Abstract
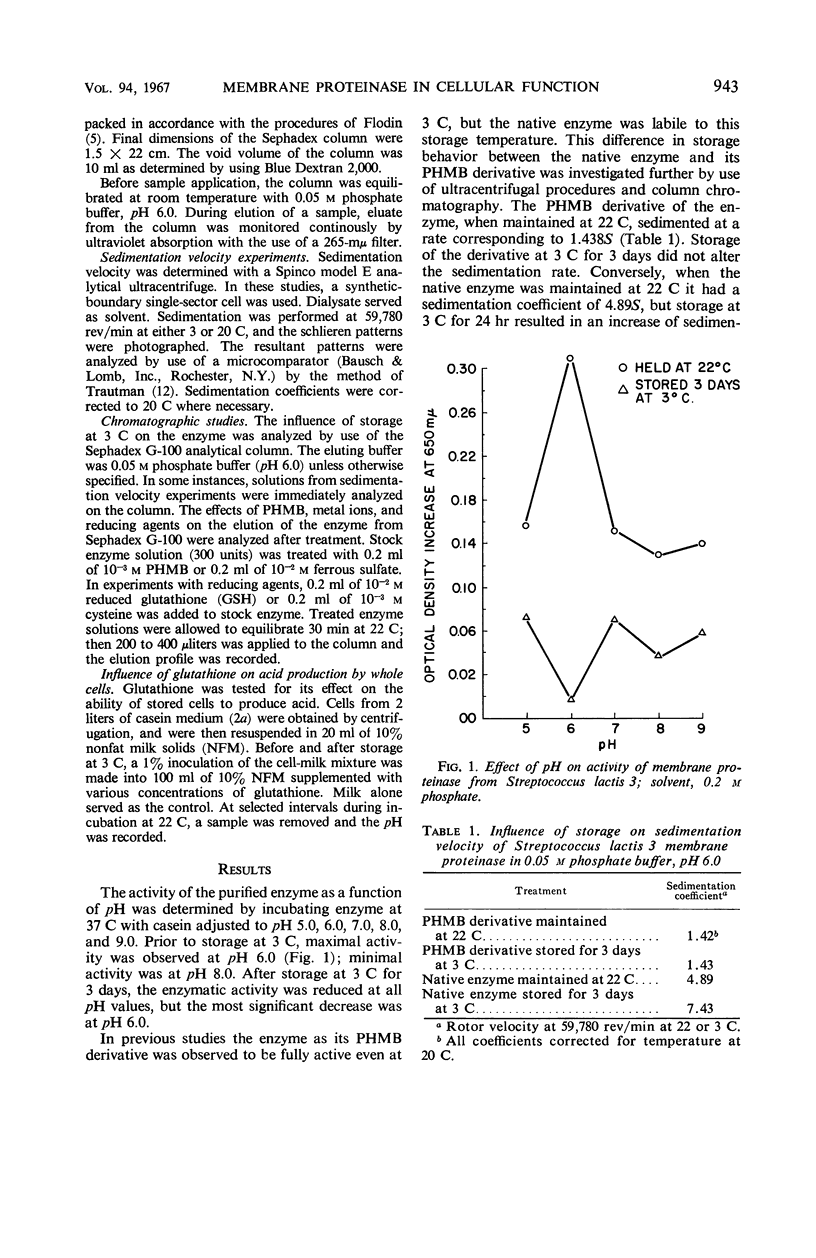
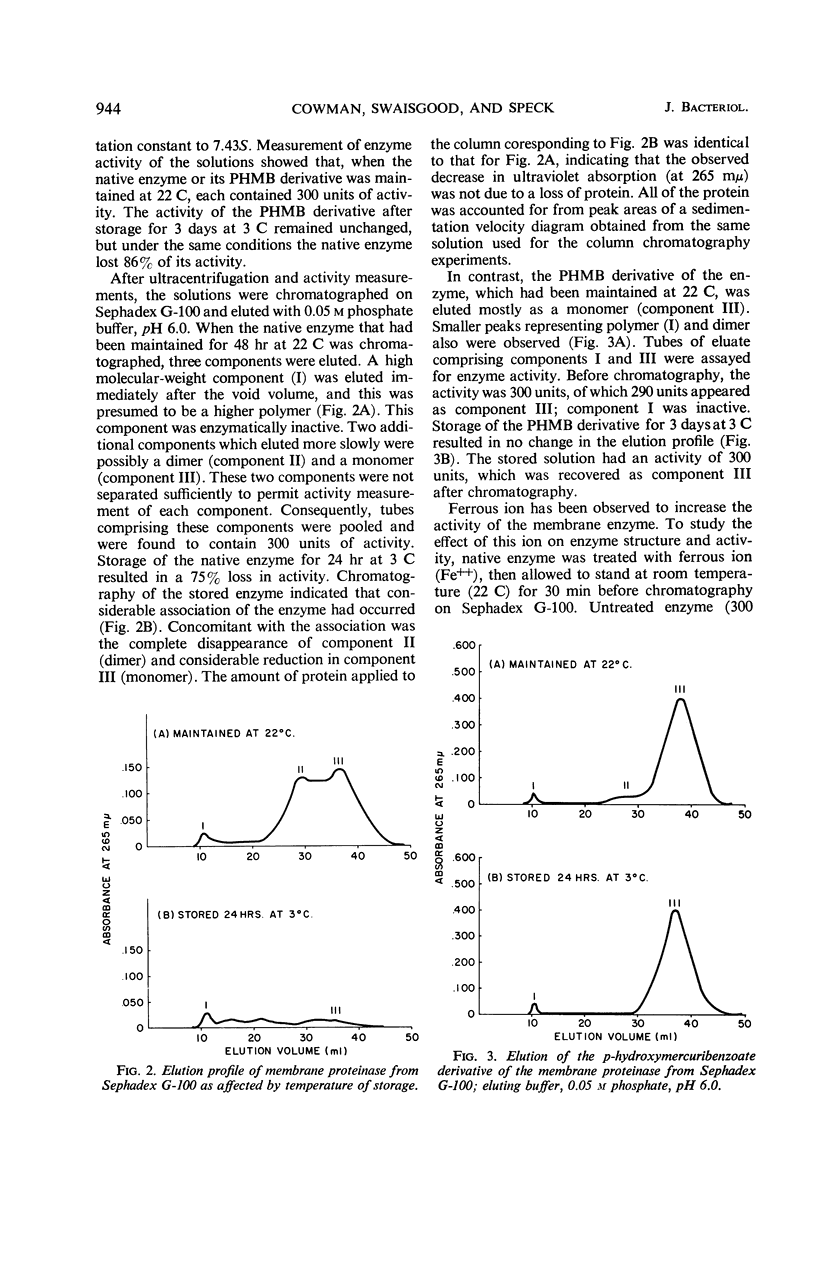
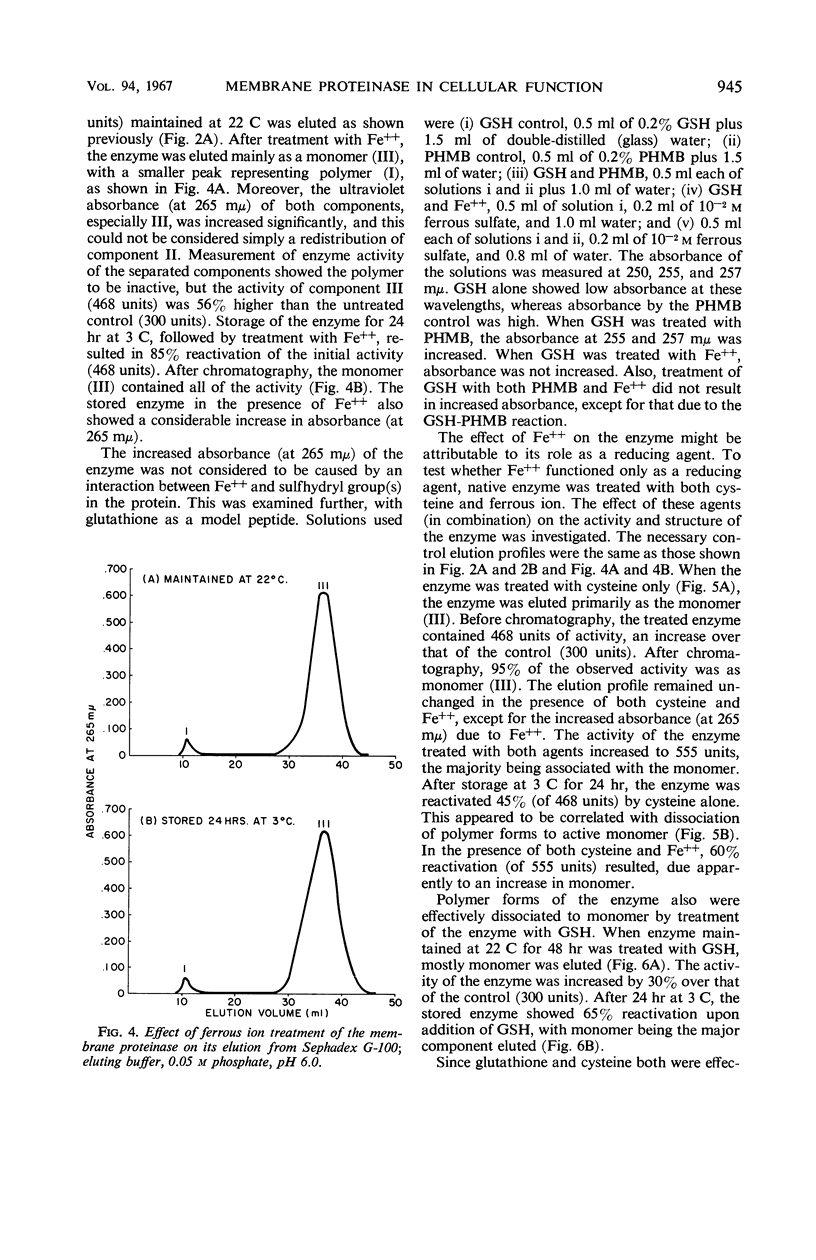
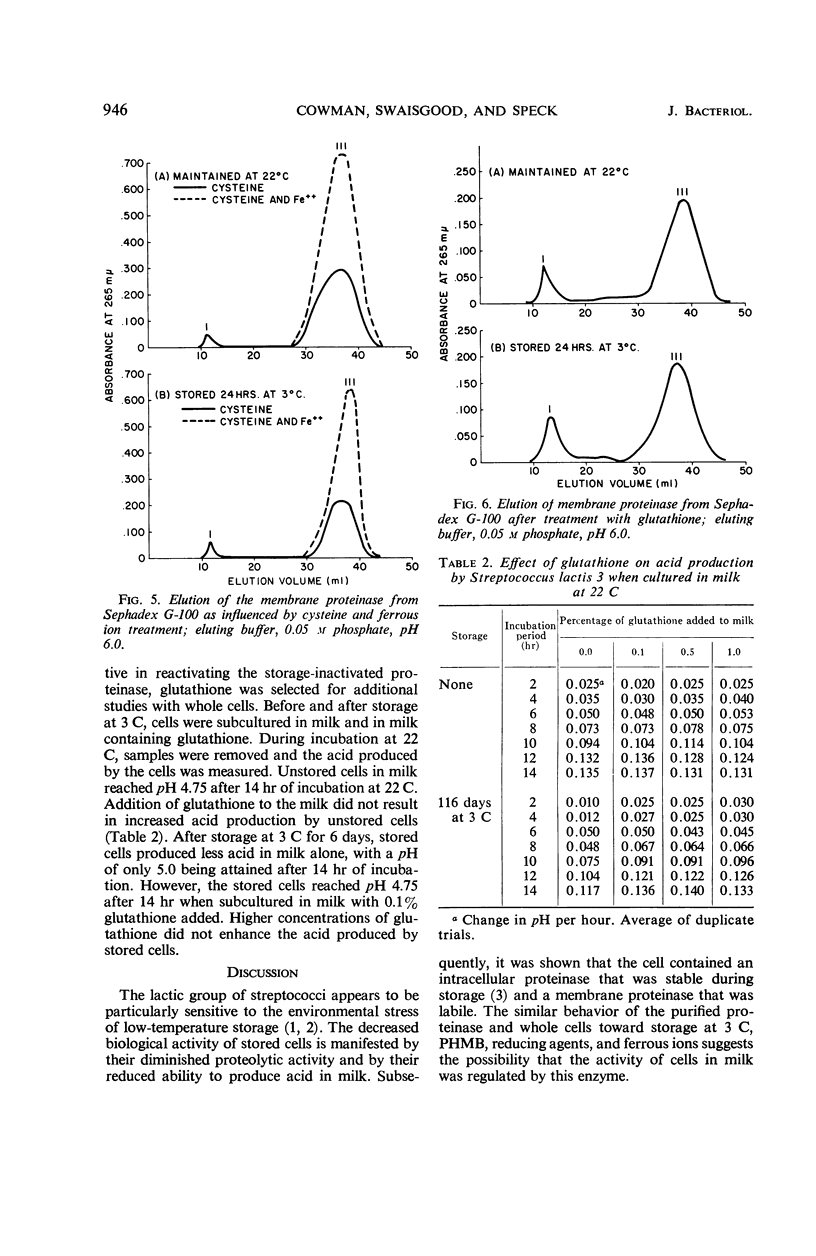
The effect of several environmental conditions on the structure and activity of a membrane-associated proteinase from Streptococcus lactis was investigated. The activity of the enzyme varied with pH. Before storage at 3 C, maximal activity occurred at pH 6.0, but was minimal at this pH after storage. At all pH values tested, the enzyme was inactivated after storage. After storage at 3 C, the enzyme showed gross structural alterations with a concomitant loss of activity. Gel filtration and sedimentation velocity data indicated that inactivation of the enzyme was the result of aggregation to higher molecular weight forms. p-Hydroxymercuribenzoate prevented inactivation of the enzyme during storage by preventing aggregation. Activity was correlated with disaggregation of polymer forms of the enzyme to an active monomer. The storage-inactivated enzyme could be reactivated by treatment of the enzyme with cysteine, glutathione, or ferrous ion. Glutathione enabled stored cells to produce acid at their original rate when subcultured in milk. This was attributed to the effect of glutathione on the membrane proteinase. The data suggested that the biological activity of stored cells may be dependent upon the activity of the membrane proteinase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowman R. A., Speck M. L. Activity of lactic streptococci following storage at refrigeration temperatures. J Dairy Sci. 1965 Nov;48(11):1441–1444. doi: 10.3168/jds.S0022-0302(65)88495-8. [DOI] [PubMed] [Google Scholar]
- Cowman R. A., Speck M. L. Proteinase enzyme system of lactic streptococci. I. Isolation and partial characterization. Appl Microbiol. 1967 Jul;15(4):851–856. doi: 10.1128/am.15.4.851-856.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman R. A., Speck M. L. Ultra-low temperature storage of lactic streptococci. J Dairy Sci. 1965 Nov;48(11):1531–1532. doi: 10.3168/jds.s0022-0302(65)88514-9. [DOI] [PubMed] [Google Scholar]
- HASS L. F., LEWIS M. S. ALKALI-INDUCED STRUCTURAL CHANGES IN MUSCLE ALDOLASE. Biochemistry. 1963 Nov-Dec;2:1368–1376. doi: 10.1021/bi00906a032. [DOI] [PubMed] [Google Scholar]
- HAVIR E. A., TAMIR H., RATNER S., WARNER R. C. BIOSYNTHESIS OF UREA. XI. PREPARATION AND PROPERTIES OF CRYSTALLINE ARGININOSUCCINASE. J Biol Chem. 1965 Jul;240:3079–3088. [PubMed] [Google Scholar]
- MUKATIS W. A., NIEMANN C. VARIATION OF THE CONFORMATION OF THE ACTIVE SITE OF ALPHA-CHYMOTRYPSIN WITH HYDROGEN ION CONCENTRATION. II. Proc Natl Acad Sci U S A. 1964 Mar;51:397–402. doi: 10.1073/pnas.51.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADANA J. C., RITTENBERG D. IRON REQUIREMENT FOR THE HYDROGENASE OF DESULFOVIBRIO DESULFURICANS. Arch Biochem Biophys. 1964 Nov;108:255–257. doi: 10.1016/0003-9861(64)90384-4. [DOI] [PubMed] [Google Scholar]
- SCRUTTON M. C., UTTER M. F. PYRUVATE CARBOXYLASE. 3. SOME PHYSICAL AND CHEMICAL PROPERTIES OF THE HIGHLY PURIFIED ENZYME. J Biol Chem. 1965 Jan;240:1–9. [PubMed] [Google Scholar]
- SHUSTER C. W., DOUDOROFF M. A cold-sensitive D(-) beta-hydroxybutyric acid dehydrogenase from Rhodospirillum rubrum. J Biol Chem. 1962 Feb;237:603–607. [PubMed] [Google Scholar]
- WANG J. H., GRAVES D. J. THE RELATIONSHIP OF THE DISSOCIATION TO THE CATALYTIC ACTIVITY OF GLYCOGEN PHOSPHORYLASE A. Biochemistry. 1964 Oct;3:1437–1445. doi: 10.1021/bi00898a008. [DOI] [PubMed] [Google Scholar]



