Abstract
Non-protein-coding RNAs (ncRNAs) play critical roles on many levels of cellular information processing and pervasive expression of ncRNAs in the nervous system could help explain brain complexity. NcRNAs are enriched in the central nervous system and are associated with specific neuroanatomical regions. Additionally, several recent publications have revealed an important role for deregulation of ncRNAs in various human neuropathologies, such as Alzheimer's disease, Parkinson's disease and Fragile X mental retardation. Herein, we summarize reports on functional ncRNA molecules involved in cellular stress response, particularly related to Alzheimer's disease. We conclude that ncRNAs have a prominent role in maintaining precise physiological levels of gene products directly implicated in Alzheimer's disease pathology.
Introduction
Accumulating evidence suggests that non-protein-coding RNAs (ncRNAs) play transformative roles as molecular information processors to enhance the performance of systems throughout the cell. This evidence led to the proposal that ncRNAs articulate high-density information flows between the many layers of functional networks, facilitating refined integration and concert of action among diverse molecular machineries 1. Across the expanse of cellular physiology, ncRNAs have the capacity, in principle, for information coupling between otherwise disparate systems, decisively impacting the behavioral dynamics of cells, tissues, and organisms.
At critical junctures in evolutionary history, genomic expansions of non-coding elements appeared simultaneously with large increases in organismal complexity. The transition from unicellular to multi-cellular organisms that occurred approximately 700 million years ago, the so called Precambrian Explosion, may only have been possible by the widespread incorporation of ncRNAs into the genomes of early multicellular organisms 2-5. The same features that drove intensive incorporation of ncRNAs in the developmental pathways of multicellular organisms later facilitated their use in the mammalian stress response. An emerging theme now links the unique information processing features of ncRNAs to stress response and the onset of chronic diseases 6.
In neurophysiology, simultaneous stresses of many different types are the rule, not the exception. The wide dynamic ranges required for signaling and plasticity create a constant stream of challenges for molecular machineries, such as thermal dissipation in restricted microregions, and maintenance of order among rapidly changing synaptic inventories of molecular materials. The interplay between these phenomena and the demands of synaptic homeostasis has emerged as a focal point in the early stages of Alzheimer's disease pathogenesis. A mosaic of recent findings demonstrating functions of ncRNAs in stress response 6 together with their specific expression in the nervous system 7 now indicate important roles for ncRNAs in neurodegeneration. In this review, we provide a framework for discussion of this emerging theme in neurodegeneration, and we highlight recently discovered examples of ncRNA pathways in the molecular etiology of Alzheimer's disease. We seek to provide a glimpse of a still mysterious additional dimension of information flow through nervous system tissues, orthogonal to both electrophysiology and canonical protein interaction networks.
NcRNAs orchestrate complex nervous system functions
Even after almost a century of intensive investigations of the molecular architecture of neurons and synapses, ncRNAs had gone virtually unnoticed. Suddenly though, high throughput studies from multiple platforms have provided evidence for a striking nervous system wide pattern of ncRNA expression 7. Whole transcriptome cDNA libraries from hundreds of mouse and human tissues, created by the FANTOM Consortium 8-10, facilitated the search for spatially defined transcript expression in the nervous system 11. Using this resource, Mercer et al. have shown widespread expression of long ncRNAs in mouse brain. They showed that the majority of ncRNAs, 849 out of 1328 examined, are expressed in the adult mouse brain in specific neuroanatomical regions, cell types, or sub-cellular compartments, suggesting that the majority of ncRNAs are expressed in the brain and play an important role in nervous system complexity 7. Although some of these RNAs may ultimately prove to result from the alternative splicing of previously unannotated 3′ UTRs12, the Mattick laboratory subjected input RNAs to a rigorous filter to eliminate these and other artifacts. Other studies have used in situ hybridization techniques to demonstrate articulate nervous system expression of many miRNAs 13. While low copy number and cell specific expression of ncRNAs result in sequence depth and localization issues, the advent of next generation sequencing technology now provides an improved approach to ncRNA discovery and expression mapping. The miRNA atlas has confirmed enhanced expression of many human miRNAs in brain tissue 14. Studies by Berezikov and colleagues also revealed hundreds of brain specific novel miRNAs, utilizing massively parallel sequencing approach 15. They also compared the miRNA between human and chimpanzee brains, and suggested that some of the new miRNAs are not conserved beyond primates, indicating their recent origin, and species specificity 15. The discovery of Human Accelerated RNA (HAR1) suggests very recent expansion events for functional ncRNAs in the human lineage, and illustrates important principles driving their evolution 16. This RNA, along with several dozen others identified, was strongly conserved from the chicken to the chimpanzee. In contrast, human HAR1 contains 14 base changes compared to chimpanzee, 8 of which are A to G transitions 16. HAR1 expression by in-situ hybridization mirrors that of Reelin in Cajal-Retzius cells suggesting a functional role (although not confirmed in the original publication by Northern blotting). Considering the rapid growth in nervous system complexity, the example of HAR1 highlights how non-conserved ncRNAs can serve important functions in rapidly evolving nervous system architectures 16.
NcRNAs as information mediators
Overall estimates of the number of distinct ncRNAs in the human genome have ranged from 450,000 to over 1,000,000 (Mattick, J.S. personal communication and 17, many of which may not have function 18, or await future data on function. Several informative recent reviews cover the expanding topic of ncRNA expression and function in the nervous system 1,19,20, including the intriguing topic of ncRNA based intercellular communication in the nervous system 21,22, and transport of glial cell derived mRNAs to adjacent neuronal synaptic regions for translation 23. While the nervous system environment features a spectacular range of diversity, functionality, and throughput, homeostasis hinges on the ability to maintain macromolecular order even under high stress levels. The unique information-coding and computational features of ncRNAs make them prime candidates for use in this demanding context. Unlike most proteins, ncRNAs usually function purely in the acquisition, processing, or delivery of information. Their rapidly evolvable information content couples analog to digital within the same molecule, allowing their interaction with protein networks through their secondary structure (analog information content), and with other nucleic acids through primary sequence homology (digital information content). In effect, ncRNAs serve as a bridge between the digital information universe of nucleic acids and the analog universe of cellular protein interactions 1. Furthermore, ncRNAs can rapidly adapt to sensory inputs and alterations in their microenvironment by reversibly changing their secondary structure, so that their exposure to recent events orchestrates combinatorial changes in the presentation of their information content 1. For example, in response to increased temperature, Heat Shock RNA (HSR) changes its secondary structure, allowing it to bind to Heat Shock factor (HSF) with high affinity, thereby activating the Heat Shock response. Cells treated with siRNA to HSR fail to induce a heat shock response even upon acute temperature shock 24. Another illuminating and recently discovered example comes from the 3′ UTR non-coding region of the vascular endothelial growth factor-A (VEGF-A) mRNA 25. This region forms a functional switch between two different secondary structures, using analog information to sense the local concentrations of RNA binding proteins. The interaction of this non-coding region with one of its two cognate proteins forms an “AND NOT” circuit that only translates VEGF-A protein when the logical condition is satisfied 25.
The typical long RNA binds co-transcriptionally to dozens, sometimes hundreds of RNA binding proteins, allowing a wide range of combinatorial interactions as the mature RNA emerges into its proper sub-cellular localization. Complex temporal and spatial interactions can occur between different sets of RNA binding or signaling proteins and information containing RNA binding motifs. These mechanisms create the potential for complex memory states, since each of the ncRNA–protein complexes within a cell can acquire, accumulate, process, and transmit an amount of information that only depends on the number of allowable states of the complex. Together the evidence now accumulating suggests that ncRNAs and their RNA–protein complexes compose a broad computational matrix that pervades all other layers of information processing within the cell. Using these features, ncRNAs increase the spatial and temporal density of macromolecular information processing in the limited space of the nervous system 1.
Alzheimer's disease-modulated ncRNAs influence the diversity of LTP and LTD
With such a spectrum of computational features, ncRNAs can play a constructive role by increasing useful variation and semantic complexity, wherever increased information content might benefit biological networks. The regulatory networks that control synaptic plasticity provide a useful example, one that may localize to the epicenter of Alzheimer's disease. Finely tuned regulation of thousands of genes controls the spatio-temporally modulated patterns required for the coding of memory. Synaptic plasticity modulates the sizes, the locations, the macromolecular content, and other parameters of synapto-dendritic landscapes, producing changes in the finely tuned heterogeneous connectivity landscape characteristic of memory and learning. Long-term potentiation (LTP) and long-term depression (LTD) operate prominently in the entorhinal cortex and the dentate gyrus during the acquisition of memory. Deregulation occurs early in these regions during the cascade of Alzheimer's neurodegeneration, often resembling a loss of control in plasticity. Recent work demonstrated that synaptic release of beta amyloid (Aβ) soluble dimers produces reversible effects practically indistinguishable from LTD 26, consolidating a variety of previous findings on the early specific effects of Aβ. Soluble Aβ oligomers bind to a number of important targets in synaptic regions, producing specific and reversible effects including NMDA receptor endocytosis, excessive calcium influx 27,28, tau phosphorylation 29-31 and loss of mitochondrial potential 32,33. These events initiate a series of consequences that parallel the LTD pathway 26, strengthening an emerging hypothesis that Alzheimer's pathology begins with early events modulating synaptic plasticity.
With the discovery that components of LTP require local protein translation, a more holistic view of synaptic mechanisms and functions came into focus. Work by Susan Lindquist 34, Joel Richter 35, Eric Kandel 36 and others have suggested the possible existence of carefully modulated synaptic translational machinery, that responds to environmental and activity dependant inputs to regulate mRNA translation levels at each synapse. Translational scaffolds may contain RNA binding proteins such as FMRP, CPEB, and possibly prions, and likely interact with core elements of the Alzheimer's disease pathway. The cytoplasmic C-terminus of membrane bound APP binds to CPEB, thereby serving as a tethering and regulatory element for these highly programmed differential translation systems 37. Cleavage of APP then implies the un-tethering and eventual relocation of these complexes. On another level, APP mRNA itself associates with FMRP within these scaffolds, resulting in the regulation of its level of translation 38. Such a well articulated, interlocking servo loop (a control architecture using feedback from system output back to the input, to achieve a purpose, usually including a stable pattern of system behavior) likely influences the information content of individual synaptic regions, and utilizes this information to define individualized protein expression profiles at each synapse. With different synapses potentially composed of different concentrations of synaptic proteins, LTP and LTD can take on broadly heterogeneous characteristics, yielding a wider range of functional responses to stimuli. Some of the proteins translated by these machineries are likely themselves RNA binding proteins, whom in turn can capture different ncRNAs to establish information feedback mechanisms and provide changing information signals at different synapses.
The reversible effects of Aβ at synaptic sites occur well in advance of plaque formation, linking Alzheimer's disease onset downstream from the accumulation of unresolved stress in the machineries of synaptic plasticity. The ncRNA enhanced information flow between layers of these networks would allow system wide tuning, providing a compelling solution to stress challenges. A recent finding that ncRNA BC200 is significantly up-regulated in Alzheimer's disease highlights the potential nexus of connections between ncRNAs, synaptic plasticity, and Alzheimer's disease 39. In contrast to normal aging, Alzheimer's disease-affected brain regions show consistently higher levels of BC200 RNA than in age-matched controls. Expression levels corresponded proportionally to the progression of the disease state, suggesting a reactive or compensatory role for BC200 in Alzheimer's disease triggered synaptodendritic deterioration 39. BC1 RNA, the possible functional analog of BC200 RNA, locates to synaptic regions, interacts with key proteins in translational scaffold complexes, and regulates the synaptic translation of mRNAs critical for synaptic function, such as dopamine D2 receptor 40. BC1's modulation of the multiplexed synaptic translation scaffolding system has broad implications for homeostatic synaptic plasticity, and LTD/LTP (Figure-1). The mechanisms of action of the BC1 and BC200 RNAs illustrate the power and flexibility of ncRNAs in cellular information processing in general, and specifically in the modulation of translational scaffolding systems in synaptic micro-regions.
Figure-1. Synaptic protein translation scaffolds contain heterogeneous and multiplexed information content; role of BC200 ncRNA.
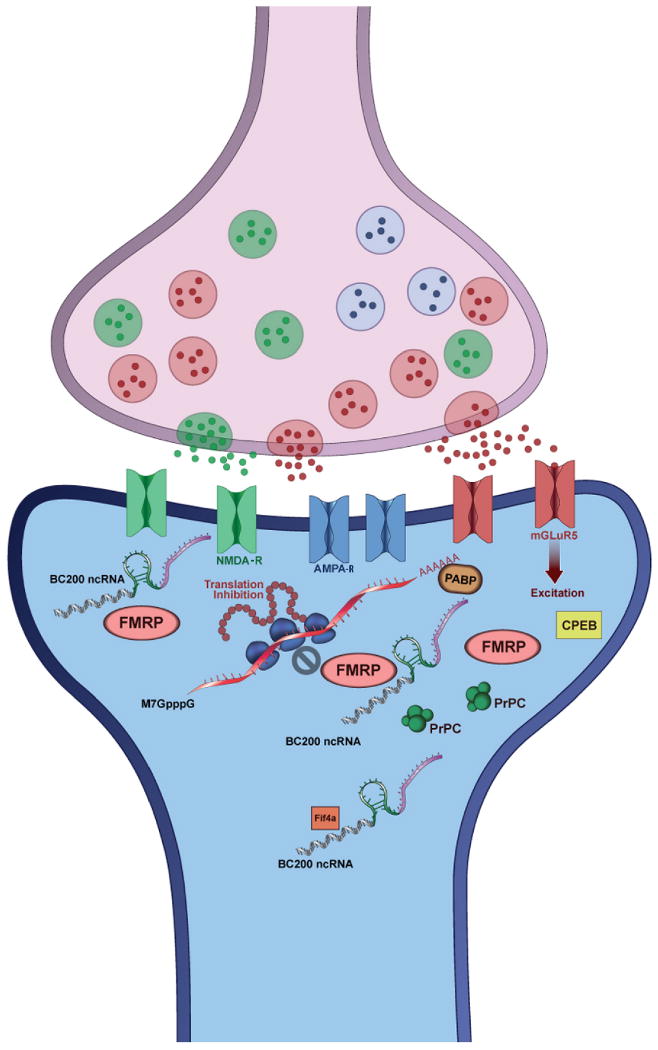
BC1 and BC200 ncRNAs, which suggested as local translational regulators, are selectively localized in somatodendritic domains of neurons. Originally discovered in 1991, by Tiedge and coworkers 76, BC1 and BC200 RNAs occupy a strategic position within the molecular machineries of synaptic plasticity. These RNAs regulate protein translation in the synaptic region through three proposed mechanisms of action. In the first mechanism, the analog information content of the RNA allows it to bind to Fragile X mental retardation protein (FMRP), while its digital information allows it to base pair with certain FMRP target mRNAs, achieving translational inhibition. In the second proposed mechanism the RNAs bind to Poly “A” binding protein (PABP) to inhibit translation initiation. In the third mechanism, BC1 binds to eif4a, a prototypical member of the Dead box RNA helicase family of proteins. Eif4a comprises two globular domains, the ATPase domain, and the helicase domain, and is required for translation of a certain subset of mRNAs that harbor significant secondary structure in their 5′ UTRs. Tiedge and colleagues have shown that BC1 disrupts a mechanical coupling between these two domains, thereby depriving the helicase domain of the free energy of ATP cleavage.
Since primate BC200 is derived from member of the large family of ALU element derived RNAs known to undergo copious ADAR editing 41, their secondary structures (analog information content) must cluster into large related families, suggesting that ALU derived RNAs other than BC200 could interact with scaffolds and regulate translation of specific subsets of synaptic mRNAs. Such machineries would integrate information content from synaptic activity, axonal transport, RNA processing, and transcriptional regulation, offering a molecular circuit for differentially shaping the contours of individual synaptic proteomes. So intriguing is the potential for ncRNAs to add to the semantic complexity of memory processing, that some authors have argued that ncRNAs must function directly in the process of memory formation 42. While this suggestion remains open for future studies, the involvement of ncRNAs in local synaptic translation underlines the complexity of synaptic plasticity associated servo loop systems involved, the intriguing roles ncRNAs play in these servo loops, and the overlap between such mechanisms and actions of Aβ at the synapse. The dynamics and mechanisms of servo loop deregulation represent key questions for understanding the etiology of Alzheimer's disease.
NcRNA based servo loops in the service of nervous system robustness
When connected to molecular sensors and other components, servo loops serve a vital function for avoiding disease pathogenesis and the achievement of biological “robustness.” The Systems Biology concept of robustness defines a quality of responsiveness to changing environmental conditions and activity levels; the more timely and accurate the information moving through the servo loops, the greater the robustness of a complex system. As Kitano points out, information content marshaled for the purpose of enhancing robustness often overcomes known forms of network perturbations, such as thermal noise 43 to maintain homeostasis. The benefits include better articulation and fine-tuning of network functional resources (systems) under known conditions. The costs include greater sensitivity to unusual perturbations not previously presented to the systems during their evolution. In the case of sporadic Alzheimer's disease, these unusual perturbations can include lipid metabolic effects, chronic alterations in glucose signaling, and aging related antagonistic pleiotropy 44,45, among others.
Robustness in turn permits expansion of the dynamic range of a system, which, when taken to its natural limit, evolves into an increasing capability for dynamic plasticity. A system that has exceeded its static dynamic range may resort to temporarily augmenting its physical extent in response to peak demand. The ability of macromolecular systems to achieve these levels of functionality depends on their precise temporal and spatial coupling of energy and information. We argue that a correspondence exists between these advantageous features of higher complexity and the unique molecular information coding features of ncRNAs, leading to their recruitment and incorporation into existing gene expression machineries and protein interaction pathways 4,46,47.
With the ability to form complex computational modules and interact with multiple different layers of protein machineries in the cell, ncRNAs serve to increase information flow into feedback loops, and to extend the cell's ability to maintain homeostasis. The heterogeneous and distributed functions of miRNA machineries provide an excellent example of servo loops and systems control in neurophysiological systems. We recently reported details of one such servo loop: miRNA-mediated feedback loop that function to maintain NMDA receptor homeostasis 48. In this case, administration of an acute antagonist to NMDA receptors rapidly reduces the synaptic concentration of miR-219. MiR-219 targets CAMKIIgamma mRNA among others, a known direct downstream effector of the NMDA signaling cascade. Treatment with acute antagonist reduces miR-219 repression of target mRNAs, causing an increase in CAMKIIgamma protein, and an increase in the NMDA receptor signaling cascade set point 48. Transient blockade of miR-219 by specific anti-miR application very rapidly reproduces the same increase in the set point of the NMDA receptor cascade.
Another useful example further illustrates the strong partnership between miRNAs and adaptive feedback loops. Alcohol stress responsive miRNA (miR-9) rapidly increases after alcohol treatment of neurons and strongly regulates ALCOREX exon inclusion, leading to alcohol adaptation in the large-conductance calcium- and voltage-activated potassium channel (BK) channels, known as an important element in behavioral and molecular alcohol tolerance 49. The short timescale of response illustrates a key principle in ncRNA function in the nervous system. The unique sensory and information processing features of ncRNAs enhance their participation in multiple layered feedback loops focused on homeostasis, stress response, and the maintenance of robustness of physiological resources. Thus while these molecules are not the cause of disease pathology, their function includes the early response to stress vectors that drive disease onset if not adequately resolved or fully neutralized by the response machineries.
In synaptic tissues, achieving homeostasis is only the beginning of the challenge. The demands of spike activity and synaptic plasticity require control architecture able to evaluate functional states far beyond the range of homeostasis. Perturbations of miRNAs in disease onset may represent regulatory signals designed to preserve nervous system function. Numbers of key transcripts in Alzheimer's disease pathways are regulated by miRNAs, such as APP 50, BACE1 51,52, and others. Non-dividing cells maintain tighter miRNA control of over translation than their rapidly dividing peers, due to their global transcription profiles. Indeed, mRNAs in these transcriptomes contain significantly longer 3′UTRs than those of dividing cells, which in turn serve as targets for many more miRNAs 53. The tendency for Alzheimer's disease neurons to change their expression landscapes towards cell division may also produce a transcriptome less regulated by miRNAs. The early events in the etiology of Alzheimer's disease involve synaptic changes in the parameters of plasticity networks, suggesting a connection between plasticity, stress, and robustness on the pathway to the onset of Alzheimer's disease.
Non-coding-RNAs mediate key stress response pathways in Alzheimer's disease
By using servo loop information flow to make intelligent defensive changes, biological robustness establishes barriers to the onset of chronic diseases. As long as the information flow remains uncompromised, the ongoing computation seeks to optimize the responses necessary for neutralization of the acute reversible phases of stress before they emerge as chronic irreversible phases. In this fashion cells and tissues avoid the progressive, irreversible loss of information content from cellular networks, and the subsequent corruption of functional systems, especially those of robustness and stress response themselves, that can occur as a prelude to chronic disease. Nervous system tissues, in particular, require a veritable arsenal of stress response pathways to move functional networks back into reversible zones of phase space, where behavior, regulation, and homeostasis are robust.
Accumulated stress often produces saturation of protein interaction pathways, making them no longer able to absorb new information 54,55 and thus unable to properly process or communicate information to downstream effectors. In these situations, ncRNAs permit enhanced information flow to stress response systems in the face of ongoing perturbations. Roles for ncRNAs in cellular stress responses include sensory transduction, integration, and distribution of regulatory signals. For a relatively low entropic cost, ncRNAs add orthogonal information content to these networks, permitting among other things; the fine-tuned and carefully measured responses to stresses and perturbations encountered by the organism. As the examples discussed below demonstrate, sensing the onset and accumulation of stress in macromolecular networks represents a particularly critical role for ncRNAs in the pathways of disease.
A number of groundbreaking examples have brought ncRNA mechanisms to the forefront of mammalian stress response. Sensory transduction roles of ncRNAs in cellular stress responses include evaluating temperature levels in the heat shock response by Heat Shock RNA (HSR) 24, sensing DNA damage 56, and regulating the hypoxia response through HIF1alpha 57,58. The 3′UTR of human VEGF-A mRNA contains an RNA switch to integrate signals from stressors such as interferon gamma and hypoxia, and regulate VEGF-A translation 25. Other ncRNAs such as hsr-omega transcripts function in neurodegeneration and stress response pathways in Drosophila 59, while vault RNAs in humans appear to participate in detoxification and innate immunity 60. In the inflammation system a variety of RNA sensing proteins such as RIG, NOD, PKR, and OAS play roles suggestive of RNAs as endogenous sensors of molecular order, with regulatory and signaling impact of broad significance, orthogonal to any pathogenic RNAs.
Consistent with their usefulness in stress response, recent evidence implies that ncRNAs mediate key events in neuropathology. We documented ncRNA-mediated regulation of PTEN induced putative kinase 1 (PINK1), a key signaling protein in Parkinson's disease 61. Our group and others previously reported the deregulation of long ncRNA's in Fragile X mental retardation and an associated neurological disease, fragile X-associated tremor ataxia syndrome (FXTAS) 62,63. Many brain disorders result in dysregulation of miRNAs, such as Tourette's syndrome 64, schizophrenia 65 and mental disorders 66.
An excellent example of ncRNA involvement in both nervous system stress and Alzheimer's pathology comes from our recent report on natural antisense-mediated regulation of beta-secretase-1 (BACE1) in Alzheimer's disease pathogenesis 6. This somewhat surprising result highlights ncRNA involvement in Alzheimer's disease, illustrates how ncRNA features facilitate higher order signal integration in this disease, and provides insight on how ncRNAs could play decisive roles in Alzheimer's disease pathology (Figure-2). The BACE1 mediated cleavage of APP represents a defining early step in the onset of Alzheimer's disease. Yet, loss of BACE1 results in numerous behavioral and physiological deficits, including memory loss 67, emotional deficits 68, myelination defects in peripheral nerves 69,70 and loss of synaptic plasticity 68. BACE1 activity increases in response to a series of specific neuronal stresses including Aβ itself. In effect, the subtle but crucial boundaries between BACE1 physiology and pathology indicate that BACE1 expression must be tightly regulated, allowing the enzyme to perform its physiological functions while avoiding the serious consequences of over- or under-expression. In contrast to the classic siRNA and miRNA mechanisms, the natural antisense transcript, BACE1-AS, forms a duplex with its cognate BACE1 mRNA, and stabilizes the mRNA for increased translation 6. The example illustrates a growing diversity of mechanisms for ncRNAs that provide fined tuned regulation of neurodegeneration-associated pathways of gene expression.
Figure-2. Elevated antisense RNA up-regulates β-secretase-1 in Alzheimer's disease.
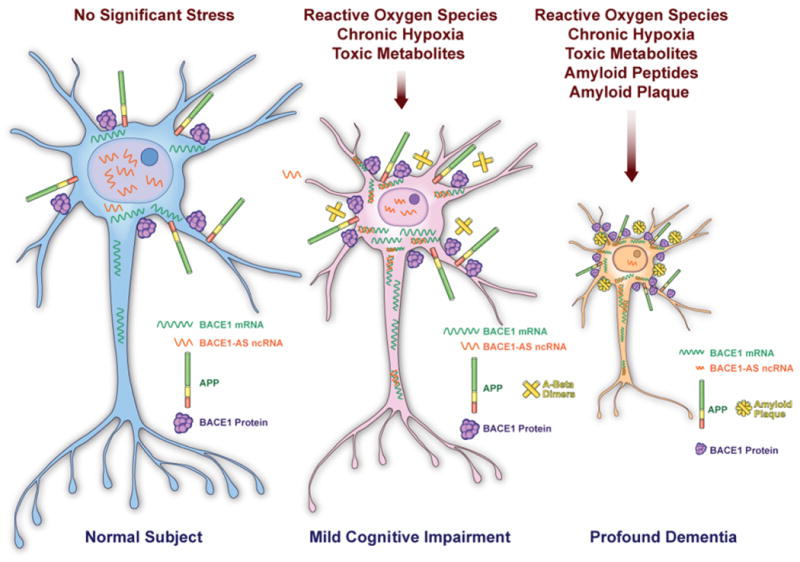
BACE1-AS is a non-protein-coding antisense RNA that regulates β-secretase-1 (BACE1). BACE1, a β-site amyloid precursor protein (APP)-cleaving enzyme, has been implicated in the pathogenesis of Alzheimer disease. BACE1 is involved in the production of the amyloid β (Aβ) peptides that form plaques in the brains of individuals with Alzheimer disease.
(a) BACE1-AS is enriched in the nucleus in the physiologic conditions, when neurons producing basal levels of BACE1 mRNA and protein. (b) Exposure of the neuronal cells to various cell stressors, such as reactive oxygen species, chronic hypoxia and toxic metabolites, may cause cytosolic translocation of BACE1-AS transcripts. BACE1-AS can possibly form a duplex RNA with the BACE1 mRNA, leading to stabilization of this transcript and up-regulation of BACE1 protein. BACE1 protein up-regulation, in turn can produce more Aβ peptides and drive the generation of amyloid plaques. (c) Amyloid peptides and plaques are known cellular stressors and could provoke more stress to neuronal cells and lead to over-expression and release of BACE1-AS. This later event possibly drives a feed-forward mechanism resulting in neuronal cells loss. The expression of BACE1-AS is elevated in Alzheimer's disease and induces feed-forward regulation of BACE1 6. It has also been shown that BACE1-AS induces production of Aβ peptides, which leads to increased expression of BACE1, which in turn further amplifies Aβ production 6. Subsequently, increasing Aβ levels can further boost that BACE1-AS expression, thereby accelerating the onset of Alzheimer's disease. We suggest that therapeutic targeting of BACE1-AS could mediate the transition between the essential physiological functions of BACE1 and its pathological malfunction in early Alzheimer's disease 6.
Other stress responsive genes also use antisense transcripts to respond to stress, such as HIF1alpha 71, Neuronal nicotinic receptor (CHRNA3 & CHRNA5) 72 and neuronal nitric oxide synthase (nNOS) 73. Growth arrested DNA-damage inducible gene 7 (gadd7) represents a stress responsive ncRNA involved in a feed forward loop 74. Similar to BACE1-AS, Gadd7 appears to aggravate palmitate induced ROS accumulation and subsequent ER stress during conditions of lipotoxic stress. BACE1-AS, Gadd7, and HIF1alpha-AS exhibit commonalities in their functions, and together illustrate a ncRNA systems biology principle: as long as the cell remains within normal ranges of environmental variation, multi-layered regulatory systems take advantage of information flows from ncRNAs to increase robustness and enhance functional diversity. However, these systems proceed at the expense of stability when variation falls outside the intended range of functionality.
A systems perspective on Alzheimer's disease from the non-coding transcriptome
The above results imply that certain types of changes in synaptic conditions, loosely labeled “stress” by biologists, modulate the cleavage of APP and the production of Aβ, through a ncRNA mechanism. In turn Aβ rapidly modulates a series of synaptic parameters that appear to have physiological importance for stress response, as they establish a feed forward loop. With many other ncRNAs active early in stress response, and with their multilayered impact on cellular networks, interesting issues arise regarding integration and synthesis of these signals. In a dynamic synaptic environment, potentially hundreds of RNA mediated information flows interact to produce a resolved set of signals that will define phase state changes. The coupling and resolution of disparate signals may represent a challenge that may not always have a happy ending in complex networks. The resolution of disparate signals may depend on some type of internetwork coherence established as a result of redundancy in information flows from many sources. In this regard, information accumulated in existing ncRNA - protein complexes may allow real time comparisons with previously registered information flows. Logical conflicts that arise may trigger sensors or signal alarms to recruit resolution machineries. In this context, the idea of “internetwork coherence”, or coupled coherence of networks 75, proposes that in healthy neurophysiology, these conflicts are routinely resolved by systems of information coupling and synthesis. The result would include a resonance between these multilayered networks, such that the loss of information from one or a few of them, would be compensated for by the other network elements containing the same or resonant information (a little like a hologram).
The above-cited studies on the multilayered and finely tuned regulation of BACE1, together with many others at different gene loci, increase our awareness of the importance of multiple nested layers of feed forward, and feedback servo loops in the biology of neurodegeneration. ncRNAs enhance the connectivity of biological networks, and the information flow constantly occurring between them. The higher the quality of information flow, the greater the dynamic range, plasticity, and robustness of the system itself. The high performance environment of the mammalian nervous system features an increased range of diversity, greater functionality, and higher throughput than most other tissues and organisms. These features require the maintenance of order of synaptic molecular materials even under the increased stress levels typical of synaptic environments. Alzheimer's disease now appears to recapitulate some of these patterns, where the participation of many more ncRNAs in these processes may be expected.
Conclusion
The advent of systems biology marks the culmination of a century of reductionism, and the beginning of an era of conceptual integration in disease biology. During this transition, the non-coding transcriptome will continue to play a formative role by emphasizing the flow of information through large macromolecular networks, as well as the continuing impact of information on their dynamic behavior during stress response. The classic aqueous biochemical behavior of protein complexes occupies a smaller part of a larger context where information theory and computational integration define the state of functional systems. Disease onset occurs only when accumulated stresses on molecular machineries overpower the capabilities of multiple layers of servo loops supporting system robustness. As the servo loops falter, their dysfunctionality results in distortion and corruption of information flows, which propagate radially like waves, across the interconnected macromolecular networks from the epicenter of disorder. The health of the tissue fails as a function of the loss of molecular order, the loss of quality in its information content. A progressive loss of information quality represents an unfortunate consequence of multiple nested servo loops pushed beyond their robustness limits. Understanding ncRNAs brings with it clarity for these servo loops and the map of their evolved information flows.
The establishment and maintenance of synaptic machineries in precise spatio-temporal coordinates require intricate order and robust control. While servo loops provide a useful and widely used architecture for achieving these requirements, they depend on the flow of information content often provided by ncRNAs. Thus ncRNAs highlight the information content of molecular machineries. The complexity of the Alzheimer's disease process reaches many cellular pathways from the dendritic spine, to the cell body, and back to the presynaptic terminal. Expanding the repertoire of ncRNAs associated with Alzheimer's disease will lead the way to a systems biology understanding of this complex disease. New insights into the unique and multifaceted functions of ncRNAs will reveal how complex systems use digital and analog information content to make molecular computations, which enhance robustness, and respond to stress. Perhaps with these insights Alzheimer's disease will be remapped as a descending cascade of corrupted information content within a landscape of information flows, servo loops, and multilayered control functions dedicated to robust synaptic functionality and plasticity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.St Laurent G, 3rd, Wahlestedt C. Noncoding RNAs: couplers of analog and digital information in nervous system function? Trends Neurosci. 2007;30:612–21. doi: 10.1016/j.tins.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Gagen MJ, Mattick JS. Accelerating, hyperaccelerating, and decelerating networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;72:016123. doi: 10.1103/PhysRevE.72.016123. [DOI] [PubMed] [Google Scholar]
- 3.Taft RJ, Pheasant M, Mattick JS. The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays. 2007;29:288–99. doi: 10.1002/bies.20544. [DOI] [PubMed] [Google Scholar]
- 4.Mattick JS. A new paradigm for developmental biology. J Exp Biol. 2007;210:1526–47. doi: 10.1242/jeb.005017. [DOI] [PubMed] [Google Scholar]
- 5.Mattick JS. RNA regulation: a new genetics? Nat Rev Genet. 2004;5:316–23. doi: 10.1038/nrg1321. [DOI] [PubMed] [Google Scholar]
- 6.Faghihi MA, et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–30. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105:716–21. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama S, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–6. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki Y, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–73. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 10.Kiyosawa H, Yamanaka I, Osato N, Kondo S, Hayashizaki Y. Antisense transcripts with FANTOM2 clone set and their implications for gene regulation. Genome Res. 2003;13:1324–34. doi: 10.1101/gr.982903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 12.Moucadel V, Lopez F, Ara T, Benech P, Gautheret D. Beyond the 3′ end: experimental validation of extended transcript isoforms. Nucleic Acids Res. 2007;35:1947–57. doi: 10.1093/nar/gkm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson PT, Wang WX, Rajeev BW. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008;18:130–8. doi: 10.1111/j.1750-3639.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berezikov E, et al. Diversity of microRNAs in human and chimpanzee brain. Nat Genet. 2006;38:1375–7. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- 16.Pollard KS, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–72. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- 17.Willingham AT, Gingeras TR. TUF love for “junk” DNA. Cell. 2006;125:1215–20. doi: 10.1016/j.cell.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Brosius J. Waste not, want not--transcript excess in multicellular eukaryotes. Trends Genet. 2005;21:287–8. doi: 10.1016/j.tig.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–20. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 20.Mehler MF, Mattick JS. Non-coding RNAs in the nervous system. J Physiol. 2006;575:333–41. doi: 10.1113/jphysiol.2006.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smalheiser NR. Synaptic enrichment of microRNAs in adult mouse forebrain is related to structural features of their precursors. Biol Direct. 2008;3:44. doi: 10.1186/1745-6150-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinger ME, Mercer TR, Mattick JS. RNAs as extracellular signaling molecules. J Mol Endocrinol. 2008;40:151–9. doi: 10.1677/JME-07-0160. [DOI] [PubMed] [Google Scholar]
- 23.Eyman M, et al. Local synthesis of axonal and presynaptic RNA in squid model systems. Eur J Neurosci. 2007;25:341–50. doi: 10.1111/j.1460-9568.2007.05304.x. [DOI] [PubMed] [Google Scholar]
- 24.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–60. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 25.Ray PS, et al. A stress-responsive RNA switch regulates VEGFA expression. Nature. 2009;457:915–9. doi: 10.1038/nature07598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–8. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 28.Chen C. beta-Amyloid increases dendritic Ca2+ influx by inhibiting the A-type K+ current in hippocampal CA1 pyramidal neurons. Biochem Biophys Res Commun. 2005;338:1913–9. doi: 10.1016/j.bbrc.2005.10.169. [DOI] [PubMed] [Google Scholar]
- 29.Esposito G, et al. CB1 receptor selective activation inhibits beta-amyloid-induced iNOS protein expression in C6 cells and subsequently blunts tau protein hyperphosphorylation in co-cultured neurons. Neurosci Lett. 2006;404:342–6. doi: 10.1016/j.neulet.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–85. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Iqbal K, Liu F, Gong CX, Alonso AD, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu D, et al. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26:11111–9. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115:879–91. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- 35.Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 36.Huang YY, Kandel ER. Age-related enhancement of a protein synthesis-dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus. Learn Mem. 2006;13:298–306. doi: 10.1101/lm.166906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Q, Huang YS, Kan MC, Richter JD. Amyloid precursor proteins anchor CPEB to membranes and promote polyadenylation-induced translation. Mol Cell Biol. 2005;25:10930–9. doi: 10.1128/MCB.25.24.10930-10939.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mus E, Hof PR, Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:10679–84. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centonze D, et al. The brain cytoplasmic RNA BC1 regulates dopamine D2 receptor-mediated transmission in the striatum. J Neurosci. 2007;27:8885–92. doi: 10.1523/JNEUROSCI.0548-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levanon K, Eisenberg E, Rechavi G, Levanon EY. Letter from the editor: Adenosine-to-inosine RNA editing in Alu repeats in the human genome. EMBO Rep. 2005;6:831–5. doi: 10.1038/sj.embor.7400507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattick JS, Mehler MF. RNA editing, DNA recoding and the evolution of human cognition. Trends Neurosci. 2008;31:227–33. doi: 10.1016/j.tins.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Kitano H, Oda K. Robustness trade-offs and host-microbial symbiosis in the immune system. Mol Syst Biol. 2006;2:2006 0022. doi: 10.1038/msb4100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campisi J. Aging, tumor suppression and cancer: high wire-act! Mech Ageing Dev. 2005;126:51–8. doi: 10.1016/j.mad.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Sempere LF, et al. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sempere LF, Cole CN, McPeek MA, Peterson KJ. The phylogenetic distribution of metazoan microRNAs: insights into evolutionary complexity and constraint. J Exp Zoolog B Mol Dev Evol. 2006;306:575–88. doi: 10.1002/jez.b.21118. [DOI] [PubMed] [Google Scholar]
- 48.Kocerha J, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A. 2009;106:3507–12. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietrzykowski AZ, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–87. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hebert SS, et al. MicroRNA regulation of Alzheimer's Amyloid precursor protein expression. Neurobiol Dis. 2009;33:422–8. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Wang WX, et al. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28:1213–23. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boissonneault V, Plante I, Rivest S, Provost P. MicroRNA-298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1. J Biol Chem. 2009;284:1971–81. doi: 10.1074/jbc.M807530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–7. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nardai G, Vegh EM, Prohaszka Z, Csermely P. Chaperone-related immune dysfunction: an emergent property of distorted chaperone networks. Trends Immunol. 2006;27:74–9. doi: 10.1016/j.it.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Szalay MS, Kovacs IA, Korcsmaros T, Bode C, Csermely P. Stress-induced rearrangements of cellular networks: Consequences for protection and drug design. FEBS Lett. 2007;581:3675–80. doi: 10.1016/j.febslet.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–30. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida T, et al. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem. 2004;279:14871–8. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 58.Cayre A, Rossignol F, Clottes E, Penault-Llorca F. aHIF but not HIF-1alpha transcript is a poor prognostic marker in human breast cancer. Breast Cancer Res. 2003;5:R223–30. doi: 10.1186/bcr652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savvateeva-Popova E, Medvedeva A, Popov A, Evgen'ev M. Role of non-coding RNAs in neurodegeneration and stress response in Drosophila. Biotechnol J. 2008;3:1010–21. doi: 10.1002/biot.200800120. [DOI] [PubMed] [Google Scholar]
- 60.Steiner E, et al. The major vault protein is responsive to and interferes with interferon-gamma-mediated STAT1 signals. J Cell Sci. 2006;119:459–69. doi: 10.1242/jcs.02773. [DOI] [PubMed] [Google Scholar]
- 61.Scheele C, et al. The human PINK1 locus is regulated in vivo by a non-coding natural antisense RNA during modulation of mitochondrial function. BMC Genomics. 2007;8:74. doi: 10.1186/1471-2164-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khalil AM, Faghihi MA, Modarresi F, Brothers SP, Wahlestedt C. A novel RNA transcript with antiapoptotic function is silenced in fragile x syndrome. PLoS ONE. 2008;3:e1486. doi: 10.1371/journal.pone.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ladd PD, et al. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007;16:3174–87. doi: 10.1093/hmg/ddm293. [DOI] [PubMed] [Google Scholar]
- 64.Abelson JF, et al. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–20. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 65.Beveridge NJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–68. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 66.Perkins DO, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma H, et al. Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:8167–72. doi: 10.1073/pnas.0609521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laird FM, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–5. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 70.Willem M, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–6. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 71.Thrash-Bingham CA, Tartof KD. aHIF: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst. 1999;91:143–51. doi: 10.1093/jnci/91.2.143. [DOI] [PubMed] [Google Scholar]
- 72.Solda G, et al. In vivo RNA-RNA duplexes from human alpha3 and alpha5 nicotinic receptor subunit mRNAs. Gene. 2005;345:155–64. doi: 10.1016/j.gene.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Korneev SA, Park JH, O'Shea M. Neuronal expression of neural nitric oxide synthase (nNOS) protein is suppressed by an antisense RNA transcribed from an NOS pseudogene. J Neurosci. 1999;19:7711–20. doi: 10.1523/JNEUROSCI.19-18-07711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The Non-coding RNA gadd7 Is a Regulator of Lipid-induced Oxidative and Endoplasmic Reticulum Stress. J Biol Chem. 2009;284:7446–54. doi: 10.1074/jbc.M806209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon YK, Cho KH. Coherent coupling of feedback loops: a design principle of cell signaling networks. Bioinformatics. 2008;24:1926–32. doi: 10.1093/bioinformatics/btn337. [DOI] [PubMed] [Google Scholar]
- 76.Tiedge H, Fremeau RT, Jr, Weinstock PH, Arancio O, Brosius J. Dendritic location of neural BC1 RNA. Proc Natl Acad Sci U S A. 1991;88:2093–7. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]


