Abstract
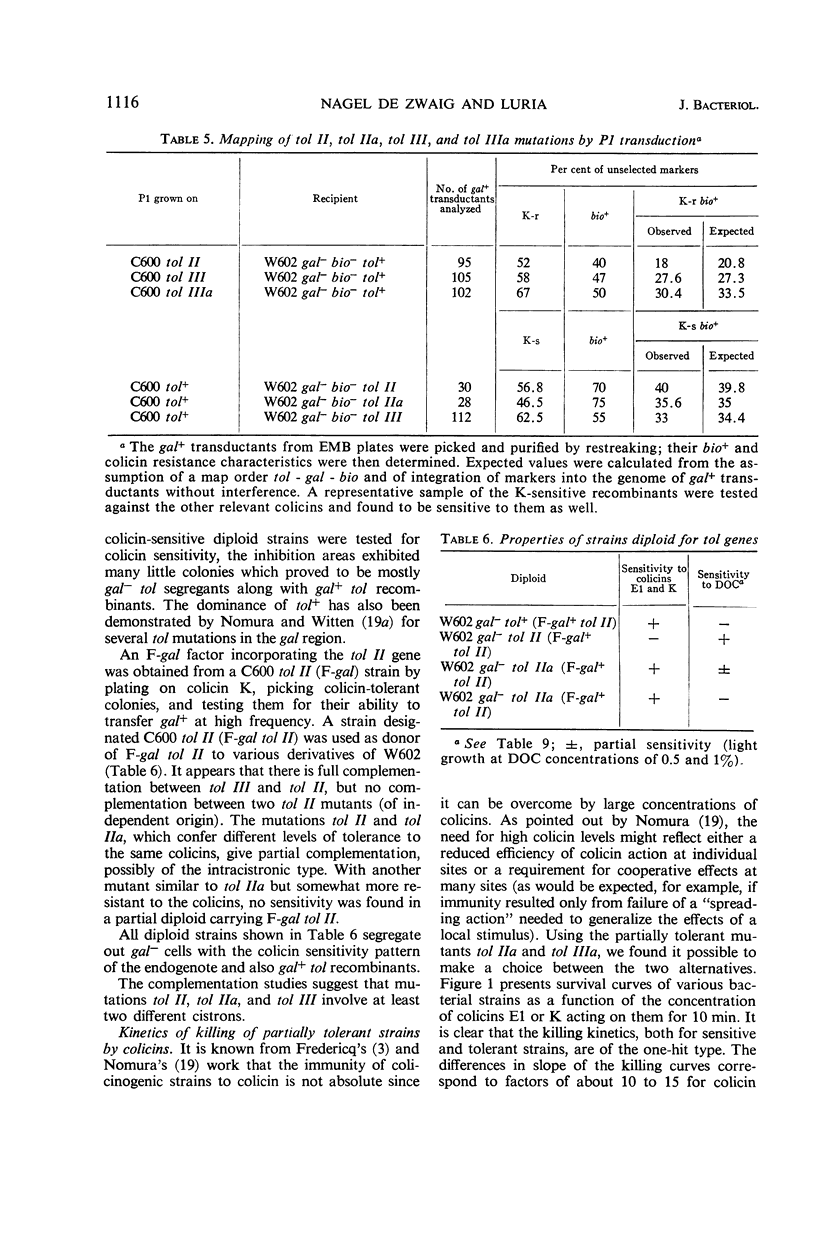
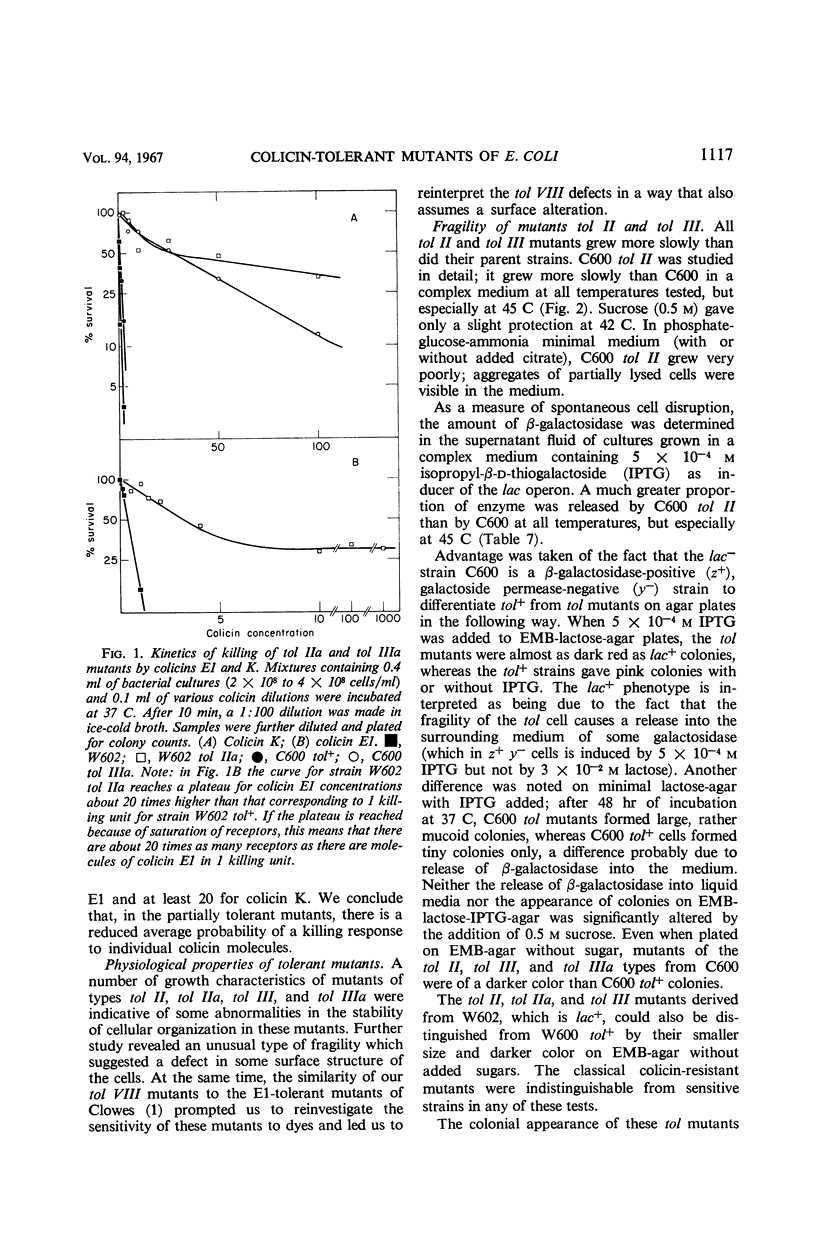
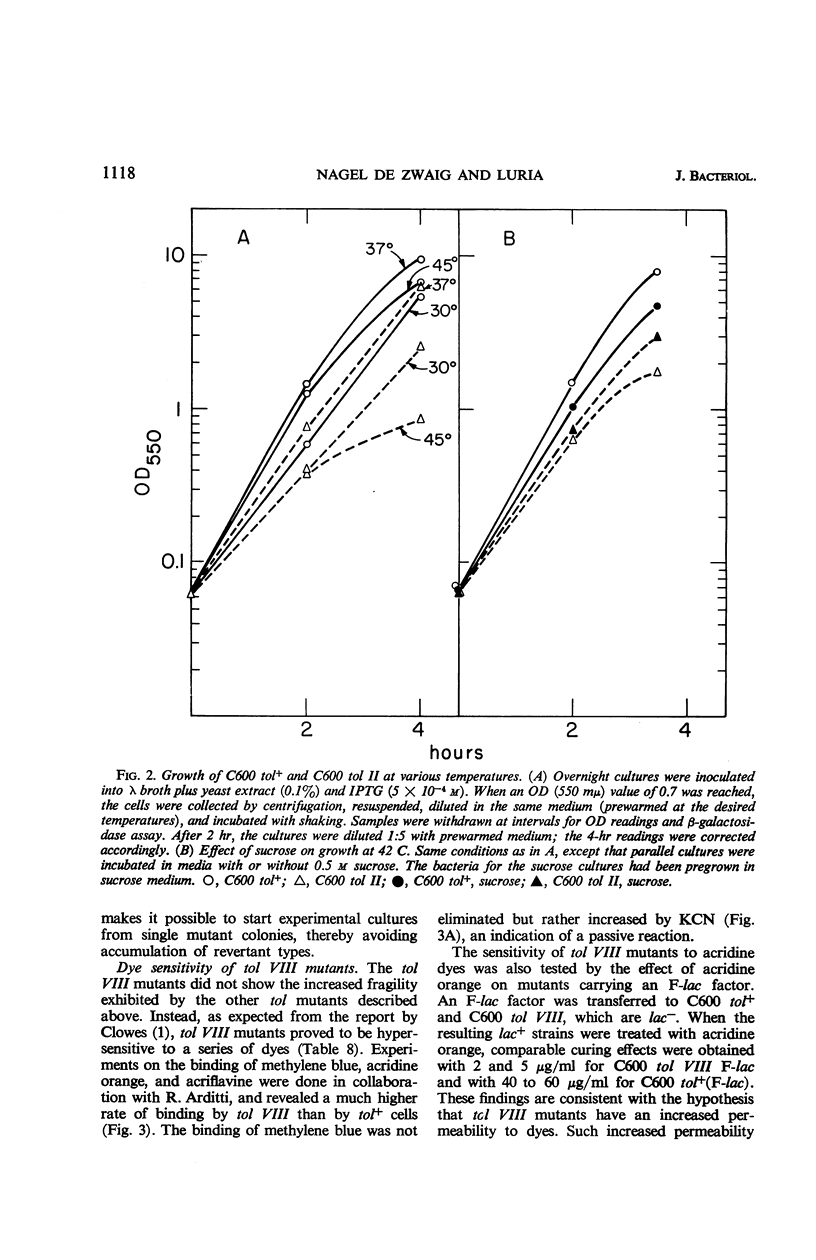
A series of colicin-tolerant (tol) mutants of Escherichia coli K-12, which adsorbed colicins but were not killed by them, were isolated and studied genetically and physiologically. Three major classes of mutants were found: tol II, tolerant to colicins A, E1, E2, E3, and K; tol III, tolerant to A, E2, E3, and K; and tol VIII, tolerant to E1 only. The sites of tol II and tol III mutations mapped near the gal region (gene order: tol-gal-bio) and were cotransduced with gal by P1. In heterozygous diploids, tol+ was dominant over tol; tol II and tol III gave full complementation. All the tol mutations that mapped near gal rendered the bacteria more fragile during growth and hypersensitive to deoxycholate and to ethylenediaminetetraacetic acid. The tol VIII mutation mapped between str and his. These mutants were extremely sensitive to deoxycholate and were also hypersensitive to methylene blue, acridines, and various other compounds. The sensitivity is attributed to increased uptake due to selective alteration of the permeability barrier. The colicin-tolerant mutations are interpreted as affecting some components of the cytoplasmic membrane which mediate between the adsorbed colicin molecules and the target sites of their biochemical effects in the bacterial cell.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FREDERICQ P. Colicins and colicinogenic factors. Symp Soc Exp Biol. 1958;12:104–122. [PubMed] [Google Scholar]
- GRATIA J. P. R'ESISTANCE 'A LA COLICINE B CHEZ E. COLI. RELATIONS DE SP'ECIFICIT'E ENTRE COLICINES B, I ET V ET PHAGE T-4. ETUDE G'EN'ETIQUE. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:151. [PubMed] [Google Scholar]
- GREEN D. E., HECHTER O. ASSEMBLY OF MEMBRANE SUBUNITS. Proc Natl Acad Sci U S A. 1965 Feb;53:318–325. doi: 10.1073/pnas.53.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., Ryter A., Cuzin F. On the association between DNA and membrane in bacteria. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):267–278. doi: 10.1098/rspb.1966.0029. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Lederberg J. Detection of Fermentative Variants with Tetrazolium. J Bacteriol. 1948 Nov;56(5):695–695. doi: 10.1128/jb.56.5.695-695.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkres K. D., Woodward D. O. On the genetics of enzyme locational specificity. Proc Natl Acad Sci U S A. 1966 May;55(5):1217–1224. doi: 10.1073/pnas.55.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M. MECHANISM OF ACTION OF COLICINES. Proc Natl Acad Sci U S A. 1964 Dec;52:1514–1521. doi: 10.1073/pnas.52.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Acriflavine-binding capacity of Escherichia coli in relation to acriflavine sensitivity and metabolic activity. J Bacteriol. 1966 Nov;92(5):1447–1452. doi: 10.1128/jb.92.5.1447-1452.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Gene-Controlled Resistance to Acriflavine and Other Basic Dyes in Escherichia coli. J Bacteriol. 1965 Jul;90(1):8–14. doi: 10.1128/jb.90.1.8-14.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J., Azoulay E., Pichinoty F. Etude génétique d'une mutation à effet pléiotrope chez l'Escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1967 Mar 13;264(11):1507–1509. [PubMed] [Google Scholar]
- REVEL H. R., LURIA S. E., ROTMAN B. Biosynthesis of B-D-galactosidase controlled by phage-carried genes. I. Induced beta-D-galactosidase biosynthesis after transduction of gene z-plus by phage. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1956–1967. doi: 10.1073/pnas.47.12.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P. Mutants resistant to colicin CA42-E2: cross resistance and genetic mapping of a special class of mutants. Aust J Exp Biol Med Sci. 1966 Jun;44(3):301–315. doi: 10.1038/icb.1966.29. [DOI] [PubMed] [Google Scholar]
- Rothman J. L. Transduction studies on the relation between prophage and host chromosome. J Mol Biol. 1965 Jul;12(3):892–912. doi: 10.1016/s0022-2836(65)80336-9. [DOI] [PubMed] [Google Scholar]
- Sugino Y. Mutants of Escherichia coli sensitive to methylene blue and acridines. Genet Res. 1966 Feb;7(1):1–11. doi: 10.1017/s0016672300009423. [DOI] [PubMed] [Google Scholar]



