Abstract
Interactions of gene therapy vectors with human blood components upon intravenous administration have a significant effect on vector efficacy and patient safety. Here we describe methods to evaluate these interactions and their effects in whole human blood, using baculovirus vectors as a model. Opsonisation of baculovirus particles by binding of IgM and C3b was demonstrated, which is likely to be the cause of the significant blood cell-associated virus that was detected. Preventing formation of the complement C5b-9 (membrane attack) complex maintained infectivity of baculovirus particles as shown by studying the effects of two specific complement inhibitors, Compstatin and a C5a receptor antagonist. Formation of macroscopic blood clots after 4 hours were prevented by both complement inhibitors. Pro- and anti-inflammatory cytokines Il-1β, IL-6, IL-8 and TNF-α were produced at variable levels between volunteers and complement inhibitors showed patient-specific effects on cytokine levels. Whilst both complement inhibitors could play a role in protecting patients from aggressive inflammatory reactions, only Compstatin maintained virus infectivity. We conclude that this ex vivo model, used here for the first time with infectious agents, is a valuable tool in evaluating human innate immune responses to gene therapy vectors or to predict the response of individual patients as part of a clinical trial or treatment. The use of complement inhibitors for therapeutic viruses should be considered on a patient-specific basis.
Keywords: Baculovirus, autographa californica, gene therapy, complement, Compstatin, C5a Receptor Antagonist
Introduction
The first environment that foreign particles or infectious agents encounter after intravenous inoculation is the innate immune system. For gene therapy strategies that involve vector administration into the bloodstream, this is likely to result in vector inactivation and eradication from the body before it has reached its target site. Immune activation could also cause significant side effects for gene therapy patients (Raper et al., 2003) and variable responses are expected between individuals. It is not possible to evaluate patient-specific responses in animal models and a further limitation is that adenovirus is predominantly cell-associated in human blood, whilst the opposite is true in mouse blood (Lyons et al., 2006). This has implications not only for differences in immune system activation, but also for the likelihood of the vector reaching its target tissue.
Viruses that are not pathogenic in humans, such as the baculovirus (BV), are becoming more attractive for use in gene therapy protocols because of the lack of pre-existing memory immune responses upon first administration. However, the complement response is activated by many viral and non-viral vectors. All three pathways of complement activation lead to generation of the opsonin C3b/iC3b, to target microbes for phagocytosis, and the membrane attack complex (MAC; C5b-9), which causes osmotic lysis of infected cells. The pro-inflammatory soluble C5b-9 (sC5b-9) and the anaphylatoxins C3a, C4a and C5a are also released.
Major advantages of BV vectors are their large insert capacity and increased safety because of an inability to replicate in mammalian cells (Stanbridge et al., 2003;van Oers, 2006). The most commonly used BV strain, Autographa californica multiple nuclear polyhedrosis virus (AcMNPV), can transduce a wide variety of tissues in vivo, if protected from the complement system (Sarkis et al., 2000;Airenne et al., 2000;Pieroni et al., 2001;Hüser et al., 2001). Complement inhibitors such as soluble complement receptor type 1 (sCR1), anti-C5 antibody and cobra venom factor (CVF, which consumes all C3 in plasma) can protect BV from inactivation in human plasma, serum or whole blood (Hofmann and Strauss, 1998;Hofmann et al., 1999;Hoare et al., 2005).
In the present study, an ex vivo whole-blood model was used to assess the role of the human innate immune response in the inactivation of BV. This model was originally developed to evaluate compatibility between blood and various biomaterials, single cells and tissues (Gong et al., 1996;Nilsson et al., 1998;Moberg et al., 2003;Goto et al., 2004). The protective effects of two novel complement inhibitors were assessed; Compstatin, a 13-residue cyclic peptide (Ac-I[CVWQDWGAHRTC]T-NH2) that inhibits the cleavage of native C3 by the C3 convertase (Sahu et al., 1996;Mallik et al., 2005) and the small cyclic hexapeptide (AcF-[OPdChaWR]) that acts as a selective C5a receptor antagonist (C5aRA) (Finch et al., 1999). The aim of using two inhibitors that act at different stages of the complement cascade was to narrow down the critical steps involved in BV inactivation.
The aims of this study were to (i) further investigate and dissect the complement pathway inactivation of BV and (ii) to demonstrate the usefulness of a human blood model to develop therapeutic strategies to abrogate destruction of systemically administered vectors. While we recognise that it is also extremely important to assess new vectors in a whole organism, the ex vivo blood loop system could even use venous blood harvested from patients a few weeks prior to their involvement in a clinical trial to get a more personalised profile for predicted responses.
Materials and Methods
Preparation of virus
The BacVector 1000 kit (Novagen) was used according to the manufacturer’s instructions with the custom-made pBAC64:CMV-EGFP transfer plasmid (pBAC4X-1 (Novagen) backbone with polh promoter and gp64 gene from pBACsurf-1 (Novagen) and the cytomegalovirus (CMV) immediate early promoter driving expression of EGFP (BD Biosciences Clontech)). Recombinant viruses were plaque purified twice and high-titre stocks were grown in sf21 insect cells, cultured in Grace’s Insect cell medium supplemented with 10% FCS (G10). These stocks were concentrated by ultracentrifugation at 24,000 rpm for 90 min at 4°C using a Beckman SW28 rotor and purified by ultracentrifugation through a sucrose gradient at 24,000 rpm in a Beckman SW41 rotor. Virus particles were washed and resuspended in PBS (approximately 1/500 starting volume). As a non-viral control for this experiment, culture supernatant from a flask of sf21 insect cells was also harvested and centrifuged according to the conditions used for virus concentration.
Tubing Loop Model
The blood donors used in this study were healthy volunteers, with fully informed consent. Incubation of whole blood with test reagents and virus was carried out in 50cm lengths of heparin coated PVC tubing (Corline, Uppsala, Sweden) with a diameter of 4mm (internal surface area 62.83cm2), closed into a loop with heparinised metal connectors. Pre-coated PVC tubing was prepared by washing with physiological saline (15mM NaCl) for at least 5 min. Using a heparin-coated tip, 4.5ml of fresh non-anticoagulated blood from one of three healthy volunteer donors was transferred into each of 6 washed PVC tubing loops, one containing Compstatin and one containing C5aRA to make final concentrations of 50μM and 5μM, respectively. Four additional 0.5ml aliquots of blood were added into polypropylene tubes containing 10μl 0.34M EDTA, one containing Compstatin and one C5aRA to mimic loop concentrations. Loops were incubated with blood in the presence of complement inhibitors for 3 min before addition of virus. Twenty-eight microlitres of test sample (either virus or cell debris suspended in PBS or PBS alone) was added to each loop and incubated on a rotating wheel that immersed the loops in a 37°C water bath. The final concentration of the virus in blood was 4.36 × 107 pfu/ml. After 15, 30, 60, 90, 120 and 240 min incubation, 500μl blood was removed and mixed with 10μl of 0.34M EDTA. After 240 min, the remaining blood was analysed by eye for the presence of macroscopic clots. Cell numbers were evaluated using a Beckman Coulter AcT diff analyzer and samples were centrifuged at 3500rpm at 4°C for 20 min to collect the plasma which was stored at −80°C.
Complement and Coagulation Analysis
Plasma concentrations of C3a and C5b-9 were evaluated after 0–90 min of incubation using a sandwich ELISA as described previously (Mollnes et al., 1985;Nilsson-Ekdahl et al., 1992). Levels of thrombin:antithrombin complex (TAT) were measured every 60 min using sandwich ELISA, based on the Enzyme Research Laboratories kit (Cat# TAT-EIA).
Antibody Binding to Baculovirus Particles
Binding of serum IgG and IgM antibodies to virus particles was detected by ELISA. Maxisorp 96 well plates (Nunc) were coated with 1.5 × 107 pfu of recombinant CMV-EGFP BV in G10 medium per well overnight at 4°C. Following 5 washes in PBS, the plate was blocked using 1% BSA (Fraction V) in PBS (1% blocking buffer) at room temperature for 1 hour. Human serum was diluted 1:50 with 1% blocking buffer and added to the plate for 1 hour at room temperature. Following 5 washes in PBS, anti-human IgM-HRP (1:50,000) or anti-human IgG-HRP (1:10,000), diluted with 1% blocking buffer, were added for 1 hour at room temperature. The plate was washed 5 times with PBS and 100μl per well of Sureblue TMB reagent (Insight Biotechnology) was added for 30 min at room temperature. The reaction was stopped by addition of 100μl of 1M HCl and the absorbance read at 450nm.
Opsonisation of Baculovirus Particles
Opsonisation by C3b/iC3b was detected by a combination of ELISA and PCR methods. Maxisorp 96 well plates (Nunc) were coated overnight at 4°C with anti-C3c antibody (Dako, detects the C3c portions of C3b and iC3b) diluted 1:1000 with PBS. Following 5 washes in PBS, blocking buffer was added for 2 hours at room temperature. Plasma extracted from virus-incubated whole blood (described in ‘virus survival in blood/serum’ section, below) was diluted 1:20 with blocking buffer and added to the plate for 1 hour at room temperature. Following 5 washes in PBS, 50μl of virus lysis buffer (10mM Tris-HCl pH 8.3, 100μg/ml gelatin, 0.45% Triton X-100, 0.45% Tween 20, 50mM KCl, 60μg/ml proteinase K) was added and incubated at 65°C for 1 hour, then 95°C for 10 min. BV particles were detected by PCR using primers that amplify 308 bp of the virus PCNA gene (AcMNPV PCNA for: 5′ GTGTGCGTTACGGTTTGCTT 3′, AcMNPV PCNA rev: 5′ GATGTGATGGCGTTCGTGTT 3′). Each PCR reaction contained 1 x reaction buffer (Invitrogen), 2.5mM MgCl2, 100μM dNTPs, 0.5μM for and rev primers, 1 unit of Taq polymerase (Invitrogen) and 2μl of the lysed samples. Reactions were denatured at 94°C for 5min followed by 35 cycles of 94°C 30sec, 62°C 30sec, 72°C 30sec, and a final elongation step of 7 min. Products were electrophoresed through 2% agarose.
Baculovirus association with Human Blood Cells
Virus particle location within blood in the presence and absence of Compstatin was determined using semi-quantitative PCR on DNA extracted from separated blood components. Freshly drawn whole blood from volunteer C was mixed with 20μg/ml Hirudin Fragment 54–65 non-sulfated (Sigma H6769) to prevent coagulation. Compstatin was added to one 2.5ml aliquot of the blood to a final concentration of 50μM and to this, and a further 2.5ml aliquot of blood, 2.2 × 108 pfu/ml BV was added. A further 2.5ml of blood served as a negative control. Each sample was incubated for 1 hour whilst rotating. Two millilitres of each sample was diluted 1:1 with PBS, layered onto 3ml Lymphocyte Separation Medium (LSM, ICN) and centrifuged at 400g for 30 min at room temperature. The plasma layer was retained separately. The mononuclear cell layer was washed twice in PBS and resuspended in 1ml PBS (half original blood volume). The erythrocyte/granulocyte layer was resuspended in 4ml PBS (twice original blood volume). Twenty microlitres of each sample was mixed with 200μl Instagene™ Matrix (Bio-Rad) and incubated at 56°C for 20 min, then 100°C for 8 min. Following centrifugation at 13,000 rpm for 1 min in a microfuge, supernatant was harvested and stored at 4°C. PCR was performed using primers that amplify 195 bp of the viral vp39 gene (AcMNPV VP39 for 5′ ATGCCTAGCGATCGTCAT 3′, AcMNPV VP39 rev 5′ CGCTGCATTTTCGCGTCCAT 3′), using the same reaction conditions as described for PCNA except that only 30 cycles were performed and the annealing step was performed at 50°C. Ten microlitres of each reaction were electrophoresed through 2% agarose containing ethidium bromide, visualised using a GeneGenius Bioimaging System and analysed using GeneTools (both Syngene) to measure band intensity (peak height).
Baculovirus Vector Inactivation by Whole Blood
The presence of infectious BV in plasma was assessed using plaque assay. Serial dilutions (10−2 to 10−5) of plasma extracted from the loops after 15, 90 and 240 min were made in G10 medium containing 100U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml Fungizone and added to triplicate plates containing 1×106 sf21 cells. The average number of visible plaques after 5 days was used to determine the number of plaque forming units remaining in the plasma.
Cytokine Release
Concentrations of pro- and anti-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-a) were measured in undiluted plasma harvested from the 2 and 4 hour timepoint samples using the CBA human inflammation kit (BD) according to the manufacturers instructions.
Results
The BV preparations used in this study were gradient purified but may still contain low levels of insect cell-derived contaminants. To control for this, we looked at immune activation by extracts of conditioned insect cell supernatant, similar to that from which the virus was harvested. Total protein content was measured in the stocks of gradient purified BV and cell debris using the Coomassie (Bradford) Protein Assay Kit (Pierce), which confirmed that there was 5 times more protein in the cell debris control than in the BV stock solution (results not shown). Therefore, any increase in immune activation by BV compared to cell debris was not due to higher concentration of the BV preparation.
Baculovirus Activates the Complement Cascade within 15 minutes
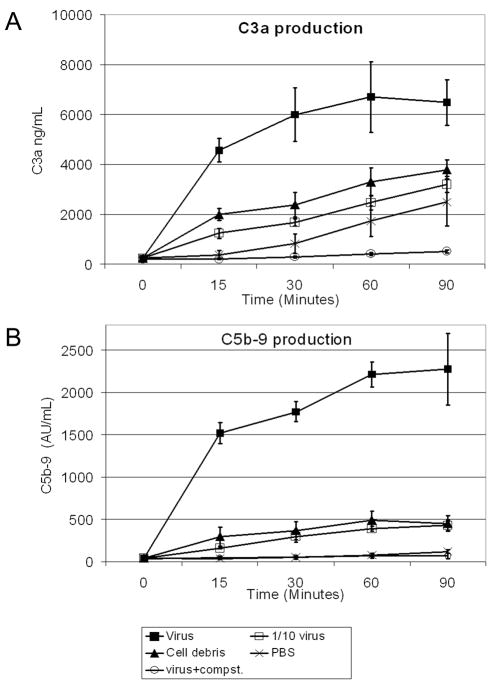
Activation of complement was investigated by measuring plasma concentrations of the C3 cleavage product, C3a. C3a was produced in response to the higher concentration of virus within 15 minutes and increased during the 90 minutes of incubation (Figure 1a). Students T-test carried out at the 30 minute timepoint showed that in comparison to the cell debris control, C3a production was significantly higher in the loops inoculated with virus alone (p<0.01) and significantly lower in the loops inoculated with virus+Compstatin (p<0.005). The lower (1/10) concentration of virus did not activate complement above the level shown by the cell debris control. C3a levels increased in all loops at a similar rate after the initial 15 minute timepoint, except for the loop containing Compstatin, which increased at a much slower rate.
Figure 1.
Complement activation by baculovirus as determined by ELISA for C3a (A) and C5b-9 (B). Each data point represents the mean of three volunteers and the error bars represent 1 standard deviation. Filled squares = virus; open squares = 10-fold diluted virus; triangles = cell debris; crosses = PBS; open circles = virus plus Compstatin (Compst).
The final step in the complement pathway is the formation of the membrane attack complex (sC5b-9). sC5b-9 was produced in response to virus within 15 minutes and increased during the 90 minutes of incubation. BV-induced sC5b-9 production was inhibited in the presence of Compstatin. Students T-test carried out on the data from the 30 minute timepoint showed a significant increase in sC5b-9 in the loops containing virus compared to the cell debris control (p<0.01) and a decrease in the virus+Compstatin containing loop (p<0.05).
Baculovirus Particles Interact with Human Blood Proteins and Cells
When a virus is injected intravenously, it comes into contact with the many cells and proteins that constitute whole blood. It is therefore essential to look at direct interaction of the virus with blood components which could affect virus trafficking and function, such as antibodies, opsonins and blood cells.
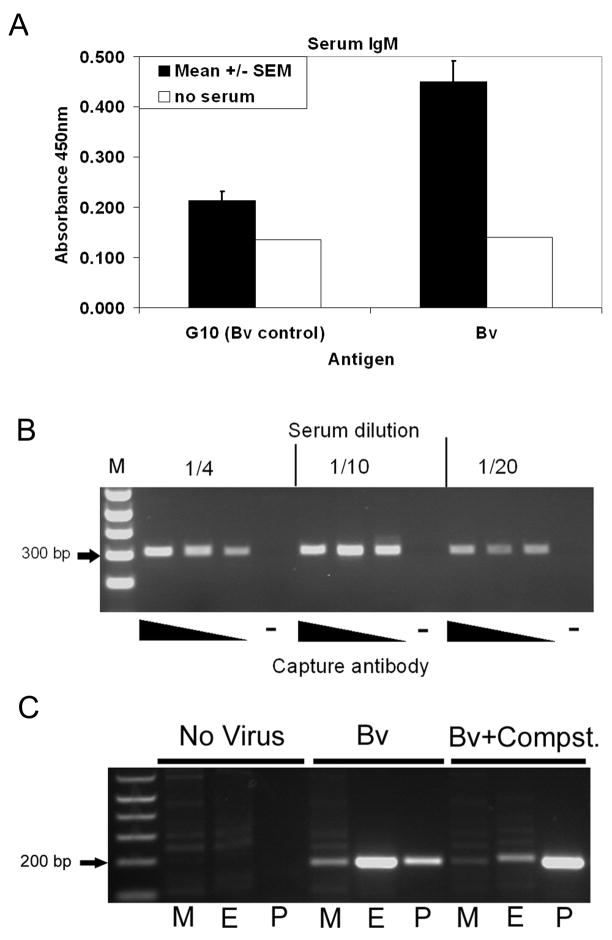
Evaluation of BV-binding IgG and IgM antibodies in the serum of three volunteers showed no IgG-binding (data not shown) but detectable IgM antibodies were detected in all three volunteers (figure 2a).
Figure 2.
(A) Presence of IgM antibodies that bind to BV particles in human serum as shown using ELISA. G10 is the medium in which BV is suspended. Bars represent the mean of three volunteers and the error bars represent 1 standard error of the mean. (B) Presence of BV particles in complex with c3b in the serum of volunteer C as detected by a combined ELISA and PCR method, using an immobilised capture antibody that binds C3b followed by BV-specific PCR. (C) Location of virus particles in separated whole blood as shown by BV-specific PCR. M=mononuclear cell fraction, E=erythrocyte fraction, P=plasma, Compst. = Compstatin.
The C3 cleavage product, C3b, can act as an opsonin, targeting foreign particles in blood for degradation by phagocytic cells such as neutrophils and monocytes/macrophages. Whether C3b binds to BV particles has not previously been shown. Figure 2b shows that BV was detected in complex with C3b in serum extracted from whole blood that had been pre-incubated with BV for 60 minutes. No binding of virus to the ELISA plate was detected in the absence of capture antibody, demonstrating an effective blocking step.
Opsonins and antibodies in combination can target pathogens to bind to erythrocytes. A semi-quantitative PCR method was used to determine the location of BV particles following 60 minutes incubation with whole human blood in the presence and absence of Compstatin. Figure 2c shows that in untreated human blood, the majority of virus particles are detected in association with the erythrocyte/granulocyte cell fraction, with a small amount free in the plasma and the smallest proportion in association with the lymphocyte/mononuclear cell fraction. In the presence of Compstatin however, the majority of virus particles are detected free in the plasma, implicating a product in the complement pathway downstream of C3 cleavage in the process of directing attachment of BV to blood cells.
Release of Inflammatory Cytokines in Response to BV
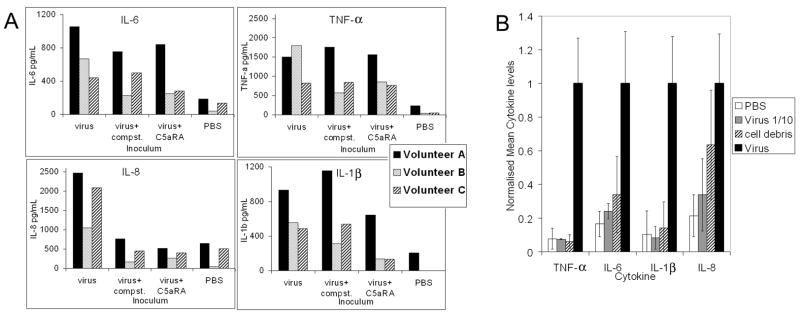
Plasma levels of IL-1β, IL-6, IL-8, IL10, IL-12p70 and TNF-α were assessed after 2 and 4 hours of virus incubation with blood, in the presence and absence of complement inhibitors (figure 3). IL-6, IL-8 and TNF-α were detectable after 2 hours and were much stronger after 4 hours in the virus-containing loops. IL-1β was also produced after 4 hours. IL-10 and IL-12p70 were not detected within the 4 hour time period of this study in any of the loops. The measured concentration of cytokines varied between volunteers and the effect of the complement inhibitors was also volunteer dependent (figure 3a). The production of IL-8 was inhibited in the presence of either complement inhibitor in all three volunteers and at both time points. In volunteer B, TNF-α was inhibited 2 to 3-fold in the presence of either complement inhibitor but was not inhibited at all in volunteers A and C. Levels of IL-6 were lower (although to varying degrees) in the presence of either complement inhibitor after 2 and 4 hours in all three volunteers, with the exception of volunteer C with Compstatin at 4 hours. IL-1β was not detected after 2 hours. At the 4 hour time point, the presence of C5aRA resulted in a reduction of IL-1β in all three volunteers to varying degrees, whereas Compstatin only had an effect on volunteer B.
Figure 3.
(A) Circulating cytokine levels 4h post-inoculation with baculovirus and control samples as detected using the CBA human inflammation kit (BD Biosciences) in the presence and absence of complement inhibitors. Levels of IL-10 and IL-12p70 were undetectable in all samples. Black bars represent data from volunteer A, spotted bars from volunteer B and striped bars from volunteer C. (B) Mean cytokine levels from all three volunteers normalised to the neat virus samples. White bars represent loops containing PBS, grey bars represent 10-fold diluted virus, striped bars represent cell debris and black bars represent virus.
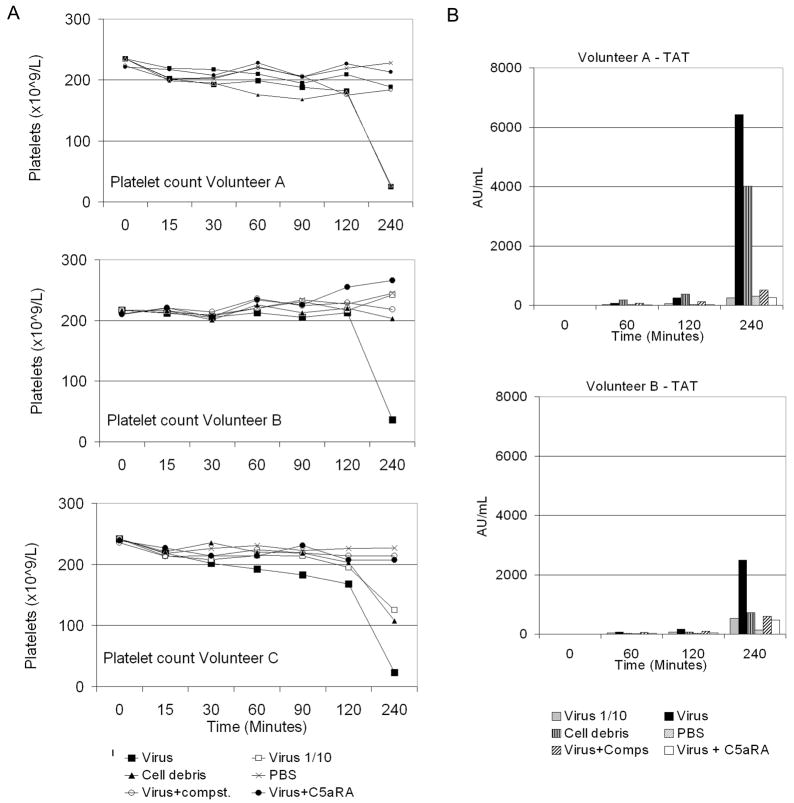
Low levels of cytokines were observed in response to the cell debris control and the 10-fold diluted virus, with no more than two-fold difference between the two samples, except in volunteer A for IL-6 and IL-8, where levels were 2.6 and 4.6-fold greater than those produced in response to 10−1 virus, respectively (results not shown). Referring to figure 4, the blood of this volunteer also clotted in response to cell debris whereas that of volunteers B and C did not.
Figure 4.
Blood clotting was assessed over time. (A) Free platelets present in the blood were counted. Large squares = virus; small squares = 10-fold diluted virus; triangles = cell debris; crosses = PBS; open circles = virus plus Compstatin (Compst.) and filled circles = virus plus C5aRA. (B) Production of thrombin:antithrombin (TAT) complexes was measured using ELISA. Grey bars represent 10-fold diluted virus; black bars = undiluted virus; vertical striped bars = cell debris; spotted bars = PBS; diagonal striped bars = virus plus Compstatin (Comps) and white bars = virus plus C5aRA. The TAT assay failed for volunteer C.
Baculovirus Activation of the Clotting Cascade is Prevented by Inhibitors of Complement
The concentration of platelets was recorded for each volunteer before adding the blood to the loops and at each sample time point in order to monitor clot formation. After 4 hours, analysis of the remaining blood by eye revealed macroscopic clots in the loops incubated with concentrated BV in all three volunteers. In the case of volunteer A, a clot was also observed in the loop containing cell debris after 4 hours. This correlates with the drop in platelet numbers observed in these loops (Figure 4a). Formation of thrombin:antithrombin (TAT) complexes was measured for volunteers A and B. Interestingly, the level of TAT complex formation was lower in volunteer B than A in response to concentrated virus (Figure 4b). Moderate production of TAT complexes was observed after 120 minutes for both volunteers in the loop containing BV incubated in the presence of Compstatin. However, by 240 minutes, levels were similar to loops containing 1/10 dilution of BV and BV plus C5aRA, all substantially lower than that in loops containing undiluted BV alone.
Baculovirus Inactivation in Human Blood is Partially Rescued by Compstatin
Plasma recovered from the loops at the various different time points was analysed for the presence of infectious virus particles by plaque assay (Table 1). The plaque assays were carried out in triplicate for each volunteer at each timepoint. The results are expressed as the percentage of input virus that is still present in the plasma and infectious at each timepoint. The data shows that very low levels of infectious virus (<0.5% input) were present in the plasma after 15 minutes incubation with whole blood, except in the presence of Compstatin where levels were markedly higher (between 7% and 50% input). The amount of infectious virus decreased over time in all measurable samples.
Table 1.
Percentage of input BV that is present in the plasma and infectious after 15, 90 and 240 minutes incubation with whole blood, in the presence and absence of complement inhibitors, as measured using plaque assay on insect sf21 cells.a
| Survival of infectious baculovirus | ||||
|---|---|---|---|---|
| Virus Survival in Blood | 15 min | 90 min | 240 min | |
| Volunteer A | Virus | 0.29% | Trace | 0% |
| Virus + Compstatin | 50.00% | 9.43% | 3.64% | |
| Virus + C5aRA | 0.05% | 0% | 0% | |
| Volunteer B | Virus | Trace | 0% | 0% |
| Virus + Compstatin | 7.36% | 2.93% | 1.85% | |
| Virus + C5aRA | Trace | Trace | Trace | |
| Volunteer C | Virus | Trace | 0% | 0% |
| Virus + Compstatin | 13.14% | 4.64% | 2.71% | |
| Virus + C5aRA | Trace | 0% | 0% | |
Trace < 0.0001%
Discussion
The use of animal models in the study of disease and therapy is extremely valuable, but notable differences between these models and humans are widely acknowledged. Whilst animal models allow the study of immunity in the setting of a whole organism, we can not expect that the results accurately reflect what would happen in humans. The use of an ex vivo human blood loop system, as described here, in conjunction with whole-organism models is a valuable tool to aid evaluation of innate immune responses to gene therapy vectors. In this study a range of innate immune responses to a novel gene therapy vector, the recombinant BV, were evaluated.
The number of virus particles (rather than plaque forming units) is likely to be critical in determining the immune response. Semi-quantitative PCR (L. J. Georgopoulos, et al, manuscript in preparation) has indicated that the particle:pfu ratio of a similar preparation of sucrose-gradient purified BV, was 250 particles per plaque forming unit (pfu). Therefore, we estimate that the loops contained approximately 1 × 1010 particles per millilitre. The highest plasma level of adenovirus that has been achieved in clinical trials is around 7.5 × 109 particles/ml (Cichon et al., 2001), i.e. comparable with the BV concentrations used in this study.
Vector inactivation in the blood remains a major hurdle to overcome in gene therapy where intravenous administration is required, such as for metastatic cancer. Complement has been shown to be a major factor in inactivation of many gene therapy vectors, therefore we have investigated the effects of two complement inhibitors on BV survival as well as inflammatory reactions and virus:blood cell interactions, upon inoculation into human blood. The two complement inhibitors used in this study could be added as co-inocula with BV. Compstatin has been systemically delivered into baboons, giving complete complement inhibition whilst not affecting heart rate or blood pressure (Soulika et al., 2000) and an analogue of Compstatin has recently been approved for clinical trials for the treatment of age-related macular degeneration. The C5aRA (AcF-[OPdChaWR]) has recently been used in a clinical trial for rheumatoid arthritis, with no serious adverse events or abnormalities in haematological findings (Vergunst et al., 2007). These two inhibitors act on different complement proteins, enabling further break-down of the critical factors involved in vector inactivation.
The classical complement pathway can be activated by IgM or IgG and our demonstration of IgM binding to BV complements earlier studies (Hoare et al., 2005), who showed that depletion of serum IgM enhanced BV survival in vitro. No BV-binding IgG was detected (data not shown), not unexpectedly, since this is a non-human pathogen and should not have been previously encountered by the immune system.
C3b/iC3b:BV immune complexes were detected in plasma extracted from whole blood that had been pre-incubated with BV. This is the first demonstration that C3b/iC3b opsonises BV particles. Since one function of C3b/iC3b is to target foreign bodies for destruction by cells, it was hypothesised that a proportion of BV is also cell-associated. This hypothesis was supported by the PCR detection of BV particles in multiple blood compartments after incubation with virus, including the erythrocyte/granulocyte cell pellet, the monocyte/platelet cell layer as well as free in the plasma. Many cell types in blood, including erythrocytes, granulocytes and monocytes, express receptors that bind immunoglobulins and C3b/iC3b, therefore opsonisation could mediate BV association with these cells in our experiments. Indeed, in the presence of the C3 inhibitor, Compstatin, a higher proportion of the virus was found to be free in the plasma rather than cell-associated, indicating that a component of the complement pathway downstream of C3 cleavage is involved in mediating a large proportion of the cell association.
To determine whether the plasma BV was infectious or not, plaque assays were carried out on plasma from which all cells had been removed by centrifugation, clearly showing that Compstatin, but not C5aRA, increases the numbers of infectious particles free in the plasma. Hofmann and Strauss (1998) previously showed that an anti-complement C5 antibody can protect BV from inactivation in human serum, but did not distinguish whether it was the actions of C5a or C5b following C5 cleavage, that was responsible for BV inactivation. Our use of a specific C5a inhibitor has shown that the actions of C5a are not responsible for inactivation of BV in human blood suggesting that it is the actions of C5b, which is involved in formation of the C5b-9 membrane attack complex, that are critical. C5a may still play a role as an anaphylatoxin in clearing any remaining virus from the bloodstream by promoting an inflammatory and immunological response, but it seems likely from our data that direct inactivation by complement is more significant in short-term circulation. Although Compstatin conferred some protection on the BV particles in blood, a substantial amount of the input infectious virus had been lost by 15 minutes in the Compstatin-treated samples, despite no activation of complement. Some explanations for this loss are offered here. When the plaque assays were carried out, the plasma itself had been through 5–7 freeze/thaw cycles after the analysis of complement and cytokines, and some virus may have been lost through this process. In addition, PCR detection showed that some BV was attached to the washed PVC tubing at the end of the 4 hour incubation (data not shown), and a small amount was found in association with blood cells after 1 hour, which could both also account for a proportion of the loss of BV over time.
It was previously shown in the absence of blood plasma that BV induces production of certain inflammatory cytokines by cultured murine peritoneal macrophages and splenic CD11c+ dendritic cells (DCs), as well as a murine macrophage cell line RAW264.7, via the Toll-like receptor 9 pathway (Abe et al., 2005). Activation of murine macrophages by BV has also been described (Abe et al., 2003). Here, in the presence of whole blood, the induction of IL-6, IL-8, IL-1β and TNF-α was found in all three volunteers. IL-8 has been shown to be intrinsically linked with coagulation (reviewed in Schoenmakers et al., 2005), which supports our data where both coagulation and IL-8 production were completely inhibited by both Compstatin and C5aRA.
After 4 hours incubation, all three individuals produced macroscopic blood clots in response to virus, except in the presence of Compstatin or C5aRA. Since clotting was prevented in the presence of C5aRA, coagulation activation is not dependent on formation of C5b-9, but a role for the anaphylatoxins in this process is implied. Since coagulation could pose a serious threat to patients, and was implicated in a death during a pilot study of gene therapy (Raper et al., 2003), use of complement inhibitors could play an essential role in protecting the patient as well as the vector.
The lower dose of virus used in this study showed substantially lower levels of immune activation. The question of patient dosage therefore has to be analysed carefully to strike the right balance between vector efficacy and immune responses, and this may be different between individuals as indicated with the small number of volunteers in this study.
This study identifies Compstatin as a promising candidate to be included in proposed clinical treatments using BV, since it not only conferred protection to infectivity of BV in human blood but also showed signs that it could protect the patient by reducing complement, inflammation and clotting. It would be interesting to incorporate the cyclic Compstatin peptide into the BV envelope gp64 protein to see whether the protective effect is sustained, in the same way as decay accelerating factor (DAF) was used previously (Hüser et al., 2001). The development and screening of these and other future vectors will benefit from the blood loop model and associated assays. We propose that this assay should be used in combination with animal models to test new vectors (viral and non-viral), alternative methods of complement inhibition or to evaluate the effects of vector modification or coating.
Also highlighted by this study was the variation in responses between volunteers, particularly with reference to the cytokine data. Therefore, this loop system will also be valuable at the clinical trial stage for pre-screening individual patients prior to vector administration, since data regarding complement activation, cytokine release and vector inactivation can be rapidly generated.
Acknowledgments
We would like to thank Rolf Larsson and Magnus Essand (Rudbeck Laboratory), for helpful discussions on the blood loop system and Jaan Hong (Rudbeck Laboratory), Alexander Turner and Mary Garthwaite (University of York) for taking blood used in the experiments. Thanks also to Susanne Lindblom and Kristina Nilsson Ekdahl (Rudbeck Laboratory) for technical help. This work was funded by grants under FP5 (PIG) and FP6 (GIANT) from the European Union, National Institute of Health grants 5-R01-EB-003968, GM-62134, GM069736, the Swedish Research Council (K2007-65X-05647-28-3; K2004-71P-15244-01A) and a program grant support (NJ Maitland) from Yorkshire Cancer Research. The blood donors used in this study were healthy volunteers, who read written information and thereafter agreed to take part.
Footnotes
Author Disclosure Statement
J. D. Lambris, along with the University of Pennsylvania, have one issued and two pending patents on Compstatin. These patents have been licensed for ophthalmic indications to Potentia Pharmaceuticals, Inc. J. D. Lambris is also a non-equity-holding member of the Scientific Advisory Board of Potentia Pharmaceuticals. No competing financial interests exist for any other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Hemmi H, Miyamato H, Moriishi K, Tamura S, Takaku H, Akira S, Matsuura Y. Involvement of the toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol. 2005;79:2847–2858. doi: 10.1128/JVI.79.5.2847-2858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y, Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- Airenne KJ, Hiltunen MO, Turunen MP, Turunen AM, Laitinen OH, Kulomaa MS, Ylä-Herttuala S. Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 2000;7:1499–1504. doi: 10.1038/sj.gt.3301269. [DOI] [PubMed] [Google Scholar]
- Cichon G, Boeckh-Herwig S, Schmidt HH, Wehnes E, Muller T, Pring-Akerblom P, Burger R. Complement activation by recombinant adenoviruses. Gene Ther. 2001;8:1794–1800. doi: 10.1038/sj.gt.3301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- Gong J, Larsson R, Ekdahl KN, Mollnes TE, Nilsson U, Nilsson B. Tubing loops as a model for cardiopulmonary bypass circuits: both the biomaterial and the blood-gas interfaces induce complement activation in an in vitro model. J Clin Immunol. 1996;16:222–229. doi: 10.1007/BF01541228. [DOI] [PubMed] [Google Scholar]
- Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77:741–747. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- Gronowski AM, Hilbert DM, Sheehan KCF, Garotta G, Schreiber RD. Baculovirus stimulates antiviral effects in mammalian cells. J Virol. 1999;73:9944–9951. doi: 10.1128/jvi.73.12.9944-9951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Waddington S, Thomas HC, Coutelle C, McGarvey MJ. Complement inhibition rescued mice allowing observation of transgene expression following intraportal delivery of baculovirus in mice. J Gene Med. 2005;7:325–333. doi: 10.1002/jgm.671. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Hüser A, Lehnert W, Strauss M. Protection of baculovirus-vectors against complement-mediated inactivation by recombinant soluble complement receptor type 1. Biol Chem. 1999;380:393–395. doi: 10.1515/BC.1999.052. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Strauss M. Baculovirus-mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 1998;5:531–536. doi: 10.1038/sj.gt.3300607. [DOI] [PubMed] [Google Scholar]
- Hüser A, Rudolph M, Hofmann C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol. 2001;19:451–455. doi: 10.1038/88122. [DOI] [PubMed] [Google Scholar]
- Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, Bazan-Peregrino M, Phipps S, Hale S, Mautner V, Seymour LW, Fisher KD. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Molecular Therapy. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Mallik B, Katragadda M, Spruce LA, Carafides C, Tsokos CG, Morikis D, Lambris JD. Design and NMR characterization of active analogs of compstatin containing non-natural amino acids. J Med Chem. 2005;48:274–286. doi: 10.1021/jm0495531. [DOI] [PubMed] [Google Scholar]
- Moberg L, Olsson A, Berne C, Felldin M, Foss A, Kallen R, Salmela K, Tibell A, Tufveson G, Nilsson B, Korsgren O. Nicotinamide inhibits tissue factor expression in isolated human pancreatic islets: implications for clinical islet transplantation. Transplantation. 2003;76:1285–1288. doi: 10.1097/01.TP.0000098905.86445.0F. [DOI] [PubMed] [Google Scholar]
- Mollnes TE, Lea T, Froland SS, Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbant assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985;22:197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- Nilsson B, Larsson R, Hong J, Elgue G, Nilsson Ekdahl K, Sahu A, Lambris JD. Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation. Blood. 1998;92:1661–1667. [PubMed] [Google Scholar]
- Nilsson-Ekdahl K, Nilsson B, Pekna M, Nilsson UR. Generation of iC3 at the interface between blood and gas. Scand J Immunol. 1992;35:85–91. doi: 10.1111/j.1365-3083.1992.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Pieroni L, Maione D, La Monica N. In vivo gene transfer in mouse skeletal muscle mediated by baculovirus vectors. Hum Gene Ther. 2001;12:871–881. doi: 10.1089/104303401750195845. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- Sarkis C, Serguera C, Petres S, Buchet D, Ridet JL, Edelman L, Mallet J. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc Natl Acad Sci U S A. 2000;97:14638–14643. doi: 10.1073/pnas.260472897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers SHHF, Retisma PH, Spek CA. Blood coagulation factors as inflammatory mediators. Blood Cells, Molecules and Diseases. 2005;34:30–37. doi: 10.1016/j.bcmd.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Soulika AM, Khan MM, Hattori T, Bowen FW, Richardson BA, Hack CE, Sahu A, Edmunds LHJ, Lambris JD. Inhibition of heparin/protamine complex-induced complement activation by Compstatin in baboons. Clin Immunol. 2000;96:212–221. doi: 10.1006/clim.2000.4903. [DOI] [PubMed] [Google Scholar]
- Stanbridge LJ, Dussupt V, Maitland NJ. Baculoviruses as vectors for gene therapy against human prostate cancer. Journal of Biomedicine and Biotechnology. 2003;2003:79–91. doi: 10.1155/S1110724303209049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers MM. Vaccines for viral and parasitic diseases produced with baculovirus vectors. Adv Virus Res. 2006;68:193–253. doi: 10.1016/S0065-3527(06)68006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergunst CE, Gerlag DM, Dinant H, Schulz L, Vinkenoog M, Smeets TJM, Sanders ME, Reedquist KA, Tak PP. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology. 2007;46:1773–1778. doi: 10.1093/rheumatology/kem222. [DOI] [PubMed] [Google Scholar]