Abstract
Opiates are potent analgesic and addictive compounds. They also act on immune responses, and morphine, the prototypic opiate, has been repeatedly described as an immunosuppressive drug. Pharmacological studies have suggested that the inhibitory action of opiates on immunity is mediated by multiple opioid receptor sites but molecular evidence has remained elusive. Recently, three genes encoding μ- (MOR), δ-, and κ-opioid receptors have been cloned. To investigate whether the μ-opioid receptor is functionally implicated in morphine immunosuppression in vivo, we have examined immune responses of mice with a genetic disruption of the MOR gene. In the absence of drug, there was no difference between wild-type and mutant mice with regard to a large number of immunological endpoints, suggesting that the lack of MOR-encoded protein has little consequence on immune status. Chronic morphine administration induced lymphoid organ atrophy, diminished the ratio of CD4+CD8+ cells in the thymus and strongly reduced natural killer activity in wild-type mice. None of these effects was observed in MOR-deficient mice after morphine treatment. This demonstrates that the MOR gene product represents a major molecular target for morphine action on the immune system. Because our previous studies of MOR-deficient mice have shown that this receptor protein is also responsible for morphine analgesia, reward, and physical dependence, the present results imply that MOR-targeted therapeutic drugs that are developed for the treatment of pain or opiate addiction may concomitantly influence immune responses.
Endogenous opioid peptides and their receptors form a neuromodulatory system that controls a number of physiological processes, including the control of pain and emotions; the response to stress, locomotion, and cognition; as well as neuroendocrine and immune functions (1). Opioid receptors are also targets for exogenous opiates, the prototype of which is morphine, and mediated their strong analgesic (2) and addictive actions (see ref. 3). Morphine remains one of the most valuable drugs for the treatment of severe pain, despite a large number of adverse side effects, which include respirator depression, constipation, tolerance, and dependence (4). Another potential drawback is the inhibitory action of morphine on immune responses. More generally, the suppressive effect of opiates on immune responses has been demonstrated both in animal models and humans (5, 6), and was shown to account for increased susceptibility to infections (6, 7).
Our present knowledge of interactions that exist between opiates and the immune system is based on pharmacological studies, and multiple mechanisms have been proposed. A number of in vivo studies show an indirect action of opiates via the central nervous system, and continuing along the hypothalamo-pituitary-adrenal axis (8, 9) or sympathetic pathways (10), while many in vitro experiments have suggested that opiates act at immune cells directly (11). In any instance, receptors that represent primary sites for opiate action have been poorly characterized. The demonstration of naloxone-sensitive effects (6) has suggested an implication of the classically described μ, δ, and κ opioid receptor sites (12). Naloxone-insensitive actions of opiates were also reported and the existence of nonclassical opioid receptors that would specifically bind the C-terminal portion of β-endorphin (13), or recognize alkaloids but not peptidic opioid ligands (14), has been proposed. The pharmacology of opiates on immune responses seems highly complex, possibly due to the implication of a wide diversity of opioid receptors sites. Therefore the molecular basis for opiate action on the immune system needs to be clarified.
Recently, three opioid receptors have been cloned from brain and the pharmacological profile of the recombinant receptors correlates well with that of the previously described μ-, δ- and κ-opioid binding sites (reviewed in ref. 15). Genes encoding these receptors are referred as to MOR (μ), DOR (δ), and KOR (κ). Their mRNAs are largely distributed throughout the nervous system (16) and some reports indicate detectable expression in immune cells also (17, 18), suggesting that they are targets for direct action. The implication of these cloned opioid receptors in opiate immunomodulation in vivo however has not been demonstrated.
Here we have used a genetic approach to correlate functional activity of the MOR gene with the known pharmacology of opiate action on immunity. We have previously constructed MOR-deficient mutant mice and shown the complete absence of μ-opioid receptor binding sites in those mice, with a concomittant loss of morphine-induced analgesia, reward, and physical dependence (19). These data have demonstrated that the MOR gene product is critically involved in these best known actions of morphine on the central nervous system. In this report, we have examined the consequences of chronic morphine administration on a variety of immune parameters in MOR-deficient mice. The comparison of morphine action in wild-type and mutant mice clearly establishes that the MOR-encoded protein is essential for morphine action on the immune system.
METHODS
Knockout Mice.
Mice deficient in the MOR gene (129 × C57BL/6 genetic background) were constructed by homologous recombination as described (19). Young adults (7 and 13 weeks) were used.
Splenocyte and Thymocyte Preparation, Staining, and Flow Cytometry.
Spleen and thymuses from wild-type and MOR-deficient mice were dissected out, weighed, and single cell suspensions were prepared. To obtain the distribution of immune cell populations within these organs, 5 × 105 cells were incubated at 0°C for 30 min with fluorescent antibodies specific for B lymphocytes (anti-IgM-fluorescein, Jackson ImmunoResearch), CD4+ (anti-CD4-phycoerythrin, Caltag, South San Francisco, CA) and CD8+ (anti-CD8a-fluorescein, Caltag) T lymphocytes and analyzed with a Profile cytofluorimeter (Coulter).
Natural Killer (NK) Assay.
Splenocytes were adjusted to 107 cells per ml in complete culture medium (DMEM supplemented with 10% fetal calf serum/2 mM l-glutamine/1 mM nonessential amino acids/5 × 10−5 M 2-mercaptoethanol, 50 units/ml penicillin/50 μg/ml streptomycin). YAC-1 cells were labeled by incubation with 51Cr for 1 hr at 37°C, washed, and adjusted to 105 per ml. Mixtures of 100 μl spleen cell suspension and 100 μl labeled YAC-1 cells were incubated for 4 hr in microplates at variable effector/target ratios (100:1, 50:1, 25:1, and 12.5:1). Plates were then centrifuged at 450 × g for 5 min and 100 μl supernatant were recovered from each well and counted. Supernatants from wells in which only YAC-1 cells were incubated served to determine spontaneous release. Maximum release was obtained by incubating target cells in 5% Triton X-100. Percent specific lysis is obtained from: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. One lytic unit corresponds to the number of spleen cells causing 20% lysis of YAC target cells.
In Vitro Lymphocyte Proliferation.
Splenocytes (5 × 105) were distributed into microtiter plates and stimulated with concanavalin A (2 μg/ml) for T lymphocytes and lipopolysaccharide (10 μg/ml) for B lymphocytes. Mitogen concentrations were adjusted to provide maximal T and B lymphocyte proliferation. Cultures, in triplicates, were pulsed with 0.5 μCi of [methyl-3H]thymidine (Amersham) during the last 18 hr of a 66 hr incubation period. Incorporation of [methyl-3H]thymidine was determined using a Packard beta counter.
Measurement of Antibody Production.
Measurement of in vitro stimulated Ig production was performed on day 3 lipopolysaccharide-stimulated cultures supernatants by ELISA using anti-mouse (IgM + IgG) and anti-mouse IgM antibodies for coating, and alkaline phosphatase-conjugated rabbit anti-(IgM+IgG) antibody for detection (Jackson 115 006 44, 715 005 020, and 315 055 044, respectively).
Chronic Morphine Treatment.
Morphine dependence was induced in mice (females, 6–14 weeks of age) by repeated i.p. injections of morphine chlorhydrate every 12 hr during 6 days. Morphine dose were progressively increased as follows: day 1, 20 mg/kg; day 2, 40 mg/kg; day 3, 60 mg/kg, day 4, 80 mg/kg, day 5, 100 mg/kg, day 6, only one injection, 100 mg/kg (19). Control mice were injected with saline under the same conditions. One hour after the last injection, lymphoid organs were removed and immune parameters determined. The number of animals per group ranged from 13 to 15.
Statistical Analysis.
Data were analyzed for statistically significant differences by the two-way ANOVA (mutation and treatment) between animals. Individual groups comparisons were made by the unpaired two-tailed Student’s t test.
RESULTS
Immune Parameters Are Not Altered in MOR-Deficient Mice.
To determine if the absence of the μ-opioid receptor itself has any influence on the immune status of mice, we have compared a number of immune parameters in untreated wild-type and mutant mice (Table 1). The size of lymphoid organ appeared undistinguishable between +/+ and −/− genotypes, independently whether size was expressed as a function of organ weight per g total body weight, or as total cell number per 100 mg organ weight (Table 1). The distribution of spleen and thymus cell populations was investigated. We observed that the percentages of splenic B, CD4+, and CD8+ T lymphocytes, as well as thymic CD4+CD8+, CD4+, and CD8+ cells were identical in mice from both genotypes, indicating that lymphoid organ cellularities are not modified in mutant mice (Table 1). In vitro splenocytes responsiveness was examined. The cytolytic function of splenic NK cells, and proliferative responses of T and B cells were unaltered in MOR-deficient mice. Also in vitro Ig production and Ig serum levels were identical in MOR +/+ and MOR −/− mice. Therefore, all immune parameters that we have measured were unaffected by the absence of μ-receptors.
Table 1.
Comparable immune parameters in wild-type mice (MOR +/+) and mice lacking the μ-opioid receptor (MOR −/−)
| Immune parameters | MOR +/+ | MOR −/− | ||
|---|---|---|---|---|
| Lymphoid organ size | Spleen | Weight, mg/g body weight | 4.1 ± 0.03 (11) | 4.6 ± 0.4 (10) |
| Cells/100 mg spleen (× 107) | 9.7 ± 1.0 (24) | 11.6 ± 1.2 (23) | ||
| Thymus | Weight, mg/body weight | 4.0 ± 0.4 (11) | 4.2 ± 0.4 (11) | |
| Cells/100 mg thymus (× 107) | 19.5 ± 1.7 (17) | 20.5 ± 1.8 (18) | ||
| Cell distribution | Spleen | % B lymphocytes | 45.6 ± 1.8 (15) | 48.2 ± 1.5 (14) |
| % CD4+ T lymphocytes | 27.2 ± 1.9 (10) | 26.1 ± 2.4 (8) | ||
| % CD8+ T lymphocytes | 13.7 ± 1.3 (11) | 9.8 ± 0.6 (10) | ||
| Thymus | % CD4+ lymphocytes | 14.3 ± 4.0 (6) | 14.1 ± 1.4 (6) | |
| % CD8+ lymphocytes | 5.5 ± 2.1 (6) | 4.6 ± 0.5 (6) | ||
| % CD4+CD8+ lymphocytes | 77.9 ± 6.4 (6) | 78.9 ± 2.2 (6) | ||
| Splenocyte responsiveness | NK activity | LU/107 cells | 11.1 ± 1.0 (10) | 14.1 ± 1.5 (10) |
| T lymphocytes | In vitro proliferation (P.I.) | 18.7 ± 1.9 (10) | 17.7 ± 2.9 (10) | |
| B lymphocytes | In vitro proliferation (P.I.) | 11.9 ± 1.1 (10) | 10.0 ± 0.6 (10) | |
| In vitro Ig production, μg/ml | 7.8 ± 0.9 (10) | 9.8 ± 1.6 (10) | ||
| Serum immunoglobulins | Total Ig, mg/ml | 3.4 ± 0.5 (12) | 3.4 ± 0.3 (12) | |
| IgM, mg/ml | 0.24 ± 0.03 (12) | 0.21 ± 0.03 (12) | ||
NK activity was measured by a 51Cr release assay and is expressed as lytic units (LU) per 107 effector cells. In vitro splenic T and B lymphocyte proliferation was assessed after mitogenic stimulation and is presented as proliferation indices (P.I.), calculated as ratios of cpm incorporated in the presence of mitogen to cpm incorporated in the absence of mitogen. Total Ig production by in vitro LPS stimulated splenocytes as well as serum total Ig and IgM levels were determined using ELISA. Results are shown as means ± SEM. Numbers in parentheses represent the number of mice analyzed. Data from both genotypes were compared using the two-tailed Student’s t test. P values were >0.05 for all measured parameters.
Morphine Induces Immunosuppression in Wild-Type But Not in MOR-Deficient Mice.
We have investigated the immunosuppressive action of morphine in both MOR+/+ and MOR−/− mice after chronic treatment. We administered daily injections of escalating doses of drug, a treatment that we showed highly effective to induce morphine physical dependence in the mouse strain under study (19). All immune parameters that we have measured in nontreated mice (Table 1) were reevaluated in the chronically morphine-treated animals. Some immune characteristics, including in vitro T cell proliferative activity and B cell functions, appeared unchanged for both mouse genotypes (data not shown). In contrast, other parameters were markedly modified in wild-type mice, indicating that the chronic morphine regimen had successfully altered several immune functions.
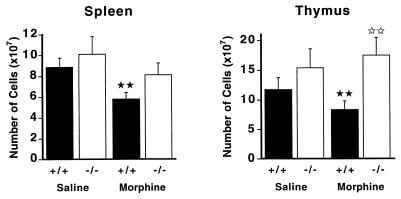
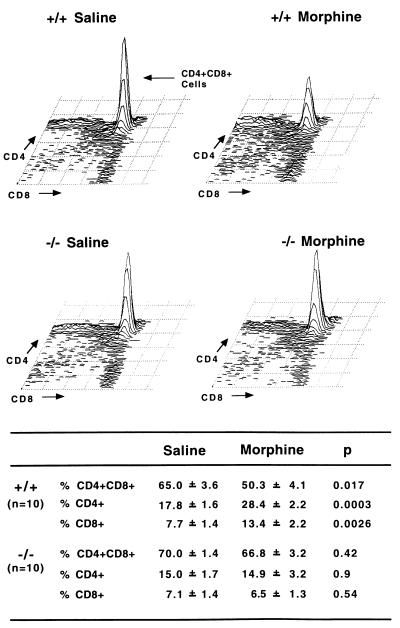
In wild-type mice, morphine administration induced atrophy of both spleen and thymus (Fig. 1), in accordance with previous work reporting a morphine-induced reduction of lymphoid organ weights in other mouse strains (20–23). We further investigated cellular changes associated with this phenomenon. In spleen, the relative contents of B cells, CD4+ and CD8+ T lymphocytes were not modified after morphine treatment, suggesting that there was no depletion of a particular splenic cell subpopulation, but rather an overall reduction in cell numbers (data not shown). In contrast, the reduction of cell content in the thymus was found associated with an alteration of cellular distribution (Fig. 2). The percentage of double positive CD4+CD8+ thymocytes was diminished by 23% while ratios of single positive cells concomitantly increased (CD4+, +60%; CD8+, +74%). This reduction of immature thymocytes content correlates well with the reported apoptosis of double positive cells after chronic morphine treatment in another mouse strain (24).
Figure 1.
Lymphoid organ atrophy in MOR +/+ but not in MOR −/− mutant mice after chronic morphine administration. Mice were treated with increasing doses of morphine for 6 days (see Materials and Methods). The total number of cells in spleen and thymus were determined from each individual animal (13–15 mice per group) and results are expressed as means ± SEM. For both organs there is a significant difference between saline and morphine-treated wild-type animals, as indicated by ★★ for P < 0.01. No significant effect of morphine treatment is observed in mutant mice. In the thymus there is a significant difference between morphine-treated wild-type and mutant animals, as shown by II for P < 0.01 (thymus).
Figure 2.
Altered distribution of thymic cell populations in MOR +/+ but not in MOR −/− mutant mice after chronic morphine treatment. On top, a representative fluorescence-activated cell sorter analysis of thymocytes stained with fluorescent anti-CD4 and -CD8 antibodies highlights the decrease in CD4+CD8+ double positive cells and increase in single positive CD4+ and CD8+ cells after morphine in +/+ mice. The bottom table shows FACS analyses of thymocytes stained with fluorescent anti-CD4 and -CD8 antibodies for 10 individuals within each group. Results are expressed as mean ± SEM. The decrease in CD4+CD8+ double positive cells and increase in single positive CD4+ and CD8+ cells are significant in morphine-treated wild-type mice compared with saline-treated animals. Morphine administration does not induce any significant change in the cellular distribution of mutant mice.
In mutant mice, morphine did not induce any modification of lymphoid organs. We found no significant reduction in the total cell numbers for both spleen and thymus after chronic morphine administration (Fig. 1). In addition, there was no alteration in the distribution of cell populations in the thymus (Fig. 2) and spleen (not shown) of MOR −/− mutant mice. Thus, morphine-induced alteration of lymphoid organs is absent in mice lacking the μ-opioid receptor.
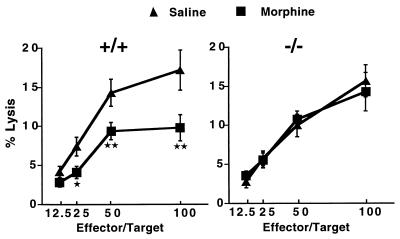
Another consequence of the chronic morphine treatment was a marked reduction of splenic NK activity in wild-type mice. Cytotoxicity decreased by 43%, 35%, 45%, and 33% at 100:1, 50:1, 25:1, and 12.5:1 effector/target ratios, respectively (Fig. 3 Left), an effect that is among the best characterized immunosuppressive action of morphine in rodents (21, 22, 25) In contrast, mice devoid of μ-receptor exhibited identical NK cell activity after saline or morphine treatment (Fig. 3 Right). This indicates that chronic morphine administration does not depress the cytotoxic activity of NK cells in the absence of μ-receptors.
Figure 3.
Reduced NK activity in MOR +/+ but not in MOR −/− mutant mice after chronic morphine administration. Wild-type and MOR-deleted mice were chronically treated with saline or morphine (see Materials and Methods). Splenic NK cell activity was measured in the presence of YAC target cells using a 51Cr assay (see Materials and Methods). Data are expressed as mean percentages of YAC cell lysis (± SEM) at four different effector/target ratios. The number of mice per group ranged from 14 to 15. Significant differences are obtained between saline- and morphine-treated wild-type animals, and are indicated as follows: ★ for P < 0.05 and ★★ for P < 0.01.
DISCUSSION
It is believed that the endogenous opioid system regulates immune functions (6), a hypothesis that mainly relies on the observation of immunoregulatory effects of opioid peptides in in vitro experiments. However the question of whether opioid peptides tonically regulate immune responses in vivo is unclear. Indeed, data obtained from the administration of opioid antagonists in vivo have provided controversial results because the opioid antagonists naloxone and naltrexone have been shown to exert enhancing (26) inhibitory effects (20, 22) or no effect (reviewed in ref. 6) on immune function. Furthermore, the lack of receptor subtype selectivity of those compounds does not allow to conclude on the specific involvement of μ-opioid receptor sites in the regulation immune function under physiological conditions. Here we show that immune parameters in MOR-deficient mice are unchanged. These results suggest that endogenous activation of the MOR-encoded receptor is not critical for the maintenance of immune capacities. Alternatively, one cannot exclude that the apparent absence of modifications in MOR −/− mutant mice results from the development of yet undiscovered (19) compensatory mechanisms. One should note that mice used in this part of the study were not exposed to any treatment or threatening environmental stimuli. Future studies involving in vivo immunization or infection may reveal modifications of immune responses in the absence of μ-opioid receptors. Also, studies performed under circumstances that specifically recruit the endogenous opioid system, such as stressful conditions or inflammatory pain, may evoke different responses in wild-type or MOR-deficient mice.
Morphine reduces immunocompetence in vivo by molecular mechanisms that remain to be established. Morphine has been shown to influence the immune system in both naloxone-sensitive and insensitive manners (6). Some effects, including lymphoid organs atrophy, could be reversed partially or totally by the opioid antagonists naloxone and naltrexone (20, 27). In addition, morphine-induced reduction of NK activity was found to be differentially antagonized by μ-selective antagonists. The inhibition of NK cytotoxicity was reverse by β-funaltrexamine (μ1- and μ2-selective) but not by naloxonazine (μ1-selective), suggesting that a subpopulation of μ-receptor sites only would be involved in morphine immunosupression for this specific action of morphine (28). Altogether, pharmacological studies suggest that morphine immunosuppression is mediated by multiple mechanisms without providing any clue on the exact nature of receptor sites that mediate morphine action at the molecular level. In our study we have observed lymphoid organ atrophy as well as strong reduction of NK cytotoxicity in wild-type hybrid 129/sv × C57BL/6 mice after chronic morphine administration, as described in other mouse strains (6). Most importantly, these two well-described immunosuppressive actions of morphine are absent in MOR-deficient mice. This unequivocally demonstrates that the MOR gene product is an essential molecular target for morphine immunosuppression.
Former pharmacological studies have proposed that the activation of all three μ-, δ-, and κ-opioid receptors results in immunomodulation (29, 30). The complete absence of morphine immunosuppression in the MOR-deficient mice in this study suggests that the δ- and κ-opioid receptors, or other receptor sites, are not critically involved in the action of morphine on the immune system. This is likely due to the fairly good μ-selectivity of morphine, which would activate the MOR-encoded receptor exclusively in wild-type mice, under our conditions of chronic administration. In agreement, binding studies performed on brain tissues (31) and in cell lines expressing the recombinant receptors (32), have shown that morphine binds at μ-receptors with a two-order-of-magnitude higher affinity compared with δ- and κ-receptors. Additional investigations including the use of δ- and κ-specific agonists in MOR-deficient mice, and the analysis of DOR- and KOR-deficient mice, will clarify the specific contribution of DOR and KOR genes in opiate immunomodulation.
In conclusion, morphine-induced analgesia (19, 33), reward (19), physical dependence (19), and immunosuppression (this study) are abolished in MOR-deficient mice. Altogether these results demonstrate that the same receptor protein is responsible for both the main desired and adverse actions of morphine in vivo. This result has important implications in the search for appropriate strategies to treat pain or opiate addiction.
Acknowledgments
We wish to thank C. Zilliox, C. Waltzinger, and C. Ebel for technical assistance and J. F. Poirier and N. Schallon for animal care. We thank P. Chambon and J. C. Stoclet for constant support. This work was funded by the Centre National de la Recherche Scientifique, Association pour la Recherche sur le Cancer, The Ministère de la Recherche et de la Technologie, The Institut Universitaire de France, and the European Community (Biomed-2).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MOR, μ-opioid receptor; NK, natural killer.
References
- 1.Olson G A, Olson R D, Kastin A J. Peptides. 1996;17:1421–1466. doi: 10.1016/s0196-9781(96)00225-2. [DOI] [PubMed] [Google Scholar]
- 2.Pasternak G W. Clin Pharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Koob G F. Trends Biol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 4.Shug S A, Zech D, Grond S. Drug Safety. 1992;7:200–213. doi: 10.2165/00002018-199207030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Rouveix B. Therapie. 1992;47:503–512. [PubMed] [Google Scholar]
- 6.Bryant H U, Holaday J W. In: Opioids in Immunologic Processes. Herz A, editor. 104/II. Berlin: Springer; 1993. pp. 361–335. [Google Scholar]
- 7.Donahoe R M. Adv Neuroimmunol. 1993;3:31–46. [Google Scholar]
- 8.Pruett S B, Han Y-C, Fuchs B A. J Pharmacol Exp Ther. 1992;262:923–928. [PubMed] [Google Scholar]
- 9.Sei Y, Yoshimoto K, McIntyre T, Skolnick P, Arora P K. J Immunol. 1991;146:194–198. [PubMed] [Google Scholar]
- 10.Carr D J, Mayo S, Gebhardt B M, Porter J. J Neuroimmunol. 1994;53:53–63. doi: 10.1016/0165-5728(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 11.Sibiga N E, Goldstein A. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein A, Naidu A. Mol Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- 13.Van den Bergh P, Rozing J, Nagelkerken L. Lymphokine Cytokine Res. 1994;13:63–69. [PubMed] [Google Scholar]
- 14.Stefano G, Scharrer B, Smith E M, Hughes T K J, Magazine H L, Bilfinger T V, Hartman A R, Fricchione G L, Liu Y, Makman M H. Crit Rev Immunol. 1996;16:109–144. doi: 10.1615/critrevimmunol.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- 15.Kieffer B L. In: The Pharmacology of Pain. Dickinson A, Besson J, editors. Vol. 130. Berlin: Springer; 1997. pp. 281–303. [Google Scholar]
- 16.Mansour A, Fox C A, Akil H, Watson S J. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 17.Gavériaux C, Peluso J, Simonin F, Laforet J, Kieffer B. FEBS Lett. 1995;369:272–276. doi: 10.1016/0014-5793(95)00766-3. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Loh H H. Neurochem Res. 1996;21:1375–1386. doi: 10.1007/BF02532379. [DOI] [PubMed] [Google Scholar]
- 19.Matthes H W D, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, et al. Nature (London) 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 20.Bussiere J L, Adler M W, Rogers T R, Eisenstein T K. Immunopharmacol Immunotoxicol. 1992;14:657–673. doi: 10.3109/08923979209005416. [DOI] [PubMed] [Google Scholar]
- 21.Pacofoci R, Bacosi A, Caronna A, Di Carlo S, Pichini S, Zuccaro P. Immunopharmacol Immunotoxicol. 1992;14:355–381. doi: 10.3109/08923979209005399. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava H N, Thomas P T, Thorat S, House R V. Brain Res. 1994;642:1–10. doi: 10.1016/0006-8993(94)90899-0. [DOI] [PubMed] [Google Scholar]
- 23.Bryant H U, Bernton E W, Holaday J W. J Pharmacol Exp Ther. 1988;245:913–920. [PubMed] [Google Scholar]
- 24.Fuchs B A, Pruett S B. J Pharmacol Exp Ther. 1993;266:417–423. [PubMed] [Google Scholar]
- 25.Lysle D T, Coussons M E, Watts V J, Bennett E H, Dykstra L A. J Pharmacol Exp Ther. 1993;265:1071–1078. [PubMed] [Google Scholar]
- 26.Carr D j J, Blalock J E. Psychoneuroendocrinology. 1991;16:407–415. doi: 10.1016/0306-4530(91)90005-e. [DOI] [PubMed] [Google Scholar]
- 27.Eisenstein T K, Bussiere J L, Rogers T J, Adler M W. Adv Exp Med Biol. 1993;335:41–52. doi: 10.1007/978-1-4615-2980-4_7. [DOI] [PubMed] [Google Scholar]
- 28.Carr D J, Gebhardt B M, Paul D. J Pharmacol Exp Ther. 1993;264:1179–1186. [PubMed] [Google Scholar]
- 29.Guan L, Townsend R, Eisenstein T K, Adler M W, Rogers T J. Brain Behav Immun. 1994;8:229–240. doi: 10.1006/brbi.1994.1021. [DOI] [PubMed] [Google Scholar]
- 30.Shahabi N A, Sharp B M. J Pharmacol Exp Ther. 1995;273:1105–1113. [PubMed] [Google Scholar]
- 31.Corbett A D, Paterson S J, Kosterlitz H W. In: Opioids I. Herz A, editor. 104/I. Berlin: Springer; 1993. pp. 645–673. [Google Scholar]
- 32.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell G I, Reisine T. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- 33.Sora I, Takahashi N, Funada M, Ujike H, Revay R S, Donovan D M, Miner L L, Uhl G R. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]