Abstract
The C-1027 enediyne antitumor antibiotic from Streptomyces globisporus possesses an (S)-3-chloro-5-hydroxy-β-tyrosine moiety, the chloro- and hydroxy- substituents of which are installed by a flavin-dependent halogenase SgcC3 and monooxygenase SgcC, respectively. Interestingly, a single flavin reductase, SgcE6, can provide reduced flavin to both enzymes. Bioinformatics analysis reveals that, similar to other flavin reductases involved in natural product biosynthesis, SgcE6 belongs to the HpaC-like subfamily of the Class I flavin reductases. The present study describes the steady-state kinetic characterization of SgcE6 as a strictly NADH- and FAD-specific enzyme.
Keywords: C-1027, enediyne, flavin reductase, halogenase, monooxygenase, SgcE6
Introduction
The enediynes are a potent family of antitumor antibiotics possessing an enediyne core to which several peripheral moieties are attached. The enediyne core can undergo Bergman or Myer-Saito cyclization to generate a benzenoid diradical capable of abstracting protons from DNA, causing double-strand breaks and cross-linking that ultimately leads to cell death (Galm et al., 2005; Van Lanen & Shen, 2008). Although the enediyne core is the bioactive warhead of the molecule, the peripheral moieties also play important functional roles. For example, the (S)-3-chloro-5-hydroxy-β-tyrosine moiety of C-1027 enediyne antitumor antibiotic modulates the reactivity of the enediyne core, and C-1027 analogs lacking the chloro- or hydroxy-substituents of this moiety exhibit modified bioactivity (Kennedy et al., 2007a; Kennedy et al., 2007b).
Biosynthesis of the β-tyrosine moiety of C-1027 in Streptomyces globisporus proceeds from tyrosine via an aminomutase reaction, adenylation, then chlorination and hydroxylation prior to being appended to the enediyne core by a condensation enzyme (Figure 1) (Lin et al., 2009). The chlorination and hydroxylation reactions have been characterized, and each is catalyzed by a two-component system comprised of either a flavin-dependent halogenase (SgcC3) (Lin et al., 2007) or monooxygenase (SgcC) (Lin et al., 2008) component that obtains reduced flavin (FADH2) from a separate flavin reductase component (SgcE6). Moreover, the E. coli flavin reductase (Fre) could also be substituted for SgcE6 without impacting the activities of SgcC3 or SgcC, implying that SgcC/C3 obtain FADH2 by diffusion rather than delivery by SgcE6 via specific protein-protein interactions. Diffusion of reduced flavin has been proposed in the HpaB-HpaC two-component system to be coordinated by a high intracellular concentration and flavin-binding affinity of the oxygenase component (Louie et al., 2003), while evidence for protein-protein interaction has been obtained in the PrnF-PrnD system (Lee & Zhao, 2007). The production of diffusible FADH2 as opposed to the requirement of specific protein-protein interactions during C-1027 biosynthesis is consistent with the observation that SgcE6 is the only flavin reductase in the gene cluster and therefore must serve all flavin-dependent enzymes for the C-1027 pathway in S. globisporus.
Figure 1.
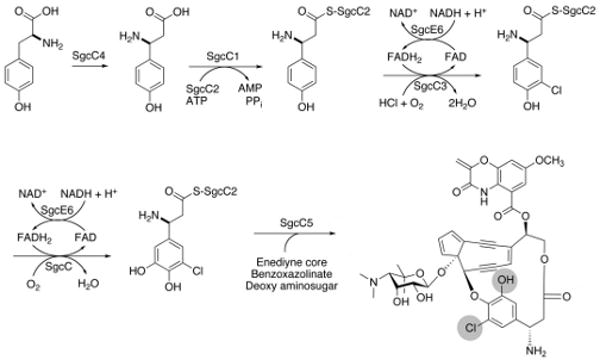
The biosynthetic transformation of L-tyrosine into the (S)-3-chloro-5-hydroxy-β-tyrosine moiety of the C-1027 enediyne chromophore in S. globisporus.
Flavin reductases use NAD(P)H to generate reduced FAD, FMN, or riboflavin. Although flavin reduction is often coupled with substrate oxidation in a single active site, there are many examples of two component systems that use a discrete flavin reductase enzyme (van Berkel et al., 2006). Flavin reductases have been classified based on whether flavin is used as a substrate or is tightly bound to the enzyme as a prosthetic group (Galan et al., 2000). The former group, Class I flavin reductases, is further divided into at least three families each exemplified by (i) E. coli Fre, (ii) FRase I from Vibrio fischeri, and (iii) E. coli HpaC (Galan et al., 2000). The HpaC-like subfamily was only recently discovered and possesses two consensus motifs (i) a Ser, Thr, or Cys prior to a pair of Pro residues near the N-terminus and (ii) a GDH motif located near the C-terminus (Galan et al., 2000). Interestingly, several flavin reductases proposed to be involved in antibiotic biosynthesis belong to the HpaC-like subfamily, and a crystal structure has been described (van den Heuvel et al., 2004).
Our previous work demonstrated that SgcE6 functions to supply diffusible FADH2 to SgcC3 and SgcC (Lin et al., 2007; Lin et al., 2008), but the ability of SgcE6 to use FMN or NADPH was not investigated. Herein we (i) investigate the substrate preference and steady-state kinetics of SgcE6 and (ii) use bioinformatics analysis to assign SgcE6 to the HpaC-like subfamily of Class I flavin reductases.
Materials and Methods
Chemicals
Flavin adenine dinucleotide disodium salt (FAD), flavin mononucleotide sodium salt (FMN), β-nicotinamide adenine dinucleotide reduced disodium salt (NADH), and NADPH were purchased from Sigma-Adrich (St. Louis, MO).
Proteins
SgcE6 was overproduced in E. coli and purified as previously described (Lin et al., 2007).
SgcE6 Activity Assays
All assays were performed in 10 mM Tris-Cl, pH 7.5. The specific activity of the enzyme towards different substrates was examined using 160 μM NAD(P)H, 100 μM FAD or FMN (if added), and 0.5 μg of SgcE6 at room temperature in a final volume of 100 μL. The oxidation of NAD(P)H was detected by monitoring the absorbance at 340 nm versus time (ε340 6,220 M-1 cm-1) (Fontecave et al., 1987). Steady-state kinetic assays were performed under the same conditions but using 176 nM SgcE6 and using variable concentrations of either FAD or NADH. The steady-state kinetic parameters were obtained by non-linear regression to fit the Michaelis-Menten equation to a plot of the initial velocity versus substrate concentration data using the Kaleidagraph software (Synergy Software, Reading, PA).
Bioinformatics Analysis
Amino acid sequences of HpaC-like enzymes and SgcE6 were retrieved from the NCBI server and subjected to multiple sequence alignment using the Clustal X software version 1.83. The percent sequence identity and similarity of selected flavin reductases with SgcE6 were determined by pairwise BLAST alignment on the NCBI server.
Results and Discussion
Bioinformatics Analysis
Amino acid sequence alignment with several other flavin reductases revealed that SgcE6 shares low sequence homology with Fre from E. coli (11% identity and 23% similarity) or FRaseI from V. fischeri (11% identity and 22% similarity), but significant sequence identity (>25%) with members of the HpaC-like family (Figure 2). Moreover, SgcE6 possesses the S/T/CxxPP and GDH consensus motifs characteristic of the HpaC-like subfamily of the Class I flavin reductases. Therefore SgcE6 was annotated as a member of the HpaC-like subfamily of the Class I flavin reductases, a subfamily which includes several other flavin reductases involved in the biosynthesis of antibiotics.
Figure 2.
Multiple amino acid sequence alignment of several Class I flavin reductases. Abbreviations, GenBank accession numbers, and % sequence identity/similarity to SgcE6 are as follows: SgcE6 (AAL06698); PheA2, phenol 2-hydroxylase component B from B. thermoglucosidasius A7 (AAF66547, 35/56); EC-HpaC, 4-hydroxyphenylacetate hydroxylase component C from E. coli (CAA82322, 26/46); SV-VlmR, flavin reductase involved in valanimycin biosynthesis in Streptomyces viridifaciens (AAC45645, 28/46); SC-ActVB, flavin reductase component B involved in actinorhodin biosynthesis in Streptomyces coelicolor (CAA45048, 40/51); RebF, flavin reductase involved in rebeccamycin biosynthesis (CAC93720, 34/48); KtzS, flavin reductase involved in kutzneride biosynthesis (ABV56599, 33/46); BC-TftC, flavin reductase component of chlorophenol 4-hydroxylase from Burkholderia cepacia (AAC23547, 27/43); EC-Fre, E. coli flavin reductase (AAA23806, 11/23); VF-FRaseI, flavin reductase from Vibrio fischeri (BAA04595, 11/22). Shaded residues are conserved among the HpaC-like subfamily, and the boxed regions denote subfamily consensus motifs.
Substrate Preference of SgcE6
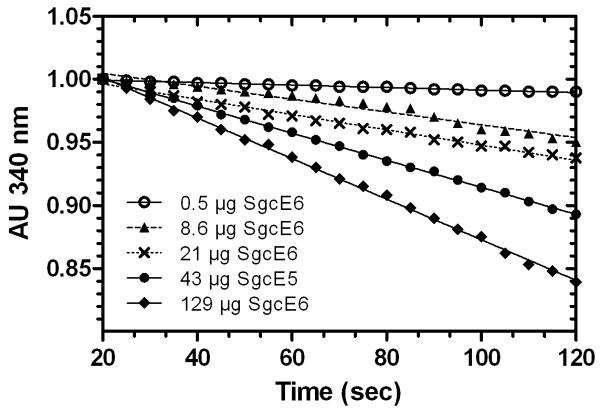
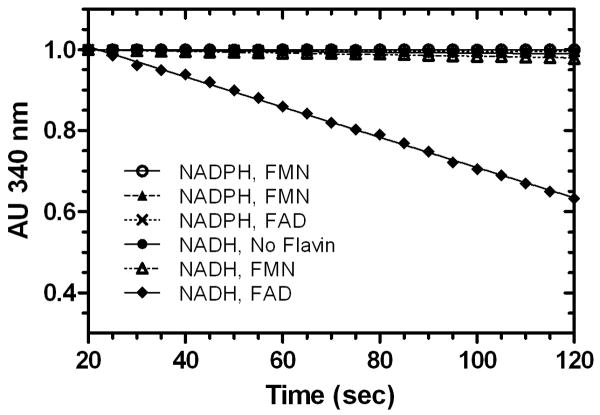
The rate of consumption of NADH in the presence of FAD was shown to be dependent on SgcE6 concentration, consistent with enzymatic catalysis (Figure 3). The substrate preference of SgcE6 was then probed by comparing the relative activities toward each possible combination of reductant (NADH or NADPH) and oxidized flavin (FAD or FMN). As shown in Figure 4, NADH and FAD was the only combination that produced a detectable time-dependent decrease in absorbance at 340 nm, indicating that SgcE6 is specific for these substrates. Although high selectivity for NADH versus NADPH is common among Class I flavin reductases, such strict flavin specificity is not (Galan et al., 2000). Indeed, the specificity constants for FAD versus FMN consumption typically vary within one order of magnitude [HpaC, 3-fold (Galan et al., 2000); RebF, 2-fold (Yeh et al., 2005); PrnF, 13-fold (Lee & Zhao, 2007); VlmR, 3-fold (Parry & Li, 1997); ActVB, 5-fold (Kendrew et al., 1995); TcpX, 3-fold (Belchik & Xun, 2008); TftC, 2-fold (Gisi & Xun, 2003); NmoB, 3-fold (Deng et al., 2007)]. Although the assay was only performed using a single enzyme concentration, our inability to detect activity for FMN suggests that SgcE6 is much more than 10-fold less active towards this substrate relative to FAD (Figure 4). This apparently strict flavin preference of SgcE6 may represent a unique opportunity to study flavin selectivity determinants in Class I enzymes.
Figure 3.
The rate of NADH oxidation with varying SgcE6 concentrations. NADH oxidation was monitored by following the decrease in absorbance at 340 nm versus time in the presence of 160 μM NADH and 0.5, 8.6, 21, 43, or 129 μg of SgcE6.
Figure 4.
Substrate preference of SgcE6. Reactions were performed with 160 μM NADH, 100 μM flavin (if added), and 0.5 μg of SgcE6.
Steady-state Kinetics
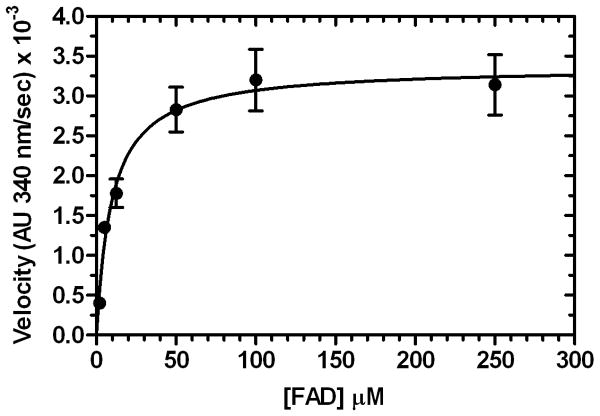
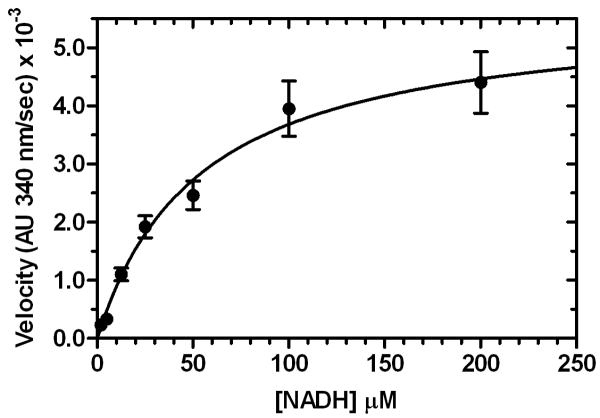
The steady-state kinetic parameters for NADH oxidation by SgcE6 were determined using both NADH and FAD in varying concentrations, yielding Km = 53 ± 8 μM for NADH and Km = 8.2 ± 0.9 μM for FAD. A higher Km for NADH versus FAD is typical for related flavin reductases. As expected, the kcat values were nearly identical, with kcat = 3.1 ± 0.1 s-1 for NADH and kcat = 4.5 ± 0.6 s-1 for FAD (Figure 5). Therefore production of FADH2 by SgcE6 is about two orders of magnitude faster than its consumption by its monooxygenase partner SgcC (Lin et al., 2008), and the difference appears to be even greater with respect to the halogenase SgcC3 (Lin et al., 2007). This finding suggests that SgcE6 can adequately supply reduced flavin under saturating substrate conditions to support SgcC3 and SgcC catalysis during C-1027 biosynthesis.
Figure 5.
Steady-state kinetic analysis of SgcE6 (176 nM) with A) 160 μM NADH and variable FAD concentrations, and B) 100 μM FAD and variable NADH concentrations. Each point represents triplicate measurements with a standard error of <10%.
In summary, bioinformatics and biochemical studies have provided new insight into SgcE6, the flavin reductase from the C-1027 biosynthetic pathway in S. globisporus. Sequence alignment has revealed that SgcE6 is a member of the HpaC-like subfamily of Class I flavin reductases. Members of this recently-described subfamily are involved in a variety of biological processes including oxidation of aromatic compounds (Galan et al., 2000; van den Heuvel et al., 2004) and antibiotic biosynthesis (Kendrew et al., 1995; Lin et al., 2007; Lin et al., 2008; Parry & Li, 1997; Yeh et al., 2005). Biochemical studies demonstrated that SgcE6 only accepts NADH and FAD as substrates, and the apparently strict FAD requirement is unusual for a Class I flavin reductase. Finally, the steady-state kinetic parameters for FAD and NADH substrates were determined, showing relative Km values typical of flavin reductase enzymes.
Acknowledgments
We thank Dr. Y. Li, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences, Beijing, China for the wild-type S. globisporus strain. This work is supported in part by NIH grants CA78747 and CA113297. SVL is the recipient of an NIH postdoctoral fellowship (CA1059845). GPH is the recipient of an NSERC postdoctoral fellowship.
References
- Belchik SM, Xun LY. Functions of flavin reductase and quinone reductase in 2,4,6-trichlorophenol degradation by Cupriavidus necator JMP134. J Bacteriol. 2008;190:1615–1619. doi: 10.1128/JB.01697-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng DY, Li XB, Fang XP, Sun GP. Characterization of two components of the 2-naphthoate monooxygenase system from Burkholderia sp strain JT1500. FEMS Microbiol Lett. 2007;273:22–27. doi: 10.1111/j.1574-6968.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- Fontecave M, Eliasson R, Reichard P. NAD(P)H-Flavin Oxidoreductase of Escherichia Coli - a Ferric Iron Reductase Participating in the Generation of the Free-Radical of Ribonucleotide Reductase. J Biol Chem. 1987;262:12325–12331. [PubMed] [Google Scholar]
- Galan B, Diaz E, Prieto MA, Garcia JL. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin: NAD(P)H reductase subfamily. J Bacteriol. 2000;182:627–636. doi: 10.1128/jb.182.3.627-636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galm U, Hager MH, Van Lanen SG, Ju JH, Thorson JS, Shen B. Antitumor antibiotics: Bleomycin, enediynes, and mitomycin. Chem Rev. 2005;105:739–758. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- Gisi MR, Xun LY. Characterization of chlorophenol 4-monooxygenase (TftD) and NADH: flavin adenine dinucleotide oxidoreductase (TftC) of Burkholderia cepacia AC1100. J Bacteriol. 2003;185:2786–2792. doi: 10.1128/JB.185.9.2786-2792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrew SG, Harding SE, Hopwood DA, Marsh ENG. Identification of a Flavin:NADH Oxidoreductase Involved in the Biosynthesis of Actinorhodin - Purification and Characterization of the Recombinant Enzyme. J Biol Chem. 1995;270:17339–17343. doi: 10.1074/jbc.270.29.17339. [DOI] [PubMed] [Google Scholar]
- Kennedy DR, Ju J, Shen B, Beerman TA. Designer enediynes generate DNA breaks, interstrand cross-links, or both, with concomitant changes in the regulation of DNA damage responses. Proc Natl Acad Sci U S A. 2007a;104:17632–17637. doi: 10.1073/pnas.0708274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DR, Gawron LS, Ju JH, Liu W, Shen B, Beerman TA. Single chemical modifications of the C-1027 enediyne core, a radiomimetic antitumor drug, affect both drug potency and the role of ataxia-telangiectasia mutated in cellular responses to DNA double-strand breaks. Cancer Res. 2007b;67:773–781. doi: 10.1158/0008-5472.CAN-06-2893. [DOI] [PubMed] [Google Scholar]
- Lee JK, Zhao HM. Identification and characterization of the Flavin: NADH reductase (PrnF) involved in a novel two-component arylamine oxygenase. J Bacteriol. 2007;189:8556–8563. doi: 10.1128/JB.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Van Lanen SG, Shen B. Regiospecific chlorination of (S)-β-Tyrosyl-S-Carrier protein catalyzed by SgcC3 in the biosynthesis of the enediyne antitumor antibiotic C-1027. J Am Chem Soc. 2007;129:12432–12438. doi: 10.1021/ja072311g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Van Lanen SG, Shen B. Characterization of the two-component, FAD-dependent monooxygenase SgcC that requires carrier protein-tethered substrates for the biosynthesis of the enediyne antitumor antibiotic C-1027. J Am Chem Soc. 2008;130:6616–6623. doi: 10.1021/ja710601d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Van Lanen SG, Shen B. A free-standing condensation enzyme catalyzing ester bond formation in C-1027 biosynthesis. Proc Natl Acad Sci U S A. 2009;106:4183–4188. doi: 10.1073/pnas.0808880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie TM, Xie XS, Xun LY. Coordinated production and utilization of FADH(2) by NAD(P)H-flavin oxidoreductase and 4-hydroxyphenylacetate 3-monooxygenase. Biochemistry. 2003;42:7509–7517. doi: 10.1021/bi034092r. [DOI] [PubMed] [Google Scholar]
- Parry RJ, Li WY. An NADPH:FAD oxidoreductase from the valanimycin producer, Streptomyces viridifaciens - Cloning, analysis, and overexpression. J Biol Chem. 1997;272:23303–23311. doi: 10.1074/jbc.272.37.23303. [DOI] [PubMed] [Google Scholar]
- van Berkel WJH, Kamerbeek NM, Fraaije MW. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J Biotechnol. 2006;124:670–689. doi: 10.1016/j.jbiotec.2006.03.044. [DOI] [PubMed] [Google Scholar]
- van den Heuvel RHH, Westphal AH, Heck AJR, Walsh MA, Rovida S, van Berkel WJH, Mattevi A. Structural studies on flavin reductase PheA2 reveal binding of NAD in an unusual folded conformation and support novel mechanism of action. J Biol Chem. 2004;279:12860–12867. doi: 10.1074/jbc.M313765200. [DOI] [PubMed] [Google Scholar]
- Van Lanen SG, Shen B. Biosynthesis of enediyne antitumor antibiotics. Curr Top Med Chem. 2008;8:448–459. doi: 10.2174/156802608783955656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Garneau S, Walsh CT. Robust in vitro activity of RebF and RebH, a two-component reductase/halogenase, generating 7-chlorotryptophan during rebeccamycin biosynthesis. Proc Natl Acad Sci U S A. 2005;102:3960–3965. doi: 10.1073/pnas.0500755102. [DOI] [PMC free article] [PubMed] [Google Scholar]