Abstract
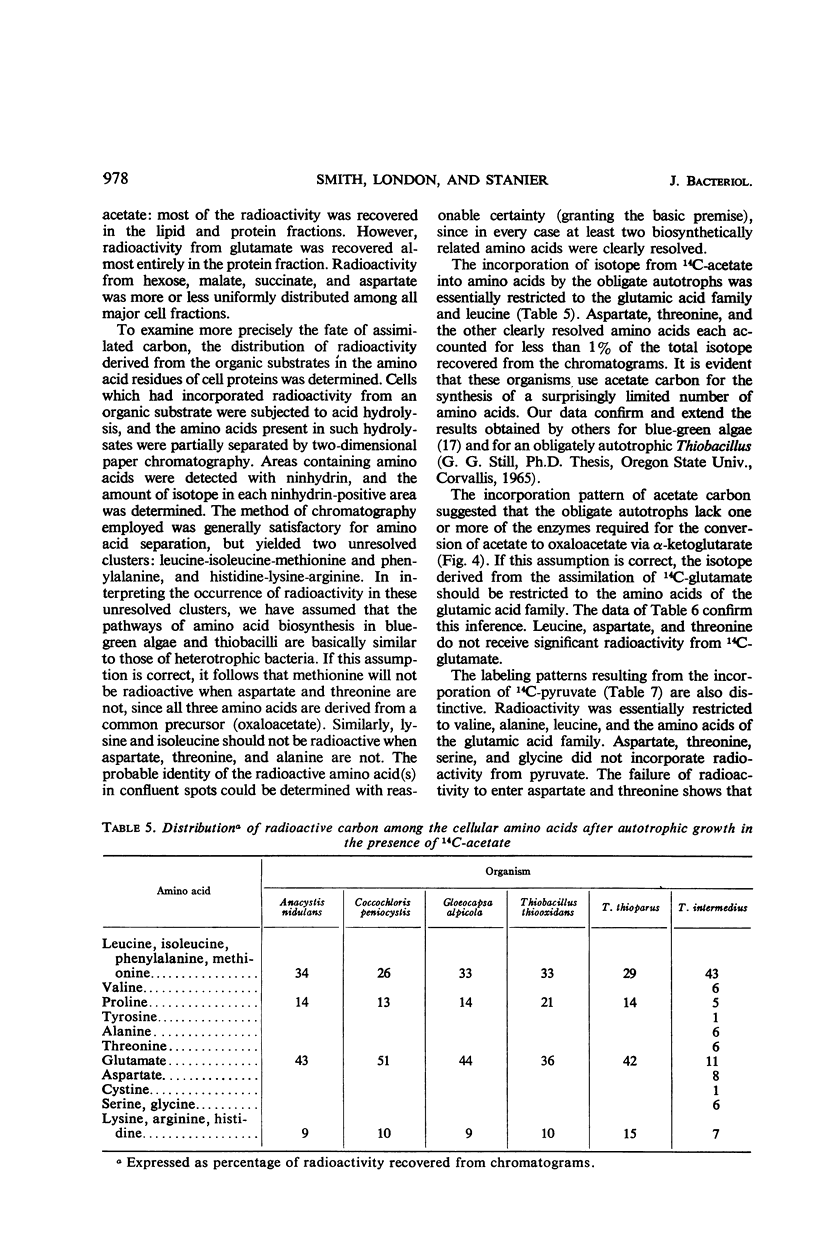
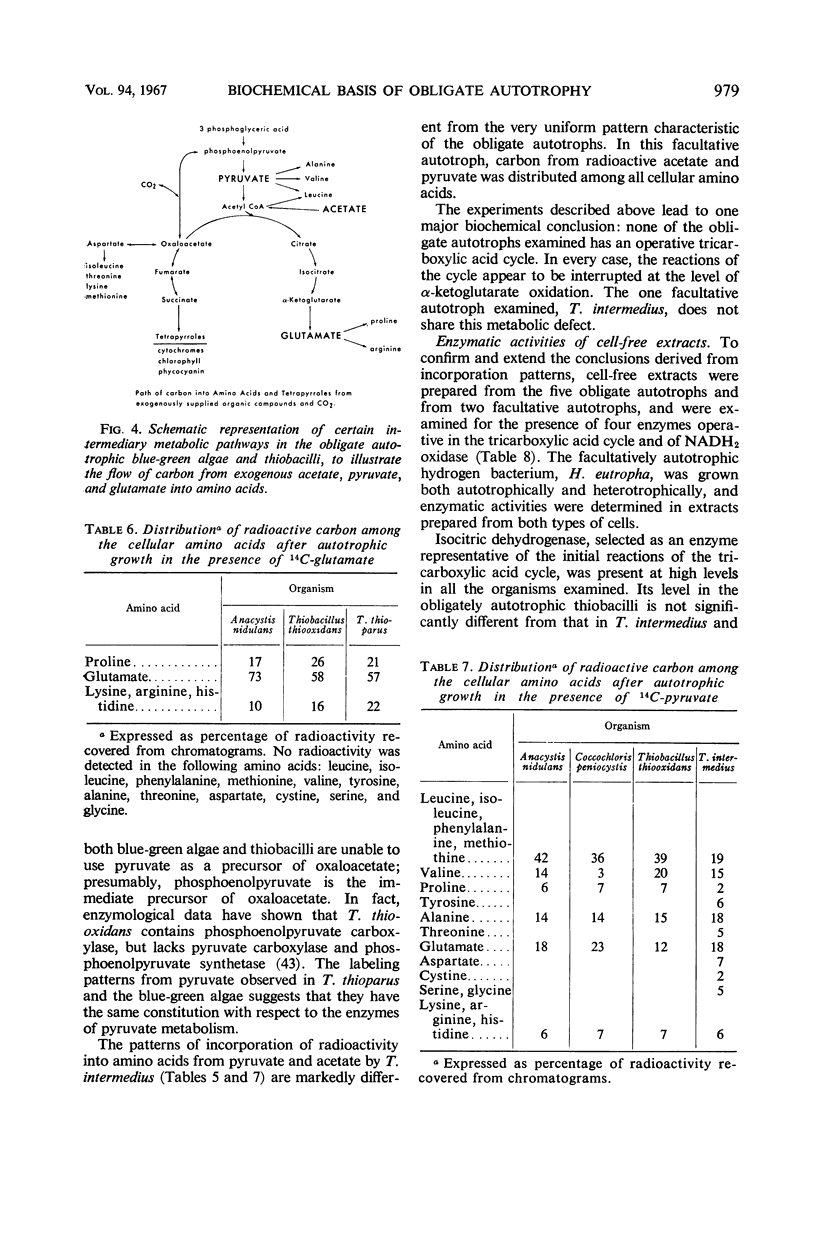
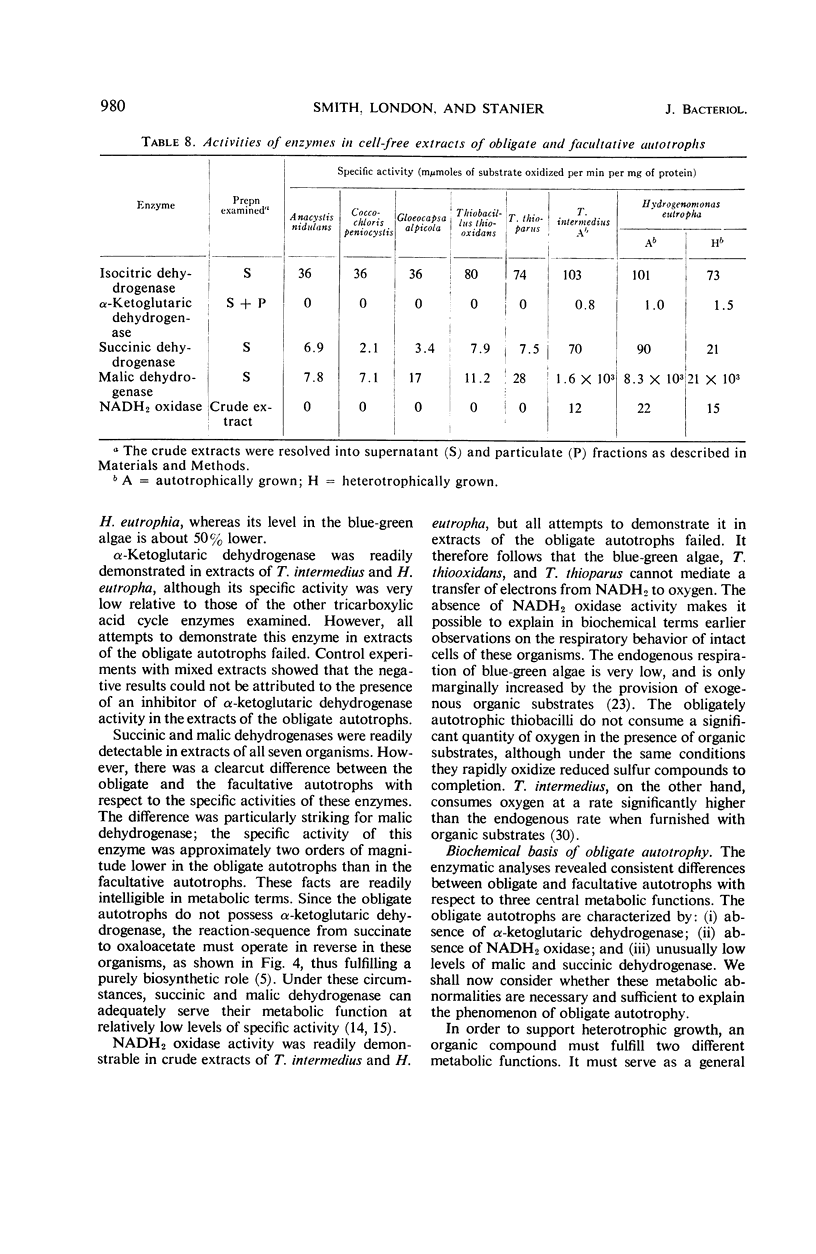
Differential rates of incorporation of sugars, organic acids, and amino acids during autotrophic growth of several blue-green algae and thiobacilli have been determined. In obligate autotrophs (both blue-green algae and thiobacilli), exogenously furnished organic compounds make a very small contribution to cellular carbon; acetate, the most readily incorporated compound of those studied, contributes about 10% of newly synthesized cellular carbon. In Thiobacillus intermedius, a facultative chemoautotroph, acetate contributes over 40% of newly synthesized cellular carbon, and succinate and glutamate almost 90%. In the obligate autotrophs, carbon from pyruvate, acetate, and glutamate is incorporated into restricted groups of cellular amino acids, and the patterns of incorporation in all five organisms are essentially identical. These patterns suggest that the tricarboxylic acid cycle is blocked at the level of α-ketoglutarate oxidation. Enzymatic analyses confirmed the absence of α-ketoglutarate dehydrogenase in the obligate autotrophs, and also revealed that they lacked reduced nicotinamide adenine dinucleotide oxidase, and had extremely low levels of malic and succinic dehydrogenase. These enzymatic deficiencies were not manifested by the two facultative chemoautotrophs examined. On the basis of the data obtained, an interpretation of obligate autotrophy in both physiological and evolutionary terms has been developed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON R. K., SKIPPER H. E., REID M. R., SHORT W. A., HOGAN G. L. Studies on the photosynthetic reaction. I. The assimilation of acetate by Nostoc muscorum. J Biol Chem. 1953 Sep;204(1):197–205. [PubMed] [Google Scholar]
- ARNON D. I. Conversion of light into chemical energy in photosynthesis. Nature. 1959 Jul 4;184:10–21. doi: 10.1038/184010a0. [DOI] [PubMed] [Google Scholar]
- Aleem M. I. Generation of reducing power in chemosynthesis. 3. Energy-linked reduction of pyridine nucleotides in Thiobacillus novellus. J Bacteriol. 1966 Feb;91(2):729–736. doi: 10.1128/jb.91.2.729-736.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem M. I. Path of carbon and assimilatory power in chemosynthetic bacteria. I. Nitrobacter agilis. Biochim Biophys Acta. 1965 Aug 24;107(1):14–28. doi: 10.1016/0304-4165(65)90384-3. [DOI] [PubMed] [Google Scholar]
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- BRALEY S. A., Sr, KINSEL N. A., LEATHEN W. W. Ferrobacillus ferrooxidans: a chemosynthetic autotrophic Bacterium. J Bacteriol. 1956 Nov;72(5):700–704. doi: 10.1128/jb.72.5.700-704.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. G., Umbreit W. W. Absorption and utilization of organic matter by the strict autotroph, Thiobacillus thiooxidans, with special reference to aspartic acid. J Bacteriol. 1966 Feb;91(2):661–666. doi: 10.1128/jb.91.2.661-666.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Growth response of Nitrosomonas europaea to amino acids. J Bacteriol. 1967 Apr;93(4):1302–1308. doi: 10.1128/jb.93.4.1302-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Uptake and utilization of amino acids by resting cells of Nitrosomonas europaea. J Bacteriol. 1967 Apr;93(4):1309–1315. doi: 10.1128/jb.93.4.1309-1315.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C. C., Finstein M. S. Carbon and Energy Sources for the Nitrifying Autotroph Nitrobacter. J Bacteriol. 1965 Jul;90(1):102–107. doi: 10.1128/jb.90.1.102-107.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H. Oxidation and evolution of molecular hydrogen by microorganisms. Bacteriol Rev. 1954 Mar;18(1):43–73. doi: 10.1128/br.18.1.43-73.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- HUGHES E. O., GORHAM P. R., ZEHNDER A. Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol. 1958 Jun;4(3):225–236. doi: 10.1139/m58-024. [DOI] [PubMed] [Google Scholar]
- Hoare D. S., Moore R. B. Photoassimilation of organic compounds by autotrophic blue-green algae. Biochim Biophys Acta. 1965 Nov 29;109(2):622–625. doi: 10.1016/0926-6585(65)90192-5. [DOI] [PubMed] [Google Scholar]
- Ida S., Alexander M. Permeability of Nitrobacter agilis to Organic Compounds. J Bacteriol. 1965 Jul;90(1):151–156. doi: 10.1128/jb.90.1.151-156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN H. L. Effect of organic compounds on nitrosomonas. Nature. 1950 Jun 17;165(4207):974–974. doi: 10.1038/165974a0. [DOI] [PubMed] [Google Scholar]
- Kratz W. A., Myers J. Photosynthesis and Respiration of Three Blue-Green Algae. Plant Physiol. 1955 May;30(3):275–280. doi: 10.1104/pp.30.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSEN H. On the culture and general physiology of the green sulfur bacteria. J Bacteriol. 1952 Aug;64(2):187–196. doi: 10.1128/jb.64.2.187-196.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London J. Cytochrome in Thiobacillus thiooxidans. Science. 1963 Apr 26;140(3565):409–410. doi: 10.1126/science.140.3565.409. [DOI] [PubMed] [Google Scholar]
- London J., Rittenberg S. C. Effects of organic matter on the growth of Thiobacillus intermedius. J Bacteriol. 1966 Mar;91(3):1062–1069. doi: 10.1128/jb.91.3.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL H. G., PFENNIG N. [Enriched culture for various purple sulfur bacteria]. Arch Mikrobiol. 1961;38:1–39. [PubMed] [Google Scholar]
- SUZUKI I., WERKMAN C. H. Chemoautotrophic carbon dioxide fixation by extracts of Thiobacillus thiooxidans. I. Formation of oxalacetic acid. Arch Biochem Biophys. 1958 Jul;76(1):103–111. doi: 10.1016/0003-9861(58)90124-3. [DOI] [PubMed] [Google Scholar]
- Sadler W. R., Stanier R. Y. THE FUNCTION OF ACETATE IN PHOTOSYNTHESIS BY GREEN BACTERIA. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1328–1334. doi: 10.1073/pnas.46.10.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey R. L. Cultivation of Organisms Concerned in the Oxidation of Thiosulfate. J Bacteriol. 1934 Oct;28(4):365–386. doi: 10.1128/jb.28.4.365-386.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBREIT W. W. Symposium on autotrophy. II. The comparative physiology of autotrophic bacteria. Bacteriol Rev. 1962 Jun;26:145–150. [PMC free article] [PubMed] [Google Scholar]



