Abstract
Objectives
The aim of this cross-sectional study was to investigate the association between bone mineral density (BMD) and lifestyle factors, as well as the influence of vitamin D receptor (VDR) gene polymorphism, in adult male workers.
Methods
The subjects were 524 male employees aged 23–49 years (37.3 ± 5.4 years, mean ± standard deviation) working at a large-scale integrated manufacturing facility in Japan. BMD was measured at the nondominant radius by dual-energy X-ray absorptiometry. Lifestyle information was obtained by a questionnaire at the same time, and genomic DNA was isolated from peripheral leukocytes.
Results
The genotype frequencies of VDR gene polymorphism detected by Taq I digestion were 81.3%, 17.9%, and 0.8% for TT, Tt, and tt, respectively. BMD was 0.56 ± 0.06 g/cm2. Analysis of covariance with adjustment for age and body mass index (BMI) revealed that subjects who had a past history of exercise, current exercise from 3 to 7 days a week or daily alcohol intake showed significantly higher BMD than subjects without these features (0.56 ± 0.06 versus 0.54 ± 0.06, 0.58 ± 0.06 versus 0.55 ± 0.06, and 0.57 ± 0.06 versus 0.55 ± 0.06, respectively) (P < 0.05). Subjects who ate only 2 meals a day or smoked ≥21 cigarettes a day showed significantly lower BMD if they had the Tt or tt genotype than if they had the TT genotype (0.51 ± 0.04 versus 0.56 ± 0.06 and 0.51 ± 0.05 versus 0.57 ± 0.06, respectively) (P < 0.05).
Conclusions
These findings suggest that the influence of lifestyle on BMD differs according to VDR gene polymorphism in adult male workers.
Keywords: Bone mineral density, Gene polymorphism, Lifestyle, Vitamin D receptor
Introduction
Osteoporosis is characterized by low bone mass and microarchitectural deterioration of the bone tissue, leading to enhanced bone fragility and consequent increase in the risk of fracture [1]. Osteoporosis is less common in men than in women, but the mortality rate after osteoporotic fracture is higher in men [2–4]. However, male osteoporosis is substantially underdiagnosed, undertreated, and underreported, as well as being inadequately researched [5].
Recently, the American College of Physicians issued new guidelines [5] based on the available evidence about risk factors and screening tests for osteoporosis in men obtained from a systematic review of 269 studies. The guidelines recommend that physicians periodically perform individual assessment of risk factors in older men and test the bone mineral density (BMD) of men who have an increased risk of developing osteoporosis. Thus, the importance of strategies for prevention of osteoporosis in men has been increasingly emphasized.
According to the 2006 Japanese guidelines for the prevention and treatment of osteoporosis [6], BMD can be affected by various environmental factors, such as physical activity, smoking, and alcohol. A diagnosis of osteoporosis is made by measuring the BMD and the most popular method for doing so is dual-energy X-ray absorptiometry (DXA) [7]. Low BMD is an important risk factor for osteoporotic fracture [8, 9]. Each person’s BMD condition is determined by a complex interplay between genetic and environmental factors, including lifestyle habits [10]. Krall et al. estimated that 46–62% of the variation in BMD was attributable to heredity [11]. At least 30 genes are thought to be associated with the development of osteoporosis [12]. Among these, polymorphism of the vitamin D receptor (VDR) gene has been most widely studied [13], and a meta-analysis [14] has suggested that BMD is influenced by VDR gene polymorphism. However, most studies have investigated the effects of VDR gene polymorphism and lifestyle factors on BMD separately, so their combined effect is not well understood. Better understanding of this combined effect may allow us to identify a high-risk group for osteoporosis and lead to more effective osteoporosis prevention and to improvement of public health. The purpose of this cross-sectional study was to investigate the association between BMD and lifestyle factors, as well as the influence of VDR gene polymorphism, in adult male workers.
Methods
Subjects and methods
The 1,029 men were recruited from employees working at a large-scale integrated manufacturing facility in Japan. The criteria for entry into this study were no previous diagnosis of osteoporosis, no systemic disease, and no medications known to influence bone or calcium metabolism. Of the 1,029 participants, 841 (81%) agreed to genotyping. Among these 841 participants, we analyzed 524 who were aged 49 years or younger for VDR genotyping, lifestyle, body measurements, and laboratory data. Their ages ranged from 23 to 49 years (mean 37.3 ± 5.4 years). They were engaged in office work (49%) or light manual work (51%). Among the participants doing light manual work, 87.6% were on night shift. Night-shift workers accounted for 77.4% of those aged 23–29 years, 59.6% of those aged 30–39 years, and 31.9% of those aged 40–49 years.
Height, weight, BMD, urinary deoxypyridinoline (DPD), and serum bone alkaline phosphatase (BAP) were measured during a comprehensive health check. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. Lifestyle assessment was performed at the same time as the above measurements. We followed the ethical guideline for human genome/gene analysis research [15] endorsed by the Japanese government. The study protocol was approved by the ethics committee of Kumamoto University Graduate School of Medical Sciences, and all subjects provided written informed consent.
Bone metabolic markers
Serum BAP and urinary DPD concentrations were analyzed by enzyme immunoassay. Urinary creatinine (Cr) concentration was measured by colorimetric assay. DPD excretion was expressed as a ratio of urinary Cr concentration. DPD has been validated as a useful marker of bone resorption, while BAP is a marker of bone formation [16].
Measurement of bone mineral density (BMD)
BMD was measured at the distal 1/3 site of the radius on the nondominant side using DXA (Osteometer DTX200) according to the manufacturer’s protocol (precision error <1.0% CV in vivo). Quality control was carried out in accordance with the manufacturer’s guidance. The BMD of the distal 1/3 site of the radius has been validated as being highly predictive of fracture risk [17].
Lifestyle assessment
A self-reporting questionnaire was used to collect the following information: past history of exercise (no, yes), current exercise (no, 1–2 days/week, 3–7 days/week), night-shift work (no, yes), sleeping time (hours), frequency of milk intake (no, sometimes, every day), number of meals per day (3 meals, 2 meals), history of dieting (no, yes), smoking status (no, 1–20 cigarettes/day, ≥21 cigarettes/day), and alcohol intake (no, sometimes, every day). Past history of exercise was determined from exercise habits until the age of 20 years.
Genotyping
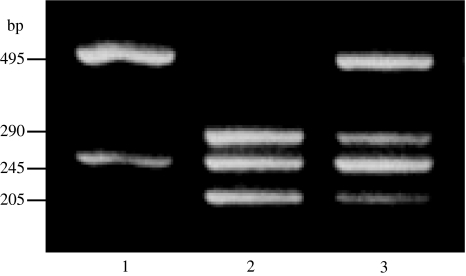
Genomic DNA was extracted from peripheral blood leukocytes by using a DNA Extractor WB Kit (Wako Pure Chemical Industries, Osaka, Japan). Taq I polymorphism of the VDR gene was determined by the polymerase chain reaction (PCR) method of Riggs et al. [18]. A 740-base-pair (bp) fragment was generated by PCR with primers located on intron 8 and exon 9. The primer sequences were 5′ cag agc atg gac agg gag caa 3′ (forward) and 5′ gca act cct cat ggc tga ggt ctc 3′ (reverse). PCR was performed for 35 cycles using Taq polymerase (Perkin Elmer Co., Ltd., NJ, USA) under the following conditions: denaturation at 94°C for 1 min, annealing at 57°C for 1 min, and extension at 72°C for 1 min. Then 10 μl of the PCR products were subjected to digestion with Taq I (Takara Shuzo Co., Ltd., Kyoto, Japan) at 65°C for 3 h and were separated on 3% Nusieve agarose gel (FMC Bioproducts, Rockland, ME, USA) [19]. The presence of a C > T substitution at position 3 on codon 352 in exon 9, which codes for isoleucine, is associated with loss of the Taq I restriction site. The resulting alleles are designated as T (Taq I site absent; 2 fragments of 495 bp and 245 bp) or t (Taq I site present; 3 fragments of 290 bp, 245 bp, and 205 bp). The subjects were therefore classified as TT, Tt or tt (Fig. 1) [19].
Fig. 1.
Restriction fragment length polymorphism (RFLP) analysis of the product of a polymerase chain reaction (PCR) targeting VDR. Pattern of fragments for the three possible genotypes after Taq I digestion of the PCR product, a 740-bp amplified region of the VDR gene. The 245-bp fragment is constant in all genotypes, being created by cleavage at a nonpolymorphic Taq I site within the range of amplification. Lane 1TT genotype (2 fragments of 495 and 245 bp), Lane 2tt genotype (3 fragments of 290, 245, and 205 bp), Lane 3Tt genotype (4 fragments of 495, 290, 245, and 205 bp)
Statistical analysis
Results are presented as mean ± standard deviation (SD), and categorical variables are expressed as frequencies. The chi-square test was used to verify Hardy–Weinberg equilibrium of genotype frequencies. Analysis of variance (ANOVA) with a post hoc Tukey’s test or Student’s t test was used to assess the differences between age groups and between VDR gene polymorphism (TT versus Tt plus tt genotypes), respectively. Mann–Whitney U test was used to compare lifestyle differences between two categorical variables. Analysis of covariance (ANCOVA) was used to adjust BMD for the covariates age and BMI. We then confirmed that these interactions were not significant. The level of statistical significance was set at p < 0.05. All analyses were done by using SPSS 15.0 software.
Results
The characteristics of the subjects in each age group are presented in Table 1. Although the age range of the study population was from 23 to 49 years, approximately 50% of the subjects were aged 30–39 years. Compared with the Japan Health and Welfare Statistics (2006) [20], the mean height (169.9 ± 5.8 cm) was approximately the same as the national average (171.9, 171.4, and 170.2 cm for subjects in their 20s, 30s, and 40s, respectively). Although there was not much difference, the average weight (67.7 ± 9.8 kg) of our subjects was approximately 2% lower than the national average (67.4, 70.5, and 69.7 kg for subjects in their 20s, 30s, and 40s, respectively). Mean BMI was significantly higher in the 40–49 age group than in the 23–29 age group. No significant differences among the age groups were seen with respect to height, weight, BMD, DPD or BAP.
Table 1.
Characteristics of the subjects in each age group
| Variable | All (n = 524) | 23–29 years (n = 53) | 30–39 years (n = 280) | 40–49 years (n = 191) |
|---|---|---|---|---|
| Age (years) | 37.3 ± 5.4 | 26.5 ± 2.1 | 35.8 ± 2.7 | 42.6 ± 2.3 |
| Height (cm) | 169.9 ± 5.8 | 170.1 ± 6.1 | 170.2 ± 5.9 | 169.4 ± 5.7 |
| Weight (kg) | 67.7 ± 9.8 | 65.3 ± 12.7 | 67.4 ± 10.0 | 68.6 ± 8.5 |
| BMI (kg/m2) | 23.4 ± 3.0 | 22.5 ± 3.7 | 23.2 ± 3.1 | 23.9 ± 2.6* |
| BMD (g/m2) | 0.56 ± 0.06 | 0.55 ± 0.06 | 0.56 ± 0.06 | 0.56 ± 0.06 |
| DPD (nmol/mmol CRE) | 3.7 ± 1.2 | 4.0 ± 1.0 | 3.6 ± 1.2 | 3.7 ± 1.2 |
| BAP (U/L) | 25.5 ± 8.0 | 26.2 ± 6.4 | 25.2 ± 7.1 | 25.8 ± 9.5 |
Values are mean ± SD
BMI body mass index, BMD bone mineral density, DPD deoxypyridinoline, BAP bone alkaline phosphatase
Data were analyzed by analysis of variance (ANOVA) and Tukey’s test
* P < 0.05 compared with 23–29 years
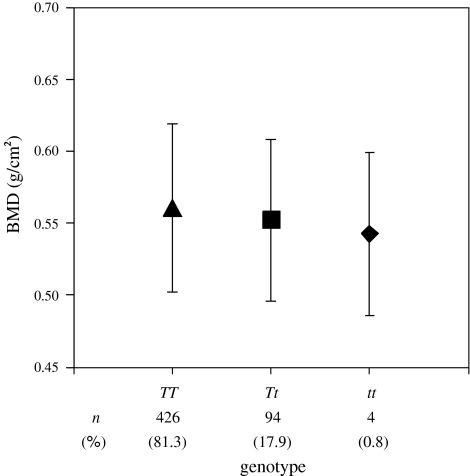
Figure 2 shows the frequencies of VDR gene polymorphism detected by Taq I digestion in relation to the BMD (mean ± standard deviation) (TT 0.56 ± 0.06, Tt 0.55 ± 0.06, and tt 0.54 ± 0.06). VDR gene polymorphism had a distribution that followed Hardy–Weinberg equilibrium (P = 0.63). Similar to a previous Japanese report [21], the genotype frequencies of VDR gene polymorphism were 81.3%, 17.9%, and 0.8% for TT, Tt, and tt, respectively. The characteristics of the subjects stratified according to VDR gene polymorphism are shown in Table 2. It was necessary to combine the heterozygous (Tt) and homozygous (tt) genotypes when comparing characteristics due to the low frequency of the tt genotype in the Japanese population. When high levels of DPD and BAP are detected, presence of metastatic bone tumor, metabolic bone disease or abnormal calcium metabolism can be suspected, but there were no significant differences in relation to VDR gene polymorphism. In addition, no significant differences of VDR gene polymorphism were seen in relation to age, height, weight or BMI.
Fig. 2.
Genotype frequencies of vitamin D receptor gene polymorphism detected by Taq I digestion and mean bone mineral density ± standard deviation
Table 2.
Characteristics of the subjects stratified according to vitamin D receptor gene polymorphism
| Variable | All (n = 524) | TT (n = 426) | Tt + tt (n = 98) |
|---|---|---|---|
| Age (years) | 37.3 ± 5.4 | 37.5 ± 5.5 | 36.6 ± 5.0 |
| Height (cm) | 169.9 ± 5.8 | 169.8 ± 5.9 | 170.4 ± 5.7 |
| Weight (kg) | 67.7 ± 9.8 | 67.9 ± 10.0 | 66.6 ± 9.0 |
| BMI (kg/m2) | 23.4 ± 3.0 | 23.5 ± 3.1 | 22.9 ± 2.6 |
| DPD (nmol/mmol CRE) | 3.7 ± 1.2 | 3.7 ± 1.2 | 3.6 ± 1.1 |
| BAP (U/L) | 25.5 ± 8.0 | 25.6 ± 8.3 | 25.1 ± 6.9 |
Values are mean ± SD
BMI body mass index, DPD deoxypyridinoline, BAP bone alkaline phosphatase
Table 3 shows the BMD according to VDR gene polymorphism stratified by BMI. In the 30–39 age group, mean BMD was significantly lower among subjects with the Tt or tt genotypes than in those with the TT genotype. Subjects were stratified into two groups according to their mean BMI (<23.0 kg/m2 or ≥23.0 kg/m2). Among those in the 30–39 age group with BMI ≥23.0 kg/m2, mean BMD was significantly lower for subjects with the Tt or tt genotypes than for those with the TT genotype.
Table 3.
Mean bone mineral density according to vitamin D receptor gene polymorphism by quintile of BMI
| Age group (years) | All | TT | Tt + tt | ||||
|---|---|---|---|---|---|---|---|
| n | BMD (mean ± SD) | n | BMD (mean ± SD) | n | BMD (mean ± SD) | ||
| All subjects | All ages | 524 | 0.56 ± 0.06 | 426 | 0.56 ± 0.06 | 98 | 0.55 ± 0.06 |
| 23–29 | 53 | 0.55 ± 0.05 | 39 | 0.55 ± 0.06 | 14 | 0.55 ± 0.04 | |
| 30–39 | 280 | 0.56 ± 0.06 | 226 | 0.56 ± 0.06 | 54 | 0.55 ± 0.06* | |
| 40–49 | 191 | 0.56 ± 0.06 | 161 | 0.56 ± 0.06 | 30 | 0.56 ± 0.05 | |
| BMI by quintile (kg/m2) | |||||||
| <23.0 | All ages | 237 | 0.54 ± 0.06 | 188 | 0.54 ± 0.06 | 49 | 0.54 ± 0.06 |
| 23–29 | 29 | 0.53 ± 0.04 | 20 | 0.52 ± 0.04 | 9 | 0.54 ± 0.03 | |
| 30–39 | 139 | 0.54 ± 0.06 | 109 | 0.54 ± 0.06 | 30 | 0.54 ± 0.06 | |
| 40–49 | 69 | 0.55 ± 0.06 | 59 | 0.55 ± 0.06 | 10 | 0.55 ± 0.05 | |
| ≥23.0 | All ages | 287 | 0.57 ± 0.06 | 238 | 0.58 ± 0.06 | 49 | 0.56 ± 0.05 |
| 23–29 | 24 | 0.57 ± 0.06 | 19 | 0.57 ± 0.07 | 5 | 0.57 ± 0.05 | |
| 30–39 | 141 | 0.58 ± 0.05 | 117 | 0.58 ± 0.05 | 24 | 0.56 ± 0.06* | |
| 40–49 | 122 | 0.57 ± 0.06 | 102 | 0.57 ± 0.06 | 20 | 0.57 ± 0.05 | |
Values are mean ± SD
BMI body mass index, BMD bone mineral density, DPD deoxypyridinoline, BAP bone alkaline phosphatase
Data were analyzed by Student’s t test
* P < 0.05 compared with TT
Table 4 shows the combined effects of lifestyle factors and VDR gene polymorphism on BMD. Only variables that presented a statistically significant difference are displayed. According to ANCOVA adjustment for age and BMI, subjects with past history of exercise, current exercise 3–7 days a week or daily alcohol intake had significantly higher BMD than those without these factors. Among subjects who ate 2 meals a day or smoked ≥21 cigarettes a day, BMD was significantly lower if they had the Tt or tt genotype than if they had the TT genotype. On the other hand, there was no significant association between other lifestyle factors (night-shift work, sleeping time, milk intake, and dieting) and BMD (data not shown).
Table 4.
Mean bone mineral density according to lifestyle factors and vitamin D receptor gene polymorphism
| Characteristic | All (n = 524) | TT (n = 426) | Tt + tt (n = 98) | |||
|---|---|---|---|---|---|---|
| n (%) | BMD (mean ± SD) | n (%) | BMD (mean ± SD) | n (%) | BMD (mean ± SD) | |
| Past history of exercise | ||||||
| No | 104 (19.9) | 0.54 ± 0.06 | 82 (19.3) | 0.54 ± 0.06 | 22 (22.7) | 0.53 ± 0.06 |
| Yes | 418 (80.1) | 0.56 ± 0.06a | 343 (80.7) | 0.57 ± 0.06b | 75 (77.3) | 0.56 ± 0.05c |
| Current exercise | ||||||
| No | 278 (54.2) | 0.55 ± 0.06 | 220 (52.8) | 0.55 ± 0.06 | 58 (60.4) | 0.55 ± 0.05 |
| 1–2 days/week | 180 (35.1) | 0.56 ± 0.06 | 149 (35.7) | 0.56 ± 0.06 | 31 (32.3) | 0.56 ± 0.06 |
| 3–7 days/week | 55 (10.7) | 0.58 ± 0.06a | 48 (11.5) | 0.58 ± 0.05b | 7 (7.3) | 0.55 ± 0.07 |
| Number of meals daily | ||||||
| 3 meals/day | 427 (81.5) | 0.56 ± 0.06 | 347 (81.5) | 0.56 ± 0.06 | 80 (81.6) | 0.56 ± 0.06 |
| 2 meals/day | 97 (18.5) | 0.55 ± 0.06 | 79 (18.5) | 0.56 ± 0.06 | 18 (18.4) | 0.51 ± 0.04d,e |
| Smoking status | ||||||
| No | 200 (38.7) | 0.56 ± 0.06 | 160 (38.1) | 0.56 ± 0.06 | 40 (41.2) | 0.55 ± 0.05 |
| 1–20 cigarettes/day | 254 (49.1) | 0.56 ± 0.06 | 204 (48.6) | 0.56 ± 0.06 | 50 (51.6) | 0.55 ± 0.06 |
| ≥21 cigarettes/day | 63 (12.2) | 0.56 ± 0.06 | 56 (13.3) | 0.57 ± 0.06 | 7 (7.2) | 0.51 ± 0.05f |
| Alcohol intake | ||||||
| No | 155 (30.4) | 0.55 ± 0.06 | 129 (31.3) | 0.55 ± 0.06 | 26 (26.5) | 0.55 ± 0.06 |
| Sometimes | 141 (27.6) | 0.55 ± 0.06 | 116 (28.2) | 0.56 ± 0.06 | 25 (25.5) | 0.54 ± 0.06 |
| Every day | 214 (42.0) | 0.57 ± 0.06a | 167 (40.5) | 0.57 ± 0.06 | 47 (48.0) | 0.56 ± 0.05 |
Data are mean ± SD
BMD bone mineral density
BMD was adjusted for age and BMI by analysis of covariance (ANCOVA)
aP < 0.05 compared with “No” in all subjects
bP < 0.05 compared with “No” in TT subjects
cP < 0.05 compared with “No” in Tt plus tt subjects
dP < 0.05 compared with 2 meals/day in TT subjects
eP < 0.05 compared with 3 meals/day in Tt plus tt subjects
fP < 0.05 compared with ≥21 cigarettes/day in TT subjects
The characteristics of the subjects are presented according to daily number of meals in Table 5. Subjects who ate only 2 meals a day (skippers) had significantly lower age, weight, and BMI than subjects who ate 3 meals a day. In addition, skippers tended to perform less physical activity, had a low intake of milk, and smoked more.
Table 5.
Characteristics of the subjects according to daily number of meals
| 3 meals/day (n = 427) | 2 meals/day (n = 97) | P | |
|---|---|---|---|
| Age (years) | 37.6 ± 5.3 | 36.1 ± 5.8 | <0.001 |
| Height (cm) | 170.1 ± 5.8 | 169.1 ± 5.9 | NS |
| Weight (kg) | 68.3 ± 9.8 | 65.2 ± 9.6 | <0.001 |
| BMI (kg/m2) | 23.6 ± 3.0 | 22.8 ± 2.9 | <0.001 |
| BMD (g/m2) | 0.56 ± 0.06 | 0.55 ± 0.06 | NS |
| DPD (nmol/mmol CRE) | 3.7 ± 1.2 | 3.8 ± 1.2 | NS |
| BAP (U/L) | 25.6 ± 8.2 | 25.0 ± 7.4 | NS |
| Lifestyle | |||
| Past history of exercise (%) | |||
| No | 19.3 | 22.7 | NS |
| Yes | 80.7 | 77.3 | |
| Current exercise (%) | |||
| No | 51.1 | 67.7 | <0.05 |
| Yes | 48.9 | 32.3 | |
| Smoking status (%) | |||
| No | 42.3 | 22.9 | <0.001 |
| Yes | 57.7 | 77.1 | |
| Daily alcohol intake (%) | |||
| No | 58.9 | 54.3 | NS |
| Yes | 41.1 | 45.7 | |
| Daily milk intake (%) | |||
| No | 78.9 | 88.7 | <0.05 |
| Yes | 21.1 | 11.3 | |
Values are the mean ± SD
BMI body mass index, BMD bone mineral density, DPD deoxypyridinoline, BAP bone alkaline phosphatase
Data were analyzed by Student’s t test or Mann–Whitney U test
There was no significant association between lifestyle factors and DPD or BAP.
Discussion
In the present study, we found that subjects who only ate 2 meals a day or smoked ≥21 cigarettes a day had a significantly lower BMD if they possessed the Tt or tt genotype than if they possessed the TT genotype. VDR gene polymorphism has been reported to be associated with BMD in Japanese females [21], but no previous studies have exclusively targeted Japanese men. To our knowledge, this is the first report to reveal an association between BMD and lifestyle factors or VDR gene polymorphism in Japanese adult men. Vitamin D plays a central role in calcium homeostasis by regulating calcium absorption, bone resorption, bone cell differentiation, and parathyroid hormone secretion [13], and the VDR gene regulates bone turnover as the receptor for vitamin D [22].
Subjects who had past history of exercise showed significantly higher BMD than those without such a history, and this relationship was unrelated to VDR genotype. Subjects with current exercise 3–7 days/week had significantly higher BMD than those without exercise among all subjects and in the case of the TT genotype. The American College of Sport Medicine has developed guidelines for exercise to improve bone health; their recommendation is to exercise 3–5 times per week [23]. A meta-analysis of 8 studies has shown that exercise has a beneficial effect on BMD [24]. In particular, past history of exercise was reported to have a strong influence on BMD [6]. In a recent study of 263 Japanese subjects (127 boys and 136 girls) aged 12–15 years, exercise performed two or more times weekly during adolescence was associated with higher BMD [25]. A review of studies performed on European and American men and women to assess the effect of exercise on BMD over the long term suggested that the most beneficial effect of exercise on BMD is obtained during growth [26]. Bone formation is known to be stimulated by mechanical stress [27] and this effect is especially important during periods of growth [23]. However, much remains uncertain about the mechanism of bone formation by mechanical stress.
There have been no previous studies investigating the influence of VDR gene polymorphism and past or current exercise on BMD in men. Japanese women performing continuous exercise from the age of 12 years display a positive effect on bone metabolism irrespective of VDR gene polymorphism [21]. In addition, a study that assessed the effect of high-impact exercise on BMD in premenopausal Finnish women aged 35–45 years who participated in an 18-month exercise program suggested that such exercise was beneficial for bones irrespective of VDR genotype [28]. These data show that BMD is not only influenced by calcium metabolism but also by other factors such as mechanical stress. However, these studies were all performed on women, so further epidemiological studies are needed to determine the effect of exercise on BMD in men. In this study, there was no significant association between current exercise and BMD in the Tt plus tt group. It should be noted that we did not assess the duration or intensity of exercise. Therefore, further research is needed to explore the interaction between physical activity (current exercise) and VDR genotypes.
In the Tt plus tt group, subjects who ate 2 meals a day had significantly lower BMD than those who ate 3 meals. Furthermore, among subjects who ate 2 meals daily, BMD was significantly lower if they had the Tt or tt genotype than if they had the TT genotype. While approximately 80–90% of bone mineral is comprised of calcium and phosphorus, other dietary components, such as protein, magnesium, zinc, copper, iron, fluoride, and vitamins D, A, C, and K are required for normal bone metabolism [29]. According to the 2002 National Nutrition Survey in Japan, intake of these nutrients required for normal bone metabolism was lower in skippers than in nonskippers [30]. In addition, higher incidence of hip fracture was associated with low intake of vitamins D and K in both men and women [31]. Based on these studies, we speculate that skipping meals may cause nutritional deficiencies that affect bone metabolism. We also found that skippers had lower weight and BMI, tended to perform less physical activity, had a low intake of milk, and smoked more. These unhealthy behaviors may have had an adverse effect on bone metabolism and resulted in poor physique.
On the other hand, there were no such adverse effects on the bone in subjects with the TT genotype. This difference might be mediated through VDR gene polymorphism, because vitamin D and calcium deficiency alter the efficiency of calcium absorption [32, 33]. Further studies will be required to determine how nutritional status affects BMD, including the influence of VDR gene polymorphism.
In this study, there were no significant differences of BMD in relation to smoking status among all subjects. Among heavy smokers (≥21 cigarettes daily), however, BMD was significantly lower in subjects with the Tt or tt genotype than in those with the TT genotype. A previous population-based study of 1,068 young men showed that smokers had significantly lower BMD than nonsmokers [34]. Hagiwara et al. suggested that smoking is a risk factor for osteoporosis after they investigated 1,736 male Japanese office workers [35]. Though the mechanism by which smoking affects bone metabolism remains unclear, several studies have detected a decrease of intestinal calcium absorption in smokers [36] and the serum level of 25-hydroxyvitamin D has been shown to be lower in smokers than in nonsmokers [37, 38]. Furthermore, a study of 402 elderly men and women revealed that calcium absorption was lower in smokers, and those smoking at least 20 cigarettes daily had the lowest mean absorption rate [36]. Although the exact mechanism underlying the relation between a lower BMD and the Tt or ttVDR genotypes remains unclear, these genotypes seem to have a more adverse impact on BMD.
Subjects who drank alcohol every day had significantly higher BMD than nondrinkers. A previous study of 632 elderly Japanese women suggested that regular alcohol intake was associated with higher BMD [39], but the influence of alcohol in men has not been investigated until now. Alcoholism is a risk factor for osteoporotic fracture and low BMD, but the effects of moderate alcohol consumption on bone are unknown. A recent meta-analysis revealed that persons of both sexes with moderate alcohol intake (0.5–1.0 drinks per day) have lower risk of hip fracture compared with abstainers or heavier drinkers [40]. However, the precise mechanism by which moderate alcohol intake alters bone metabolism is still unknown. Thus, further detailed studies about the association between alcohol consumption and BMD are required.
Vitamin D is produced endogenously when ultraviolet rays in sunlight strike the skin and trigger its synthesis [41]. Night work is associated with lack of sunlight, but there was no influence of the shift work pattern. In a study of 220 young Finnish men, vitamin D status (serum level of 25-hydroxyvitamin D) varied according to season and was lower in winter, with lower serum levels of 25-hydroxyvitamin D being associated with low BMD [42]. However, the subjects were from North Europe, which receives fewer hours of sunlight, and there is no evidence of this effect in the Japanese population.
Our study had several limitations. First, there was no questionnaire to assess environmental factors in detail or for complete assessment of factors related to osteoporosis. However, the questionnaire was designed to cover the well-known risk factors for fracture [6]. We should develop a reliable and valid questionnaire to assess environmental factors contributing to genetic susceptibility to osteoporosis. Also, our study subjects were relatively young. We could not find a relationship between VDR gene polymorphism and bone loss. Clearly, further studies will be needed to define the relation between bone loss and VDR gene polymorphism.
In conclusion, our results suggest that the influence of lifestyle factors on BMD depends on VDR gene polymorphism in adult male workers. Genetic diagnosis of VDR gene polymorphism may be helpful for researching the risk of, and identifying individuals susceptible to, osteoporosis. Such studies could lead to more effective prevention of osteoporosis and improvement of public health. A large-scale prospective study will be needed to define the relation between BMD and lifestyle factors, as well as the influence of VDR gene polymorphism.
Acknowledgments
This work was supported in part by the Advanced Education Program for Integrated Clinical, Basic, and Social Medicine of the Graduate School of Medical Sciences at Kumamoto University (Support Program for Improving Graduate School Education, MEXT, Japan).
References
- 1.Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–41. [DOI] [PubMed]
- 2.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–82. [DOI] [PubMed]
- 3.Nakamura T. Epidemiological study on hip fractures in Tottori Prefecture. Nippon Seikeigeka Gakkai Zasshi. 1993;67:189–200. (in Japanese). [PubMed]
- 4.Aharonoff GB, Koval KJ, Skovron ML, Zuckerman JD. Hip fractures in the elderly: predictors of one year mortality. J Orthop Trauma. 1997;11:162–5. [DOI] [PubMed]
- 5.Qaseem A, Snow V, Shekelle P, Hopkins R Jr, Forciea MA, Owens DK, et al. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2008;148:680–4. [DOI] [PubMed]
- 6.The guidelines committee for prevention and treatment of osteoporosis. In: Orimo H et al., editors. Guidelines for the prevention and treatment of osteoporosis (2006 Edition). Tokyo (Japan): Life Science Publishing; 2006. pp 34, 38.
- 7.Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–7. [DOI] [PubMed]
- 8.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9. [DOI] [PMC free article] [PubMed]
- 9.Fujiwara S, Kasagi F, Masunari N, Naito K, Suzuki G, Fukunaga M. Fracture prediction from bone mineral density in Japanese men and women. J Bone Miner Res. 2003;18:1547–53. [DOI] [PubMed]
- 10.Giguère Y, Rousseau F. The genetics of osteoporosis: ‘complexities and difficulties’. Clin Genet. 2000;57:161–9. [DOI] [PubMed]
- 11.Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8:1–9. [DOI] [PubMed]
- 12.Liu YZ, Liu YJ, Recker RR, Deng HW. Molecular studies of identification of genes for osteoporosis: the 2002 update. J Endocrinol. 2003;177:147–96. [DOI] [PubMed]
- 13.Ralston SH. The genetics of osteoporosis. OJM. 1997;90:247–51. [DOI] [PubMed]
- 14.Gong G, Stern HS, Cheng SC, Fong N, Mordeson J, Deng HW, et al. The association of bone mineral density with vitamin D receptor gene polymorphisms. Osteoporos Int. 1999;9:55–64. [DOI] [PubMed]
- 15.Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; and Ministry of Economy, Trade and Industry. Ethics guidelines for human genome/gene analysis research (in Japanese). 2001. http://www.mhlw.go.jp/topics/bukyoku/seisaku/kojin/dl/161228genomu.pdf. Accessed 15 April 2009.
- 16.Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, et al. Committee on the guidelines for the use of biochemical markers of bone turnover in osteoporosis Japan Osteoporosis Society. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J Bone Miner Metab. 2005;23:97–104. [DOI] [PubMed]
- 17.Bouxsein ML, Palermo L, Yeung C, Black DM. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int. 2002;13:358–65. [DOI] [PubMed]
- 18.Riggs BL, Nguyen TV, Melton LJ 3rd, Morrison NA, O’Fallon WM, Kelly PL, et al. The contribution of vitamin D receptor gene alleles to the determination of bone mineral density in normal and osteoporotic women. J Bone Miner Res. 1995;10:991–6. [DOI] [PubMed]
- 19.Hamasaki T, Inatomi H, Katoh T, Ikuyama T, Matsumoto T. Clinical and pathological significance of vitamin D receptor gene polymorphism for prostate cancer which is associated with a higher mortality in Japanese. Endocr J. 2001;48:543–9. [DOI] [PubMed]
- 20.Ministry of Health, Labour and Welfare. Statistical Database System (in Japanese). 2001. http://wwwdbtk.mhlw.go.jp/toukei/youran/indexyk_2_1.html. Accessed 15 April 2009.
- 21.Fujita Y, Katsumata K, Unno A, Tawa T, Tokita A. Factors affecting peak bone density in Japanese women. Calcif Tissue Int. 1999;64:107–11. [DOI] [PubMed]
- 22.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–49. [DOI] [PubMed]
- 23.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;1985–96. [DOI] [PubMed]
- 24.Kelley GA, Kelley KS, Tran ZV. Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol. 2000;88:1730–6. [DOI] [PubMed]
- 25.Tamaki J, Ikeda Y, Morita A, Sato Y, Naka H, Iki M. Which element of physical activity is more important for determining bone growth in Japanese children and adolescents: the degree of impact, the period, the frequency, or the daily duration of physical activity? J Bone Miner Metab. 2008;26:366–72. [DOI] [PubMed]
- 26.Karlsson MK. Physical activity, skeletal health and fractures in a long term perspective. J Musculoskelet Neuronal Interact. 2004;4:12–21. [PubMed]
- 27.Morinobu M, Ishijima M, Rittling SR, Tsuji K, Yamamoto H, Nifuji A, et al. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J Bone Miner Res. 2003;18:1706–15. [DOI] [PubMed]
- 28.Järvinen TL, Järvinen TA, Sievänen H, Heinonen A, Tanner M, Huang XH, et al. Vitamin D receptor alleles and bone’s response to physical activity. Calcif Tissue Int. 1998;62:413–7. [DOI] [PubMed]
- 29.Ilich JZ, Kerstetter JE. Nutrition in bone health revisited: a story beyond calcium. J Am Coll Nutr. 2000;19:715–37. [DOI] [PubMed]
- 30.Ministry of Health, Labour and Welfare, Japan. The national nutrition survey in Japan, 2001. Tokyo (Japan): Daiichi Shuppan Publishing; 2002. p. 106 (in Japanese).
- 31.Yaegashi Y, Onoda T, Tanno K, Kuribayashi T, Sakata K, Orimo H. Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. Eur J Epidemiol. 2008;23:219–25. [DOI] [PubMed]
- 32.Allen LH. Calcium bioavailability and absorption: a review. Am J Clin Nutr. 1982;35:783–808. [DOI] [PubMed]
- 33.Barger-Lux MJ, Heaney RP, Lanspa SJ, Healy JC, DeLuca HF. An investigation of sources of variation in calcium absorption efficiency. J Clin Endocrinol Metab. 1995;80:406–11. [DOI] [PubMed]
- 34.Lorentzon M, Mellström D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab. 2007;92:497–503. [DOI] [PubMed]
- 35.Hagiwara S, Tsumura K. Smoking as a risk factor bone mineral density in the heel of Japanese men. J Clin Densitom. 1999;2:219–22. [DOI] [PubMed]
- 36.Krall EA, Dawson-Hughes B. Smoking increases bone loss and decrease intestinal calcium absorption. J Bone Miner Res. 1999;14:215–20. [DOI] [PubMed]
- 37.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, et al. An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7. [DOI] [PubMed]
- 38.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53:920–6. [DOI] [PubMed]
- 39.Muraki S, Yamamoto S, Ishibashi H, Oka H, Yoshimura N, Kawaguchi H, et al. Diet and lifestyle associated with increased bone mineral density: cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J Orthop Sci. 2007;12:317–20. [DOI] [PubMed]
- 40.Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–18. [DOI] [PMC free article] [PubMed]
- 41.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. [DOI] [PubMed]
- 42.Välimäki VV, Alfthan H, Lehmuskallio E, Löyttyniemi E, Sahi T, Stenman UH, et al. Vitamin D status as a determinant of peak bone mass in young Finnish men. J Clin Endocrinol Metab. 2003;89:76–80. [DOI] [PubMed]