Abstract
Natural killer (NK) cells express C-type lectin-like receptors, encoded in the NK gene complex, that interact with major histocompatibility complex class I and either inhibit or activate functional activity. Human NK cells express heterodimers consisting of CD94 and NKG2 family molecules, whereas murine NK cells express homodimers belonging to the Ly-49 family. The corresponding orthologues for other species, however, have not been described. In this report, we used probes derived from the expressed sequence tag database to clone C57BL/6-derived cDNAs homologous to human NKG2-D and CD94. Among normal tissues, murine NKG2-D and CD94 transcripts are highly expressed only in activated NK cells, including both Ly-49A+ and Ly-49A− subpopulations. Additionally, mNKG2-D is expressed in murine NK cell clones KY-1 and KY-2, whereas mCD94 expression is observed only in KY-1 cells but not KY-2. Last, we have finely mapped the physical location of the Cd94 (centromeric) and Nkg2d (telomeric) genes between Cd69 and the Ly49 cluster in the NK complex. Thus, these data indicate the expanding complexity of the NK complex and the corresponding repertoire of C-type lectin-like receptors on murine NK cells.
Natural killer (NK) cells are a distinct lymphocytic lineage that functions as a critical component of innate immunity against a wide variety of intracellular and parasitic pathogens and may also mediate tumor surveillance and influence hematopoiesis (1, 2). The activity of NK cells is controlled by inhibitory surface receptors for major histocompatibility complex (MHC) class I molecules (3, 4). Two structural types of NK receptors for MHC class I have been described—type I integral membrane Ig-like killer inhibitory receptors and type II integral-membrane C type lectin-like disulfide-linked dimers, including the human (h) CD94/NKG2 family of heterodimers and the murine (m) Ly-49 family of homodimers (5–8). Both types of NK cell receptors for MHC class I transmit potent inhibitory signals that are dependent upon the presence of immunoreceptor tyrosine-based inhibitory motifs (ITIM) consisting of the consensus sequence I/VXYXXV/L in the cytoplasmic domains (9, 10). Receptor cross-linking appears to lead to tyrosine phosphorylation of the ITIM and the subsequent recruitment of the SHP-1 intracellular tyrosine phosphatase that then presumably dephosphorylates tyrosine residues on molecules involved in the activation cascade.
Among the C-type lectin-like receptors, cross-linking of hCD94 with a mAb either inhibited cytolytic activity or induced redirected lysis of various NK clones, leading to confusion about its function in NK cells (11–14). Remarkably, the cDNA sequence of CD94 reveals an extremely short cytoplasmic domain that contains no consensus sequences involved in cell signaling (15). The phenotypic differences observed with CD94 engagement has been recently clarified in studies demonstrating that CD94 forms heterodimers with NKG2 molecules (16, 17). At least five NKG2 family members have been described in humans: NKG2-A/B, -C, -D, -E, and -F (18–20). NKG2-A/B, -C, and -E show 94–95% amino acid homology in the extracellular domain, whereas NKG2-D is less related (21% amino acid homology overall) (20). Functional studies of human NK cell clones revealed that NKG2-A forms a disulfide-linked heterodimer with CD94 that inhibits cytotoxicity toward targets expressing HLA-A, -B, -C, and -G and virus-encoded MHC class I-like homologues (21–25). Notably, NKG2-A contains two ITIMs in its cytoplasmic domain that associate with SHP-1 (26). On the other hand, NKG2-C, which also forms heterodimers with CD94, lacks ITIM sequences and delivers activating signals (26, 27). In mouse NK cells, Ly-49A belongs to a family of highly related molecules that bear significant amino acid identity to each other (65–89%) (28, 29) but are distinct from human CD94 and NKG2 (<30% identity), suggesting that Ly-49 and NKG2/CD94 are not orthologous. This is also highlighted by previous studies demonstrating that Ly-49 molecules form homodimers rather than heterodimers and that inhibitory Ly-49 molecules bear only one ITIM in the cytoplasmic domain rather than two ITIMs (10, 29). Nevertheless, Ly-49A interacts with H-2Dd and H-2Dk, resulting in inhibition of NK cytotoxicity and secretion of cytokines (30, 31). Other Ly-49 members, such as Ly-49C and Ly-49G, also transmit inhibitory signals after engagement of specific MHC class I ligands (32, 33). However, the Ly-49D receptor, which lacks cytoplasmic ITIM (29), appears to be a stimulatory NK cell receptor (34). With orthologues for Ly-49 receptors in humans yet to be reported, one hypothesis to reconcile these observations is that the murine Ly-49 receptors are functional orthologues of human CD94/NKG2 molecules and that they substitute for the other in their corresponding species. Recent studies, however, indicate that rat NK cells express Ly-49, CD94, and NKG2 molecules (35–37).
The genes encoding the Ly-49 family of receptors reside in the NK complex (NKC) on mouse chromosome 6 (5, 38). Our laboratory has mapped genes encoding other C-type lectins expressed on NK cells, including Nkrp1 and Cd69, within a 2-megabase region by constructing a contig of overlapping yeast artificial chromosomes (YACs) containing NKC DNA from C57BL/6 mice (39). In humans, the NKG2 family members and CD94, as well as orthologues to murine Nkrp1 and Cd69, are located on human chromosome 12p12.3–13.1, a region syntenic to mouse chromosome 6, indicating that the NKC has been conserved across species (15, 40–42). Because the NKC contains a very large genomic region in which gene order has been conserved, and recent studies of rat NK cells indicate the presence of NKG2 and CD94 genes (35–37), we hypothesized that orthologues for human NKG2 and CD94 genes were also encoded in the mouse NKC. In this report, we identified cDNA clones for mouse Nkg2d and Cd94 genes, determined their expression, and mapped their physical positions within the NKC. Because an individual NK cell can express multiple receptors simultaneously, these studies indicate the complexity of the C-type lectin-like NK cell receptors.
MATERIALS AND METHODS
Animals.
C57BL/6J and BALB/cJ mice were purchased from the Jackson Laboratory, whereas C57BL/6T mice were purchased from Taconic Farms. All mice were maintained in a pathogen-free facility at Washington University.
Cell Lines.
KY-1 and KY-2 murine NK cell clones were as described (43). Both lines were grown in R10 medium consisting of RPMI 1640 medium (GIBCO/BRL) supplemented with l-glutamine (300 μg/ml), penicillin (100 units/ml), streptomycin (100 μg/ml), 2-mercaptoethanol (50 μM), 10% fetal calf serum (Harlan Breeders, Indianapolis, IN), and recombinant human interleukin 2 (IL-2; Chiron; 100 units/ml). All other cell lines were obtained from the American Type Culture Collection. C1498, EL-4, MM48T, P815, WEHI-265.1, and WR19L cells were grown in D10 medium [DMEM (GIBCO/BRL) supplemented with l-glutamine (300 μg/ml), penicillin (100 units/ml), streptomycin (100 μg/ml), 2-mercaptoethanol (50 μM), 10% fetal calf serum, sodium pyruvate (1 mM), and nonessential amino acids (0.1 mM)]. MH-S and YAC-1 were grown in R10 without IL-2.
IL-2-Activated NK Cell (LAK) Preparation.
C57BL/6-derived LAK cells were prepared as described (30). Briefly, nylon wool nonadherent splenocyte suspensions were cultured at 2–4 × 106 cells per ml in R10 medium supplemented with recombinant IL-2 (Chiron; 800 units/ml) for 8–14 days. Ly-49A+ LAK cell populations were selected by panning using the anti-Ly-49A mAb A1 (44). Nonadherent Ly-49A− LAK cells were transferred to new flasks, depleted of remaining Ly-49A+ cells with rabbit anti-mouse Ig and rabbit complement (Cedarlane Laboratories), and then harvested by centrifugation on a Lympholyte-M (Cedarlane Laboratories) density gradient. Ly-49A+ LAKs were cultured in the Ig-coated flasks briefly before passage. Both Ly-49A+ and Ly-49A− LAK cell populations were grown for 3 days in R10 with recombinant IL-2 (800 units/ml) and then analyzed for purity by flow cytometry. Flow cytometry analysis with mAb A1 showed that the Ly-49A+ and Ly-49A− LAK cells displayed 96% and 99% purity, respectively.
cDNA Cloning.
By using the tblastn algorithm available from the blast 2.0 program (National Center for Biotechnology Information), we identified murine cDNA sequences from the expressed sequence tag (EST) database (dbEST; National Center for Biotechnology Information) that contained a high degree of homology to the deduced amino acid sequences for human NKG2-D and CD94 (15, 20). One murine EST clone (I.M.A.G.E. Consortium Clone 621324; GenBank accession no. AA178606) homologous to NKG2-D, and two murine EST clones (I.M.A.G.E. Consortium Clones 598616 and 596465; GenBank accession nos. AA161895 and AA122795, respectively) homologous to CD94 were obtained from Genome Systems (St. Louis) and sequenced. A cDNA library from C57BL/6J LAK cells was constructed with the ZAP-cDNA synthesis kit (Stratagene). For each cDNA, ≈1.1 × 106 plaque-forming units were transferred to Hybond-N membranes (Amersham) and hybridized with EcoRI/NotI-digested EST-derived probes labeled with [α-32P]dCTP using the Rediprime kit (Amersham). Stringent washing was performed in 0.2× SSPE (0.18 M NaCl/10 mM sodium phosphate, pH 7.4/1 mM EDTA)/0.1% SDS at 56°C. Positive clones were purified to homogeneity on tertiary plating and then subjected to in vivo excision (Stratagene) and subcloning. Plasmids containing cDNA inserts were sequenced by PCR-based methodology (Perkin–Elmer) with an automated Applied Biosystems sequencer (Perkin–Elmer). DNA sequence analyses were performed by using the sequencher version 3.0 (Genecodes, Ann Arbor, MI) and macvector version 4.0 (International Biotechnologies) software. The GCG sequence analysis software (version 8.0; Genetics Computer Group) was also used via the computer facilities of the Frederick Biomedical Supercomputing Center, National Cancer Institute, Frederick, MD.
RNA Preparation and Northern Blot Analysis.
Total cellular RNA was prepared from LAK cell cultures, cell lines, and murine tissues by using guanidine thiocyanate essentially as described (45). Tissue culture cells at 0.5–5 × 107 cells per ml in 1 × HEBS (137 mM NaCl/5 mM KCl/0.7 mM Na2HPO4/6 mM dextrose/20 mM Hepes base) were directly extracted in 4 M guanidine thiocyanate; mouse tissues and organs were flash frozen and then homogenized in 1 × HEBS prior to RNA extraction. Lyophilized RNA pellets were resuspended in 0.2% diethyl pyrocarbonate in H2O at concentrations of 100–1,000 μg/ml. For Northern blot analyses, 10–20 μg of RNA samples were electrophoresed in a 1% agarose/2.2 M formaldehyde gel (46). RNA was transferred overnight in 20× SSC onto Hybond-N membranes (Amersham) and fixed by baking at 80°C for 2 h. Membranes were hybridized with [α-32P]dCTP-labeled probes derived from the full-length murine NKG2-D and CD94 cDNAs or from human β-actin cDNA (CLONTECH) as an internal quality and content control. Stringent washing was performed in 0.2× SSPE/0.1% SDS at 59°C for NKG2-D and human β-actin and at 50°C for CD94.
Southern Blot Analysis.
C57BL/6J and BALB/cJ genomic DNA was extracted from mouse liver in proteinase K as described (39). Samples of 2–5 μg were subjected to overnight digestion with various restriction endonucleases and then fractionated in 1% agarose. Southern blots were prepared and analyzed (46). DNA was transferred to Hybond-N (Amersham) and membranes were hybridized with [α-32P]dCTP-labeled full-length mNKG2-D or mCD94 cDNA probes. Stringent washing was performed in 0.2× SSPE/0.1% SDS at 56°C for NKG2-D and at 50°C for CD94.
YAC clones 85D11, 178C4, 95E6, 9C10, and 52A6 are derived from C57BL/6 mice and are known to contain NKC-linked DNA fragments (39). Agarose-plug YAC DNA was prepared and separated by pulsed-field gel electrophoresis as described (39) before transfer and hybridization as described above. Stringent washing was performed in 0.2× SSPE/0.1% SDS at 63°C.
RESULTS
cDNA Cloning of Murine NKG2-D and CD94.
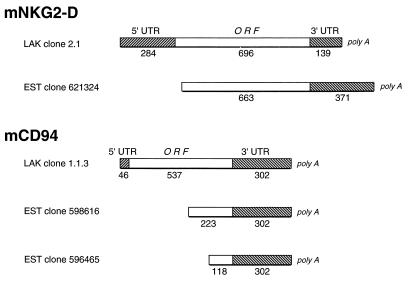
By amino acid sequence comparison of hNKG2-D and hCD94 with deduced protein sequences from the EST database (47), we identified a clone (621324) highly homologous to hNKG2-D and two clones (598616 and 596465) homologous to hCD94. With the EST clone inserts as probes, we screened a cDNA library constructed from C57BL/6J LAK cells and obtained cDNA clones 2.1 and 1.1.3 hybridizing with the mNKG2-D and mCD94 EST probes, respectively. The three EST clone inserts and the cDNA clones were sequenced on both strands, and all three ESTs were determined to be incomplete cDNA sequences when compared with the full-length human cDNAs (Fig. 1). LAK cDNA clone 2.1 contains a 284-bp 5′ untranslated region (UTR), an ORF of 696 bp, and a 139-bp 3′ UTR with a poly(A) tail. Upon comparison of this sequence to EST clone 621324 (NKG2-D-like), a region of complete identity is apparent from nucleotide position 317 and extending 3′. The EST clone, therefore, contains a 5′ truncation involving the complete 5′ UTR and the 32 nucleotides encoding the initial 11 amino acids (Fig. 1). LAK cDNA clone 1.1.3 contained a short 5′ UTR (46 bp), an intact 537-nucleotide ORF, and a 3′ UTR (321 bp) that includes a polyadenylation signal and a partial poly(A) tail. On comparison of this sequence to EST clones 598616 and 596465 (CD94-like), it is apparent that these EST clones contain 3′ regions of identity to the LAK cDNA and incomplete coding sequences at the 5′ end. Moreover, clones 598616 and 596465 have 5′ DNA segments that do not correspond to the full-length LAK cDNA. Whether these sequences represent incompletely or alternatively spliced mRNA transcripts or cloning artifacts is not currently known. Nevertheless, we have identified apparent full-length cDNAs corresponding to truncated EST clones that are homologous to hNKG2-D and CD94.
Figure 1.
Schematic diagram depicting the relationship between the murine LAK cDNA clones for mNKG2-D (Upper) and mCD94 (Lower) and the corresponding EST clones. Hatched boxes, the 5′ and 3′ UTRs; open boxes, coding sequences of the ORFs. The numbers below each section indicate length in nucleotides.
The ORF of cDNA clone 2.1 encodes a type II integral membrane protein with homology to hNKG2-D and rat NKG2-D (NKR-P2) (60% and 81% amino acid identity, respectively; Fig. 2 and Table 1). The predicted protein product contains 232 amino acids, corresponding to a predicted molecular mass of 27 kDa. Notably, as is observed for the human orthologue, the N-terminal cytoplasmic domain lacks ITIMs that are present in human inhibitory NKG2-A/B receptors (20, 26). By contrast, murine NKG2-D also contains a 13-amino acid stretch within the cytoplasmic domain that is absent in hNKG2-D (Fig. 2). Similar to hNKG2-D and hCD94, the putative 23-amino acid transmembrane domain of mNKG2-D contains charged residues, Arg78, Lys91, and Glu92. The 142-amino acid extracellular domain has two potential glycosylation sites and contains cysteine residues conserved among all of the C type lectins (48, 49), as well as cysteines that are conserved only in the NKG2/CD94 subfamily (see below).
Figure 2.
Amino acid alignment of the C type lectins in the CD94/NKG2 family and Ly-49A. Brackets indicate boundaries for the cytoplasmic, transmembrane, stalk, and carbohydrate recognition domains (CRD). Regions of identity are shaded for emphasis and include tryptophan and cysteine residues that are conserved in all C-type lectins as well as spacing (48, 49). Consensus ITIM (boldface type underlined), charged residues in transmembrane domain in mNKG2-D and mCD94 (circled), stop codon (∗), and potential N-linked glycosylation sites (♦♦♦) are shown. The 13-amino acid sequence present in the cytoplasmic domain of mNKG2-D but not hNKG2-D is doubly underlined.
Table 1.
Amino acid identity of representative C-type lectins expressed on mouse, human, and rat NK cells
| Lectin | Amino acid identity, %
|
|
|---|---|---|
| With mNKG2-D | With mCD94 | |
| Rat NKG2-D (NKR-P2) | 81 | 28 |
| hNKG2-D | 60 | 24 |
| mCD94 | 28 | 100 |
| Rat CD94 | 24 | 77 |
| hCD94 | 29 | 55 |
| hNKG2-A | 21 | 26 |
| hNKG2-C | 24 | 25 |
| hNKG2-E | 21 | 23 |
| Ly-49A | 21 | 20 |
| mNKR-P1A | 19 | 24 |
| mNKR-P1C | 21 | 24 |
| mCD69 | 21 | 23 |
LAK cDNA clone 1.1.3 contains an 179-residue ORF encoding a type II transmembrane protein (predicted molecular mass = 20.8 kDa) with high homology to rat and hCD94 (77% and 55% amino acid identity, respectively; Table 1 and Fig. 2). It also contains a single charged residue in its transmembrane domain, Lys19. Since the cytoplasmic tail is only 10 residues in all three species and the putative transmembrane domain is 23 amino acids, the extracellular domain forms the bulk (145 of 179 amino acids or 81%) of this receptor. Additionally, the external domain contains a region of homology to the C-type lectins.
Alignment of both mouse deduced sequences to human and rat CD94 and NKG2 (Fig. 2 and Table 1) highlights the similarity of mNKG2-D and mCD94 to their human and rat orthologues. Moreover, there is conserved position and spacing of invariant cysteine and tryptophan residues found in all of the NKC-linked C type lectins. Note that the cysteine at position 112 of mNKG2-D (position 58 in mCD94) is unique to the NKG2-D/CD94 subgroup and is not conserved in the Ly-49, NKR-P1, or CD69 receptors. However, mNKG2-D, like hNKG2-D, is as homologous to the other NKG2 family members as it is to NKR-P1 and Ly-49A, suggesting that it is not a member of the highly related NKG2 family (Table 1). Thus, these data indicate that we have identified full-length cDNAs for mouse NKG2-D and CD94.
Expression of mNKG2-D and mCD94.
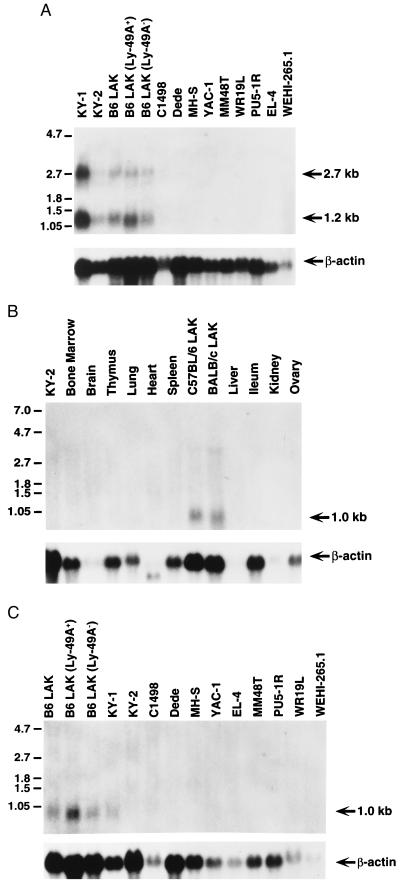
Northern blot analysis revealed the presence of two mNKG2-D transcripts (2.7 and 1.2 kb) in NK cell clones and IL-2-activated NK cells but not in a panel of other cell lines including those of lymphocyte (C1498, WR19L, YAC-1, and EL-4), monocyte/macrophage (WEHI-265.1, PU5–1R, and MH-S), and fibroblast (Dede and MM48T) origins (Fig. 3A). The NKG2-D cDNA clone 2.1 most likely represents the 1.2-kb transcript although the origin of the 2.7-kb transcript is not currently known. It could represent a transcript for a related but distinct molecule or an incompletely processed mNKG2-D transcript. The CD94 cDNA detected a 1.0-kb transcript with similar expression pattern (Fig. 3C). In addition, NKG2-D and CD94 transcripts were not detected in bone marrow, brain, thymus, lung, heart, spleen, liver, ileum, kidney, and ovary (Fig. 3B and data not shown). Thus, NKG2-D and CD94 transcripts appear to be preferentially expressed in NK cells.
Figure 3.
Northern blot analyses of mNKG2-D and mCD94 transcripts. (A) Ten micrograms of total cellular RNA from the indicated cells and cell lines was hybridized with full-length mNKG2-D cDNA. (B) Twenty micrograms of total cellular RNA from the indicated organs and cultured cells were hybridized with full-length mCD94 cDNA. (C) Twenty micrograms of total cellular RNA from the indicated LAK cell populations and cell lines were hybridized with the mCD94 cDNA. All of the blots were rehybridized with a human β-actin probe to control for RNA quality and content, as shown below.
The NKG2-D and CD94 transcripts, however, are not always expressed in NK cells. Particularly for CD94 transcripts, there appeared to be markedly enhanced expression in only one (KY-1) NK cell clone (Fig. 3 A and C), even though bulk IL-2-activated NK cells express abundant transcripts for both NKG2-D and CD94. This is not due to differences in RNA loading because hybridization with an actin probe revealed ample transcripts in both lanes. Moreover, the differences in transcript expression are unrelated to Ly-49 family expression because both NK cell clones fail to express the Ly-49 family, and Ly-49A+ and Ly-49A− LAK cells do not display differences in NKG2-D or CD94 expression (Fig. 3 A and C, respectively).
There were no marked differences in NKG2-D or CD94 expression in BALB/c LAK cells compared with C57BL/6-derived cells. However, the BALB/c CD94 transcripts were reproducibly slightly smaller than C57BL/6 transcripts, possibly reflecting allelic differences. A 3.5-kb transcript was also detected in BALB/c LAK, which may represent an incompletely spliced transcript. Interestingly, transcripts in the spleen were undetectable, perhaps reflecting a prerequisite for activation or insensitivity of Northern blots for transcripts in the very small percentage of NK cells (<2.5%) in the spleen. Thus, the data indicate that transcripts for both NKG2-D and CD94 are preferentially expressed in NK cells, although there are differences in expression among individual NK cells.
Physical Mapping of Nkg2d and Cd94 Within the NKC.
Previous studies on other C-type lectins encoded in the NKC have demonstrated that many of these genes, including Nkrp1 and Ly49a, display restriction fragment length polymorphisms (RFLP) between the C57BL/6 and BALB/c strains (50, 53). Southern blot analysis of genomic mouse DNA derived from nine inbred strains (including C57BL/6 and BALB/c) digested with multiple individual enzymes revealed RFLPs between C57BL/6 and BALB/c for both mNKG2-D and mCD94. For example, a RFLP for mNKG2-D was detected on PvuII digest, whereas RFLPs for mCD94 were detected on HindIII and EcoRI digests (data not shown). These RFLPs should be useful for genetic analysis of the NKC.
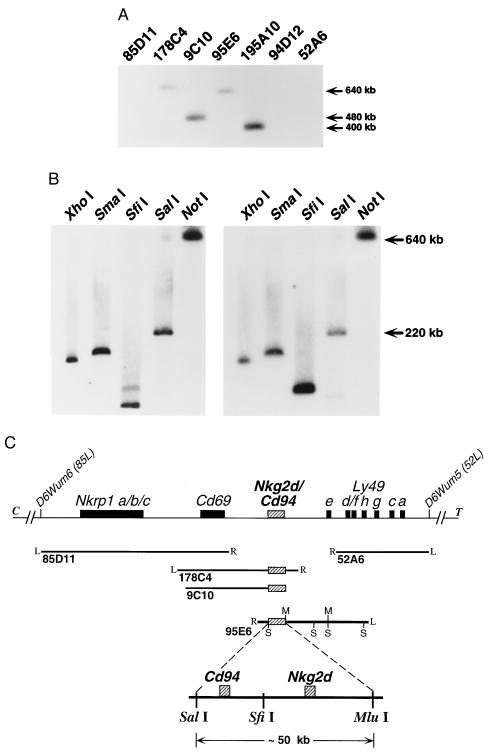
To more precisely map the location of both genes, the NKG2-D and CD94 cDNA probes were hybridized to Southern blots containing YACs representing our previously assembled >2 megabase contig of the NKC, containing 14 YACs (39). The mCD94 and the mNKG2-D probe hybridized only to YACs 9C10, 95E6, 195A10, and 178C4 (Fig. 4 and data not shown). Note that neither mNKG2-D nor mCD94 probes hybridized to the flanking YACs 85D11 and 52A6, which contain the Nkrp1 and the Ly49 gene clusters, respectively. PCR analysis using mNKG2-D and mCD94 sequence-specific primers on YAC DNA templates independently and specifically confirmed the presence of each gene within these YACs (data not shown). These data indicate that the genes for mNKG2-D and CD94 reside in the NKC between Cd69 and the Ly49 cluster.
Figure 4.
Mapping of murine Nkg2d and Cd94 within the NKC. (A) Southern blot analysis of YACs previously shown to form a contig spanning an ≈2.0-megabase region of the murine NKC on chromosome 6 (39), schematically represented in C. This blot was hybridized with both mCD94 (shown) and mNKG2-D (not shown) full-length cDNA probes; identical patterns were observed. (B) Southern blot analysis of YAC 95E6 digested with the indicated enzymes and hybridized with mNKG2-D (Left) or mCD94 (Right) cDNA probes. The murine Nkg2d and Cd94 loci are discriminated on SfiI digestion of YAC 95E6. (C) Schematic representation of the murine Nkg2d and Cd94 loci (hatched boxes) positioned between Cd69 and the Ly49 cluster (solid boxes). The positions of each YAC previously aligned within the NKC contig are shown below (solid lines). Where known, the right (R) and left (L) ends of each YAC are indicated. SalI (S) and Mlu I (M) sites present in YAC 95E6 are shown for reference. An expanded region of YAC 95E6 representing the ≈50-kb SalI–Mlu I fragment containing both loci is shown at the bottom. The exact position of the SfiI site separating the Cd94 and Nkg2d loci and the precise location of the individual genes on the fragment have not been determined.
Southern blot analyses of YAC 95E6 digested with a panel of rare-cutting restriction endonucleases revealed that mNKG2-D and mCD94 hybridized to many of the same fragments permitting localization of Nkg2d and Cd94, as well as their gene order (Fig. 4 B and C). The region of overlap was confirmed to be limited to an ≈50-kb SalI–MluI fragment present near the right end of YAC 95E6 (data not shown). In addition, gene localization and order was resolved by the enzyme SfiI (Fig. 4 B and C). The NKG2-D probe detects two bands in the SfiI lane that could represent an SfiI site within the gene or incomplete digestion of the slightly larger band. A probe specific for the right end of YAC 95E6 insert hybridized to the same fragment as did the mCD94 probe (data not shown). Because the NKG2-D probe did not hybridize to this fragment, this localized Cd94 centromeric relative to Nkg2d. Thus, we have determined the position and gene order for Nkg2d and Cd94 that map between the Cd69 locus (centromeric) and the Ly49 cluster (telomeric) on murine chromosome 6 (Fig. 4C).
DISCUSSION
We used murine ESTs homologous to hNKG2-D and CD94 to isolate cDNAs for mouse NKG2-D and CD94 from C57BL/6-derived IL-2-activated NK cells, providing another example of the utility of dbEST as a resource in molecular cloning (47). Although both cDNAs encode molecules with high degree of similarity to other murine NK cell C-type lectin-like receptors, sequence alignment demonstrated that the ORF of LAK cDNA clone 2.1 showed the highest degree of homology to hNKG2-D (60% identity). Notably, murine CD69, a monomorphic C-type lectin also mapped to the NKC (5), has a similar degree of homology to its human orthologue (58% amino acid identity) (40, 51). LAK cDNA clone 1.1.3 encodes a molecule that is related to hCD94 with similar amino acid identity and is closely related to rat CD94 (55% and 77% amino acid identity, respectively). Therefore, clone 2.1 contains the murine homologue of hNKG2-D, and clone 1.1.3 is the murine homologue of hCD94.
The deduced murine NKG2-D and CD94 polypeptides share several features with the well-characterized Ly-49 family of NK cell receptors (38). These features include structural aspects, such as type II integral membrane protein orientation and C- type lectin homology. Although human homologues of Ly-49 have yet to be described, and CD94/NKG2 molecules share functions with Ly-49, including NK cell selective expression, MHC class I specificity, and inhibitory activity, our data clearly indicate that murine NK cells can coexpress Ly-49, CD94, and NKG2-D molecules. Although definitive protein expression analysis awaits the development of specific serological reagents, the RNA expression patterns of these molecules indicate that the repertoire of murine C type lectin NK cell receptors is larger than initially appreciated.
The function of mCD94, because it also contains a short cytoplasmic domain, is likely to be similar to hCD94, which requires a partner chain (16, 17). Consistent with this hypothesis, murine CD94 contains charged residues in its transmembrane domain, as does hCD94, suggesting that it will also pair with mouse NKG2 molecules. Although we have not yet identified the mouse homologues for the other human NKG2 family members, recent studies indicate that such homologues are expressed on rodent NK cells (37). The specificity of human CD94/NKG2-A molecules has been controversial with apparent specificity for a wide range of HLA-A, -B, -C, and -G molecules (21–25). Recent studies indicate that this promiscuity may be largely explained by CD94/NKG2-A specificity for nonclassical MHC class I molecules that can bind the leader peptides of other MHC class I molecules (M. López-Botet, personal communication). Because the mouse, like the human, contains a very large number of nonclassical MHC class I molecules, this possibility will require further detailed examination.
Recent molecular genetic studies suggest that the hNKG2-D has distinct features from the other NKG2 molecules, including its position within another functional gene (18). Furthermore, the degree of homology of mNKG2-D to the other NKG2 family members, including hNKG2-A, hNKG2-C, and hNKG2-E, is similar to other unrelated C type lectins (21–24% identity), suggesting that NKG2-D may not belong to the NKG2 family, as noted (36). Unlike hNKG2-A and hNKG2-C, murine NKG2-D contains charged residues in its transmembrane domain that are incompatible in the heterodimeric configuration with the charged residue in CD94. Although it has not yet been shown that hNKG2-D molecules can be expressed on the cell surface, Northern blot analysis demonstrates it is expressed in NK cells that lack CD94 expression, suggesting it may pair with another partner chain. Like the human and rat orthologues, the cytoplasmic domain of mNKG2-D lacks conserved ITIM sequence motifs, indicating that mNKG2-D may not function as an inhibitory receptor but rather may be stimulatory, as has been demonstrated for hNKG2-C (26, 36). Thus, these data suggest that murine NKG2-D molecules may function as activation receptors that are independent of CD94.
Nkg2d and Cd94 are positioned within ≈50 kb of each other in the mouse NKC, indicating the presence of another C-type lectin cluster. This is supported by data that demonstrate the human NKG2A/B, -C, -D, -E, and -F genes are tightly clustered with human CD94 within the NKC on chromosome 12p (54). This predicts that additional members of the mouse NKG2 family will reside in this cluster, which we are currently investigating. Presently, we have shown that the murine Nkg2d and Cd94 loci are located in the NKC between Cd69 and the Ly49 gene cluster. Dissen et al. (35, 55) have reported an essentially identical syntenic position for rat Cd94 in the NKC on rat chromosome 4. Additionally, the human NKG2A, -C, and -E and CD94 loci have been mapped to the NKC by using gene-specific primers and PCR (56). Therefore, the identification of the CD94/NKG2 gene cluster further illustrates the conservation and complexity of the NKC.
Importantly, several phenotypic traits have been genetically linked to the NKC. In rats, the lymphocyte alloreactivity is mediated by NK cells that can apparently recognize MHC alloantigens (55). This gene, termed Nka, is linked to the rat NKC and segregates with rat Ly49 separately from rat Nkrp1, Cd94, and Nkg2d, raising the possibility that the Ly-49 activation or inhibitory receptors may be responsible for recognition in this system. In mice, three additional phenotypic markers have been linked to the NKC. Chok encodes the capacity of C57BL/6-derived NK cells to kill Chinese hamster ovary target cells that is lacking in BALB/c-derived cells (A. Idris and W.M.Y., unpublished results). Cmv1 controls NK cell-mediated host resistance to otherwise lethal murine cytomegalovirus infection (57). Similarly, Rmp1 controls host resistance to mousepox virus (58). CD94 and/or NKG2D may be related to any of these traits not only because they map physically and genetically to the same region but also because they are known to contribute toward NK cell target recognition and effector function. Therefore, the identification of murine CD94 and NKG2-D contributes toward understanding the complex nature of NK cell regulation and function.
Acknowledgments
We thank Beatrice Plougastel for helpful discussions and critical review of this manuscript. We gratefully acknowledge the use of the Frederick Biomedical Supercomputing Center, National Cancer Institute, Frederick, MD. This work was supported by grants from the National Institutes of Health and Barnes–Jewish Research Foundation. E.L.H. is a recipient of the American Heart Association Medical Student Fellowship (Grant 95005120). J.W.H. is a fellow of the Howard Hughes Medical Institute. M.G.B. is a recipient of a National Research Service Award from the National Institute of Allergy and Infectious Diseases. W.M.Y. is an investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- EST
expressed sequence tag
- h
human
- IL-2
interleukin 2
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- NK
natural killer
- LAK
IL-2-activated NK cell
- m
mouse
- MHC
major histocompatibility complex
- NKC
natural killer complex
- YAC
yeast artificial chromosome
- UTR
untranslated region
- RFLP
restriction fragment length polymorphism
Note Added in Proof
The EST cloning of murine CD94 recently was described by Vance et al. (59).
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF054819 and AF0547714).
References
- 1.Trinchieri G. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancroft G J. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama W M. Proc Natl Acad Sci USA. 1995;92:3081–3085. doi: 10.1073/pnas.92.8.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colonna M. Curr Opin Immunol. 1996;8:101–107. doi: 10.1016/s0952-7915(96)80112-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown M G, Scalzo A A, Matsumoto K, Yokoyama W M. Immunol Rev. 1997;155:53–65. doi: 10.1111/j.1600-065x.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 6.Lanier L L, Corliss B, Phillips J H. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 7.Long E O, Wagtmann N. Curr Opin Immunol. 1997;9:344–350. doi: 10.1016/s0952-7915(97)80080-5. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Botet M, Perezvillar J J, Carretero M, Rodriguez A, Melero I, Bellon T, Llano M, Navarro F. Immunol Rev. 1997;155:165–174. doi: 10.1111/j.1600-065x.1997.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 9.Burshtyn D N, Long E O. Trends Cell Biol. 1997;7:473–479. doi: 10.1016/S0962-8924(97)01167-7. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M C, Niemi E C, Fisher M J, Shultz L D, Seaman W E, Ryan J C. J Exp Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Villar J J, Melero I, Rodriguez A, Carretero M, Aramburu J, Sivori S, Orengo A M, Moretta A, Lopez-Botet M. J Immunol. 1995;154:5779–5788. [PubMed] [Google Scholar]
- 12.Aramburu J, Balboa M A, Rodriguez A, Melero I, Alonso M, Alonso J L, Lopez-Botet M. J Immunol. 1993;151:3420–3429. [PubMed] [Google Scholar]
- 13.Aramburu J, Balboa M A, Ramirez A, Silva A, Acevedo A, Sanchez-Madrid F, De Landazuri M O, Lopez-Botet M. J Immunol. 1990;144:3238–3247. [PubMed] [Google Scholar]
- 14.Aramburu J, Balboa M A, Izquierdo M, Lopez-Botet M. J Immunol. 1991;147:714–721. [PubMed] [Google Scholar]
- 15.Chang C, Rodriguez A, Carretero M, Lopez-Botet M, Phillips J H, Lanier L L. Eur J Immunol. 1995;25:2433–2437. doi: 10.1002/eji.1830250904. [DOI] [PubMed] [Google Scholar]
- 16.Carretero M, Cantoni C, Bellon T, Bottino C, Biassoni R, Rodriguez A, Perezvillar J J, Moretta L, Moretta A, Lopez-Botet M. Eur J Immunol. 1997;27:563–567. doi: 10.1002/eji.1830270230. [DOI] [PubMed] [Google Scholar]
- 17.Lazetic S, Chang C, Houchins J P, Lanier L L, Phillips J H. J Immunol. 1996;157:4741–4745. [PubMed] [Google Scholar]
- 18.Plougastel B, Trowsdale J. Eur J Immunol. 1997;27:2835–2839. doi: 10.1002/eji.1830271114. [DOI] [PubMed] [Google Scholar]
- 19.Adamkiewicz T V, McSherry C, Bach F H, Houchins J P. Immunogenetics. 1994;39:218. doi: 10.1007/BF00241264. [DOI] [PubMed] [Google Scholar]
- 20.Houchins J P, Yabe T, McSherry C, Bach F H. J Exp Med. 1991;173:1017–1020. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretta A, Vitale M, Sivori S, Bottino C, Morelli L, Augugliaro R, Barbaresi M, Pende D, Ciccone E, Lopez-Botet M, et al. J Exp Med. 1994;180:545–555. doi: 10.1084/jem.180.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivori S, Vitale M, Bottino C, Marcenaro E, Sanseverino L, Parolini S, Moretta L, Moretta A. Eur J Immunol. 1996;26:2487–2492. doi: 10.1002/eji.1830261032. [DOI] [PubMed] [Google Scholar]
- 23.Phillips J H, Chang C W, Mattson J, Gumperz J E, Parham P, Lanier L L. Immunity. 1996;5:163–172. doi: 10.1016/s1074-7613(00)80492-6. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Villar J J, Melero I, Navarro F, Carretero M, Bellon T, Llano M, Colonna M, Geraghty D E, Lopez-Botet M. J Immunol. 1997;158:5736–5743. [PubMed] [Google Scholar]
- 25.Reyburn H T, Mandelboim O, Vales-Gomez M, Davis D M, Pazmany L, Strominger J L. Nature (London) 1997;386:514–517. doi: 10.1038/386514a0. [DOI] [PubMed] [Google Scholar]
- 26.Houchins J P, Lanier L L, Niemi E C, Phillips J H, Ryan J C. J Immunol. 1997;158:3603–3609. [PubMed] [Google Scholar]
- 27.Brooks A G, Posch P E, Scorzelli C J, Borrego F, Coligan J E. J Exp Med. 1997;185:795–800. doi: 10.1084/jem.185.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan J, Mager D, Jefferies W, Takei F. J Exp Med. 1994;180:2287–2295. doi: 10.1084/jem.180.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith H R C, Karlhofer F M, Yokoyama W M. J Immunol. 1994;153:1068–1079. [PubMed] [Google Scholar]
- 30.Karlhofer F M, Ribaudo R K, Yokoyama W M. Nature (London) 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 31.Daniels B F, Karlhofer F M, Seaman W E, Yokoyama W M. J Exp Med. 1994;180:687–692. doi: 10.1084/jem.180.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason L H, Ortaldo J R, Young H A, Kumar V, Bennett M, Anderson S K. J Exp Med. 1995;182:293–303. doi: 10.1084/jem.182.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoneman E R, Bennett M, An J, Chesnut K A, Wakeland E K, Scheerer J B, Siciliano M J, Kumar V, Mathew P A. J Exp Med. 1995;182:305–313. doi: 10.1084/jem.182.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason L H, Anderson S K, Yokoyama W M, Smith H R C, Winklerpickett R, Ortaldo J R. J Exp Med. 1996;184:2119–2128. doi: 10.1084/jem.184.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dissen E, Berg S F, Westgaard I H, Fossum S. Eur J Immunol. 1997;27:2080–2086. doi: 10.1002/eji.1830270836. [DOI] [PubMed] [Google Scholar]
- 36.Berg S F, Dissen E, Westgaard I H, Fossum S. Int Immunol. 1998;10:379–385. doi: 10.1093/intimm/10.4.379. [DOI] [PubMed] [Google Scholar]
- 37.Berg S F, Dissen E, Westgaard I H, Fossum S. Eur J Immunol. 1998;28:444–450. doi: 10.1002/(SICI)1521-4141(199802)28:02<444::AID-IMMU444>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama W M, Seaman W E. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 39.Brown M G, Fulmek S, Matsumoto K, Cho R, Lyons P A, Levy E R, Scalzo A A, Yokoyama W M. Genomics. 1997;42:16–25. doi: 10.1006/geno.1997.4721. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Cabrera M, Santis A G, Fernandez-Ruiz E, Blacher R, Esch F, Sanchez-Mateos P, Sanchez-Madrid F. J Exp Med. 1993;178:537–547. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanier L L, Chang C, Phillips J H. J Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- 42.Yabe T, McSherry C, Bach F H, Fisch P, Schall R P, Sondel P M, Houchins J P. Immunogenetics. 1993;37:455–460. doi: 10.1007/BF00222470. [DOI] [PubMed] [Google Scholar]
- 43.Karlhofer F M, Orihuela M M, Yokoyama W M. J Exp Med. 1995;181:1785–1795. doi: 10.1084/jem.181.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagasawa R, Gross J, Kanagawa O, Townsend K, Lanier L L, Chiller J, Allison J P. J Immunol. 1987;138:815–824. [PubMed] [Google Scholar]
- 45.Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T J. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 47.Boguski M S, Lowe T M, Tolstoshev C M. Nat Genet. 1993;4:332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- 48.Spiess M. Biochemistry. 1990;29:10009–10018. doi: 10.1021/bi00495a001. [DOI] [PubMed] [Google Scholar]
- 49.Drickamer K. Curr Opin Struct Biol. 1993;3:393–400. [Google Scholar]
- 50.Yokoyama W M, Ryan J C, Hunter J J, Smith H R, Stark M, Seaman W E. J Immunol. 1991;147:3229–3236. [PubMed] [Google Scholar]
- 51.Ziegler S F, Ramsdell F, Hjerrild K A, Armitage R J, Grabstein K H, Hennen K B, Farrah T, Fanslow W C, Shevach E M, Alderson M R. Eur J Immunol. 1993;23:1643–1648. doi: 10.1002/eji.1830230737. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama W M, Jacobs L B, Kanagawa O, Shevach E M, Cohen D I. J Immunol. 1989;143:1379–1386. [PubMed] [Google Scholar]
- 53.Yokoyama W M, Kehn P J, Cohen D I, Shevach E M. J Immunol. 1990;145:2353–2358. [PubMed] [Google Scholar]
- 54.Plougastel, B. & Trowsdale, J. (1998) Genomics, in press. [DOI] [PubMed]
- 55.Dissen E, Ryan J C, Seaman W E, Fossum S. J Exp Med. 1996;183:2197–2207. doi: 10.1084/jem.183.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renedo M, Arce I, Rodriguez A, Carretero M, Lanier L L, Lopez-Botet M, Fernandez-Ruiz E. Immunogenetics. 1997;46:307–311. doi: 10.1007/s002510050276. [DOI] [PubMed] [Google Scholar]
- 57.Scalzo A A, Lyons P A, Fitzgerald N A, Forbes C A, Yokoyama W M, Shellam G R. Genomics. 1995;27:435–441. doi: 10.1006/geno.1995.1074. [DOI] [PubMed] [Google Scholar]
- 58.Delano M L, Brownstein D G. J Virol. 1995;69:5875–5877. doi: 10.1128/jvi.69.9.5875-5877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vance R E, Tanamachı̄ D M, Hanker T, Raulet D H. Eur J Immunol. 1997;27:3236–3241. doi: 10.1002/eji.1830271222. [DOI] [PubMed] [Google Scholar]