Abstract
Background:
γ-Tocopherol (γ-T) has unique properties that may be beneficial in sustaining optimal human health, but hepatic vitamin E metabolism enhances γ-T turnover.
Objective:
Our aim was to determine the extent to which dietary sesame lignans alter human α- and γ-T metabolism and elimination as carboxyethyl hydroxychromanols (CEHCs).
Design:
Healthy participants (n=5, each men and women) in a randomized, crossover study (4 w washout) consumed muffins prepared with either corn oil or unrefined sesame oil (sesamin, 94 mg; sesamolin, 42 mg), along with a capsule containing a 1:1 molar ratio of deuterium-labeled d6-α- and d2-γ-T acetates (~50 mg each). Plasma and urine were collected up to 72 h; unlabeled and labeled T and CEHC concentrations were determined by LC-MS.
Results:
Sesame oil muffin consumption in men, but not in women, decreased (P<0.05) areas under plasma d2-γ-CEHC concentration-time curves (AUCs) and maximum concentrations. However, in both genders urinary d2-γ-CEHCs were decreased for 24 h following sesame oil muffin consumption.
Conclusion:
In humans, γ-T metabolism can be inhibited by the simultaneous consumption of γ-T and sesame lignans. The observed differences between men and women with respect to vitamin E metabolism warrant further investigation.
Keywords: carboxyethyl hydroxychromanols, CEHC, gender differences, kinetics, lignans, metabolism, sesame, sesamin, sesamolin, tocopherols, vitamin E.
INTRODUCTION
γ-Tocopherol (γ-T) has unique properties that may be beneficial in sustaining optimal human health and preventing disease (1). For example, γ-T as a result of the unsubstituted 5-position on the chromanol ring can scavenge reactive nitrogen species (RNS) (2). RNS damage in humans has been substantiated by the detection of higher 5-nitro-γ-T concentrations in smokers (3) and Alzheimer patients (4). γ-T reportedly inhibits thrombogenesis (5), decreases inflammation (6), as well as reduces cancer cell proliferation (7,8). Moreover, γ-T, but not α-T levels, were reduced in coronary heart disease patients (9-11), suggesting that there may be benefits in raising plasma γ-T concentrations (5,12).
Although the liver expresses the α-T transfer protein (α-T TP), which is responsible for salvaging α-T from the excretory pathway and returning to the liver, hepatic metabolism appears to be a key factor in the discrimination between tocopherols (13). In the fruit fly Drosophila melanogaster because the fly lacks α-TTP, the selective accumulation of α-T has been attributed to metabolism of non-α-Ts (14). Vitamin E forms are metabolized to side-chain truncated, water-soluble carboxyethyl hydroxychromans (CEHCs) (15-18) and are excreted in urine (19-21) and bile (22,23). Cytochrome P450 (CYP) enzymes catalyze the initial ω-hydroxylation of the side-chain prior to its shortening by enzymes of the β-oxidation pathway (17,18). Importantly, these processes have a higher catalytic activity towards the non-α-vitamers (15,17).
Sesamin, an abundant lignan in sesame seeds and oils, when fed to rats substantially increased plasma γ-T, but not α-T concentrations (24,25). In HepG2 cells and primary rat hepatocytes, sesamin inhibited the degradation of non-α-Ts to their corresponding CEHC metabolites (18,26). Consistently, dietary sesame seeds or sesame lignans (sesamin and sesaminol) reduced the urinary excretion of γ-CEHC in rats (21). Increased blood γ-T concentrations in response to sesame lignan intake have also been reported in two controlled human studies (27,28). Thus, the lack of γ-T retention in blood is greatly influenced by hepatic metabolism.
Our previous studies using deuterium-labeled α- and γ-Ts demonstrated that not only does γ-T turnover faster than does α-T, but that γ-CEHC and γ-T disappear from the plasma at the same rates (29). Moreover, women had a faster γ-T disappearance rate than did men. These data suggested that vitamin E metabolism is critical in regulating plasma γ-T concentrations in humans. To evaluate the hypothesis that the simultaneous consumption of vitamin E with sesame oil containing sesame lignans could would decrease vitamin E metabolism, and thus increase plasma γ-T concentrations, a randomized, cross-over study was carried out in both men and women. The participants consumed deuterium labeled α- and γ-tocopheryl acetates along with a breakfast containing either sesame or corn oil muffins; after a 4 week washout, the subjects repeated the study with the opposite muffin.
METHODS
Materials
α-5,7-(C2H3)2 tocopheryl acetate (d6-α-TAc) was a gift from Dr. James Clark of Cognis Nutrition and Health (LaGrange, IL, USA). γ-3,4-(2H) tocopheryl acetate (d2-γ-TAc) was prepared from γ-T as described (30). The d6-α- and d2-γ-TAc were diluted in tocopherol-stripped corn oil at a 1:1 molar mixture, and gelatin capsules containing approximately 50 mg of each α- and γ-TAc, were prepared. The d6-α- to d2-γ-T molar ratio was determined by liquid chromatography/mass spectrometry (LC/MS) to be 0.98. The internal standard, α-5,7,8-(C2H3)3 tocopheryl acetate (d9-α-TAc), was provided by Dr. Carolyn Good of The Bell Institute of Health and Nutrition (Minneapolis, MN, USA), and was synthesized by Isotec, Inc. (Miamisburg, OH, USA). Cold-pressed, unrefined sesame oil (a gift from Henry Lamotte GmbH, Bremen, Germany) contained sesamin, 607 mg/100 g oil and sesamolin, 269 mg/100 g oil. For use as standards 2,5,7,8-tetramethyl-2-(2′-carboxyethyl)-6-hydroxychroman (α-CEHC) and 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman (γ-CEHC) were gifts from W.J. Wechter of Loma Linda University (Loma Linda, CA, USA). Trolox, γ-T, ascorbic acid, butylated hydroxy toluene (BHT), potassium hydroxide (KOH), lithium perchlorate, and β-glucuronidase (type H-1, contains minimum 300,000 U/g β-glucuronidase activity and minimum 10,000 U/g sulfatase activity) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Diethyl ether was obtained from Mallinckrodt Baker, Inc. (Phillipsburg, NJ, USA), and HPLC-grade methanol, ethanol, hexane, and glacial acetic acid were from Fisher (Fair Lawn, NJ, USA).
Subjects
Healthy participants (5 women, 5 men) were recruited and written informed consent was obtained prior to inclusion in the trial. Participants were not smokers, did not take any dietary supplements at least 3 weeks prior to and during the study, and restricted their physical activity to <5 h/wk. Routine blood serum chemistry assays were performed at Good Samaritan Hospital (Corvallis, OR, USA) and were within the normal limits for all subjects. For subject characteristics see Table 1. The study protocol was approved by the Institutional Review Board at Oregon State University.
Table 1.
Subject characteristics and blood lipids at screening.
|
Women (n=5) |
Men (n=5) |
|
|---|---|---|
| Age (y) | 25.4 ± 1.8 | 30.6 ± 2.6 |
| BMI (kg/m2) | 23.7 ± 0.6 | 24.9 ± 1.0 |
| Plasma lipids (mmol/L) | ||
| Total cholesterol | 4.40 ± 0.22* | 4.97 ± 0.11 |
| LDL cholesterol | 2.40 ± 0.17# | 3.14 ± 0.13 |
| VLDL cholesterol | 0.33 ± 0.06 | 0.52 ± 0.08 |
| HDL | 1.67 ± 0.16 | 1.31 ± 0.11 |
| Triacylglycerols | 0.72 ± 0.15 | 1.12 ± 0.18 |
| Total Lipids | 5.1 ± 0.2* | 6.1 ± 0.3 |
| Plasma tocopherols | ||
| α-Tocopherol (μmol/L) | 19.4 ± 3.4 | 18.3 ± 2.4 |
| γ-Tocopherol (μmol/L) | 1.8 ± 0.5* | 3.5 ± 1.5 |
| α-Tocopherol per lipids(mmol/mol) | 3.8 ± 0.3 | 3.0 ± 0.2 |
| γ-Tocopherol per lipids(mmol/mol) | 0.34 ± 0.4 | 0.57 ± 0.10 |
Shown are the baseline serum lipids, plasma tocopherols, and the tocopherol/lipid ratios (mean ± SEM). Lipids are defined as the sum of the serum total cholesterol and triglycerides, expressed as mmol/L. Statistical differences between values of women and men were calculated by an unpaired Student's t-test; significantly different at:
P<0.05,
P<0.01.
Experimental diets and study design
Corn and sesame oils used for the preparation of the muffins were equalized with respect to concentrations of α-T (17.9 mg/100 g corn oil; 15.6 mg/100 g sesame oil) and of γ-T (84.9 mg/100 g corn oil; 84.0 mg/100 g sesame oil). The two muffins made with either oil contained approximately 13.0 mg α-T and 2.5 mg γ-T. The two sesame oil muffins provided 135.4 mg sesame lignans (sesamin 93.8 mg; sesamolin, 41.6 mg). Sesame oil or corn oil (with equalized vitamin E concentrations in each oil) was used to prepare muffins from 650 g wheat flour, 250 g sugar, 25 g baking powder, 10 g salt, 130 g whole egg, 440 g milk, and 275 g oil by mixing all ingredients and weighing 50 g of the resulting batter into paper baking cups. Muffins were baked for 25 min at 205°C and stored at −20°C until used in the trials.
Subjects were randomly assigned to one of two treatments in a crossover design with a four-week washout period between treatments. A standard dinner was provided at 1800 on the evening before the trial and subjects were instructed to abstain from all food and beverages with the exception of water overnight. In the morning, blood samples were drawn from the antecubital arm vein into tubes containing 0.05 mL 15% K3 EDTA (Vacutainer, Becton Dickinson, Franklin Lakes, NJ, USA). Subsequently, a standardized breakfast was served, including two sesame oil or corn oil (control) muffins and a capsule containing ~50 mg each deuterated d6-α- and d2-γ-tocopheryl acetates.
Blood samples were drawn 3, 6, 9, 12, 24, 36, 48, and 72 h after treatment. Meals were standardized during the period of 12 h before to 12 h after the dietary intervention and the subjects recorded their food consumption during the 2 consecutive days. For separation of blood plasma, samples were centrifuged (500 × g, 15 min, 4 °C), the plasma snap frozen in liquid nitrogen and stored at −80°C until analyzed. Urine was collected from 1 h prior to treatment on the first day of the trial; the urine bottles were exchanged at 0, 6, 12, 24, 36, 48, and 72 h after treatment. Urine volumes were recorded and 15 mL aliquots for each period were stored at −20°C until analyzed. After a 4-week washout period, treatments (sesame or corn oil muffins) were switched and the above procedures repeated.
Quantification of plasma labeled and unlabeled tocopherols
Tocopherols were extracted from plasma according to the method of Podda et al. (31). Briefly, plasma was saponified with saturated alcoholic KOH and the tocopherols extracted with hexane. An appropriate aliquot was dried under N2-gas, the residue re-suspended in MeOH:EtOH (1:1, v:v) containing d9-α-T as internal standard and injected into the LC system. For LC-MS analysis of labeled and unlabeled tocopherols, a Waters (Milford, MA, USA) 2690 Separations Module equipped with a Symmetry LC-18 column (Waters; 4.6 × 75 mm, 3.5 μm particle size; mobile phase, 100% methanol; flow rate, 1 mL/min) and a negative atmospheric pressure chemical ionization probe was used as previously described (32).
Quantification of plasma and urine labeled and unlabeled CEHC metabolites
Plasma and urinary CEHCs were extracted using a modified method of Lodge et al. (33). In brief, a known amount of trolox (internal standard) was added to either 0.5 mL plasma or 1 mL urine, before incubation for 30 min at 37°C with 100 μL β-glucuronidase (10 mg/mL dissolved in 0.01 M potassium phosphate buffer, pH 6.8), and 20 μL of H2O containing 1% ascorbic acid. After incubation, the samples were acidified by the addition of 10 μL 12 M HCl. CEHCs were extracted with 5 mL of diethyl ether, dried under nitrogen gas and re-suspended in H2O:MeOH (1:1, v:v), containing 0.1% (v:v) acetic acid, and 10 μL injected into the LC system described above equipped with a SymmetryShield™ RP-18 column (Waters; 3.0 × 150 mm, 3.5 μm particle size) and the solvent was delivered by a modified gradient method of Himmelfarb et al. (34). The system was first equilibrated with 50:50 H2O:MeOH (both containing 0.1% acetic acid) for 1 min, followed by a linear gradient to 80% MeOH at 6 min at a flow rate of 0.25 mL/min. These conditions were maintained for 15 min, followed by a 5 min wash period with 95% MeOH, at which time original conditions were returned to and run for 5 min prior to injection of the proceeding sample. Samples were detected using a Micromass (Manchester, UK) ZQ 2000 single-quadrupole mass spectrometer with an electrospray ionization source (capillary voltage, 2.5 V; sample cone voltage, −30 V; desolvation temperature, 150°C; desolvation gas (nitrogen) flow, 160 L/h; nebulizer gas (nitrogen) pressure, 80 psi; cone gas (nitrogen) flow, 50 L/h), dwell time per compound, 0.20 s. Single ion recording mass-to-charge (m/z) ratios for molecular ions were as follows: d0-α-CEHC, m/z 277; d6-α-CEHC, m/z 283; d0-γ-CEHC, m/z 263; d2-γ-CEHC, m/z 265, and trolox, m/z 249. Sample CEHC concentrations were calculated from the ratio of the peak area of the corresponding ion to that of the internal standard trolox peak; deuterated CEHCs were calculated using the corresponding non-deuterated CEHC standards.
Quantification of plasma triacylglycerols and cholesterol and urinary creatinine
Plasma triacylglycerols and cholesterol were determined using the respective ThermoDMA Kits (Louisville, CO, USA). Urinary creatinine was quantified spectrophotometrically at a wavelength of 500 nm after reaction with picric acid according to the Jaffé reaction (35).
Statistical analyses
The maximal concentrations (Cmax) and the time to reach maximal concentrations (Tmax) were identified by visual inspection of each individual's plasma concentration data. Areas under the curve (AUC) were calculated using the trapezoidal rule. Fractional disappearance rates (FDRs) of d6-α- and d2-γ-tocopherols and d6-α- and d2-γ-CEHCs were calculated separately for each individual as described previously (36). Repeated measures MANOVA was performed using JMP Statistical Discovery Software (SAS Institute, Cary, NC) to evaluate effects attributed to oil type and to gender. When significant interactions were found then an unpaired Student's t-test for between gender comparisons or a one-tailed, paired Student's t-test for comparisons of the two treatments within the same subjects was carried out. Differences were considered significant at a p<0.05 level. Data are expressed as means ± SEM unless otherwise noted.
RESULTS
Participant Characteristics
All participants had normal blood levels of analytes measured in routine chemistry panels (data not shown) and were within the normal range for BMI and blood lipids (Table 1). Men, as compared with women, had significantly higher plasma γ-T concentrations, as well as serum total and LDL cholesterol at the time of screening; plasma α-T concentrations were similar in all subjects (Table 1). Neither plasma α-T nor γ-T concentrations were different between men and women, when expressed per serum total lipids (sum of cholesterol and triacylglycerols) because total lipid concentrations were lower in women than in men (p <0.05).
No significant differences in the dietary intakes of macro- and micronutrients, including vitamin E, were observed between treatment groups or between men and women throughout the trials (data not shown).
Plasma tocopherols and CEHCs
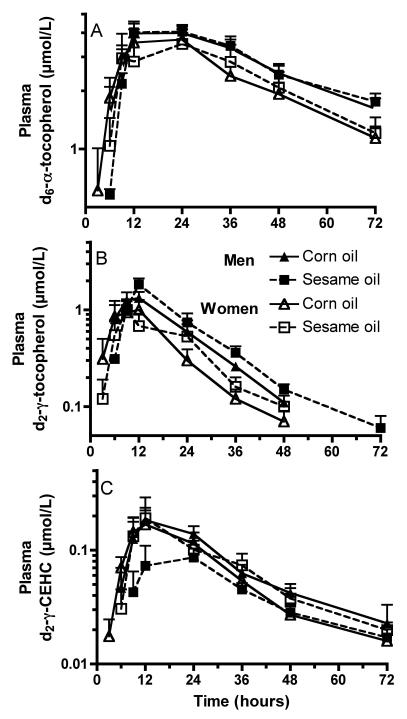
With respect to plasma d6-α-T concentrations and calculated kinetic parameters, sesame oil compared with corn oil-containing muffins had no significant effects on any of these parameters (Figure 1A, Table 2). Specifically, plasma d6-α-T AUC, Cmax, and Tmax values did not differ between the treatments (Table 2). Participants' plasma unlabeled (d0)-α-T and d0-γ-T concentrations were unresponsive to the type of muffin consumed (data not shown).
Figure 1. Plasma d6-α and d2-γ-tocopherol and d2-γ-CEHC concentrations.
Plasma d6-α-T (A), d2-γ-T (B) and d2-γ-CEHC (C) concentrations (μmol/L, mean + SEM) following ingestion of muffins prepared from unrefined sesame oil (squares, dotted lines) or corn oil (triangles, solid lines) simultaneously with a capsule containing 50 mg each d6-α- and d2-γ-TAcs in men (n= 5, filled symbols) and in women (n= 5, open symbols). Plasma samples were collected periodically for 72 h. By 72 h, the d2-γ-T (B) concentrations were below levels of detection for many subjects, thus the means are not shown.
Table 2.
Kinetic parameters calculated from plasma d6-α-T, d2-γ-T and d2-γ-CEHC concentrations in subjects consuming d6-α-TAc and d2-γ-TAc together with sesame oil or corn oil muffins, respectively.
| d6-α-Tocopherol | d2-γ-Tocopherol | d2-γ-CEHC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| corn oil |
sesame oil |
oil | gender | Oil × gender |
corn oil |
sesame oil |
oil | gender | Oil × gender |
corn oil | sesame oil |
oil | gender | Oil × gender |
|
| AUC (μmol/L·h) | |||||||||||||||
| Women (n=5) |
164 ± 19 |
162 ± 27 |
NS | NS | NS | 20 ± 5 | 21 ± 3 | NS | p<0.07 | NS | 4.8 ± 1.0 |
4.9 ± 0.8 |
p<0.05 | NS | p<0.03 |
| Men (n=5) |
194 ± 24 |
193 ± 16 |
29 ± 3 | 34 ± 5 | 5.0 ± 1.0 |
2.6 ± 0.8*,+ |
|||||||||
| Cmax (μmol/L) | |||||||||||||||
| Women (n=5) |
4.2 ± 0.5 |
4.8 ± 0.4 |
NS | NS | NS | 1.3 ± 0.3 |
1.3 ± 0.2 |
NS | p<0.05 | NS | 0.18 ± 0.05 |
0.29 ± 0.08 |
NS | NS | p<0.01 |
| Men (n=5) |
4.3 ± 0.6 |
4.3 ± 0.4 |
1.8 ± 0.3 |
2.0 ± 0.2 |
0.21 ± 0.04 |
0.10 ± 0.03*,+ |
|||||||||
| Tmax (h) | |||||||||||||||
| Women (n=5) |
16 ± 3 | 16 ± 3 | NS | NS | NS | 10 ± 2 | 16 ± 3 | p<0.03 | NS | NS | 13 ± 3 | 19 ± 5 | p<0.005 | NS | NS |
| Men (n=5) |
19 ± 3 | 17 ± 3 | 10 ± 1 | 11 ± 1 | 14 ± 2 | 22 ± 2 | |||||||||
| FDR (pools/day) | |||||||||||||||
| Women (n=5) |
0.57 ± 0.01 |
0.53 ± 0.02 |
NS | P<0.002 | NS | 2.15 ± 0.14 |
1.94 ± 0.14 |
NS | NS | NS | 1.38 ± 0.09 |
1.48 ± 0.08 |
NS | NS | NS |
| Men (n=5) |
0.45 ± 0.01 |
0.42 ± 0.01 |
1.49 ± 0.04 |
1.46 ± 0.05 |
1.2 ± 0.0 |
1.0 ± 0.1 |
|||||||||
Shown are mean ± SEM. No d6-α-CEHC was detected in plasma. Repeated measures MANOVA was performed using JMP Statistical Discovery Software (SAS Institute, Cary, NC) to evaluate effects attributed to oil type and to gender. Where there were significant interactions, post-hoc t-tests were performed;
P<0.05 for paired comparisons of corn vs sesame oil within each gender.
P<0.05 for unpaired comparisons between men and women within each treatment group.
In contrast, consumption of sesame oil compared with corn oil muffins delayed the time of the peak (Tmax) in the plasma kinetics of concurrently ingested d2-γ-T in both men and women (Figure 1B, Table 2). The dietary treatments did not significantly alter any of the other parameters of d2-γ-T kinetics (Table 2).
Differences between plasma α- and γ-T concentrations have been attributed in part to increased γ-T metabolism (13). Therefore, we sought to evaluate the efficacy with which sesame oil might alter vitamin E metabolism by measuring plasma CEHCs derived from the administered deuterated α- and γ-Ts. Similar to our previous studies, no d6-α-CEHC was detected in plasma or urine (29), suggesting that the dose of d6-α-T was insufficient to be metabolized and detectable as plasma d6-α-CEHC.
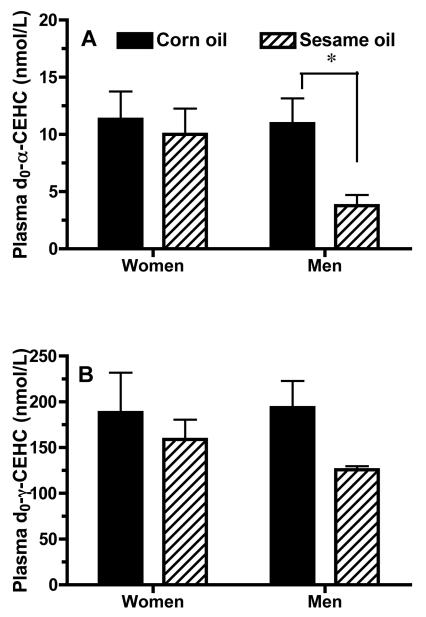
In the present study, differences in d2-γ-CEHC concentrations were observed with respect to the sesame oil treatment in men, but not in women (significant interactions between oil and gender, for Cmax and AUC). Sesame oil consumption significantly (P<0.05) decreased both plasma Cmax and d2-γ-CEHC AUCs (Figure 1C,Table 2). Thus, sesame oil consumption in men not only delayed the Tmax of plasma d2-γ-T concentrations (discussed above), it also halved the maximum plasma d2-γ-CEHC concentrations and the AUCs. Remarkably, sesame oil consumption also decreased (p<0.05) mean concentrations over 72 h of both plasma d0-α- and d0-γ-CEHCs in men, but not women (Figure 2).
Figure 2. Sesame oil consumption in men decreased plasma d0-α and d0-γ-CEHC concentrations.
Plasma d0-α-CEHC (A) and d0-γ-CEHC (B) concentrations (nmol/L, mean + SEM) from all samples from 3 to 72 h were averaged for each subject (n=5 men, 5 women) following ingestion of sesame oil (solid bar) or corn oil (hatched bar) muffins. Repeated measures MANOVA was performed using JMP Statistical Discovery Software (SAS Institute, Cary, NC) to evaluate effects attributed to oil type and to gender. Sesame oil consumption decreased plasma d0-α-CEHC concentrations (interaction P < 0.01, only in men, P<0.05). There was a main effect of oil type on plasma d0-γ-CEHC concentrations (P< 0.05).
Some notable differences in vitamin E kinetics were observed that were related only to gender differences. Women had significantly (p<0.002, main effect) greater fractional disappearance rates (FDR) of plasma d6-α-T concentrations than did men (Figure 1A, Table 2). Similarly, when the d2-γ-T FDRs were averaged for the two dietary treatments for each subject, women compared with men also had greater d2-γ-T FDRs (2.0 ± 0.1 vs 1.5 ± 0.0 pools per day, p<0.05). When concentrations were adjusted for serum total lipids at each time point, and kinetics similarly calculated, women's plasma d6-α-T/lipids FDRs were also greater than men's (main effect, p<0.01, not shown).
Urinary CEHC excretion
We observed that urinary d2-γ-CEHC concentrations during the first 24 h of the study were significantly decreased by consumption of sesame oil muffins both in women and in men (Table 3). Since sesame lignans and their metabolites appear to be eliminated from the body and excreted in urine within 24 h following consumption by humans (37), any potential physiological effects would only be expected during this interval. Thus, it is not surprising that sesame oil consumption did not have effects in the subsequent urine collections (data not shown). No d6-α-CEHC was detectable in the urine samples (data not shown).
Table 3.
Urinary excretion of d0-α-CEHC, d0-γ-CEHC and d2-γ-CEHC (nmol/g creatinine) over 24 h in subjects consuming d6-α-TAc and d2-γ-TAc together with sesame oil or corn oil muffins, respectively.
| d0-α-CEHC | d0-γ-CEHC | d2-γ-CEHC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| corn oil |
sesame oil |
oil | gender | Oil × gender |
corn oil |
sesame oil |
oil | gender | Oil × gender |
corn oil | sesame oil |
oil | gender | Oil × gender |
|
| Women (n=5) |
0.87 ± 0.15 |
0.92 ± 0.44 |
NS | P<0.008 | NS | 2.00 ± 0.26 |
1.83 ± 0.60 |
NS | P<0.008 | NS | 1.15 ± 0.24 |
0.81 ± 0.31 |
P<0.030 | P<0.055 | NS |
| Men (n=5) |
0.23 ± 0.04 |
0.11 ± 0.03 |
1.00 ± 0.21 |
0.59 ± 0.17 |
0.56 ± 0.16 |
0.27 ± 0.12 |
|||||||||
Shown are mean ± SEM. Repeated measures MANOVA was performed using JMP Statistical Discovery Software (SAS Institute, Cary, NC) to evaluate effects attributed to oil type and to gender.
Both d0-α- and d0-γ-CEHC urinary excretion were higher in women than in men, but sesame oil consumption had no significant effect on their excretion.
DISCUSSION
This study was designed to test the hypothesis that humans consuming sesame lignans (sesamin and sesamolin) from unrefined sesame oil would have increased plasma γ-T concentrations likely as a result of inhibiting the metabolism of γ-T to γ-CEHC. These expectations are in accordance with previously published findings from human (27,28) and rat (24,25,38,39) studies that showed markedly increased plasma γ-T concentrations after dietary intervention with sesame seeds, sesame oil, or isolated sesame lignans. Although sesame oil consumption delayed the peak in d2-γ-T concentrations in both men and women (Table 2), none of the other d2-γ-T kinetic parameters were altered by sesame oil. However, we found that plasma d2-γ-CEHC AUCs, and Cmaxs were significantly lower in men after consuming sesame oil muffins compared with corn oil muffins (Figure 1C, Table 2), suggesting that sesame lignans interfered with d2-γ-T metabolism. Surprisingly, similar decreases in response to sesame oil consumption were not found for women's plasma d2-γ-CEHC concentrations, although sesame delayed the peak in the d2-γ-CEHC concentrations in both men and women.
In conjunction with the significant decrease in the plasma d2-γ-CEHC AUCs, and Cmaxs, a reduction in urinary d2-γ-CEHC excretion was observed in both genders during the first 24 h of the sesame oil trial. It is likely that women's vitamin E metabolism was affected by sesame lignans, as observed previously (27), but did not result in differences in their plasma d2-γ-CEHC concentrations. This speculation is supported by the findings that overall urinary excretion of unlabeled α- and γ-CEHC and d2-γ-CEHC was higher in women than in men (Table 3).
The percentage of d2-γ-T dose recovered over 72 h as urinary d2-γ-CEHC in men was nearly doubled during the corn oil (2.1% ± 0.6%) intervention compared with the sesame intervention (1.2% ± 0.4%), but these differences did not reach statistical significance; in women the overall percentage of dose excreted was 2.5% ± 0.7% and 2.3% ± 0.5%, during the corn and sesame oil trials, respectively. Parker and co-workers (26) previously demonstrated in cells in culture that the sesame lignan, sesamin, at very low concentrations (1 μM) almost completely inhibited the formation of the side-chain truncated metabolite γ-CEHC from γ-T. They proposed the involvement of cytochrome P450 (CYP) enzymes in the initial ω-hydroxylation of the terminal methyl group of the tocopherol side-chain (18) and its inhibition by sesamin (26). These findings offer a ready explanation for the pronounced decrease in plasma γ-CEHCs concentrations due to sesame lignan consumption in our study. This mechanism is further supported by reports of a reduced excretion of γ-CEHC in the urine of rats fed sesame seeds, isolated sesame lignans (sesamin or sesaminol), or ketoconazole (21). The data presented herein, however, shows in humans that γ-T metabolism can be inhibited by the simultaneous consumption of γ-T and sesame lignans. It is remarkable, that this γ-T-sparing activity was observed after the consumption of only a single oral dose of 94 mg sesamin and 42 mg sesamolin. Repeated ingestion of sesame lignans results in an even more pronounced increase in γ-T concentrations, as observed by Cooney et al. (28) in subjects consuming muffins prepared with ground sesame seeds on three consecutive days.
The potent inhibition of CYP-mediated vitamin E metabolism by sesame lignans is of particular importance with regard to clinical nutrition. Chemicals with a methylenedioxyphenyl function, such as sesamin and sesamolin, are known to form complexes with CYPs, thereby irreversibly inactivating the enzymes (40). CYPs are centrally involved in the detoxification of xenobiotics, including many pharmaceutical agents. CYP3A4, for example, metabolizes more than 50% of prescription drugs (41). Thus, simultaneously ingested sesame lignans may alter the bioavailability and biopotency of drugs by altering their in vivo-conversion to the bioactive forms or by slowing down their elimination from the body. Furthermore, phase I enzymes, such as CYPs, are involved in the activation, as well as the elimination of pro-carcinogens; thus, sesame lignans may hypothetically interfere with cancer development. In female rats, feeding of a mixture of sesamin and episesamin at 0.2% (by weight) in the diet, significantly reduced the formation of chemically-induced mammary carcinomas (42).
Throughout the current investigation, we observed considerable differences in the handling, metabolism, and excretion of tocopherols and their water-soluble metabolites between men and women. The faster plasma disappearance of both plasma d6-α-T and d2-γ-T in women compared with men (Table 2) and increased urinary excretion of γ-CEHC relative to α-CEHC is consistent with our previous results (29). In general, the production of α-CEHC from α-tocopherol is limited (17,18). Our previous study examining the relative metabolism of α-T and γ-T showed in normal individuals that plasma α-CEHC concentrations are 1/10th of those of γ-CEHC (29). Additionally, Shultz et al. (43) showed that α-tocopherol intakes in human had to be over 150 mg/day in order to detect plasma α-CEHC. We therefore did not find it surprising that d6-α-CEHC was not detectable with a single dose of 50 mg/day.
These data suggest that further studies comparing vitamin E metabolism in men and women are warranted.
ACKNOWLEDGMENTS
We express thanks to the study participants for their cooperation throughout the investigation. The skillful help of Dr. Richard Bruno and Lee Moore (LPI) with the data analyses and sample collection is gratefully acknowledged.
JF, AKE, and MGT designed the study. JF recruited and attended to the subjects and supervised the trial. JKA synthesized the labeled tocopherols. JF and SWL participated in the sample collection. JF, SEL, and SWL analyzed the samples. MGT, SEL and JF performed the calculations and statistical analyses. JF, SEL, AKE, and MGT wrote the first draft of the manuscript and all authors edited and reviewed the final manuscript.
Supported by grants to MGT (NIH DK 67930) and AK-E (Swedish Research Council; Vetenskapsrådet, project no. 621-2003-4746 ); JF was supported by a travel stipend from the Swedish University of Agricultural Sciences (Dnr 12.23-3272/02).
Footnotes
None of the authors had a known conflict of interest.
REFERENCES
- 1.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-Tocopherol - An Underestimated Vitamin? Ann Nutr Metab. 2004;48:169–88. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 2.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci USA. 1993;90:1771–5. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard SW, Bruno RS, Paterson E, et al. 5-Nitro-γ-tocopherol increases in human plasma exposed to cigarette smoke in-vitro and in-vivo. Free Radic. Biol. Med. 2003;38:813–9. doi: 10.1016/j.freeradbiomed.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Williamson KS, Gabbita SP, Mou S, et al. The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide. 2002;6:221–7. doi: 10.1006/niox.2001.0399. [DOI] [PubMed] [Google Scholar]
- 5.Hensley K, Benaksas EJ, Bolli R, et al. New Perspectives on Vitamin E: γ-Tocopherol and Carboxyethylhydroxychroman Metabolites in Biology and Medicine. Free Radic Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q, Ames BN. γ-Tocopherol, but not α-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–22. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 7.Gysin R, Azzi A, Visarius T. γ-Tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002;16:1952–4. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 8.Galli F, Stabile AM, Betti M, et al. The effect of α- and γ-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch Biochem Biophys. 2004;423:97–102. doi: 10.1016/j.abb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Öhrvall M, Sundlöf G, Vessby B. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary heart disease patients. J Intern Med. 1996;239:111–7. doi: 10.1046/j.1365-2796.1996.410753000.x. [DOI] [PubMed] [Google Scholar]
- 10.Kristenson M, Zieden B, Kucinskiene Z, et al. Antioxidant state and mortality from coronary heart disease in Lithuanian and Swedish men: concomitant cross sectional study of men aged 50. BMJ. 1997;314:629–33. doi: 10.1136/bmj.314.7081.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kontush A, Spranger T, Reich A, Baum K, Beisiegel U. Lipophilic antioxidants in blood plasma as markers of atherosclerosis: the role of α-carotene and γ-tocopherol. Atherosclerosis. 1999;144:117–22. doi: 10.1016/s0021-9150(99)00044-1. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Christen S, Shigenaga MK, Ames BN. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–22. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 13.Traber MG. Vitamin E regulatory nechanisms. Annu Rev Nutr. 2007;27:347–62. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 14.Parker RS, McCormick CC. Selective accumulation of alpha-tocopherol in Drosophila is associated with cytochrome P450 tocopherol-omega-hydroxylase activity but not alpha-tocopherol transfer protein. Biochem Biophys Res Commun. 2005;338:1537–41. doi: 10.1016/j.bbrc.2005.10.124. [DOI] [PubMed] [Google Scholar]
- 15.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113–8. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 16.Birringer M, Drogan D, Brigelius-Flohe R. Tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic Biol Med. 2001;31:226–32. doi: 10.1016/s0891-5849(01)00574-3. [DOI] [PubMed] [Google Scholar]
- 17.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their ω-oxidation by tocopherol-ω-hydroxylase. J Lipid Res. 2007;48:1090–8. doi: 10.1194/jlr.M600514-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–6. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 19.Parker RS, Swanson JE. A novel 5′-carboxychroman metabolite of gamma-tocopherol secreted by HepG2 cells and excreted in human urine. Biochem Biophys Res Commun. 2000;269:580–3. doi: 10.1006/bbrc.2000.2319. [DOI] [PubMed] [Google Scholar]
- 20.Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun. 2000;277:531–4. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda S, Tohyama T, Yamashita K. Dietary sesame seed and its lignans inhibit 2,7,8-trimethyl- 2(2′-carboxyethyl)-6-hydroxychroman excretion into urine of rats fed γ-tocopherol. J Nutr. 2002;132:961–6. doi: 10.1093/jn/132.5.961. [DOI] [PubMed] [Google Scholar]
- 22.Hattori A, Fukushima T, Imai K. Occurrence and determination of a natriuretic hormone, 2,7,8-trimethyl-2-(beta-carboxyethyl)-6-hydroxy chroman, in rat plasma, urine, and bile. Anal Biochem. 2000;281:209–15. doi: 10.1006/abio.2000.4566. [DOI] [PubMed] [Google Scholar]
- 23.Kiyose C, Saito H, Kaneko K, et al. Alpha-tocopherol affects the urinary and biliary excretion of 2,7,8-trimethyl-2 (2′-carboxyethyl)-6-hydroxychroman, gamma-tocopherol metabolite, in rats. Lipids. 2001;36:467–72. doi: 10.1007/s11745-001-0744-2. [DOI] [PubMed] [Google Scholar]
- 24.Kamal-Eldin A, Pettersson D, Appelqvist LÅ. Sesamin (a compound from sesame oil) increases tocopherol levels in rats fed ad libitum. Lipids. 1995;30:499–505. doi: 10.1007/BF02537023. [DOI] [PubMed] [Google Scholar]
- 25.Kamal-Eldin A, Frank J, Razdan A, Tengblad S, Basu S, Vessby B. Effects of dietary phenolic compounds on tocopherol, cholesterol, and fatty acids in rats. Lipids. 2000;35:427–35. doi: 10.1007/s11745-000-541-y. [DOI] [PubMed] [Google Scholar]
- 26.Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun. 2000;277:531–4. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 27.Lemcke-Norojärvi M, Kamal-Eldin A, Appelqvist LÅ, Dimberg LH, Öhrvall M, Vessby B. Corn and sesame oils increase serum γ-tocopherol concentrations in healthy Swedish women. J Nutr. 2001;131:1195–1201. doi: 10.1093/jn/131.4.1195. [DOI] [PubMed] [Google Scholar]
- 28.Cooney RV, Custer LJ, Okinaka L, Franke AA. Effects of dietary sesame seeds on plasma tocopherol levels. Nutr Cancer. 2001;39:66–71. doi: 10.1207/S15327914nc391_9. [DOI] [PubMed] [Google Scholar]
- 29.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med. 2005;38:857–66. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Lei HS, Atkinson J. Hydrogen-deuterium exchange during the reductive deuteration of alpha- and gamma-tocopherol chromenes. J Labelled Comp & Radiopharm. 2001;44:215–223. [Google Scholar]
- 31.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 32.Vaule H, Leonard SW, Traber MG. Vitamin E delivery to human skin: studies using deuterated alpha-tocopherol measured by APCI LC-MS. Free Radic Biol Med. 2004;36:456–63. doi: 10.1016/j.freeradbiomed.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Lodge JK, Traber MG, Elsner A, Brigelius-Flohe R. A rapid method for the extraction and determination of vitamin E metabolites in human urine. J Lipid Res. 2000;41:148–54. [PubMed] [Google Scholar]
- 34.Himmelfarb J, Kane J, McMonagle E, et al. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–91. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 35.Jaffé MZ. [Über den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine Reaktion des Kreatinins.] Zeitschrift Für Physiologische Chemie. 1886;10:391–400. [Google Scholar]
- 36.Traber MG, Ramakrishnan R, Kayden HJ. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-α-tocopherol. Proc. Natl. Acad. Sci. USA. 1994;91:10005–10008. doi: 10.1073/pnas.91.21.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moazzami AA, Andersson RE, Kamal-Eldin A. Quantitative NMR analysis of a sesamin catechol metabolite in human urine. J Nutr. 2007;137:940–4. doi: 10.1093/jn/137.4.940. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita K, Iizuka Y, Imai T, Namiki M. Sesame seed and its lignans produce marked enhancement of vitamin E activity in rats fed a low alpha-tocopherol diet. Lipids. 1995;30:1019–28. doi: 10.1007/BF02536287. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita K, Nohara Y, Katayama K, Namiki M. Sesame seed lignans and gamma-tocopherol act synergistically to produce vitamin E activity in rats. J Nutr. 1992;122:2440–6. doi: 10.1093/jn/122.12.2440. [DOI] [PubMed] [Google Scholar]
- 40.Murray M. Mechanisms of inhibitory and regulatory effects of methylenedioxyphenyl compounds on cytochrome P450-dependent drug oxidation. Curr Drug Metab. 2000;1:67–84. doi: 10.2174/1389200003339270. [DOI] [PubMed] [Google Scholar]
- 41.Cholerton S, Daly AK, Idle JR. The role of individual human cytochromes P450 in drug metabolism and clinical response. Trends Pharmacol Sci. 1992;13:434–9. doi: 10.1016/0165-6147(92)90140-2. [DOI] [PubMed] [Google Scholar]
- 42.Hirose N, Doi F, Ueki T, et al. Suppressive effect of sesamin against 7,12-dimethylbenz[a]-anthracene induced rat mammary carcinogenesis. Anticancer Res. 1992;12:1259–65. [PubMed] [Google Scholar]
- 43.Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohé R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J. Clin. Nutr. 1995;62(suppl):1527S–1534S. doi: 10.1093/ajcn/62.6.1527S. [DOI] [PubMed] [Google Scholar]