Abstract
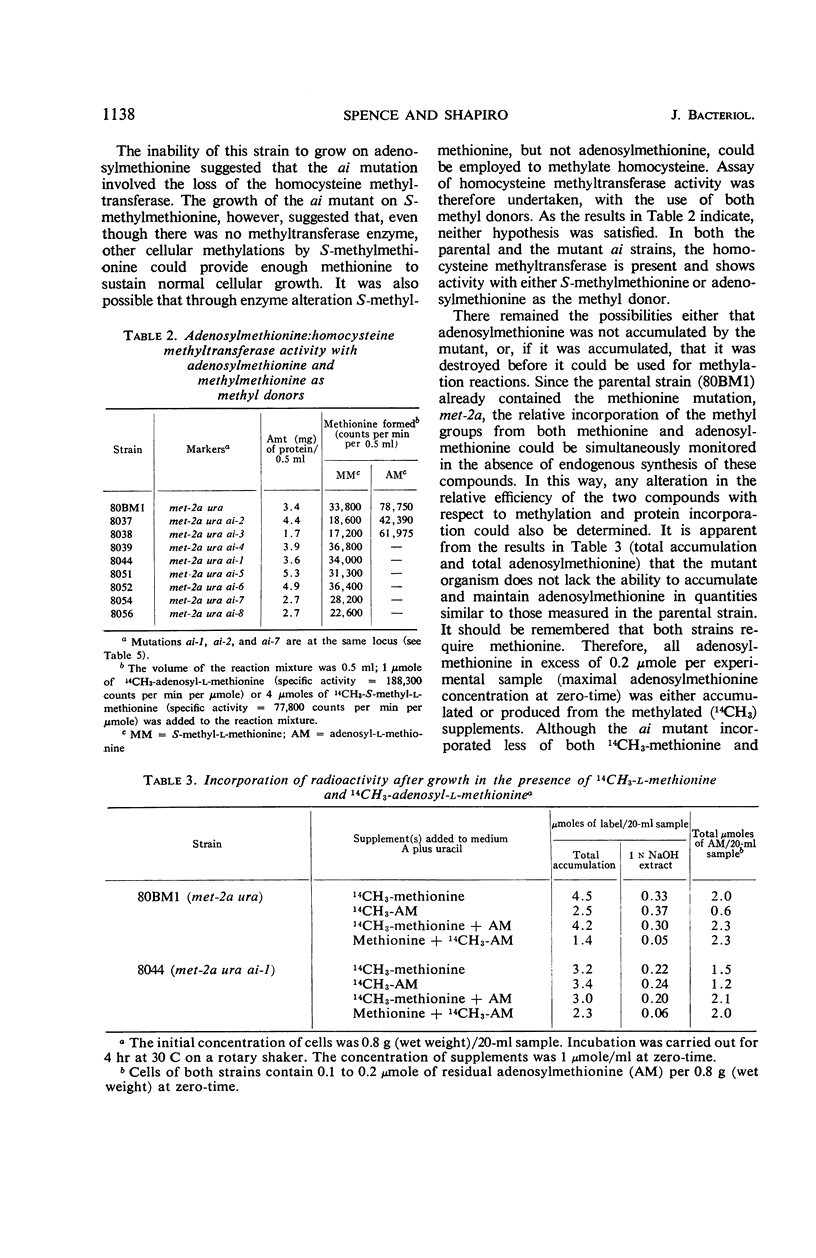
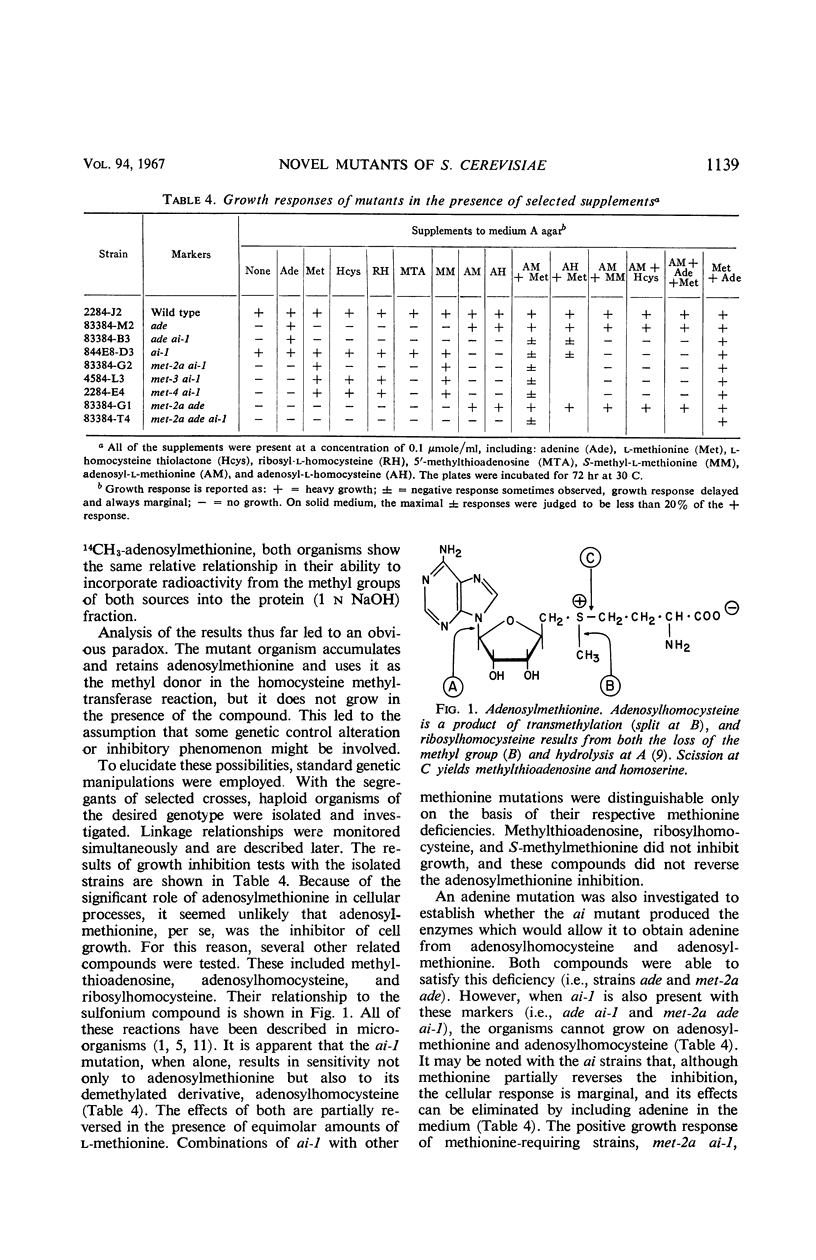
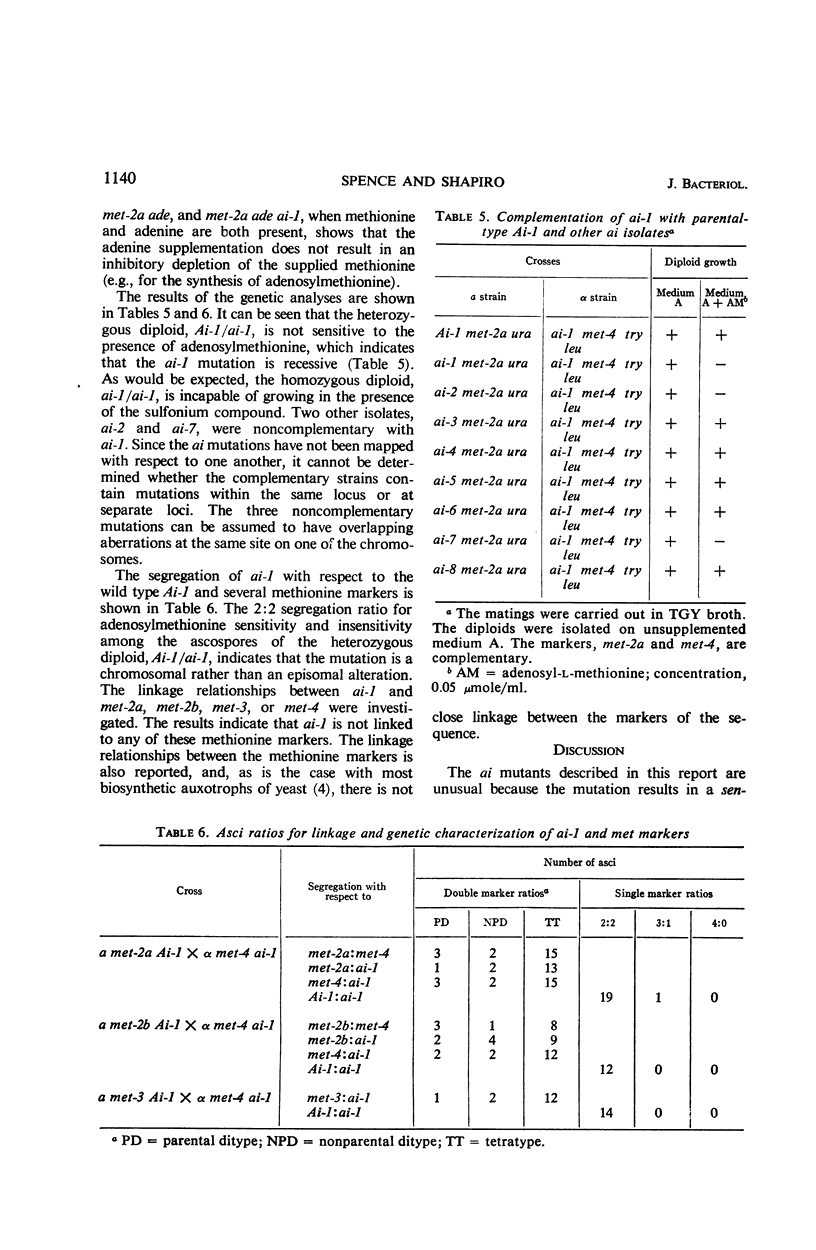
These investigations have established the existence of a novel type of non-nutritional mutant (ai) which is inhibited in the presence of two naturally occurring cellular compounds. The inhibition is complete at an extracellular concentration at least as low as 0.05 μmole/ml of either adenosylhomocysteine or adenosylmethionine. It is suggested that adenosylhomocysteine is the true inhibitor. The ai mutants are phenotypically indistinguishable from the wild type in the absence of inhibitors. The results have shown that, if any direct effect on the methionine biosynthetic pathway exists, it is a secondary rather than the primary effect of the inhibitors. The ai mutation does not involve the loss of the adenosylmethionine (or methylmethionine): homocysteine methyltransferase. In addition, the ai mutants accumulate, maintain, and utilize adenosylmethionine and methionine in a manner similar to the parental strain. No genetic relationship could be detected between the ai-1 mutation and several different markers affecting methionine biosynthesis. The ai-1 mutation was also shown to be genetically recessive. Methionine partially reverses the inhibition caused by adenosylmethionine or adenosylhomocysteine. Neither methylmethionine nor homocysteine reversed the inhibition, which showed that the homocysteine methyltransferase cannot catalyze the synthesis of sufficient methionine under these conditions to simulate the effects of extracellularly supplied methionine. If adenine is present, methionine does not cause reversal of inhibition due to adenosylmethionine or adenosylhomocysteine. From the data presented, it is clear that the ai mutation involves some metabolic control mechanism, though the alteration does not appear to be associated primarily with the biosynthesis of methionine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUERRE J. A. Preparation and properties of S-adenosyl-L-homocysteine, S-adenosyl-L-homocysteine sulfoxide and S-ribosyl-L-homocysteine. Arch Biochem Biophys. 1962 Jan;96:70–76. doi: 10.1016/0003-9861(62)90453-8. [DOI] [PubMed] [Google Scholar]
- DUERRE J. A., SCHLENK F. Formation and metabolism of S-adenosyl-L-homocysteine in yeast. Arch Biochem Biophys. 1962 Mar;96:575–579. doi: 10.1016/0003-9861(62)90339-9. [DOI] [PubMed] [Google Scholar]
- MUDD S. H. Enzymatic cleavage of S-adenosylmethionine. J Biol Chem. 1959 Jan;234(1):87–92. [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIGG C. J., SORSOLI W. A., PARKS L. W. INDUCTION OF THE METHIONINE-ACTIVATING ENZYME IN SACCHAROMYCES CEREVISIAE. J Bacteriol. 1964 Apr;87:920–923. doi: 10.1128/jb.87.4.920-923.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIGG C. J., SPENCE K. D., PARKS L. W. Methionine biosynthesis in yeast. Arch Biochem Biophys. 1962 Jun;97:491–496. doi: 10.1016/0003-9861(62)90112-1. [DOI] [PubMed] [Google Scholar]
- POMPER S. A pH-sensitive, multiple mutant of saccharomyces cerevisiae. J Bacteriol. 1952 Sep;64(3):353–361. doi: 10.1128/jb.64.3.353-361.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAPIRO S. K., ALMENAS A., THOMSON J. F. BIOSYNTHESIS OF METHIONINE IN SACCHAROMYCES CEREVISIAE. KINETICS AND MECHANISM OF REACTION OF S-ADENOSYLMETHIONINE:HOMOCYSTEINE METHYLTRANSFERASE. J Biol Chem. 1965 Jun;240:2512–2518. [PubMed] [Google Scholar]
- SORSOLI W. A., SPENCE K. D., PARKS L. W. AMINO ACID ACCUMULATION IN ETHIONINE-RESISTANT SACCHAROMYCES CEREVISIAE. J Bacteriol. 1964 Jul;88:20–24. doi: 10.1128/jb.88.1.20-24.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk F. The chemistry of biological sulfonium compounds. Fortschr Chem Org Naturst. 1965;23:61–112. doi: 10.1007/978-3-7091-7139-4_3. [DOI] [PubMed] [Google Scholar]
- Schlenk F., Zydek C. R., Ehninger D. J., Dainko J. L. The production of S-adenosyl-L-methionine and S-adenosyl-L-ethionine by yeast. Enzymologia. 1965 Nov 6;29(3):283–298. [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]



