Abstract
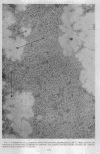
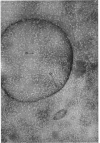
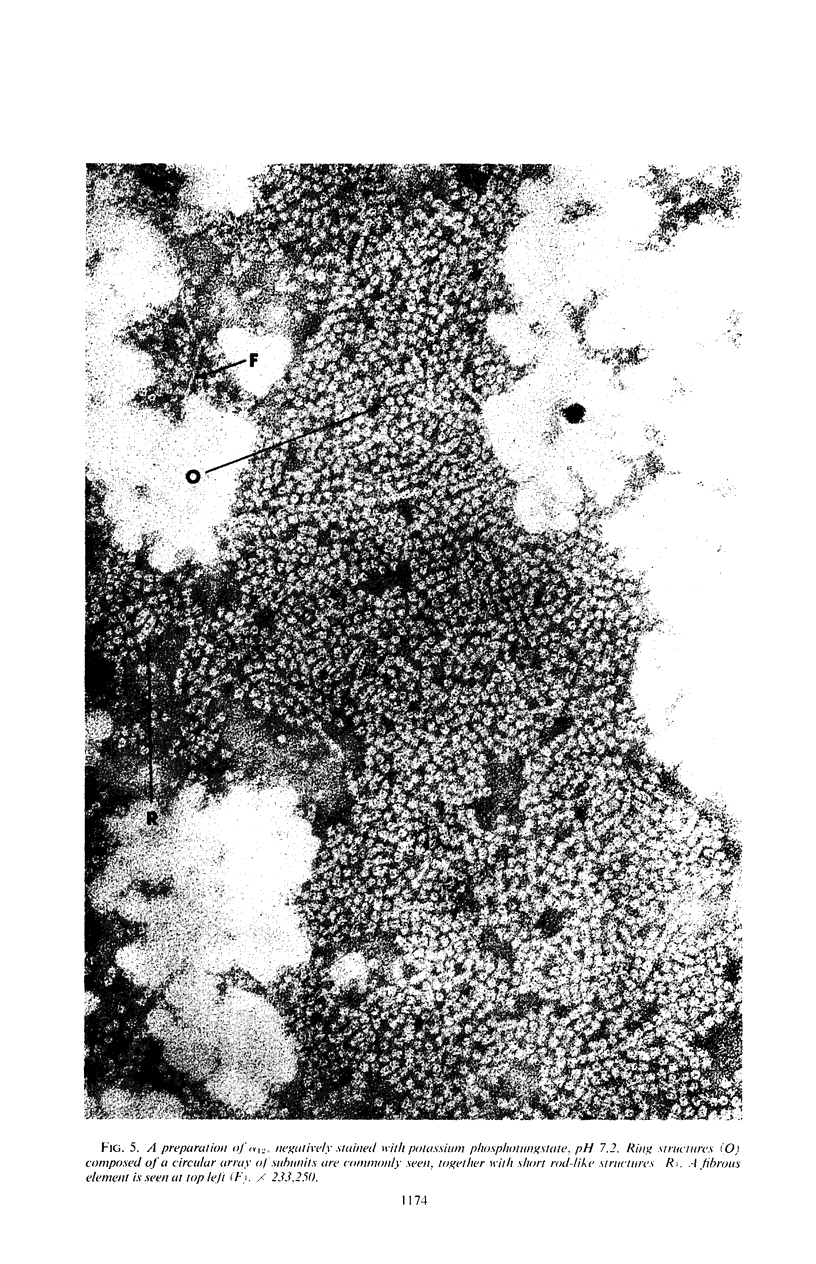
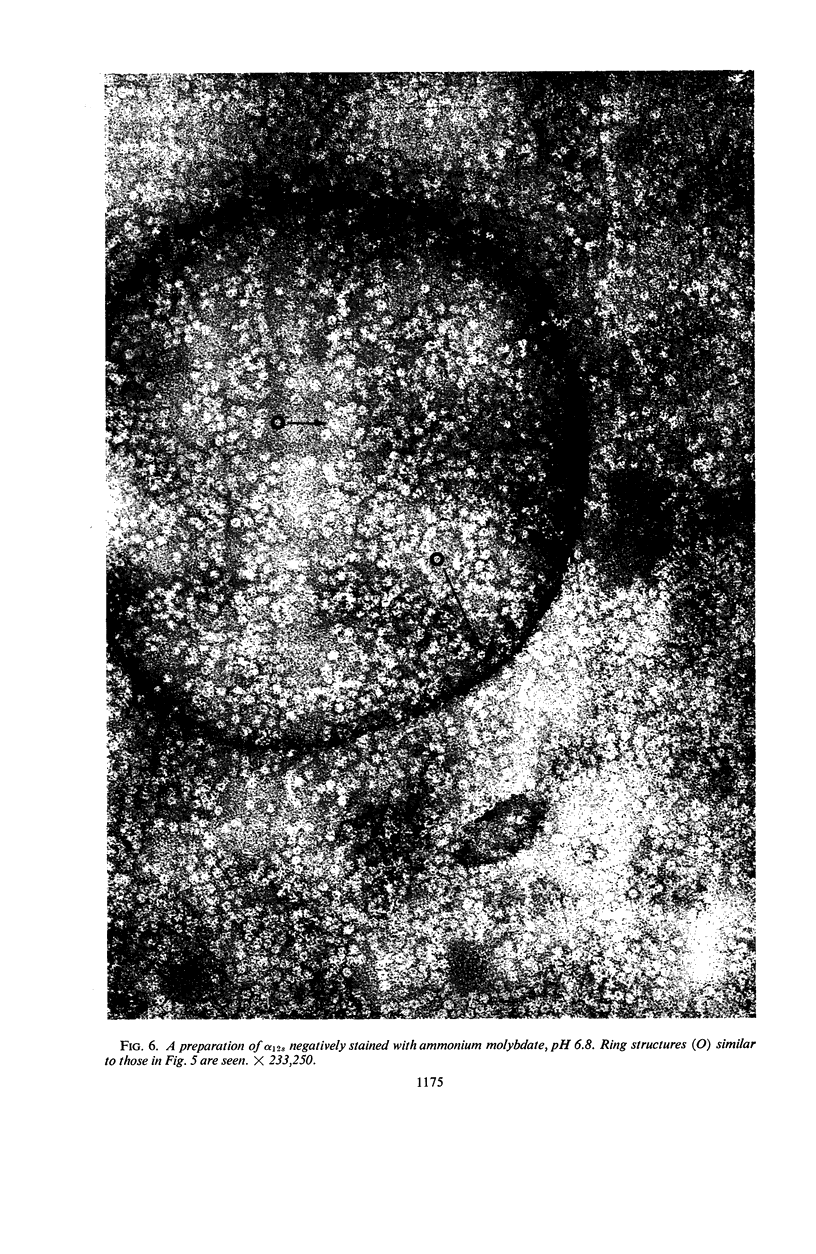
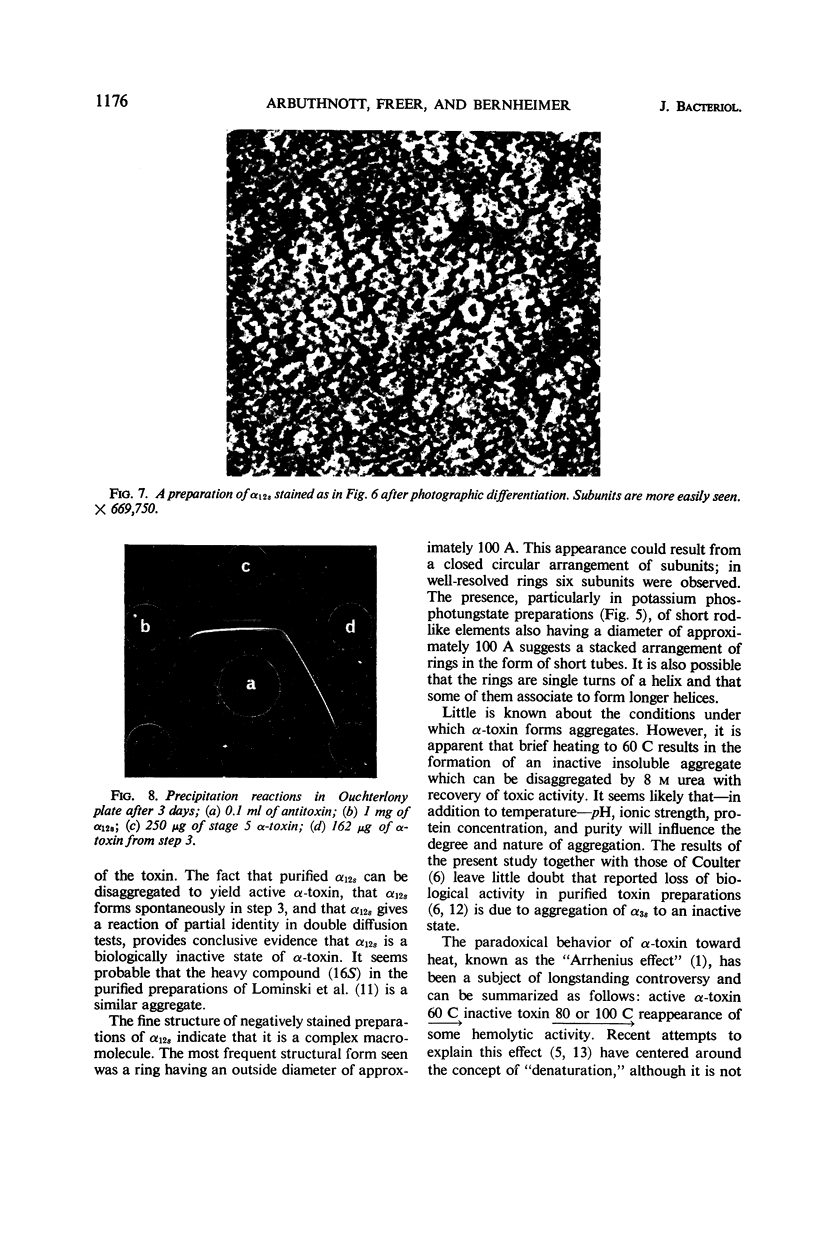
At least three different forms of staphylococcal α-toxin have been shown to exist: soluble active α-toxin (α 3S), soluble inactive α-toxin (α12s), and insoluble inactive aggregate. Aggregation to the insoluble, biologically inactive form could be induced by brief heating to 60 C. The aggregate was dissociated by treatment with 8 m urea with reappearance of biological activity. Subsequent removal of urea by dialysis resulted in some spontaneous reaggregation to the insoluble state. The supernatant fluid obtained after dialysis contained soluble active α-toxin of high specific activity, possessing physical, toxic, and immunological properties closely resembling those of native toxin. The soluble biologically inert component (α12s) was identified as a third physical state. Negatively stained preparations of this material, when examined in the electron microscope, showed rings of approximately 100 A outside diameter containing 6 ± 1 subunits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W. Staphylococcal alpha toxin. Ann N Y Acad Sci. 1965 Jul 23;128(1):112–123. doi: 10.1111/j.1749-6632.1965.tb11633.x. [DOI] [PubMed] [Google Scholar]
- Charles A. Preparation of films with holes for electron microscopy. Nature. 1966 Oct 1;212(5057):106–106. doi: 10.1038/212106a0. [DOI] [PubMed] [Google Scholar]
- Cooper L. Z., Madoff M. A., Weinstein L. Heat stability and species range of purified staphylococcal alpha-toxin. J Bacteriol. 1966 May;91(5):1686–1692. doi: 10.1128/jb.91.5.1686-1692.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter J. R. Production, purification, and composition of staphylococcal alpha toxin. J Bacteriol. 1966 Dec;92(6):1655–1662. doi: 10.1128/jb.92.6.1655-1662.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSHI K., CLUFF L. E., NORMAN P. S. Studies on the pathogenesis of staphylococcal infection. V. Purification and characterization of staphylococcal alpha hemolysin. Bull Johns Hopkins Hosp. 1963 Jan;112:15–30. [PubMed] [Google Scholar]
- Kawahara K., Tanford C. Viscosity and density of aqueous solutions of urea and guanidine hydrochloride. J Biol Chem. 1966 Jul 10;241(13):3228–3232. [PubMed] [Google Scholar]
- MADOFF M. A., WEINSTEIN L. Purification of staphylococcal alpha-hemolysin. J Bacteriol. 1962 Apr;83:914–918. doi: 10.1128/jb.83.4.914-918.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar M., Kumar S., Lindorfer R. K. Heat reactivation of the alpha-hemolytic, dermonecrotic, lethal activities of crude and purified staphylococcal alpha-toxin. J Bacteriol. 1966 May;91(5):1681–1685. doi: 10.1128/jb.91.5.1681-1685.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITHEL F. J. THE DISSOCIATION AND ASSOCIATION OF PROTEIN STRUCTURES. Adv Protein Chem. 1963;18:123–226. doi: 10.1016/s0065-3233(08)60269-7. [DOI] [PubMed] [Google Scholar]