103 (P = 0.02 compared to WT), respectively (Fig. 4). The numbers of newly generated activated microglia (BrdU+/CD68) present in the dentate SGZ were not different between WT (600 ± 82), SOD1 (706 ± 38) or SOD2 (792 ± 138) mice.
Figure 2.
Effects of irradiation on the survival of newly generated (BrdU+) cells in the dentate subgranular zone of WT and SOD KO mice. A dose of 5 Gy significantly reduced newly generated cells in WT mice but not in SOD KO mice. Each bar represents a mean of 4–5 mice; error bars are SEM. * irradiated vs. non-irradiated WT (p = <0.01); # irradiated SOD1 vs. WT (p = 0.05); + irradiated SOD2 vs. WT (p = 0.001).
Figure 3.
Effects of irradiation on the survival/differentiation of newly generated neurons (BrdU+/NeuN+) in the dentate subgranular zone of WT and SOD KO mice. How irradiation affected newly born neurons was dependent upon strain, with WT mice showing significant reductions after irradiation in contrast to either of the KO strains. After irradiation there were more newly born neurons observed in SOD KO mice relative to WT. Each bar represents a mean of 4–5 mice; error bars are SEM. * irradiated vs. non-irradiated WT (p = <0.01); # irradiated SOD1 vs. WT (p = 0.02); + irradiated SOD2 vs. WT (p = .003).
Figure 4.
Effects of irradiation on the survival/differentiation of newly born astrocytes (BrdU+/GFAP+) in the dentate subgranular zone of WT and SOD KO mice. How irradiation impacted newly born astrocytes was dependent upon strain, with KO mice showing significantly higher numbers relative to WT mice. * non-irradiated WT vs. non-irradiated SOD1 (p = <0.001); # non-irradiated WT vs. non-irradiated SOD2 (p = 0.02); + irradiated SOD1 and 2 vs. WT (p = 0.001).
Newly generated cells and SOD deficiency in irradiated mice
For both newly generated cells independent of phenotype (BrdU+ only) and newly born neurons (Brdu+/NeuN+), there was a statistically significant interaction between radiation and animal strain (P ≤ 0.01), indicating that the impact of irradiation depended on genotype. As reported previously [32], and shown again here, in WT mice a single dose of 5 Gy significantly reduced the average numbers of BrdU+ cells (Fig. 2) and BrdU+/NeuN+ cells (Fig. 3) observed in the dentate SGZ; the reduction was ~75% in both cases (P <0.01). There was no indication of an interaction between dose and strain when only the data from the 2 mutant strains were considered (P > 0.5) (Fig. 3), but the number of cells expressing BrdU+ only tended to be higher for SOD2 than SOD1 (P = 0.04). Looking only at post-irradiation data, the numbers of newly generated cells (BrdU only) and newly generated neurons were higher in KO mice than in WT after irradiation. For newly generated cells only, the significance values were P = 0.05 and P = 0.001 for SOD1 and SOD2 respectively, and for newly born neurons P = 0.02 and P = 0.003 respectively.
There was no indication of an overall dose by genotype interaction (P = 0.37) in terms of newly generated cells that differentiated into astrocytes (BrdU+/GFAP+) after irradiation (Fig. 4). When modeled without an interaction term there still was no indication of a dose effect (P = 0.89), but there was a significant difference across genotypes (P <0.001). Looking only at the KO strains, there continued to be no indication of a dose effect (P = 0.83), but there was some indication of a difference between the strains (P =0.07) (Fig. 4). Paired comparisons between WT and each of the SOD KO strains showed a significant genotype difference (P < 0.001).
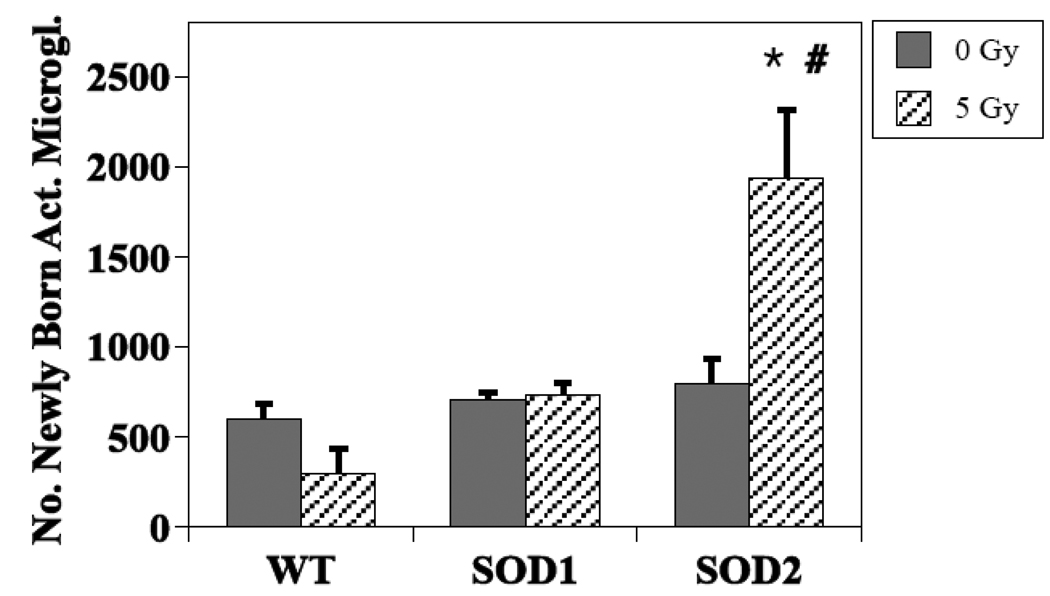
In terms of newly generated activated microglia, there was a dose by genotype interaction (P =0.003) (Fig. 5) suggesting genotype impacted this element of neuroinflammation. While a single dose of 5 Gy had no significant effect on the numbers of activated microglia in WT (P = 0.71) or SOD1 KO mice (P = 0.21), there was a significant effect in SOD2 KO (P = 0.03). When only post-irradiation values were considered, there was no significant difference between WT and SOD1 mice (P = 0.21) but a highly significant difference between WT and SOD2 (P = 0.001). When comparing the post-irradiation values for the SOD mice only, the average value obtained from SOD1 mice (792 ± 138) was significantly different (P = 0.005) from that observed in SOD2 KO mice (1939 ± 375).
Figure 5.
Effects of irradiation on the survival of newly generated (BrdU+) activated microglia in the dentate gyrus of WT and SOD KO mice. The data showed a dose by genotype interaction (P =0.003) suggesting genotype impacted this particular element of neuroinflammation. Each bar represents a mean of 3–5 mice; error bars are SEM. * Irradiated WT vs. irradiated SOD2 (P = 0.001); # irradiated SOD1 vs. irradiated SOD2 (P = 0.005).
Excess superoxide affects radiation response of neural precursor cells in culture
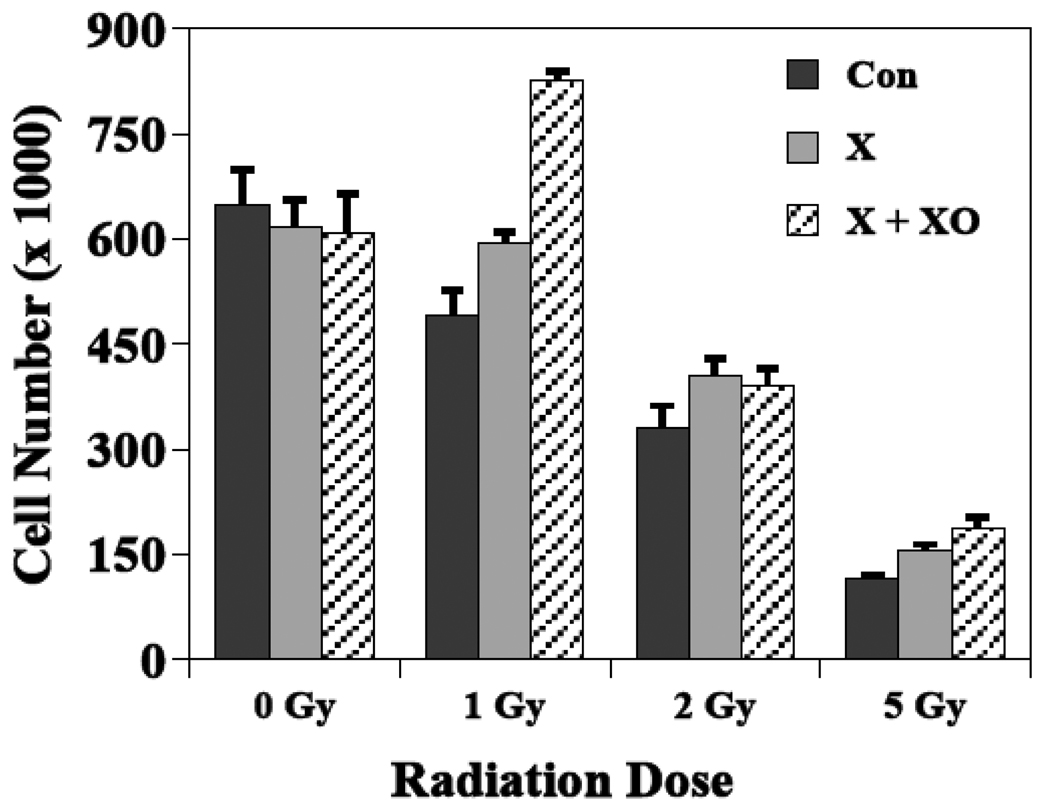
Cultured neural precursor cells subjected to a 6-day treatment of either xanthine alone or the combination of xanthine plus xanthine oxidase did not exhibit any significant cytotoxicity in the absence of irradiation (Fig. 6). After irradiation there was a highly significant dose effect for all groups (P < 0.001), but there was no indication that the dose-dependent decrease in cell number differed based on xanthine or xanthine oxidase treatment. However, regardless of radiation dose, after treatment with either xanthine alone or the combination of xanthine plus xanthine oxidase, cell numbers tended to be greater than corresponding cultures that received irradiation only (Fig. 6). The cell yields after xanthine only treatment were 121, 122 and 135% compared to cultured cells that received radiation only at doses of 1, 2 or 5 Gy, respectively. For those cultures given xanthine plus xanthine oxidase treatments, cell yields were higher by 168, 118 and 163%, after 1, 2 or 5 Gy, respectively, compared to cultures that received γ-irradiation only. An ANOVA of these data showed statistical significance after 1 and 5 Gy but not after 2 Gy. While these data are limited, they suggest that elevated superoxide levels may provide a protective effect in this system.
Figure 6.
Elevated levels of superoxide affects the radiation response of neural precursor cells in culture. Neural precursor cells grown as neurospheres and were treated before irradiation for 6 consecutive days with xanthine (X) or xanthine plus xanthine oxidase (X + XO) to generate excess levels of superoxide. Controls received neither treatment. Radiation doses of 0, 1, 2, or 5 Gy were given at the end of each treatment and cells were counted 5 days later. There was a highly significant (P < 0.001) dose effect in all treatment groups, and the cell yields after all radiation doses were higher in the X and X + XO groups relative to control. Each bar represents a mean of 3 plates and error bars are SEM.
Discussion
The main findings of the present study were: 1) before irradiation, the numbers of newly born neurons generated in the dentate SGZ were lower in SOD KO mice compared to WT mice; 2) irradiation did not reduce the numbers of newly generated neurons in SOD KO mice; 3) both before and after irradiation the numbers of newly born astrocytes in SOD KO mice were significantly elevated relative to that seen in WT mice; and 4) changes in neuroinflammation, at least in the context of newly born activated microglia, are genotype dependent. These findings confirmed our earlier observations in EC-SOD KO mice, in that after irradiation an environment lacking SOD was much more permissive in the context of hippocampal neurogenesis [32]. The mechanism(s) behind this observation are not yet known, but given that all SOD KO mice should have variable levels of increased oxidative stress, it likely will involve persistent and elevated levels of ROS.
SODs, which are critical elements of the cellular antioxidant defense mechanism [56], are oxidoreductases that remove superoxide by catalyzing the dismutation of the superoxide radical to hydrogen peroxide. Hydrogen peroxide is then metabolized to molecular oxygen and water by catalase or peroxidases. The 3 different SOD isoforms catalyze the same chemical reaction, but have different enzymatic properties and distinct subcellular localizations. Therefore, deficiency in SOD, regardless of location, should result in relatively higher levels of ROS and altered redox state, which will induce a state of persistent oxidative stress. While ROS have often been considered to be hostile or destructive entities, data also exist showing that ROS can have beneficial effects [47, 48], and in the brain, they are critically involved in a number of important processes, particularly those involved in learning and memory formation [46, 57, 58]. This information documents the paradoxical effects associated with ROS, where differing levels can either be good or bad, depending upon the circumstances [46, 57, 59–61].
Here we found that partial depletion of the SOD1 and SOD2 isoforms was associated with reduced baseline levels of neurogenesis, but was also associated with a ‘protective’ effect after irradiation. These effects were not coupled with any obvious compensatory changes in other anti-oxidant enzymes (Fig. 1), at least in the context of western blot analyses of brain extracts. Furthermore, there were no compensatory changes in SOD1 or SOD2 activities (Fig. 1). It is possible that circulating antioxidants, including ascorbic acid, tocopherol, uric acid, bilirubin, proteins and other compounds, could be increased as a compensatory mechanism, but we did not address that in the current study. The fact that a partial deficiency in the SOD1 and SOD2 isoforms as seen here, as well as a full deficiency in SOD3 reported earlier [32], all imparted a common protective effect in the hippocampus, suggests that there are common effectors throughout the cell capable of engaging prosurvival or differentiation pathways. While the precise mechanism(s) responsible for this type of response is not yet known, in a general sense this effect resembles a preconditioning [62], adaptive (reviewed in [63]), or inducible-like radioprotective response [64], where a sublethal or potentially injurious stimulus (i.e. oxidative stress) induces tolerance to a subsequent and potentially more damaging insult (irradiation). Lower levels of SOD should lead to higher levels of oxidative stress [65] which should in turn result in an increase in reactive species that are derived from superoxide, e.g. H2O2, and data exist showing that the presence of such compounds can reduce the effects of a subsequent insult [66, 67]. In the CNS, this could involve specific trophic factors and signaling molecules that favor differentiation and long-term survival of newly generated neurons and which are up-regulated or activated in the brains of irradiated SOD KO mice. Such factors could include brain derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and nitric oxide (NO), all of which have been shown to favor differentiation and survival of neurons [68–71]. Regardless of the mechanism involved, our findings clearly suggest that SOD deficient mice have developed a resistance to radiation-induced inhibition of neurogenesis that may involve some type of adaptation within the microenvironment, without, necessarily, any compensatory changes in other major intracellular antioxidants.
Astrocytes are now recognized as dynamic regulators of a variety of neuron-related functions, including neurogenesis [72–74]. In fact, it has been suggested that the astrocytes within the neurogenic niche are highly specialized and contribute to the regulation of proliferation and fate specification of neural precursor cells [74]. In the present study, before irradiation the relative abundance of newly born astrocytes was 5–9 fold higher in SOD KO mice compared to WT controls (Fig. 4), and the ratio of new neurons to new astrocytes was about 17 in WT and 1–2 in the SOD KO mice. The finding of increased numbers of newly generated astrocytes in SOD1 and SOD2 KO mice needs to be considered in the context of an increase in steady-state levels of superoxide due to a ~ 50% reduction in CuZnSOD or MnSOD. It is possible that increased steady-state levels of superoxide and downstream ROS derived from superoxide alter the differentiation pattern of neuronal progenitor cells in favor of astroglial lineage. Alternatively, it may be that astrocytes are more resistant to superoxide than neurons, leading to a better outcome in terms of long-term survival. The notion that astrocytes are more resistant to superoxide and other ROS is consistent with previous findings showing that primary astrocytes are more resistant to menadione induced apoptotic cell death than primary neuronal cultures [75] and that astrocytes are more efficient in repairing oxidative mitochondrial DNA damage mediated by menadione [76]. Astrocytes tend to produce lower steady-state levels of ROS during metabolism, and primary astrocytes derived from hippocampus have been shown to have lower steady-state level lipid peroxidation than primary neurons derived from the same region [77]. It has been shown in different experimental systems that a more oxidized cellular environment facilitates progenitor cell differentiation as opposed to proliferation [33, 61], but whether the more oxidized redox state favors differentiation toward one cell lineage over another is less clear. In our experimental system, the data suggest that the SOD deficient environment favors differentiation toward an astrocytic lineage.
It is particularly interesting to note that prior to irradiation, the average number of newly born cells that differentiated into astrocytes in SOD1 mice (1112 ± 157) was higher than that seen in SOD2 (586 ± 102), which in turn was higher than the value for SOD3 (291 ± 98; [32]). A critical redox-related factor associated with differentiation may be more predominant in the cytoplasm rather than in the mitochondria or extracellularly, but this conclusion is highly speculative. Additionally, when the number of newly born cells that differentiate into neurons prior to irradiation was analyzed between the different SOD isoforms the opposite trend was found, where SOD1, SOD2 and SOD3 deficient mice have an average of 1048 ±40, 1355 ± 290, and 1793 ± 226 [32] new neurons, respectively. These trends are provocative and it is tempting to speculate that the different cellular compartments from which the SOD isoforms evolved may contain redox-sensitive factors that play a deciding role in the lineage commitment during precursor cell differentiation [78].
Irradiation had little or no effect on newly born cells that become astrocytes, regardless of genotype, but the new neuron to new astrocyte ratio after irradiation fell to about 5 in WT mice but remained around 1–1.5 in the KO animals. Given the supportive role of astrocytes in neurogenesis [72–74], the relatively higher numbers of the newly generated astrocytes in the KO mice may have promoted the survival of newly born neurons after irradiation, although our data do no provide definitive proof of this thesis. Alternatively, the higher numbers of newly born astrocytes seen after irradiation in the SOD KO mice might simply be associated with gliosis, which has been shown to be mediated in part by reactive species [79]. Finally, there are data suggesting that the putative stem cell in the adult mammalian brain is astrocytic [80], so it may be that the SOD deficient background favors the selection/survival of stem like cells in the dentate SGZ. This latter idea is supported, in part, by data suggesting that oxidative stress and redox regulation play important roles in self-renewal and differentiation in specific precursor cell populations [33, 61]. Whether or not the differences in the astrocytic cell population is responsible for ‘protecting’ neurogenesis after irradiation, or is merely a sign of some sort of non-specific activation or process due to a persistent oxidative stress needs to be further investigated.
Previous studies have suggested that neuroinflammation, and in particular elevated numbers of activated microglia, may negatively impact neurogenesis after irradiation [12, 14, 27, 30]. However, with the wide diversity of cell types involved, including astrocytes, and differences in activation state, it is now being recognized that neuroinflammation may be supportive as well as detrimental to neurogenesis (reviewed in [81, 82]). In fact, in our previous EC-SOD study we saw that there were more activated microglia in EC-SOD KO mice than in WT mice, and concluded that in an SOD deficient background, increased numbers of activated microglia had no apparent association with neurogenesis [32]. In general, this agrees with work from others showing that microglial phenotype critically influences the ability of these cells to support or impair cell renewal processes in the adult brain [83], and suggests that microglia may respond differently to a given stimulus (irradiation) depending upon the presence of another and preceding stimulus (oxidative stress). The present data further complicate this issue inasmuch as irradiation induced a very substantial increase in numbers of newly born activated microglia in the irradiated SOD2 background, but had no apparent effect in the SOD1 background, while both showed a protective effect in the context of neurogenesis. This could mean that the numbers of newly born activated microglia per se play a limited role in the observed changes in neurogenesis, or that the deficiency of mitochondrial SOD impacts specific elements of neuroinflammation to a greater extent than cytoplasmic SOD deficiency. Identification and investigation of potential pathways and molecules that may be responsible for such observations should provide us the information to determine the significance of these findings.
Given the normal physiologic role of SOD, it seems likely that a major factor in the responses seen in the SOD KO mice relate to superoxide levels. The evolution of the different SOD isoforms must in part be related to the poor diffusion of the superoxide anion across different cellular compartments, as well as the need to remove this mildly reactive molecule from specific intracellular and extracellular spaces. Previous studies with MnSOD deficient mice (Sod2−/+) showed a significant reduction in reduced GSH and an increase in 8-oxodG in the brain, suggesting an increased steady state level of oxidative stress in SOD2 KO [50, 65]. Even though similar types of measurements have not been carried out in SOD1 KO (Sod1−/+) brains, studies focusing on liver and skeletal muscles in homozygous SOD1 KO (Sod1−/−) mice all showed increased steady state level of oxidative damage [49, 84]. Therefore, SOD deficiency should increase superoxide levels in cells; but we did not directly measure this in vivo due to a limited quantity of hippocampal tissue. Therefore, to determine if elevated superoxide has an influence on how neural precursor cells responded to irradiation, we performed an in vitro study where we could actually subject cells to elevated levels of superoxide prior to irradiation. While cells exposed to xanthine plus xanthine oxidase showed a similar dose response as controls, on average, at the doses used, there was about a 1.5-fold increase in cell number compared to that seen after irradiation only (Fig. 6). There were also increased cell numbers noted in the presence of xanthine alone (Fig. 6), suggesting that there was some endogenous xanthine oxidase activity available that led to intermediate levels of superoxide. While these in vitro data are limited, they do suggest that the presence of excess superoxide prior to irradiation has significant positive effects on the proliferation and/or survival of multipotent neural precursor cells. Further, the data are qualitatively consistent with our in vivo studies, and suggest that superoxide may act to stimulate a redox-sensitive pathway/s that ameliorates the inhibition of neurogenesis typically found after irradiation.
In summary, our findings have clearly demonstrated that SOD deficiency has paradoxical effects with respect to hippocampal neurogenesis. In the absence of irradiation, SOD deficient animals exhibited reduced baseline neurogenesis and, presumably, increased basal oxidative stress, the latter of which may have been sufficient to elicit a ‘protective’ or adaptive response following low dose radiation exposure. This effect was independent of specific isoform and whether the deficiency was complete (SOD3) [32] or partial (SOD1, 2). Further, the effect did not apparently involve compensatory responses in other major antioxidant defenses, nor was it associated with a clear trend in detrimental neuroinflammation. Interestingly, a pilot in vitro study carried out under excess superoxide levels also revealed beneficial effects in terms of increased survival and/or proliferation. Opposing trends in the yields of newly generated astroglia versus newly generated neurons in unirradiated animals deficient for specific SOD isoforms suggest that factors controlling the phenotypic fate of multipotent hippocampal precursor cells might be restricted to and/or concentrated in specific subcellular compartments. While the precise mechanism behind the neuroprotective effects of SOD deficiency is not known, further studies need to be performed to determine whether adaptation to pre-existing oxidative stress plays a key role. The use of conditional tissue-specific SOD knockout animals [85] should help delineate the time required to develop any favorable adaptation before exposure to irradiation, and continued in vitro experimentation will elucidate the redox responsive pathways for conferring the neuroprotective phenotype found in irradiated animals deficient in SOD.
Acknowledgements
This work was supported in part by NIH grant R01 NS46051 (JRF) NSCOR grant NNJ04HC90G (JRF), NIH grant AG24400 (TTH), VA Palo Alto Health Care System (TTH), the Palo Alto Institute for Research and Education (TTH), and ACS grant RSG-00-036-04-CNE (CLL)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: A dynamic process. Radiat. Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 3.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7:517–523. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 4.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 5.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 6.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J. Clin. Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 7.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 8.Surma-aho O, Niemela M, Vilkki J, Kouri M, Brander A, Salonen O, Paetau A, Kallio M, Pyykkonen J, Jaaskelainen J. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56:1285–1290. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 9.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 10.Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann. Neurol. 2004;55:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 11.Raber J, Rola R, LeFevour A, Morhardt DR, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-Induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 12.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 14.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Can. Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 15.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 16.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur. J. Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R, Brunso-Bechtold JK. Spatial Learning and Memory Deficits after Whole-Brain Irradiation are Associated with Changes in NMDA Receptor Subunits in the Hippocampus. Radiat. Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 20.Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat. Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 21.Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68:9763–9770. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat . Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 28.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 29.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg. Clin. N. Am. 2007;18:115–127. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 31.Rola R, V S, Obenaus A, Nelson GA, Otsuka S, Limoli CL, Fike JR. High LET irradiation induced inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat. Res. 2005;164:556–560. doi: 10.1667/rr3412.1. [DOI] [PubMed] [Google Scholar]
- 32.Rola R, Zou Z, Huang T-T, Fishman K, Baure J, Rosi S, Milliken H, Limoli CL, Fike JR. Lack of EC-SOD in the microenvironment impacts radiation-induced changes in neurogenesis. Free Rad. Biol. & Med. 2007;42:1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 35.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juurlink BH, Paterson PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med. 1998;21:309–334. doi: 10.1080/10790268.1998.11719540. [DOI] [PubMed] [Google Scholar]
- 38.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 40.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiation Research. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- 42.Limoli CL, Rola R, Giedzinski E, Mantha S, Huang T-T, Fike JR. Cell density dependent regulation of neural precursor cell function. PNAS. 2004;101:16052–16057. doi: 10.1073/pnas.0407065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lonergan PE, Martin DS, Horrobin DF, Lynch MA. Neuroprotective effect of eicosapentaenoic acid in hippocampus of rats exposed to gamma-irradiation. J Biol Chem. 2002;277:20804–20811. doi: 10.1074/jbc.M202387200. [DOI] [PubMed] [Google Scholar]
- 44.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 45.Huang TT, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann N Y Acad Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- 46.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slemmer JE, Shacka JJ, Sweeney MI, Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15:404–414. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- 48.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 50.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 51.Huang TT, Raineri I, Eggerding F, Epstein CJ. Transgenic and mutant mice for oxygen free radical studies. Methods Enzymol. 2002;349:191–213. doi: 10.1016/s0076-6879(02)49335-4. [DOI] [PubMed] [Google Scholar]
- 52.Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 53.Fourquet S, Huang ME, D'Autreaux B, Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid Redox Signal. 2008;10:1565–1576. doi: 10.1089/ars.2008.2049. [DOI] [PubMed] [Google Scholar]
- 54.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin ED, Brady TC, Hochrein EC, Oury TD, Jonsson LM, Marklund SL, Crapo JD. Molecular manipulations of extracellular superoxide dismutase: functional importance for learning. Behav Genet. 1998;28:381–390. doi: 10.1023/a:1021673703129. [DOI] [PubMed] [Google Scholar]
- 59.Kamsler A, Avital A, Greenberger V, Segal M. Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxid Redox Signal. 2007;9:181–189. doi: 10.1089/ars.2007.9.181. [DOI] [PubMed] [Google Scholar]
- 60.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- 62.Gori T, Forconi S. The role of reactive free radicals in ischemic preconditioning--clinical and evolutionary implications. Clin Hemorheol Microcirc. 2005;33:19–28. [PubMed] [Google Scholar]
- 63.Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev. 2006;127:436–443. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 64.Qutob SS, Multani AS, Pathak S, McNamee JP, Bellier PV, Liu QY, Ng CE. Fractionated X-radiation treatment can elicit an inducible-like radioprotective response that is not dependent on the intrinsic cellular X-radiation resistance/sensitivity. Radiat Res. 2006;166:590–599. doi: 10.1667/RR0514.1. [DOI] [PubMed] [Google Scholar]
- 65.Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363:91–97. doi: 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- 66.Spitz DR, Dewey WC, Li GC. Hydrogen peroxide or heat shock induces resistance to hydrogen peroxide in Chinese hamster fibroblasts. J Cell Physiol. 1987;131:364–373. doi: 10.1002/jcp.1041310308. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan SJ, Roberts RJ, Spitz DR. Replacement of media in cell culture alters oxygen toxicity: possible role of lipid aldehydes and glutathione transferase in oxygen toxicity. J Cell Physiol. 1991;147:427–433. doi: 10.1002/jcp.1041470307. [DOI] [PubMed] [Google Scholar]
- 68.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 69.Cardenas A, Moro MA, Hurtado O, Leza JC, Lizasoain I. Dual role of nitric oxide in adult neurogenesis. Brain Res Brain Res Rev. 2005;50:1–6. doi: 10.1016/j.brainresrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Gibbs SM. Regulation of neuronal proliferation and differentiation by nitric oxide. Mol Neurobiol. 2003;27:107–120. doi: 10.1385/MN:27:2:107. [DOI] [PubMed] [Google Scholar]
- 71.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jordan JD, Ma DK, Ming GL, Song H. Cellular niches for endogenous neural stem cells in the adult brain. CNS Neurol Disord Drug Targets. 2007;6:336–341. doi: 10.2174/187152707783220866. [DOI] [PubMed] [Google Scholar]
- 75.Harrison JF, Hollensworth SB, Spitz DR, Copeland WC, Wilson GL, LeDoux SP. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 2005;33:4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hollensworth SB, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP. Glial cell type-specific responses to menadione-induced oxidative stress. Free Radic Biol Med. 2000;28:1161–1174. doi: 10.1016/s0891-5849(00)00214-8. [DOI] [PubMed] [Google Scholar]
- 77.Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol. 2001;169:416–424. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- 78.Oberley LW, Oberley TD, Buettner GR. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Med Hypotheses. 1980;6:249–268. doi: 10.1016/0306-9877(80)90123-1. [DOI] [PubMed] [Google Scholar]
- 79.Baydas G, Tuzcu M. Protective effects of melatonin against ethanol-induced reactive gliosis in hippocampus and cortex of young and aged rats. Exp Neurol. 2005;194:175–181. doi: 10.1016/j.expneurol.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 81.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 82.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 84.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 85.Zou Y, Chen CH, Fike JR, Huang TT. A new mouse model for temporal- and tissue-specific control of extracellular superoxide dismutase. Genesis. 2009;47:142–154. doi: 10.1002/dvg.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]