Abstract
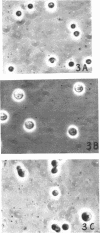
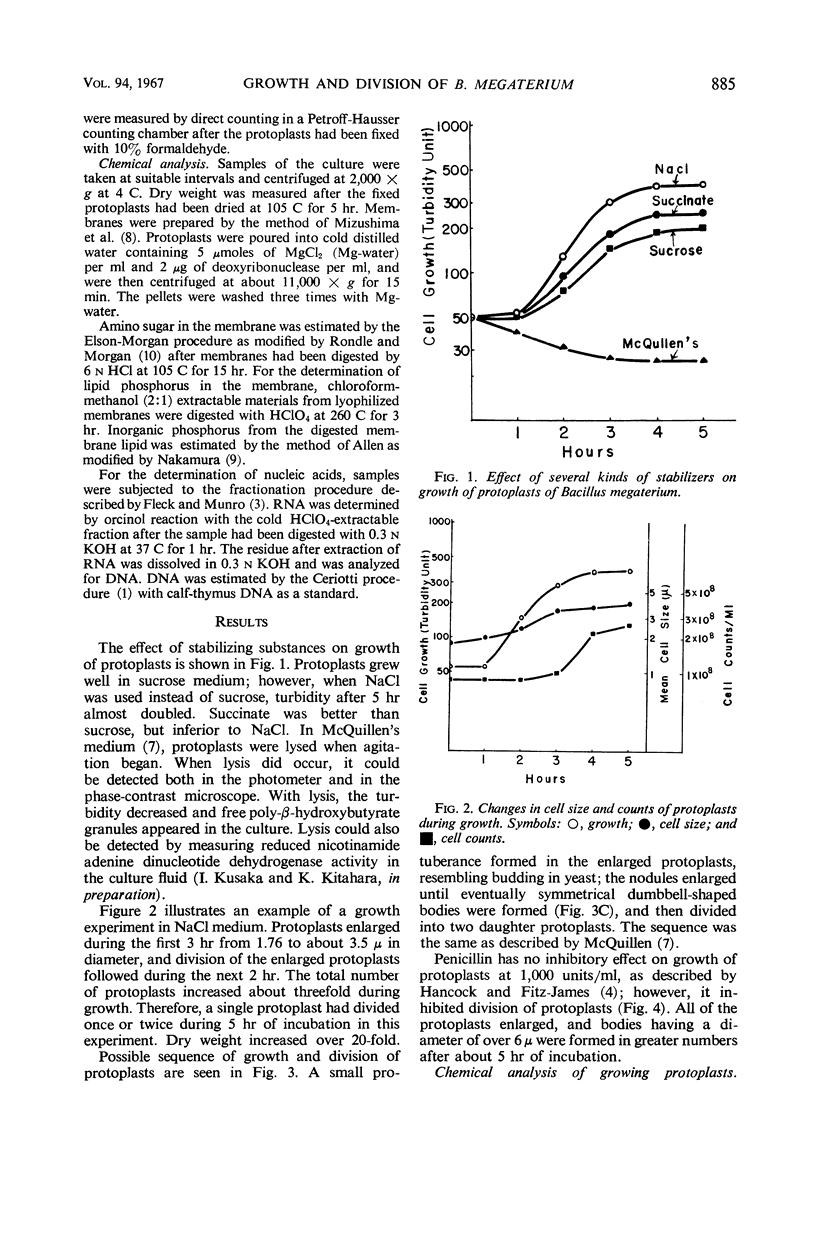
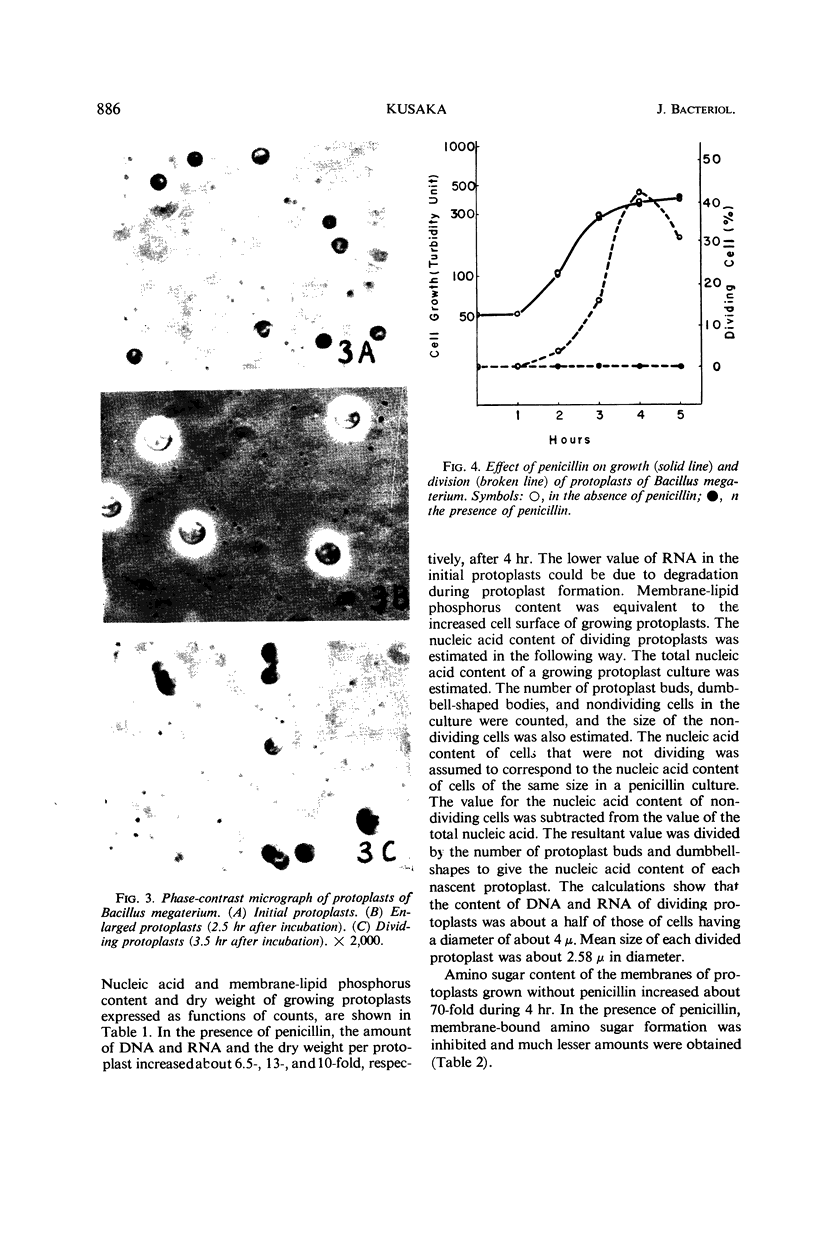
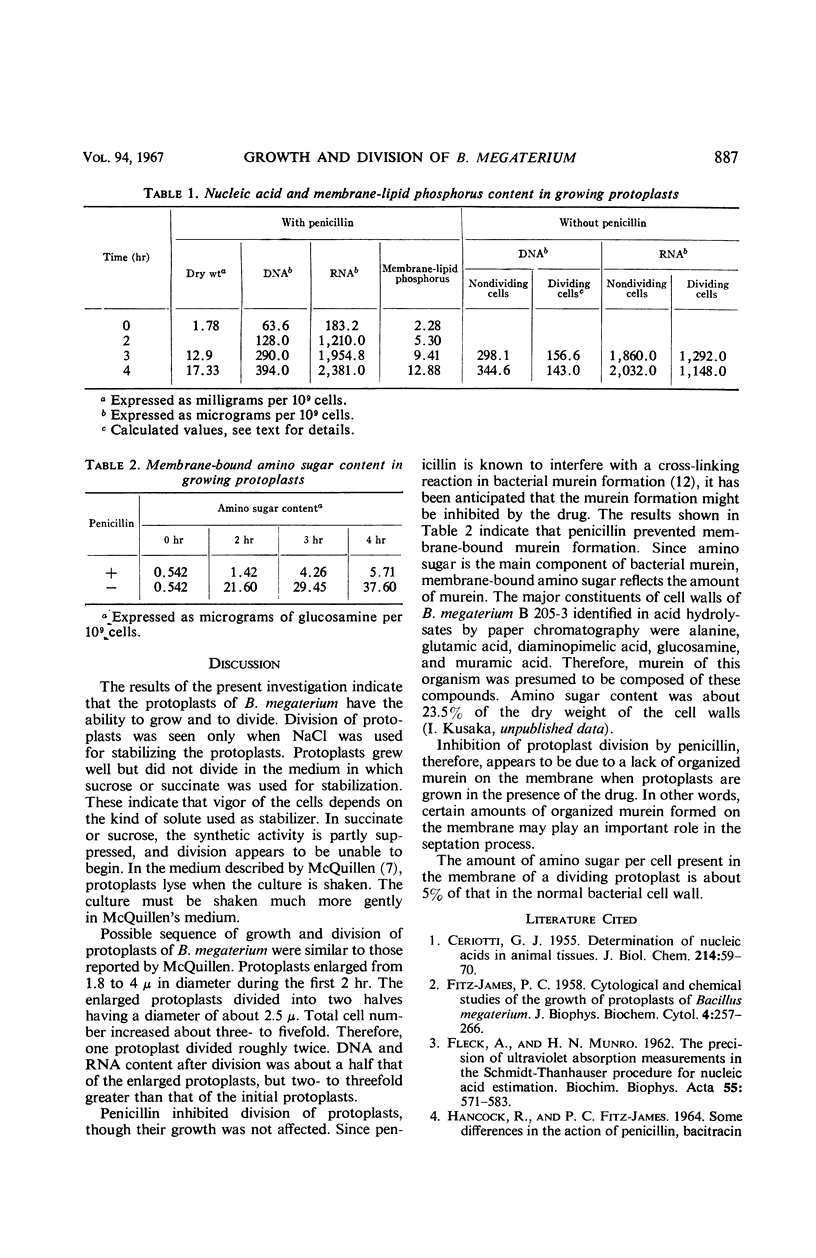
Protoplasts of Bacillus megaterium grew well and divided in nutrient broth containing 0.5 m NaCl as the stabilizer. Protoplasts also grew when sucrose or succinate was used instead of NaCl; however, no division phenomena were observed. The sequence of growth and division was similar to the results obtained by McQuillen. Protoplasts enlarged from 1.8 to 3.5 μ in diameter, and then a small protuberance formed in the enlarged cells. The nodules enlarged until eventually symmetrical dumbbell-shaped bodies were formed, which then separated into two daughter protoplasts having a diameter of about 2.5 μ. Deoxyribonucleic acid and ribonucleic acid were halved during division. Penicillin inhibited the division of protoplasts, though the growth was not influenced by the drug. Membrane-bound amino sugar content was considerably reduced when the cells were grown in the presence of penicillin. These results suggest that organized murein formed on the protoplasts membrane may play an important role in the septation process.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Cytological and chemical studies of the growth of protoplasts of Bacillus megaterium. J Biophys Biochem Cytol. 1958 May 25;4(3):257–266. doi: 10.1083/jcb.4.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- JEYNES M. H. The growth and division of bacterial protoplasts. Exp Cell Res. 1961 Aug;24:255–264. doi: 10.1016/0014-4827(61)90427-x. [DOI] [PubMed] [Google Scholar]
- LARK C., BRADLEY D., LARK K. G. FURTHER STUDIES ON THE INCORPORATION OF D-METHIONINE INTO THE BACTERIAL CELL WALL; ITS INCORPORATION INTO THE R-LAYER AND THE STRUCTURAL CONSEQUENCES. Biochim Biophys Acta. 1963 Oct 29;78:278–288. doi: 10.1016/0006-3002(63)91638-x. [DOI] [PubMed] [Google Scholar]
- MCQUILLEN K. Bacterial protoplasts: growth and division of protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1955 Nov;18(3):458–461. doi: 10.1016/0006-3002(55)90129-3. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Ishida M., Kitahara K. Chemical composition of the protoplast membrane of Bacillus megaterium. J Biochem. 1966 Apr;59(4):374–381. doi: 10.1093/oxfordjournals.jbchem.a128312. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., BECKMAN H. Growth of bacterial L forms and bacterial protoplasts. J Bacteriol. 1960 May;79:638–649. doi: 10.1128/jb.79.5.638-649.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]