Abstract
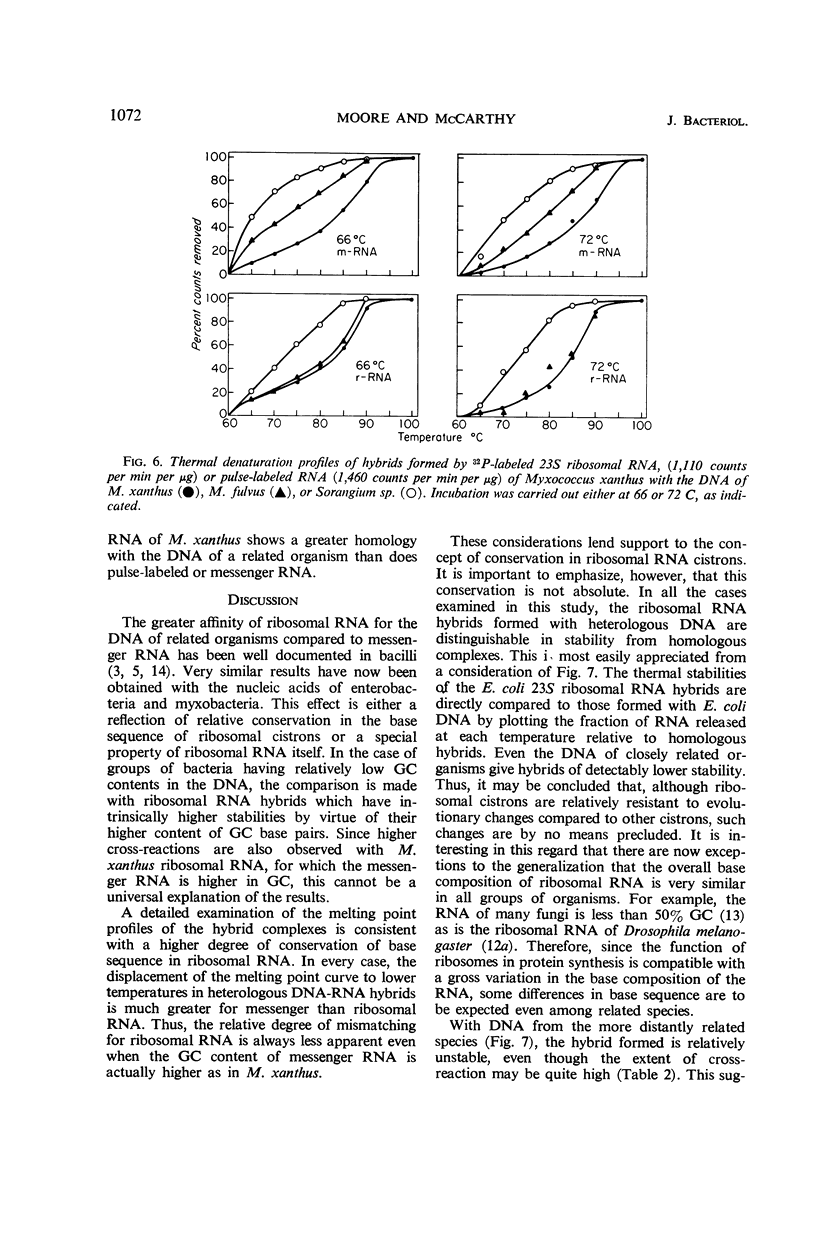
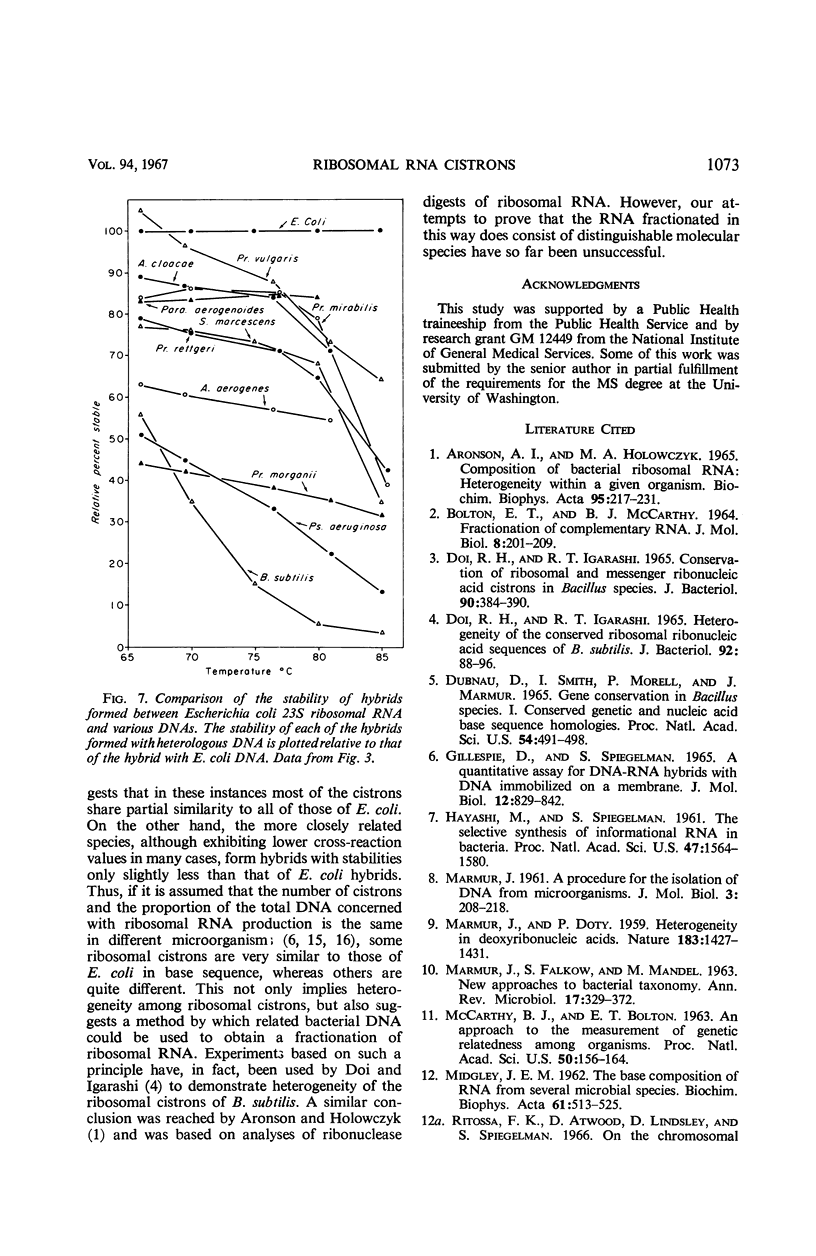
Deoxyribonucleic acid (DNA)-ribonucleic acid (RNA) hybrids are formed by Escherichia coli 16S or 23S ribosomal RNA or pulse-labeled RNA with the DNA of various species of the Enterobacteriaceae. The relative extent of hybrid formation is always greater for ribosomal RNA. These DNA-RNA hybrids have been further characterized by their stability to increasing temperature, and, in every case, the stability of pulse-labeled RNA hybrids was lower than that of the corresponding ribosomal RNA hybrids, although 16S and 23S ribosomal RNA hybrids had very similar stabilities. Therefore, ribosomal RNA showed a greater degree of apparent conservation in base sequence than pulse-labeled or messenger RNA both in the extent of cross-reaction and in the stability of hybrid structures. Similar results were obtained with Myxococcus xanthus RNA. Since in this case the base composition of the pulse-labeled or messenger RNA is richer in guanine plus cytosine than ribosomal RNA, the higher cross-reaction of ribosomal RNA is more readily attributable to conservation of base sequence in these cistrons than to its base composition. Thus, the base sequence of ribosomal RNA cistrons of bacilli, enteric bacteria, and myxobacteria is conserved relative to those of the rest of the genomes. This conservation is, however, not absolute since the stability of heterologous ribosomal RNA hybrids is always lower than that of homologous hybrids.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I., HOLOWCZYK M. A. COMPOSITION OF BACTERIAL RIBOSOMAL RNA. HETEROGENEITY WITHIN A GIVEN ORGANISM. Biochim Biophys Acta. 1965 Feb 8;95:217–231. doi: 10.1016/0005-2787(65)90487-9. [DOI] [PubMed] [Google Scholar]
- BOLTON E. T., MCCARTHY B. J. FRACTIONATION OF COMPLEMENTARY RNA. J Mol Biol. 1964 Feb;8:201–209. doi: 10.1016/s0022-2836(64)80129-7. [DOI] [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. CONSERVATION OF RIBOSOMAL AND MESSENGER RIBONUCLEIC ACID CISTRONS IN BACILLUS SPECIES. J Bacteriol. 1965 Aug;90:384–390. doi: 10.1128/jb.90.2.384-390.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H., Igarashi R. T. Heterogeneity of the conserved ribosomal ribonucleic acid sequences of Bacillus subtilis. J Bacteriol. 1966 Jul;92(1):88–96. doi: 10.1128/jb.92.1.88-96.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., SPIEGELMAN S. The selective synthesis of informational RNA in bacteria. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1564–1580. doi: 10.1073/pnas.47.10.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Heterogeneity in deoxyribonucleic acids. I. Dependence on composition of the configurational stability of deoxyribonucleic acids. Nature. 1959 May 23;183(4673):1427–1429. doi: 10.1038/1831427a0. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. An approach to the measurement of genetic relatedness among organisms. Proc Natl Acad Sci U S A. 1963 Jul;50:156–164. doi: 10.1073/pnas.50.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck R. Nucleotide composition of nucleic acids of fungi. I. Ribonucleic acids. J Bacteriol. 1965 Nov;90(5):1260–1264. doi: 10.1128/jb.90.5.1260-1264.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. Distinct cistrons for the two ribosomal RNA components. Proc Natl Acad Sci U S A. 1963 Apr;49:538–544. doi: 10.1073/pnas.49.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. II. Saturation of and competitive interaction at the RNA cistron. Proc Natl Acad Sci U S A. 1962 Aug;48:1466–1472. doi: 10.1073/pnas.48.8.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]



