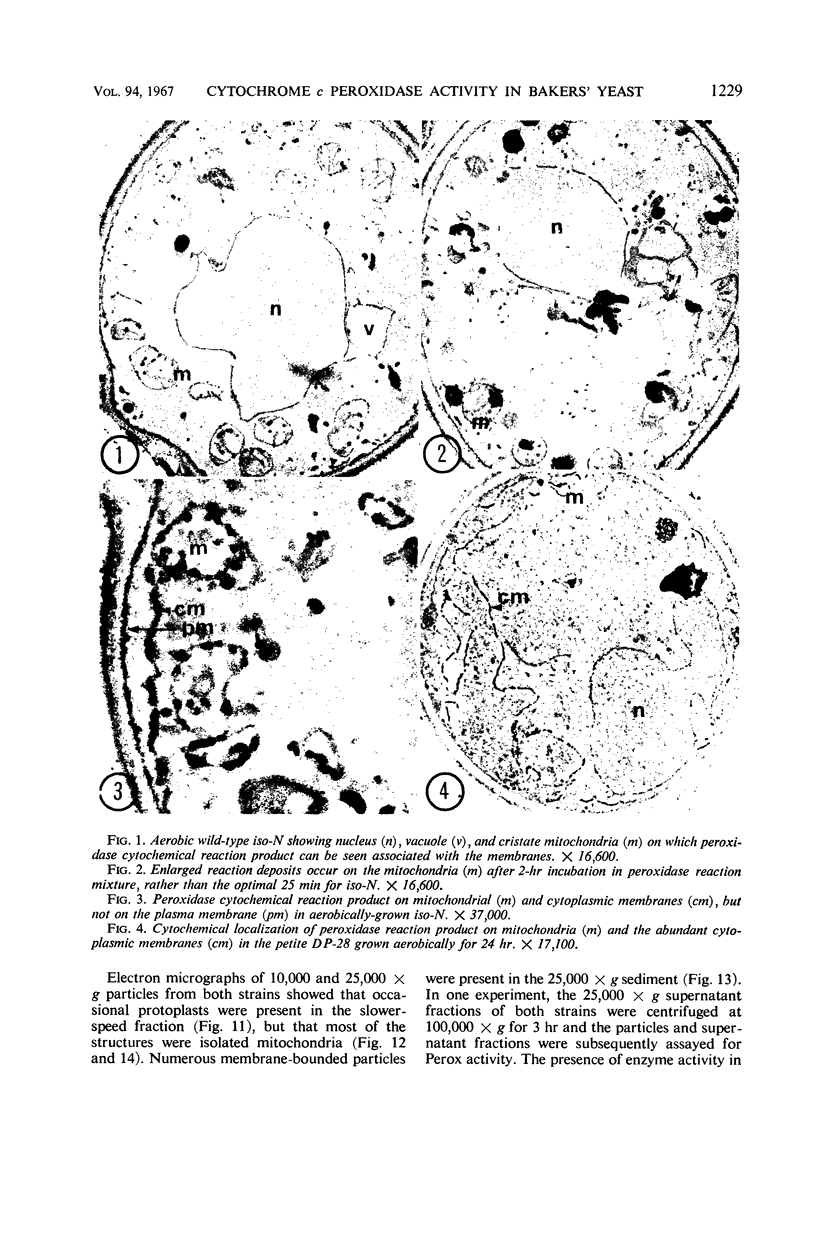
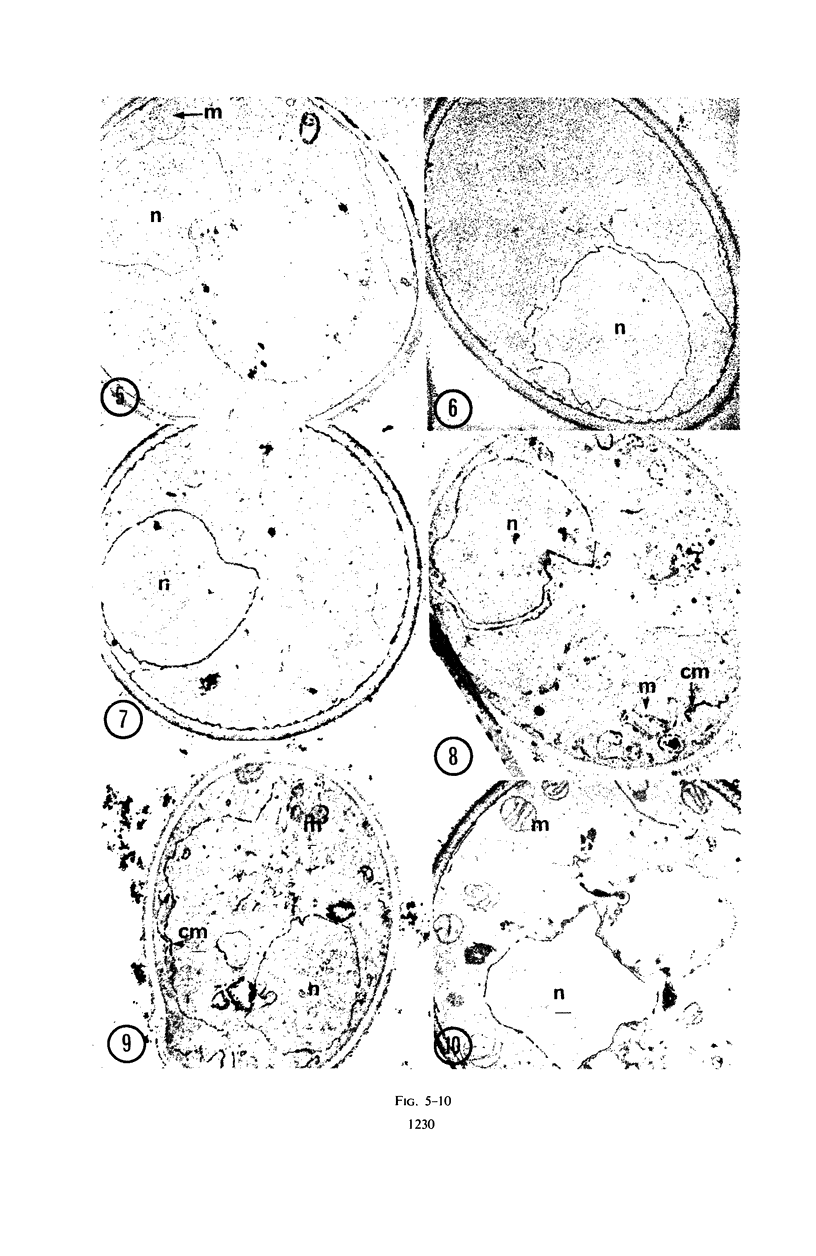
Abstract
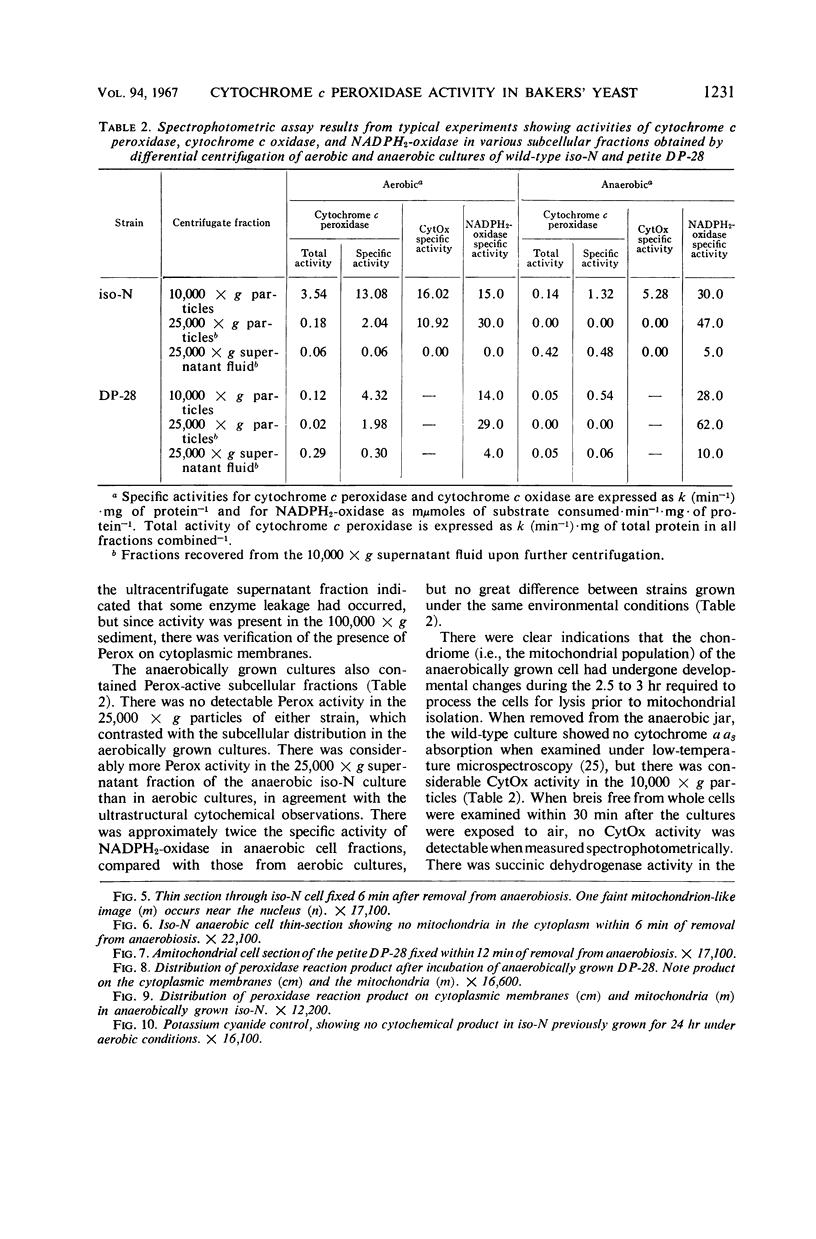
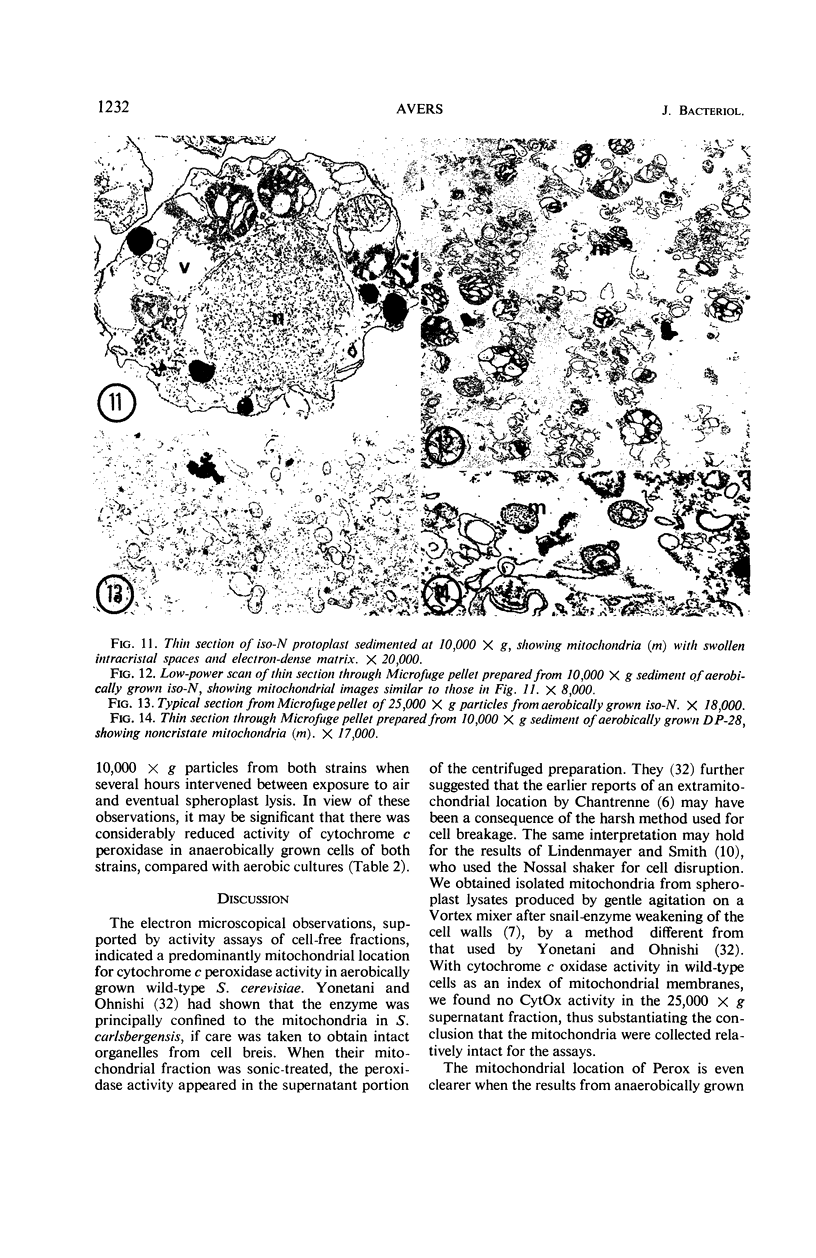
Studies of mitochondrial biogenesis in yeast have been hampered by a lack of suitable membrane markers in anaerobically grown cells subsequently grown in air. Cytochrome c peroxidase activity and subcellular location was studied to determine whether it would be a useful marker for an analysis of mitochondrial formation. Cytochemical tests revealed enzyme reaction product on all mitochondrial membranes in aerobically grown wild-type cells. Anaerobically grown wild-type and all petite cultures contained cytochrome c peroxidase cytochemical reaction deposits on abundant cytoplasmic membranes and on the few mitochondrial profiles which also were seen in the electron photomicrographs. Biochemical studies corroborated the cytochemistry because mitochondrial fractions were greatly enriched in cytochrome c peroxidase activity for aerobically grown wild-type cultures, but petite and anaerobically grown wild-type cultures showed higher enzyme activities in supernatant fractions than was present in the corresponding particulate fractions after differential centrifugation. Evidence from low-temperature microspectroscopy, spectrophotometric assays of mitochondrial enzyme activities, and electron microscopy showed mitochondrial formation during the time required for preparation and lysis of spheroplasts from anaerobically grown cultures. The data were interpreted as indicating that cytochrome c peroxidase was an oxygen-inducible enzyme, and that there was a developmental relationship between enzyme-reactive membranes of mitochondria and cytoplasm during the period of respiratory adaptation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- AVERS C. J., LIN F. H., PFEFFER C. R. HISTOCHEMICAL STUDIES OF MITOCHONDRIAL VARIATION DURING AEROBIC GROWTH OF RESPIRATION-NORMAL BAKER'S YEAST. J Histochem Cytochem. 1965 May-Jun;13:344–349. doi: 10.1177/13.5.344. [DOI] [PubMed] [Google Scholar]
- AVERS C. J., PFEFFER C. R., RANCOURT M. W. ACRIFLAVINE INDUCTION OF DIFFERENT KINDS OF "PETITE" MITOCHONDRIAL POPULATIONS IN SACCHAROMYCES CEREVISIAE. J Bacteriol. 1965 Aug;90:481–494. doi: 10.1128/jb.90.2.481-494.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avers C. J., Rancourt M. W., Lin F. H. Intracellular mitochondrial diversity in various strains of Saccgarintces cerevisiae. Proc Natl Acad Sci U S A. 1965 Aug;54(2):527–535. doi: 10.1073/pnas.54.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical demonstration of cytochrome oxidase with new amine reagents. J Histochem Cytochem. 1960 Jan;8:63–70. doi: 10.1177/8.1.63. [DOI] [PubMed] [Google Scholar]
- CHANTRENNE H. Peroxydases induites par l'oxygène chez la levure. Biochim Biophys Acta. 1955 Sep;18(1):58–62. doi: 10.1016/0006-3002(55)90008-1. [DOI] [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYMAN-BLANCHET T., OHANIANCE L., CHAIX P. EVOLUTION DE LA SYNTH'ESE DU SYST'EME RESPIRATOIRE CHEZ LES LEVURES R'ECOLT'EES 'A DIFF'ERENTS STADES DE LEUR CROISSANCE ANA'EROBIE. Biochim Biophys Acta. 1964 Mar 9;81:462–472. [PubMed] [Google Scholar]
- Kovachevich R. Comparison of oxidation rates of reduced pyridine nucleotides by normal and respiration deficient mutant yeast. Biochem Biophys Res Commun. 1964;14:48–53. doi: 10.1016/0006-291x(63)90209-2. [DOI] [PubMed] [Google Scholar]
- LINDENMAYER A., SMITH L. CYTOCHROMES AND OTHER PIGMENTS OF BAKER'S YEAST GROWN AEROBICALLY AND ANAEROBICALLY. Biochim Biophys Acta. 1964 Dec 9;93:445–461. doi: 10.1016/0304-4165(64)90329-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUCK D. F. FORMATION OF MITOCHONDRIA IN NEUROSPORA CRASSA. A STUDY BASED ON MITOCHONDRIAL DENSITY CHANGES. J Cell Biol. 1965 Mar;24:461–470. doi: 10.1083/jcb.24.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCK D. J. Formation of mitochondria in Neurospora crassa. A quantitative radioautographic study. J Cell Biol. 1963 Mar;16:483–499. doi: 10.1083/jcb.16.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukins H. B., Tham S. H., Wallace P. G., Linnane A. W. Correlation of membrane bound succinate dehydrogenase with the occurrence of mitochondrial profiles in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1966 May 25;23(4):363–367. doi: 10.1016/0006-291x(66)90734-0. [DOI] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORPURGO G., SERLUPI-CRESCENZI G., TECCE G., VALENTE F., VENETTACCI D. INFLUENCE OF ERGOSTEROL ON THE PHYSIOLOGY AND THE ULTRA-STRUCTURE OF SACCHAROMYCES CEREVISIAE. Nature. 1964 Feb 29;201:897–899. doi: 10.1038/201897a0. [DOI] [PubMed] [Google Scholar]
- McClary D. O., Bowers W. D., Jr Structural differentiation of obligately aerobic and facultatively anaerobic yeasts. J Cell Biol. 1967 Feb;32(2):519–524. doi: 10.1083/jcb.32.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHANIANCE L., CHAIX P. APTITUDES RESPIRATOIRES ET SPECTRES CYTOCHROMIQUES DES LEVURES AU COURS DE LEUR CROISSANCE A'EROBIE. Biochim Biophys Acta. 1964 Aug 19;90:221–227. [PubMed] [Google Scholar]
- Person P., Zipper H. Disruption of mitochondria and solubilization of cytochrome oxidase by a synthetic zeolite. Biochem Biophys Res Commun. 1964 Oct 14;17(3):225–230. doi: 10.1016/0006-291x(64)90388-2. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J. 1965 Oct;97(1):298–302. doi: 10.1042/bj0970298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZ G. SUBCELLULAR PARTICLES CARRYING MITOCHONDRIAL ENZYMES IN ANAEROBICALLY-GROWN CELLS OF SACCHAROMYCES CEREVISIAE. Biochim Biophys Acta. 1965 Feb 22;96:342–345. [PubMed] [Google Scholar]
- SELS A. Recherches sur le mécanisme de l'induction des hémoprotéines pendant l'aération chez Saccharomyces cerevisiae. Arch Int Physiol Biochim. 1958 Feb;66(1):127–127. [PubMed] [Google Scholar]
- SMITH L. A study of some oxidative enzymes of baker's yeast. Arch Biochem Biophys. 1954 Jun;50(2):285–298. doi: 10.1016/0003-9861(54)90044-2. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlo M., Fukuhara H. On the necessity of molecular oxygen for the synthesis of respiratory enzymes in yeast. Biochem Biophys Res Commun. 1965 May 18;19(5):587–591. doi: 10.1016/0006-291x(65)90379-7. [DOI] [PubMed] [Google Scholar]
- TUSTANOFF E. R., BARTLEY W. THE EFFECT OF GLUCOSE ON THE DEVELOPMENT OF RESPIRATION BY ANAEROBICALLY GROWN YEAST. Can J Biochem. 1964 May;42:651–665. doi: 10.1139/o64-078. [DOI] [PubMed] [Google Scholar]
- Tustanoff E. R., Bartley W. Development of respiration in yeast grown anaerobically on different carbon sources. Biochem J. 1964 Jun;91(3):595–600. doi: 10.1042/bj0910595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VITOLS E., NORTH R. J., LINNANE A. W. Studies on the oxidative metabolism of Saccharomyces cerevisiae. I. Observations on the fine structure of the yeast cell. J Biophys Biochem Cytol. 1961 Mar;9:689–699. doi: 10.1083/jcb.9.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE P. G., LINNANE A. W. OXYGEN-INDUCED SYNTHESIS OF YEAST MITOCHONDRIA. Nature. 1964 Mar 21;201:1191–1194. doi: 10.1038/2011191a0. [DOI] [PubMed] [Google Scholar]
- YOTSUYANAGI Y. [Study of yeast mitochondria. I. Variations in mitochondrial ultrastructure during the aerobic growth cycle]. J Ultrastruct Res. 1962 Aug;7:121–140. doi: 10.1016/s0022-5320(62)80031-8. [DOI] [PubMed] [Google Scholar]
- YOTSUYANAGI Y. [Study of yeast mitochondria. II. Mitochondria of respiration-deficient mutants]. J Ultrastruct Res. 1962 Aug;7:141–158. doi: 10.1016/s0022-5320(62)80032-x. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Ohnishi T. Cytochrome c peroxidase, a mitochondrial enzyme of yeast. J Biol Chem. 1966 Jun 25;241(12):2983–2984. [PubMed] [Google Scholar]