SUMMARY
Segmental kinematics were investigated in horses during overground locomotion and compared with published reports on humans and other primates to determine the impact of a large neck on rotational mobility (>20deg.) and stability (≤20deg.) of the head and trunk. Three adult horses (Equus caballus) performing walks, trots and canters were videotaped in lateral view. Data analysis included locomotor velocity, segmental positions, pitch and linear displacements and velocities, and head displacement frequencies. Equine, human and monkey skulls and cervical spines were measured to estimate eye and vestibular arc length during head pitch displacements. Horses stabilized all three segments in all planes during all three gaits, unlike monkeys and humans who make large head pitch and yaw rotations during walks, and monkeys that make large trunk pitch rotations during gallops. Equine head angular displacements and velocities, with some exceptions during walks, were smaller than in humans and other primates. Nevertheless, owing to greater off-axis distances, orbital and vestibular arc lengths remained larger in horses, with the exception of head–neck axial pitch during trots, in which equine arc lengths were smaller than in running humans. Unlike monkeys and humans, equine head peak-frequency ranges fell within the estimated range in which inertia has a compensatory stabilizing effect. This inertial effect was typically over-ridden, however, by muscular or ligamentous intervention. Thus, equine head pitch was not consistently compensatory, as reported in humans. The equine neck isolated the head from the trunk enabling both segments to provide a spatial reference frame.
Keywords: quadrupedal, bipedal, walk, trot, canter, kinematics, sensorimotor control, vision, vestibular, spatial orientation, navigation, reference frames, inertia, pitch rotation, linear displacement, velocity, Equus caballus, Homo, Hylobates, Cercopithecus, Macaca, Semnopithecus
INTRODUCTION
Locomotion, which requires stability, progression and adaptation (Das and McCollum, 1988; Patla, 1991; Patla, 1997; Shumway-Cook and Woollacott, 2001), depends upon visual, vestibular and somatosensory inputs to provide continually updated information on whole-body orientation relative to space (gravito-inertial acceleration vector) and on how to navigate through complex and cluttered natural environments without falling down or getting lost. All three types of sensory input contribute to balance and spatial orientation, and a loss of one of these inputs can be partially, if not largely, compensated through sensory re-weighting (Peterka, 2002). The visual and vestibular systems, nevertheless, take on predominant roles in locomotion. Vision is central to adapting step patterns on uneven terrain, for avoiding obstacles and arriving at pre-planned destinations. The vestibular system, responding to gravito-inertial accelerations of head rotations and translations, provides information about head orientation in space. It also, however, contributes to posture (e.g. neck and trunk orientation) and locomotion through vesribulo-collic reflexes (VCR) and other vestibulospinal mechanisms (Kleine et al., 2004; Peterson and Boyle, 2004; Wilson and Peterson, 1981), and to gaze stabilization through angular (semicircular canals), and linear (otolith organ) vestibulo-ocular reflexes (VOR) (Fuchs, 1981; Liao et al., 2008; Moore et al., 1999; Paige, 1989; Pozzo et al., 1990; Schwarz and Miles, 1991). Furthermore, vestibular (but not visual) inputs are critical to discharge patterns of forebrain–midbrain head direction cells (Stackman and Taube, 1997; Stackman et al., 2002) and hippocampal place cells (Russell et al., 2003; Stackman et al., 2002), thereby providing the nervous system with a means to continually update its internal two-dimensional surface map for navigation (Day and Fitzpatrick, 2005).
During locomotion, the head rotates and translates through space, requiring compensatory eye movements that stabilize the image on the retina for clear vision. Much of this stabilization is accomplished by eye-in-orbit rotations using VOR (Fuchs, 1981; Grossman et al., 1988; Liao et al., 2008; Moore et al., 1999; Paige, 1989; Schwarz and Miles, 1991). Laboratory studies of humans (Cromwell et al., 2001; Cromwell et al., 2004; Pozzo et al., 1990), monkeys and a gibbon (Hirasaki and Kumakura, 2004) walking bipedally overground, humans walking bipedally on a treadmill (Moore et al., 1999; Pozzo et al., 1990) and humans seated on a vertically movable apparatus (Paige, 1989), demonstrate that another mechanism is a compensatory (approximately 180deg. out-of-phase) angular head rotation accompanying vertical head translations. Closer examination of one of these studies (Pozzo et al., 1990), which presents graphs depicting typical head vertical translations overlapped with simultaneous head pitch-plane rotations, however, reveals that head pitch rotations are compensatory in some locomotor tasks more than others. Whereas, head pitch and vertical translation are, with few exceptions, approximately 180deg. out of phase during bipedal runs and hops, phase shifts appear during walks, both overground and treadmill. Thus out-of-phase compensatory head pitch rotations, while common, are not essential in all forms of locomotion.
Pozzo and colleagues, based upon laboratory investigations of human bipedal walks and a variety of other bipedal locomotor tasks (e.g. runs, hops), have proposed that the head is rotationally stabilized (≤20 deg. rotation) to provide the brain with a gravito-inertial reference for whole-body spatial orientation and gaze accommodation (Pozzo et al., 1990), Bipedal walking studies by others on humans (Cromwell and Wellmon, 2001), monkeys and gibbons (Hirasaki and Kumakura, 2004) found the head to be similarly stabilized, lending support to this proposal. From our own real-world experiences, however, we know that large head rotations (>20deg.), especially head turns in the yaw plane, are indeed avoided when we run or hop. When we walk, by contrast, our head frequently rotates through much more than 20deg. in the yaw and pitch planes, as when we visually scan our surroundings (e.g. sightseeing). Thus, if the brain depends upon the head to provide a critical stable platform for spatial orientation (Pozzo et al., 1990), how then can we rotate our head during walks and not disturb balance?
The answer may lie with the trunk. Although not explicitly taken into consideration by Pozzo and colleagues (Pozzo et al., 1990), another study revealed that the trunk remains stabilized (≤20 deg. rotation) during bipedal walks (Cromwell et al., 2001). Based on a stick figure drawing (Pozzo et al., 1990), the trunk is likely to be also stabilized during bipedal runs. This finding raises the possibility that the head may be free to move without detriment to the locomotor performance because the stabilized trunk provides the spatial reference frame for postural vertical. Indeed, whereas the vestibular apparatus and eyes provide the essential information for navigation (position, heading, velocity), trunk receptors make important contributions to spatial orientation for posture and balance. Experimental evidence indicates that the nervous system can use the trunk as the body-to-space reference in two ways: (1) relative to gravity-vertical using information from vestibular signals and neck proprioceptors in combination (Kleine et al., 2004; Mergner et al., 1983; Mergner et al., 1991; Mergner et al., 1992), and (2) relative to gravity-vertical using proprioceptive (Jakobs et al., 1985; Mittelstaedt, 1988; Taylor and McCloskey, 1990) and non-proprioceptive receptors (Mittelstaedt, 1995; Mittelstaedt, 1996; Mittelstaedt, 1997; Mittelstaedt, 1998; Vaitl et al., 1997; Vaitl et al., 2002) in the trunk itself.
The above studies focus on humans and other primates performing bipedally, raising the possibility that the observed segmental movement patterns are specific to two-point support. To address the issue for four-point support, Dunbar and colleagues conducted a study on two quadrupedal monkey species (Macaca radiata and Semnopithecus entellus) walking and galloping in the wild (Dunbar et al., 2004). Quadrupedal walks were revealed to be comparable with bipedal walks in mat the trunk remains stabilized (≤20 deg. rotation). In addition, although commonly stabilized, the head frequently rotates in the yaw and pitch planes through more than 20deg. as the monkeys view their surroundings. Laboratory studies of head and trunk movements by Macaca fuscata during overground locomotion (Hirasaki and Kumakura, 2004), and Macaca mulatto, Macaca fascicularis (Xiang et al., 2008) and Cercopithecus aethiops (Dunbar, 2004) during treadmill locomotion, also found the monkey trunk to be stabilized. By contrast, during overground quadrupedal gallops, a fast running gait characterized by unequal timing between touchdown of the limbs (Hildebrand, 1977), the trunk is not stabilized (Dunbar et al., 2004). Rather, it rotates in the pitch plane up to 50 deg., while the head remains stabilized in all planes. Based on these findings, the hypothesis was presented that either the head or the trunk will be rotationally stabilized (≤20 deg. rotation) in order to provide the brain with a reference frame for spatial orientation relative to the gravito-inertial acceleration vector (Dunbar et al., 2004). The only known exception to the hypothesis is treadmill quadrupedal locomotion, during which the head (pitch and yaw) and trunk (pitch) frequently rotate simultaneously through more than 20 deg. (Dunbar, 2004). Under these unnatural conditions, the stable visual surround and dependably smooth treadmill belt surface, and possibly sensory re-weighting of vestibular and proprioceptive inputs, may provide novel spatial reference frames that allow simultaneous head and trunk rotations to occur without balance detriment. Furthermore, head pitch-plane rotations are rarely 180 deg. out-of-phase with head vertical translations. Rather, the phase relationship shifts throughout the cycle during quadrupedal walks, as revealed in an illustration of human bipedal walks (Pozzo et al., 1990), and are primarily in-phase during gallops. Regardless, under real-world conditions, the basic proposal of Pozzo and colleagues (Pozzo et al., 1990) that the brain requires a stabilized segment for determining whole-body spatial orientation remains supported for both bipedal and quadrupedal locomotion, with the amendment that either the head or the trunk may meet that requirement (Dunbar et al., 2004).
Neither the human study by Pozzo and colleagues (Pozzo et al., 1990) nor the monkey studies (Dunbar, 2004; Dunbar et al., 2004) considered the role of the neck in stabilization. Cromwell and colleagues, however, found that the human neck rotates in the pitch plane more than either the head or trunk during bipedal walking in the laboratory but that all three segments nevertheless remained stabilized (≤20 deg. rotation) (Cromwell et al., 2001). Neck movement patterns in monkeys during natural overground locomotion remain unknown because the neck is obscured by shoulder and arm movements in lateral view, thus prohibiting reliable kinematic measurements (Dunbar et al., 2004). Anatomically, however, monkeys are similar to humans in that they have round heads and short necks. The cervical column of monkeys and humans also articulates with the inferior (ventral) surface or base of the skull, as opposed to the typical non-primate mammalian column, which has a more posterior (caudal) articulation with the skull (Ankel-Simons, 2000). In addition, whereas they are similar to other quadrupeds in allowing large ranges of flexion (ventroflexion) and extension (dorsiflexion) at the cervicothoracic joints (between the 6th cervical and 1st thoracic vertebrae), both monkeys and humans differ from other quadrupeds in that the rotational range in the pitch plane at the atlanto-occipital joint is quite restricted (Graf et al., 1995a; Graf et al., 1995b). Thus, the anatomical similarities reported above lead us to predict that the monkey neck will be rotationally stabilized in a manner similar to the human neck.
A stabilized neck could theoretically provide the spatial reference frame through a combination of signals from its proprioceptors with signals from the vestibular apparatus. At least for humans and monkeys, however, we do not believe the neck, in itself, is serving in this role because no overground locomotor pattern is known to incorporate simultaneous head and trunk rotations of more than 20 deg. in these species. The human and monkey necks may simply be too short and of too little mass compared with the head and trunk to serve in this role.
If, then, a segment must be large to adequately serve as a reference frame, what is the impact of a long and heavy neck on head and trunk movements during locomotion? In the present study, we examine this issue by investigating the kinematics of head, neck and trunk movements in horses during natural overground locomotion. We address the following basic question. Are equine head, neck or trunk movements limited to 20 deg. or less in the pitch plane relative to space (i.e. gravity vertical, earth horizontal) and, if so, does the stabilized segment change with a change in gait? Our first two hypotheses are based on the overground patterns observed in quadrupedal monkeys (Dunbar et al., 2004), Hypothesis 1 states that the equine head is free to rotate in the pitch and yaw planes on a stabilized trunk during walks. Hypothesis 2, by contrast, states that the equine trunk will pitch through more man 20 deg. on a stabilized head during canters, which are slow gallops. Hypothesis 3 states that the equine head and trunk will remain rotationally stabilized during trots. Unlike gallops, trots are characterized by the symmetrical coordination (equal timing) of contralateral hind limb-forelimb pairs and vertical trunk movements (Clayton, 2004). The monkey study did not analyze the trot, which is a slower quadrupedal running gait than the gallop, because these animals rarely practice it. In addition, large head rotations that are comparable with those seen during walks are not practiced during monkey quadrupedal gallops (Dunbar et al., 2004) or human bipedal runs (Pozzo et al., 1990), and head yaw-plane rotations during trots and other running gaits may induce unwanted lateral body sway due to a sudden increase in optic flow (Dunbar, 2004; Schubert et al., 2003). Hypothesis 4 states that the neck will not be rotationally stabilized in the pitch plane because it must make large (>20 deg.) compensatory movements to isolate the head from the rotational influences of the trunk, thereby enabling independent head stabilization relative to space. That the human neck pitches through more degrees than the head or trunk during bipedal walks (Cromwell and Wellmon, 2001) supports this hypothesis. Fig. 1 illustrates Hypotheses 1–4. Lastly, Hypothesis 5 states that, based on what is known for quadrupedal monkeys (Dunbar et al., 2004), pitch plane rotations of the equine head will not be consistently 180 deg. out-of-phase with head vertical translation. To lend context and significance to our investigation, the Discussion will include comparisons of the equine data with those published on humans (Cromwell and Wellmon, 2001; Cromwell et al., 2004; Lieberman et al., 2006; Pozzo et al., 1990) and other primates (Dunbar, 2004; Dunbar et al., 2004; Hirasaki and Kumakura, 2004; Xiang et al., 2008).
Fig. 1.
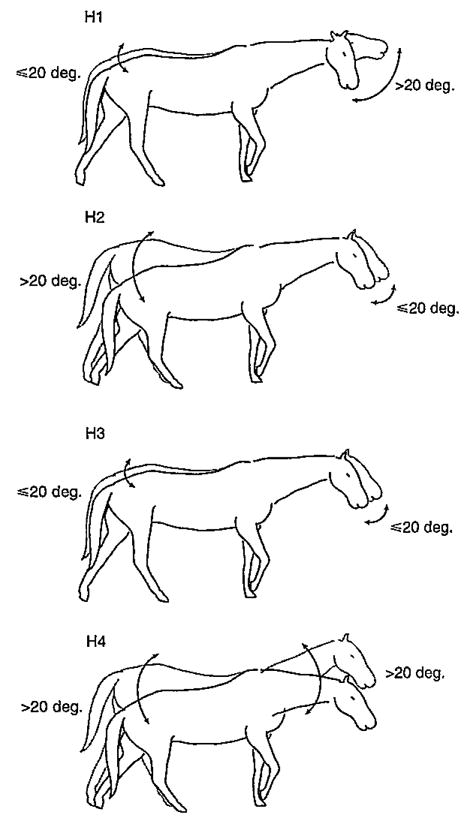
Graphic depictions of Hypotheses 1–4. Although the hypotheses refer to different gaits, a standard walking figure is used for all illustrations in order to emphasize the differences in head, neck and trunk pitch predicted by each hypothesis. Hypothesis 1 (H1); the head is free to rotate more than 20 deg. in the pitch and yaw planes on a stabilized trunk during walks. Hypothesis 2 (H2); the trunk will pitch through more than 20 deg. on a stabilized head during canters. Hypothesis 3 (H3); the head and trunk will remain rotationally stabilized (≤20 deg.) during trots. Hypothesis 4 (H4); the neck will not be rotationally stabilized in the pitch plane because it must make large (>20 deg.) compensatory movements to isolate the head from the rotational influences of the trunk, thereby enabling independent head stabilization relative to space. Arrows indicate the direction and relative magnitude of pitch rotation.
MATERIALS AND METHODS
Animals, training and videotaping
Three gelded domestic horses (Equus caballus L. 1758), 14.3±8.3 years of age, 164.6±2.7cm in height and 536.2±16.8kg in mass, were the subjects of this study. The horses were a Thoroughbred, a Warm Blood and a Thoroughbred-Warm Blood cross. The Thoroughbred is a true Hot Blood breed that is characterized by speed, stamina and athleticism but also tends to be nervous and temperamental. The Warm Blood, by contrast, is a group of horse breeds and types that were developed by crossbreeding Hot Blood breeds with large and powerful, but relatively even-tempered (Cold Blood), draft horses in an effort to produce athletic horses of reasonable temperament. Hot Bloods are commonly crossbred with Warm Bloods in order to further improve the latter for competitive sports (Edwards, 2001). Despite their different heritages, however, all three horses were of similar build, temperament, and behavior. The horse owners provided written permission to use the animals. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and conducted according to the National Institutes of Health (NIH) guidelines (USA).
The horses were videotaped while walking, trotting and cantering (slow galloping) in an outdoor arena, the support surface of which consisted of an 8cm sand mixture on a firm base. Gaits were recorded using a Sony Digital 8, 60 Hz video camera (Sony Electronics, San Diego, CA, USA) positioned approximately 28 m from the plane of action. Small squares of white masking tape (5cm×5cm) were placed on the camera side of the horse in alignment with the withers (spinous process of the 3rd thoracic vertebra) and the ear (external auditory meatus) to assist data reduction. The camera was mounted on a tripod and leveled with respect to earth horizontal. The built-in telephoto lens was used to reduce parallax and to provide data capture for a minimum of one complete stride cycle. Before and after each recording session, a 457 cm (15 ft) wooden pole was placed parallel to, and in line with, the plane of gait and also leveled. Videotape of this pole provided a horizontal reference for measurement of head, neck, and trunk position and a length calibration. Thus, measurement error was minimized during data collection by using clear landmarks, minimizing parallax with the zoom lens, and placing the beam of known length in the locomotor path. The 60 Hz sampling rate proved sufficient because the segments of interest are massive and displaced at rates slow enough to avoid or minimize blurring, even at the fastest gallops sampled.
Data were collected while the horses completed their usual exercise regimen. Following a warm-up period, each horse performed eight trials of each gait in the following sequence: walks, trots and canters. All trials were videotaped in a single exercise session. During walks and trots, an experienced handler used a loose shank to lead the horses through the plane of action. The looseness of the shank allowed unrestricted head movement, and the handler was always in front of the horses to ensure the video lens an unobstructed view. During canters, the halter and shank were removed and the horses were free to move at will. The handler encouraged cantering through handclaps and verbal prompts. At the conclusion of the exercise session, the horse was recaptured, cooled off and returned to the stable.
Data analysis
Video images were analyzed with a Peak motion analysis system using Motus 3.2 software (Vicon, formerly Peak Performance Technologies, Centennial, CO, USA) for eight walk, trot and canter cycles from each horse, for a total of 24 cycles per gait type. The only exceptions were the sample sizes for vertical head displacements and velocities (see below) during walks in horse 1, each of which consisted of six cycles. Four anatomical landmarks were digitized frame-by-frame and used to define the relevant body segments: the lower lip, the ear, the withers and the base of the tail (dock). The axis of the head segment was defined as a line passing through the lower lip and the ear, the neck axis through die ear and the withers, and the trunk axis through the withers and the dock (Fig. 2A). To minimize measurement error, only trials in which the head, neck and trunk remained aligned in the sagittal plane (the plane of progression) were analyzed quantitatively. Measurement resolution was estimated to be 1 cm for linear position and less than or equal to 1 deg. for angular position (pitch rotation) based on the length of the shortest segment (head). To maximize accuracy and consistency, one experimenter (A.Z.) who has extensive experience and training in motion data collection with the Peak system performed all the digitizing.
Fig. 2.
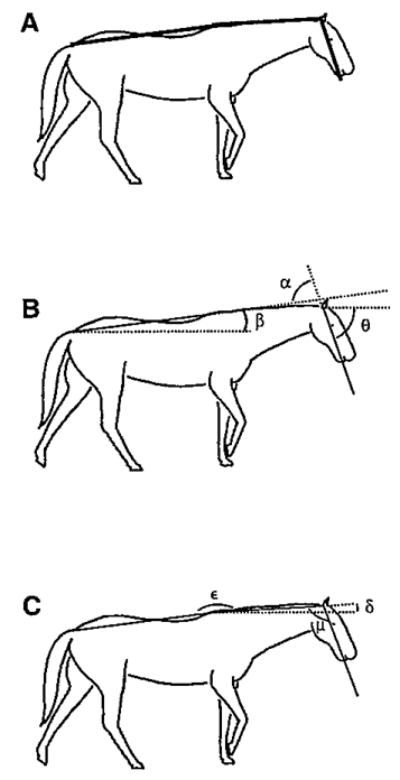
Measured axes and angles in the pitch plane. (A) head axis (black) was a line passing through the ear and the lower lip, neck axis (red) was a line passing through the withers (3rd thoracic vertebra) and the ear, and trunk axis (blue) was a line passing through the dock (tail base) and the withers. (B) Head angle (θ) and trunk angle (β) relative to space, and head angle relative to the trunk (α). (C) Neck angle relative to space (δ), head angle relative to the neck (μ) and neck angle relative to the trunk (ε). Horizontal broken lines indicate the earth-horizontal space reference.
A number of variables were computed for each of the three gaits. A gait cycle was defined as the time between the touchdown of the right hind hoof and the subsequent touchdown of the same hoof. Locomotor velocities were calculated for each horse using the horizontal displacement of the body from the beginning to the end of the gait cycle and the number of frames as a time measure. Linear variables included vertical displacement and velocity of the head (ear), neck and trunk. Angular variables of pitch rotation included head, neck and trunk displacement and velocity relative to earth horizontal and relative to each other (Fig. 2B, C). We adopted the standard rotational coordinate system whereby pitch is in the sagittal plane, yaw in the horizontal plane and roll in the frontal plane. Displacement data were smoothed at 10 Hz prior to calculating velocities.
The relationship between vertical translation and pitch rotation of the head during the various gaits was explored with Fourier analysis using Matlab software (The Mathworks Inc., Natick, MA, USA). The displacement data were smoothed (4th order low-pass filter with zero phase lag, using cutoffs of 6Hz for walks and 8 Hz for trots and canters), and the mean values subtracted for each cycle to yield change in pitch angle and vertical position. Data were then de-trended to remove any linear trends within a cycle. Using Fourier analysis, the amplitude and phase of the cosine curve fits were determined at the main frequency for head movement. A phase difference was then calculated as pitch phase minus vertical position phase such that a positive value indicates that pitch rotation precedes, or leads, vertical position.
Fourier analysis was also used to examine the relative importance of inertia in head stabilization. Peak frequencies for angular and linear displacements of the head were determined for each horse during walks, trots and canters. Frequency spectra were derived for each individual cycle, and then averaged across cycles. This technique, which was applied previously to monkeys (Dunbar et al., 2004), produced clear mean peak frequencies. Harmonics, however, were not clearly produced and, thus, were not included in the analysis. Owing to camera orientation, rotations in other planes, if present, were analyzed qualitatively.
To aid discussion of the effects of head motion during the various gaits on the signaling capacity of the visual and vestibular systems, various anatomical measures were made. Orientation of the horizontal semicircular canals in the head was estimated through a combination of horse skull illustrations depicting the canal’s alignment in the sagittal plane (DeBeer, 1947) and specific external cranial landmarks (Goody, 1983), and found to be pitched upward by 60deg. relative to the head axis used in the present study.
Both the eyes and the vestibular organs of the horse are at some distance from the main axes of head rotation, the atlanto–occipital (head–neck) and cervicothoracic (neck–trunk) joints, and will therefore experience curvilinear sagittal plane displacement during pitch rotation of the head. To aid in estimating this motion, we measured the distance of the orbit and the external auditory meatus (approximated vestibular apparatus location) from a line passing through the mid-sagittal anterior (rostral) border of the foramen magnum between the occipital condyles (approximated pitch axis) on the adult skull of one horse. For comparison, we made similar measurements on adult skulls of one human and one monkey (Macaca mulatto Zimmermann 1780). We also estimated the length of the cervical vertebral column from an anatomical drawing of an adult horse skeleton (Goody, 1983) scaled to the length of the horse skull that was used for cranial measurements. Cervical spine length was measured directly from the mounted adult skeletons of one human and one monkey (M. mulatta). For all three species, the cervical spine was measured with the skeletons in a quiet stance posture. The impact of off-axis locations of both end organs in a head rotating in pitch at the head–neck axis alone and neck–trunk axis alone were then estimated by calculating the arc length at the following measured distances: (1) linear distance to the orbit from the head–neck axis of pitch rotation – between the pitch axis and the medial border of the orbital rim; (2) linear distance of the external auditory meatus from the head–neck axis of pitch rotation – between the pitch axis and the posterior (horse) or superior (monkey, human) border of the external auditory meatus. (3) Cervical spine length – linear distance between the 1st thoracic and 1st cervical or atlas vertebrae. The cervical spine length was added to the cranial linear distances to approximate arc lengths generated by pitch at the neck-trunk axis alone.
Statistical analyses
Variables were presented as means ± standard deviation (s.d.) or means ± standard error (s.e.m.). The exception was the phase relationships between change in head angle and change in head vertical position, which were presented as means ± angular dispersion (a.d.), a measure of variability in the circular domain. The majority of variables were analyzed using two-way analysis of variance (ANOVA). Head angular and vertical mean peak frequencies, however, were analyzed using one-way ANOVA. Kruskal–Wallis ANOVA on Ranks was used if normality failed. Post hoc analyses were performed on pair-wise comparisons for significant results. Angular-linear correlation, a parametric circular statistical procedure (Johnson and Wehrly, 1977; Mardia, 1976), was employed to determine how strongly change in head pitch related to change in head vertical position in each horse for each gait. The accepted P-value for significance was equal to or less than 0.05 for both the main analyses and all post hoc pair-wise comparisons.
RESULTS
Descriptions of rotational direction below assume that the horses are moving from left to right. Thus, ‘clockwise’ rotations are forward and downward whereas ‘counter-clockwise’ rotations are backward and upward. The means and standard deviations or standard errors of the measured variables are summarized in Table 1. For ease of comparison, those values within each variable that did not differ significantly (P-value greater than 0.05) among the three gaits have been highlighted in bold type.
Table 1.
Summary of mean measurements*
| Variable | Walk | Trot | Canter |
|---|---|---|---|
| Locomotor velocity (ms−1) (±s.d.) | 1.06 (±0.02) | 2.32 (±0.04) | 5.82 (±0.36) |
| Head-to-earth horizontal mean position (deg.) (±s.e.m.) | −66 (±4) | −56 (±1) | −55 (±2) |
| Neck-to-earth horizontal mean position (deg.) (±s.e.m.) | −4(±2) | 11 (±1) | 23 (±7) |
| Trunk-to-earth horizontal mean position (deg.) (±s.e.m.) | 0(±0) | 2(±0) | 1(±0) |
| Head-to-neck mean position (deg.} (±s.e.m.) | 121 (±3) | 113(±1) | 109(±1) |
| Neck-to-trunk mean position (deg.) (±s.e.m.) | 182 (±3) | 170 (±1) | 166 (±2) |
| Head-to-trunk mean position (deg.) (±s.e.m.) | 66 (±2) | 54 (±1) | 53 (±2) |
| Head-to-space pitch displacement (deg.) (±s.d.) | 9(±4) | 5 (±2) | 10 (±4) |
| Neck-to-space pitch displacement (deg.) (±s.d.) | 10 (±3) | 4 (±2) | 8 (±3) |
| Trunk-to-space pitch displacement (deg.) (±s.d.) | 6 (±2) | 2(±1) | 8(±1) |
| Head-to-neck pitch displacement (deg.) (±s.d.) | 7 (±3) | 5 (±2) | 11 (±3) |
| Neck-to-trunk pitch displacement (deg.) (±s.d.) | 14 (±4) | 5 (±2) | 12 (±4) |
| Head-to-trunk pitch displacement (deg.) (±s.d.) | 11 (±4) | 6 (±2) | 11 (±4) |
| Head-to-space mean pitch velocity (deg. s−1) (±s.e.m.) | 20 (±1) | 15 (±1) | 41 (±3) |
| Head-to-space maximum pitch velocity (deg. s−1) (±s.e.m.) | 42 (±3) | 38 (±3) | 105 (±11) |
| Neck-to-space mean pitch velocity (deg. s−1) (±s.e.m.) | 25 (±1) | 14 (±1) | 30 (±3) |
| Neck-to-space maximum pitch velocity (deg. s−1) (±s.e.m.) | 46 (±2) | 33 (±2) | 63 (±5) |
| Trunk-to-space mean pitch velocity (deg. s−1) (±s.e.m.) | 15 (±1) | 7(±1) | 32 (±1) |
| Trunk-to-space maximum pitch velocity (deg. s−1) (±s.e.m.) | 29 (±2) | 17 (±2) | 67 (±5) |
| Head-to-neck mean pitch velocity (deg. s−1) (±s.e.m.) | 15 (±1) | 17 (±1) | 47 (±3) |
| Head-to-neck maximum pitch velocity (deg. s−1) (±s.e.m.) | 34 (±3) | 39 (±3) | 120 (±9) |
| Neck-to-trunk mean pitch velocity (deg. s−1) (±s.e.m.) | 39 (±2) | 19 (±1) | 44 (±3) |
| Neck-to-trunk maximum pitch velocity (deg. s−1) (±s.e.m.) | 70 (±3) | 44 (±3) | 101 (±9) |
| Head-to-trunk mean pitch velocity (deg. s−1) (±s.e.m.) | 26 (±3) | 15 (±1) | 41 (±3) |
| Head-to-trunk maximum pitch velocity (deg, s−1) (±s.e.m.) | 58 (±10) | 39 (±3) | 107 (±10) |
| Lower lip-to-space vertical displacement (cm) (±s.d.) | 15 (±5) | 9 (±2) | 22 (±8) |
| Ear-to-space vertical displacement (cm) (±s.d.) | 12 (±4) | 9 (±2) | 23 (±5) |
| Withers-to-space vertical displacement (cm) (±s.d.) | 7{±1) | 9 (±1) | 18 (±5) |
| Dock-to-space vertical displacement (cm) (±s.d.) | 10 (±2) | 9 (±2) | 20 (±4) |
| Lower lip-to-space mean vertical velocity (cm s−1) (±s.e.m.) | 39 (±2) | 37 (±1) | 79 (±4) |
| Ear-to-space mean vertical velocity (cm s−1) (±s.e.m.) | 31 (±1) | 38 (±1) | 80 (±3) |
| Ear-to-space maximum vertical velocity (cm s−1) (±s.e.m.) | 47 (±2) | 86 (±1) | 139 (±6) |
| Withers-to-space mean vertical velocity (cm s−1) (±s.e.m.) | 21 (±1) | 36 (±1) | 60 (±2) |
| Dock-to-space mean vertical velocity (cm s−1) (±s.e.m.) | 32 (±1) | 39 (±1) | 71 (±2) |
| Head pitch displacement peak frequency (Hz) (±s.d.) | 1.3 (±0.4) | 1.2 (±1.0) | 1.5 (±0.6) |
| Head (ear) vertical displacement peak frequency (Hz) (±s.d.) | 1.5 (±0.5) | 2.5 (±0.6) | 1.5 (±0.5) |
For each variable, values in bold are not significantly different (P>0.05).
Qualitative segmental coordination patterns
The overall characteristics of the three quadrupedal gaits analyzed in this study, in terms of the pattern and timing of footfalls, corresponded to the combined definitions and descriptions provided by others (Alexander, 1982; Hildebrand, 1966; Hildebrand, 1977; Howell, 1944). All three gaits are illustrated in Fig. 3. In walks, the timing between footfalls was nearly equal, and the support phase duration of each limb exceeded 50% of the total cycle duration. The horses used a lateral sequence walk pattern in that a hind limb touchdown was followed by touchdown of the forelimb on the same (ipsilateral) side of the body. In trots, both touchdown and liftoff of a hind limb and its opposite (contralateral) forelimb were nearly simultaneous, and the timing between footfalls of the forelimb–hind limb pairs was nearly equal. Each limb pair contacted the support for 50% or less of the total cycle duration, and airborne phases, when present, occurred between touchdowns of forelimb–hind limb pairs. In canters, the timing between footfalls was unequal, and the support phase duration of each limb was less than 50% of the total cycle duration. Sequential touchdowns of the hind limbs were followed by sequential touchdowns of the forelimbs. Two types of canters were observed in the horses: a ‘rotary’ canter, in which the ipsilateral forelimb touched down following touchdown of the leading (second) hind limb, and a ‘transverse’ canter, in which the contralateral forelimb touched down following touchdown of the leading hind limb. Head, neck and trunk movements, however, did not distinguish one canter type from the other. The canters investigated in this study occasionally lacked an airborne phase near the end of the cycle following liftoff of the leading forelimb. Whereas it is typically present in most gallops, an airborne phase is commonly absent in slow canters (Howell, 1944).
Fig. 3.
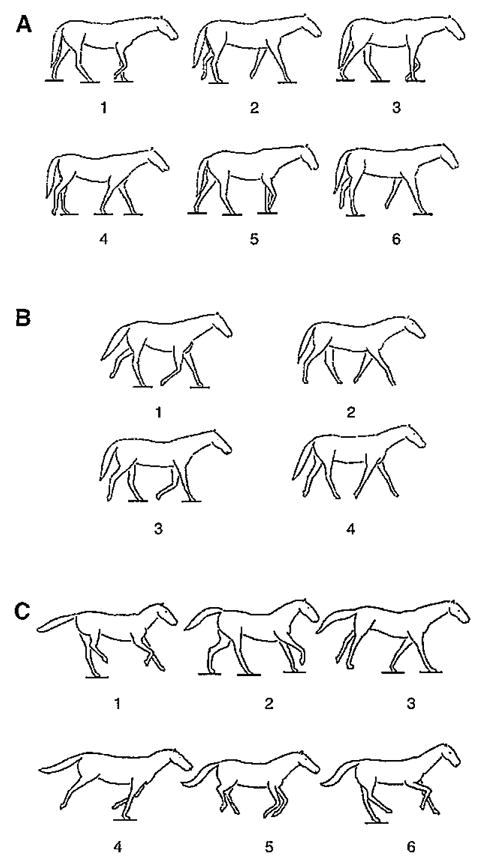
Video tracings of the walk (A), trot (B) and canter (C). Horizontal lines indicate limb contact with the ground.
Head, neck and trunk rotations appeared small in all three gaits but the coordination pattern of the segmental rotations that did occur differed among the three gaits. During walks, the head and neck pitched on the trunk as a single, combined segment. Counter-clockwise rotations in the latter portion of the support phase of each forelimb enabled the combined head and neck to assist in lifting the forelimbs off the ground at the beginning of swing phase. Oscillating the cantilevered head–neck mass in this manner also most likely assisted forward propulsion through stretch and recoil of the nuchal ligament (particularly in the cervicothoracic region) for storage and release, respectively, of potential energy (Gellman and Bertram, 2002a; Gellman and Bertram, 2002b). A second pattern occurred during trots, in which the neck and trunk formed a single, combined segment on which the head rotated (bobbed) slightly, but rapidly, in the pitch plane as the body rose and fell vertically. Yet a third pattern occurred during canters, in that all segments rotated relative to space and to their adjacent segments. This pattern allowed the head and neck to both protract and retract on the trunk during the gait cycle but for the head to also maintain its spatial orientation independently of the neck. Protraction, which involved neck-to-trunk clockwise ventroflexion and head-to-neck counter-clockwise dorsiflexion, was initiated in the latter part of the airborne phase and continued into the support phase of the forelimbs. Initiating a clockwise ventroflexion of the neck would require an angular acceleration. Following Newton’s Third Law of Motion, a clockwise, neck acceleration during the airborne phase would cause the trunk to rotate counter-clockwise (Dunbar, 1988;Dunbar, 1994; Frohlich, 1979; Frohlich, 1980), lowering the hind limbs to begin the support phase. Acceleration from ‘throwing’ the head–neck mass forward throughout much of the limb support period would also help extend the subsequent airborne phase, which lengthens overall stride length to help achieve and maintain high locomotor velocities (Hildebrand, 1974). Retraction, which involved neck-to-trunk dorsiflexion and head-to-neck ventroflexion, was initiated during touchdown of the leading forelimb and continued through the initial part of the airborne phase. Whereas retraction may have a decelerating effect on forward progression, counter-clockwise dorsiflexion of the neck on the trunk would aid vertical lift for the upcoming airborne phase.
Quantitative gait comparisons
Vertical displacements of the head (lower lip+ear landmarks) were smallest in trots, intermediate in walks and largest in canters (P<0.001) (Figs 4 and 5). Vertical displacements of the neck (ear+withers landmarks) and trunk (withers+dock landmarks) were also largest in canters (P<0.001) but similar during walks and trots. This similarity was attributed to the withers translating slightly less in walks than in trots and the dock translating about the same distance in both gaits (Table 1), thereby minimizing differences in neck and trunk vertical displacement. All three segments were stabilized (≤20 deg. rotation), as defined in this study, in all three gaits relative to space and to each other in the pitch plane (Figs 6 and 7). In addition, rotations in other planes were minimal or absent. Nevertheless, as indicated in the previous section, pitch rotations that did occur varied among the gaits and segments.
Fig. 4.
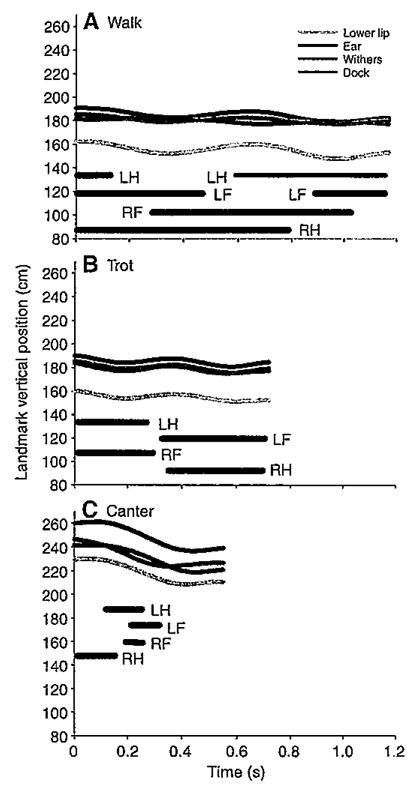
Graphs of vertical displacements of anatomical landmarks for single, representative cycles of the walk (A), trot (B) and canter (C). Thick horizontal lines depict support phases for the left hind limb (LH), right hind limb (RH), left forelimb (LF) and right forelimb (RF).
Fig. 5.
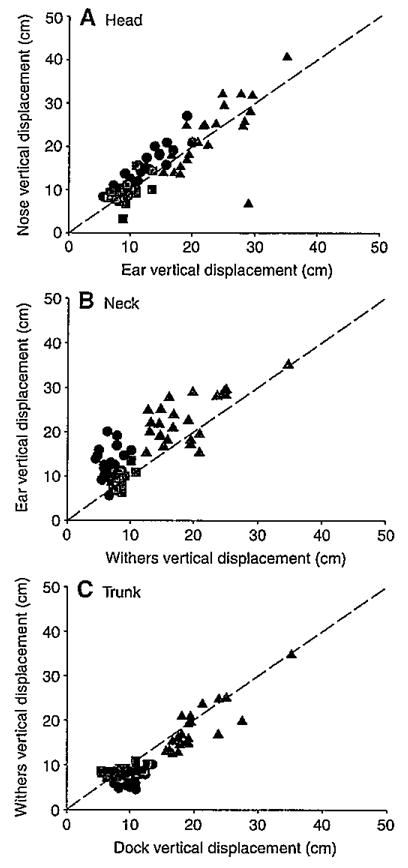
Vertical translations of the head (A), neck (B) and trunk (C) during walks (black circles), trots (red squares) and canters (blue triangles). Translation of each segment is depicted by plotting the vertical displacement of its rostral landmark (y-axis) against that of its caudal landmark (x-axis). Broken lines have a slope of 1 indicating vertical segmental translation with no rotation.
Fig. 6.
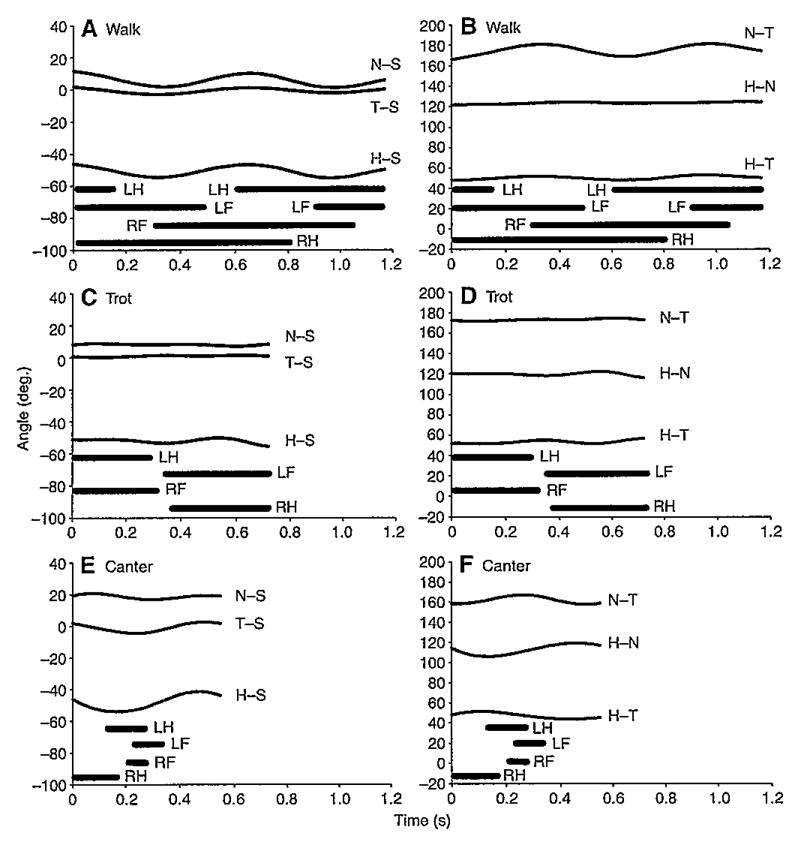
Segmental pitch displacements for single, representative cycles of the walk (A, B), trot (C, D), and canter (E, F). Left column (A, C, E) depicts head (H–S), neck (N–S) and trunk (T–S) pitch relative to space. Right column (B, D, F) depicts pitch of the head relative to the neck (H–N), the neck relative to the trunk (N–T) and the head relative to the trunk (H–T). Negative values denote head angles below earth horizontal (0 deg.). Thick horizontal lines depict support phases for the left hind limb (LH), right hind limb (RH), left forelimb (LF) and right forelimb (RF).
Fig. 7.
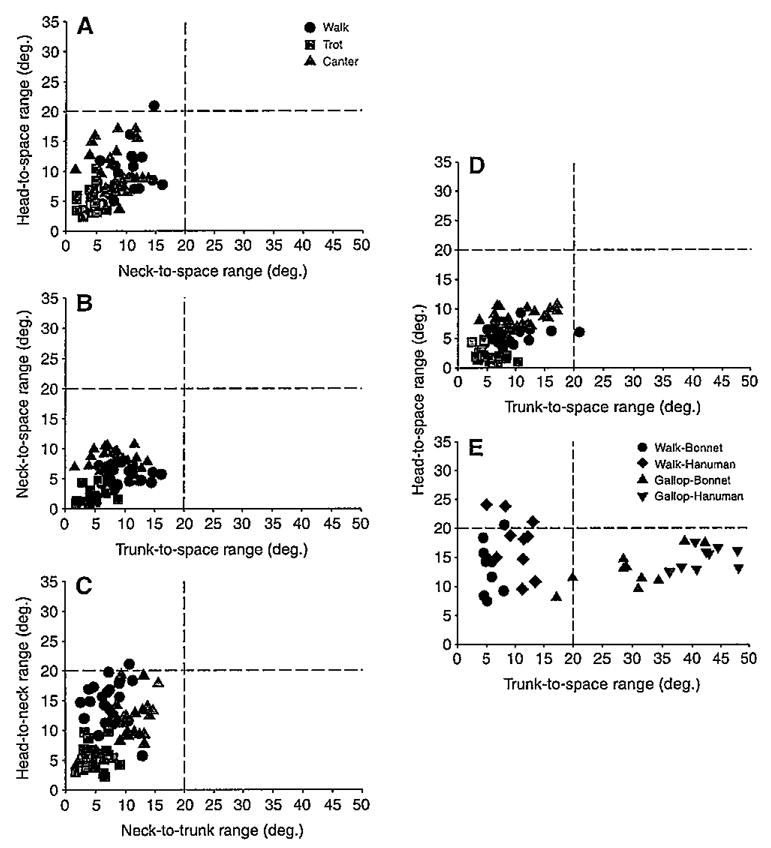
Pitch rotations during walks, trots, and canters of the head vs the neck (A), the neck vs the trunk (B) and the head vs the trunk (D) relative to space, and of the head relative to the neck vs the neck relative to the trunk (C). Pitch rotations of the head vs the trunk during overground walks and slow gallops in two monkey species – bonnet macaques (Macaca radiata) and hanuman langurs (Semnopithecus entellus) – in the wild (E) are presented for comparison with graph D. Horizontal and vertical broken lines indicate 20 deg. threshold for stabilization. Monkey data (E) are from Dunbar and colleagues (Dunbar et al. 2004) and the graph is modified from Dunbar (Dunbar, 2004).
Of the 36 gait characteristics listed in Table 1, seven variables increased progressively from walks to trots to canters. These variables included locomotor velocity, the mean position adopted by the neck relative to space, the amount of vertical displacement experienced by the withers, the mean vertical velocity of the trunk (withers+dock) and both the mean and maximum vertical velocities of the head (ear). Thus, with the transition from slower to faster gaits, neck posture shifted counter-clockwise from the horizontal towards a more vertical orientation, and the head and trunk moved vertically at an increasing rate as the pivot (withers or shoulder region) of these two segments increasingly rose and fell.
The greatest number of variables (10) had values that were least in trots, intermediate in walks and largest in canters. Vertical head (lower lip and ear) displacement fell into this group. The remaining variables were angular displacements and velocities: pitch displacement of the head relative to the neck; pitch displacement and both mean and maximum pitch velocities of the trunk relative to space; mean and maximum pitch velocities of the head relative to the trunk; and maximum pitch velocities of the neck relative to space and to the trunk. These findings indicate that the trunk pitched minimally during trots but that its rotation tripled and quadrupled during walks and canters, respectively. Head-to-neck pitch displacements followed the same gait order but the canter values were only twice the trot values. Head (ear and lower lip) vertical displacements, resulting in part from the head-to-neck pitch displacements, followed a similar pattern, with canter values between two and three times the trot values. The majority of the pitch velocities related to the neck and trunk but only one pitch velocity to the head (head-to-trunk), indicating that neck and trunk rotations were slowest in trots and increased progressively in walks and canters.
Two neck pitch displacement variables were also least in trots but differed from the other neck and trunk variables discussed in the last paragraph in that the canter values were less than the walk values: neck pitch relative to space and neck pitch relative to the trunk. These findings revealed the contrast between the neck and trunk working together as a single segment during trots (minimum displacement), and the combined neck and head segments oscillating on the trunk during walks (maximum displacement).
Only one variable had its significantly smallest value in canters, while increasing progressively in trots and walks: head-to-neck mean position. Although not statistically different between canters and trots, the same canter–trot–walk trend was nevertheless suggested by the values for neck-to-trunk and head-to-trunk position. In the context of how these intersegmental angles were measured (Fig. 2), these findings further reflect how the neck maintains a more horizontal posture during walks but how it dorsiflexes counterclockwise on the trunk to maintain a progressively more vertical orientation during trots and canters. The head, while also adopting a progressively more vertical mean posture across the walk–trot–gallop transitions, accomplishes this reorientation by ventroflexing clockwise on the neck. The head-to-trunk mean position changes reflect the result of these head-to-neck and neck-to-trunk angular re-orientations.
One displacement variable and five velocity variables had comparable values between walks and trots, but increased in canters: dock vertical displacement relative to space; both mean and maximum pitch velocities for the head relative to space and the head relative to the neck: and lower lip mean vertical velocity relative to space. Overall, these findings indicate the relatively slow pitch and vertical movements that characterized walks and trots compared with canters. Specifically, the pattern of dock vertical displacement paralleled the pattern for withers vertical displacement reported above, although statistically, the withers value was significantly less in walks than in trots. These findings reflect the substantially increased trunk pitch in canters compared with walks and trots. Likewise, the head pitch velocities and the associated mean vertical velocity of the lower lip were comparable during walks and trots but much smaller than the velocities found during canters. It should be noted, however, that although the values for head-to-space pitch velocities did not differ statistically between walks and trots, the pattern of lower trot velocities seen with the trunk-to-space and the majority of intersegmental velocities (see above) is suggested by the head-to-space mean values.
Two positional variables had comparable values between trots and canters, both of which were greater than the walk values: head and trunk horizontal mean positions relative to earth horizontal. During walks, as compared with trots and canters, the trunk had a slightly, though significantly (P<0.001), greater withers-down orientation and the head had a greater nose-down orientation (P<0.05). When the measured head values were adjusted by +60deg. (see Materials and Methods), the estimated mean position of the horizontal semicircular canals was −6 deg., 4 deg. and 5 deg. during walks, trots and canters, respectively. Thus, the horizontal semicircular canals were oriented near earth horizontal on average in all three gaits, being pitched downward slightly during walks and pitched upward slightly during trots and canters.
Two head pitch displacement variables and two neck pitch velocity variables were comparable during walks and canters but least during trots: head pitch displacements relative to space and to the trunk, and neck mean pitch velocities relative to space and to the trunk. These findings reveal that during trots, head rotations were smaller and the small neck pitch rotations that did occur (see above) were slower, than in walks and canters.
The only variables that were comparable across all three gaits were the peak frequencies for head pitch and head (ear) vertical displacement, all of which fell between 1 Hz and 2.5 Hz.
Phase relationships of head pitch to head vertical displacement
The phase relationships between the head pitch and head vertical displacements (0deg.=completely in-phase; 180deg.=completely out-of-phase) were, in general, closer to being in-phase during walks but closer to being out-of-phase during trots and canters (Figs 8 and 9). Computed phase differences, based on the estimated fits of the displacement data to cosine functions, were 21±12deg. for horse 1, 21±22deg. for horse 2 and −10±22deg. for horse 3 (means±a.d.), supporting the general in-phase walk pattern. The angular-linear correlations for walks were all strong (horse 1=0.79, horse 2=0.73, horse 3=0.75) and significant (P<0.001). Horse 3 differed from the other horses during walks in having a small phase lag for pitch relative to vertical displacement, compared with a larger phase lead in horses 1 and 2. The mean phase differences also supported the out-of-phase pattern for canters in horses 1 (135±14deg.) and 2 (108±26deg.) but not in horse 3 (41±47deg.) (Fig.9C). Although significant for all horses (P<0.001), the angular-linear correlations for canters were less strong (horse 1=0.64, horse 2=0.40, horse 3=0.48) than during walks. Phase differences and correlations are not presented for trots because head pitch in this gait was so small as to be insignificant (Fig. 9B, solid lines). As revealed by the angular dispersion values, the degree of phase differences, as well as relative timing of head pitch to head vertical displacement, varied among the individual cycles for all horses but to a much greater degree for canters in horse 3.
Fig. 8.
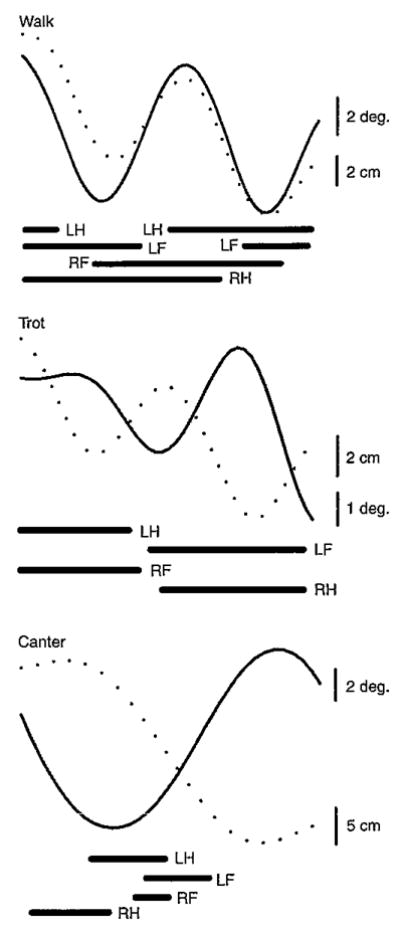
Equine head pitch rotation (solid line) and head vertical translation (broken line) during representative walk, trot, and canter cycles. Thick horizontal lines depict support phases for the left hind limb (LH), right hind limb (RH), left forelimb (LF) and right forelimb (RF). The highly in-phase relationship depicted in the walk example is characteristic of this gait. By contrast, the highly out-of-phase relationships depicted in the trot and gallop examples are common but do not occur consistently (see Fig. 9).
Fig. 9.
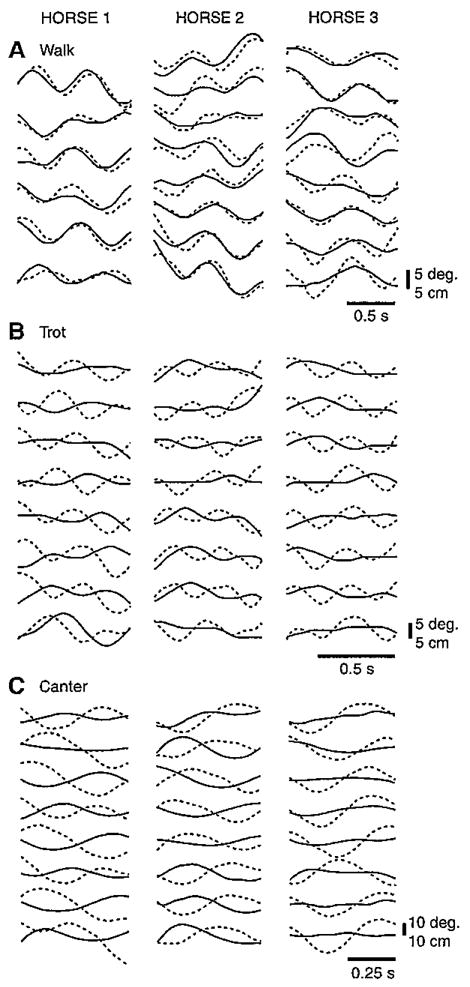
Head pitch (solid line) and head vertical (broken line) displacements during all walk (A), trot (B), and canter (C) cycles for all three horses. Data are smoothed (6 Hz cut-off frequency for walks, 8 Hz for trots and canters) and the mean values have been subtracted for each cycle to yield change in pitch cycle and vertical position. The angular and linear displacements of the head were consistently in-phase for walks but had shifting phase relationships for trots and canters.
DISCUSSION
Are equine head, neck or trunk movements limited to less than 20 deg. in the pitch plane relative to space (i.e. gravity vertical, earth horizontal) and, if so, does the stabilized segment change with a change in gait? Yes. Horses actually stabilize all three segments relative to space and to each other during walks, trots and canters; pitch rotations are much less than 20 deg., and yaw and roll rotations, based on visual observation, are minimal or absent. This finding lends further support to the hypothesis developed from the wild monkey locomotor study (Dunbar et al., 2004) that at least one axial segment (i.e. head or trunk) must be stabilized to provide the nervous system with a whole-body spatial reference frame. Furthermore, the trunk is always the most stabilized segment (2–8 deg. pitch rotation), with head (5–10 deg.) and neck (4–10 deg.) rotations being either equal to, or greater than, trunk rotations. This latter finding is in strong contrast to the movement patterns of quadrupedal monkeys (Fig. 7) and the hypothesized equine rotations (Fig. 1), where the head pitches on a stabilized trunk during walks but the trunk effectively pitches on a stabilized head during gallops (Dunbar et al., 2004). Thus, Hypothesis 1, that the equine head will rotate in the pitch and yaw planes on a stabilized trunk during walks, Hypothesis 2, that the equine trunk will pitch through more than 20 deg. on a stabilized head during canters and Hypothesis 4, that the equine neck will not be rotationally stabilized in the pitch plane, are rejected. Hypothesis 3, however, that the equine head and trunk will remain rotationally stabilized during trots, is supported.
Equine head, neck and trunk displacements and velocities
Table 2 compares our findings on segmental mean displacements in horses with those from studies on humans (Cromwell and Wellmon, 2001; Cromwell et al., 2004; Pozzo et al., 1990) and other primates (Dunbar, 2004; Dunbar et al., 2004; Hirasaki and Kumakura, 2004; Xiang et al., 2008). Vertical displacements of the equine head are larger during walks than those of humans and other primates, whether bipedal or quadrupedal. Equine head vertical displacements are also larger during canters than those of slow galloping monkeys. By contrast, equine head vertical displacements during trots are similar to those in humans during runs, the bipedal equivalent of trots in terms of the diagonal couplet coordination of upper and lower limb movements. These findings indicate that the range of head vertical displacements in horses is larger than those of humans and monkeys but that the largest human head displacements that occur during bipedal runs, overlap in magnitude with the smallest displacements of equine heads, which occur during trots.
Table 2.
Interspecific comparisons of head, neck and trunk pitch (deg.) and head vertical (cm) displacements relative to space
| Quadrupedal |
Bipedal |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Displacement | Gait | Segment | Horsea | Langurb* | Bonnetbe | Rhesuscf | Cynomolguscf | Japanesedg | Vervetch | Humandi | Humandj | Gibbondg | Japanesedg |
| Pitch rotation | Walk | Head | 9(±4) | 17(±5) | 13(±4) | 5(±2) | 6–16k | <5 | 15(±7) | 8(±2) | 9(±3) | <5 | <5 |
| Neck | 10(±3) | – | – | – | – | – | – | 14(±4) | – | – | – | ||
| Trunk | 6(±2) | 10(±3) | 13(±3) | 7(±2) | <10 | – | 6(±2) | 4(±1) | – | – | – | ||
| Trot=Bipedal run | Head | 5(±2) | – | – | – | – | – | – | – | 14(±4) | – | – | |
| Canter=Slow gallop | Head | 10(±4) | 15(±2) | 13(±3) | – | – | – | 14(±7) | – | – | – | – | |
| Trunk | 8(+1) | 42(+4) | 30(+8) | – | – | – | 25(±7) | – | – | – | – | ||
| Vertical translation | Walk | Head | 12(±4) | 7(±2) | 7(±2) | 1(±1) | <1–4k | <2 | – | – | 5–9l | ~2 | <2 |
| Trunk | 10(±2) | – | – | 2(±0.4) | – | – | – | – | – | – | – | ||
| Trot=Bipedal run | Head | 9(±2) | – | – | – | – | – | – | – | 7–16l | – | – | |
| Canter=Slow gallop | Head | 23(±5) | 19(±4) | 11 (±5) | – | – | – | – | – | – | – | – | |
Overground arena locomotion.
Overground natural terrain locomotion.
Treadmill locomotion.
Overground laboratory flat surface locomotion.
Hanuman, common or gray langur (Semnopithecus entellus); Bonnet macaque (Macaca radiata); (Dunbar et al., 2004).
Rhesus macaque (Macaca mulatta); Cynomolgus, long-tailed or crab-eating macaque (Macaca fascicularis); (Xiang et al., 2008).
Japanese macaque (Macaca fuscata); Lar, white-handed or common gibbon (Hylobates lar); (Hirasaki and Kumakura, 2004).
Vervet, grivet, savanna, or African green monkey (Cercopithecus aethiops); (Dunbar, 2004).
Lowest mean to highest mean values across Cynomolgus individuals.
Data presented as range values.
Head pitch displacements of walking horses are similar to those of humans but larger than those of other primates, walking bipedally. Equine head pitch during trots, however, is approximately one third of human head pitch during bipedal runs. When compared with the quadrupedal locomotion of non-human primates, equine head pitch displacements are consistently smaller during both walks and canters/slow gallops. In a monkey treadmill study (Xiang et al., 2008), one quadrupedal cynomolgus subject pitched its head similarly to that reported for monkey overground locomotion (Dunbar et al., 2004) and through a greater range than horses. Two other cynomolgus subjects and four rhesus subjects, however, pitched their heads through much fewer degrees, only half that of equine pitch. This variability in head pitch rotations found between and within species can be attributed to differences in support surface characteristics (e.g. flatness, unevenness, firmness), environmental stimuli (e.g. movement, sound, visual targets of interest), experimental protocol, individual choice and other factors. Regardless of this variability, the significant finding is that, with occasional exceptions during walks (Dunbar et al., 2004), head pitch is limited to 20 deg. or less (Table 2).
Dunbar and colleagues report that bonnet macaques and hanuman langurs walking overground in the wild occasionally make large (>20deg.) head yaw rotations to observe their surroundings, but not during gallops, and that head roll rotations were not notable visually during any gait. In quantitative support of this finding, Xiang and colleagues measured head yaw rotations in rhesus and cynomolgus monkeys walking quadrupedally on a treadmill (Xiang et al., 2008). Head yaw was greater than 20 deg. (i.e. 27±11 deg.) in one cynomolgus subject but less than 20 deg. (i.e. 9.1±3.9deg.) in two other cynomolgus and four rhesus macaques. Furthermore, head roll was always less than 20 deg., being 15 deg. (±5deg.) in the cynomolgus subject with large head yaw and only 5 deg. (±2 deg.) in the remaining cynomolgus and rhesus subjects. Thus, based upon the qualitative and quantitative evidence above, humans and other primates, with occasional pitch and yaw exceptions during walks, follow the equine pattern of head stabilization in all planes during locomotion.
Trunk pitch displacements in horses during walks were similar to those in bipedal humans walking on flat surfaces in the laboratory (Cromwell and Wellmon, 2001) and to quadrupedal monkeys walking on treadmills (Dunbar, 2004; Xiang et al., 2008) but smaller than in monkeys walking quadrupedally on natural terrain in the wild (Dunbar et al., 2004). These trunk pitch similarities and differences during walks can be attributed largely to variation in support characteristics. The equine arena surface resembles more closely the flat and continuous surfaces of the laboratory floor and treadmill than the irregular surfaces of natural terrain, thereby requiring less vertical displacement of the trunk for the hooves or hands and feet to clear the surface. The trunk also pitches less in horses during canters than in monkeys during either overground or treadmill gallops, due largely to species differences in segmental coordination related to differences in mass, its distribution and the resultant inertial properties of the trunk and limb segments (see below).
Neck pitch during walks is somewhat smaller in horses than in humans. Considering that the head and neck pitch largely as a unit on the trunk during walks, horses may be reducing the amount of pitch to minimize the curvilinear displacement of the eyes and vestibular apparatus relative to space (see below).
Maximum velocities of the equine head and trunk can also be compared with those of humans (Cromwell et al., 2004; Lieberman et al., 2006; Pozzo et al., 1990) and other primates (Dunbar, 2004; Dunbar et al., 2004; Hirasaki and Kumakura, 2004; Xiang et al., 2008), and are summarized in Table 3.
Table 3.
Interspecific comparisons of head and trunk maximum pitch (deg.s−1) and head vertical (cms−1) velocities relative to space
| Quadrupedal |
Bipedal |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Velocity | Gait | Segment | Horsea | Langurbe | Bonnetbc | Cynomolguscf | Japanesedg | Vervetcf | Humandl | Humandj | Humanck | Gibbondg | Japanesedg |
| Pitch rotation | Walk | Head | 42(±3) | 241(±27) | 181(±15) | >50 | ~60–100 | 194(±25) | 9(±3) | 30(±8) | – | 58 | ~70 |
| Trunk | 29(±2) | 129(±11) | 83(±7) | – | – | 87(±9) | 17(±4) | – | 68(±21) | – | – | ||
| Trot=Bipedal run | Head | 38(±3) | – | – | – | – | – | – | 72(±20) | – | – | – | |
| Trunk | 17(±2) | – | – | – | – | – | – | – | 147(±22) | – | – | ||
| Canter=Slow gallop | Head | 105(±11) | 216(±17) | 183(±13) | – | – | 202(±29) | – | – | – | – | – | |
| Trunk | 67(+5) | 356(+30) | 353(+33) | – | – | 271(±27) | – | – | – | – | – | ||
| Vertical translation | Walk | Head | 47(±2) | 55(±7) | 38(±1) | – | – | – | – | 25–35l | – | – | – |
| Trot=Bipedal run | Head | 86(±1) | – | – | – | – | – | – | 70–150l | – | – | – | |
| Canter=Slow gallop | Head | 139(±6) | 148(±9) | 85(±8) | – | – | – | – | – | – | – | – | |
Overground arena locomotion.
Overground natural terrain locomotion.
Treadmill locomotion.
Overground laboratory flat surface locomotion.
Hanuman, common or gray langur (Semnopithecus entellus); Bonnet macaque (Macaca radiata); Dunbar et al., 2004.
Cynomolgus, long-tailed or crab-eating macaque (Macaca fascicularis); Xiang et al., 2008.
Japanese macaque (Macaca fuscata); Lar, white-handed or common gibbon (Hylobates lar); Hirasaki and Kumakura, 2004.
Vervet, grivet, savanna, or African green monkey (Cercopithecus aethiops); Dunbar, 2004.
Data presented as range values.
Maximum vertical velocities of the equine head are up to two times larger than those of humans during walks but fall within the human bipedal running range during trots and parallel the findings for equine and human head displacements. During both walks and canters/slow gallops, equine head vertical velocities are consistently larger than those of bonnet macaques despite the greater inertia of the equine head. This is most probably attributed to the contributions of the equine nuchal ligament to head and neck pitch on the trunk during walks and, along with overall body movements, during gallops. By contrast, horses have smaller head vertical velocities than hanuman langurs, probably due to the relatively large vertical (and angular) displacements of the hanuman’s body segments during both walks and gallops, giving this and other langur species bounce-like gaits that distinguish them from macaques and most other monkeys (Dunbar et al., 2004).
Maximum pitch velocities of the equine head during walks are 1.5–5 times greater than those of bipedal humans but less than those of bipedal and quadrupedal monkeys. Head pitch velocities are also less in trotting horses than in humans running bipedally, and less in cantering horses than in monkeys galloping overground or on a treadmill. Thus, with the exception of humans during walks, maximum pitch velocities of the equine head are less than those of humans and other primates. These low pitch velocities are most likely attributed to the greater mass and inertial properties of the equine head and neck. The larger equine head pitch velocities compared with those in humans during walks, despite comparable ranges of head pitch displacement between the species, are most likely attributed to the equine head and neck bob during walks that stores and releases kinetic energy in the nuchal ligament to assist forward progression (Gellman and Bertram, 2002a; Gellman and Bertram, 2002b).
Maximum pitch velocities for the equine trunk are 1.7 times larger than those of humans walking overground (Cromwell et al., 2004). This finding is not surprising considering the sequential footfall of the four equine limbs, combined with the head and neck bobbing on the trunk. Humans on a treadmill (Lieberman et al., 2006), show much higher maximum pitch velocities than horses during walking and running/trotting. These findings for the faster gaits are understandable given the mechanical conditions of each gait. Humans rapidly swing their upper and lower limbs but support body mass alternately with the lower limbs, which would certainly result in rapid pitch (and probably yaw and roll) movements of the vertical trunk. Horses, by contrast, alternate diagonal hind limb–forelimb pairs to support the cranial and caudal ends of the horizontal trunk, resulting in small and slow pitch rotations of the trunk. Maximum pitch velocities of the trunk are much smaller in horses than in monkeys during both walks and canters/slow gallops, primarily due to the significantly greater mass and inertia of the equine trunk.
Curvilinear displacement of equine eyes and vestibular apparatus during pitch-plane rotations
The strategy of horses to limit the angular excursion of the head and neck segments relative to primates may relate to the particular morphology of the horse head and head-based sensors. During head pitch, the eyes and vestibular apparatus experience curvilinear displacements because both end organs are located at a distance from the head–neck and neck–trunk rotational axes. Our equine skull measurements reveal an occipital condyle-to-orbit distance of 22 cm and a pitch axis-to-external auditory meatus distance of 6 cm, both of which are two times larger than similar measurements in the human skull (10 cm and 3.5 cm, respectively) and three times larger than those in the monkey (rhesus macaque) skull (7 cm and 2 cm, respectively). Moreover, the equine cervical spine measured 55 cm, which is five times the measured length of the human spine (11.5 cm) and nine times that of the monkey (rhesus macaque) spine (6.5 cm). Thus, for 20 deg. head pitch, for example, the eye and vestibular arc lengths due to rotation at the head–neck (atlanto–occipital joint) axis alone would be greatest in horses (7.7 cm and 2.1 cm, respectively), intermediate in humans (3.5 cm and 1.2 cm, respectively) and least in monkeys (2.4 cm and 0.7 cm, respectively). These estimated head pitch arc lengths are most relevant to horse trots, in which the majority of rotation occurs at the head–neck axis. The same 20 deg. pitch of the head and neck together about the cervicothoracic joint dramatically increases the eye and vestibular arc lengths to 26.9 cm and 21.3 cm, respectively, as compared with the much smaller arc lengths in humans (7.5 cm and 5.2 cm) and monkeys (4.7 cm and 3.0 cm), respectively. These estimated neck pitch arc lengths are most relevant to equine and human walking. Considering the limited range of mobility at the base of the primate skull (Graf et al., 1995b) compared with horses (Clayton and Townsend, 1989), these combined head/neck pitch arc lengths are probably most relevant to human and monkey gaits in general. Nevertheless, for the same rotation at either the head–neck axis or neck–trunk axis alone, eye and vestibular arc lengths will be much greater in horses than in humans or monkeys. These morphological differences alone have a significant impact on how the eyes and vestibular apparatus in each species are spatially displaced during comparable gaits and most probably on the qualitative and quantitative characteristics of the sensory signals transmitted to the brain.
Horses appear to compensate in part for this morphological impact by reducing the range of pitch rotation compared to humans and monkeys. This reduction is accomplished by isolating the relatively small degree of pitch primarily to a single joint (e.g. neck–trunk during walks, head–neck during trots), or by adjusting the dorsiflexion–plantar flexion phase relationships between the head–neck and neck–trunk joints to counteract one another (e.g. canters). During walks and canters, the equine arc lengths, though reduced, nevertheless remain greater than in humans and monkeys performing comparable gaits when measured from either the skull base (head–neck axis) or cervical base (neck–trunk axis). During trots, by contrast, whereas the arc lengths from the cervical base also remain greater in horses, the equine eye (1.92 cm) and vestibular (0.52 cm) arc lengths from the skull base are actually smaller than those in running humans (2.44 cm and 0.86 cm, respectively).
Equine body mass, its distribution and segmental mobility and stability
The head, neck and trunk movements characterizing equine locomotion can be attributed, in large part, to morphological features and adaptations. Owing to the enormous stresses generated in muscles and other supportive tissues to propel such massive animals (Hill, 1950), the movement of the large and heavy axial segments is minimized, allowing the smaller and lighter distal limb segments to move rapidly through several degrees of rotation (Hildebrand, 1959; Hildebrand, 1974). The head and neck together contribute approximately 10% of total body mass (TBM) in horses (Clayton, 2004), compared with 8% in humans (Dempster, 1955) and chimpanzees (Crompton et al., 1996) and 6% in prosimian lemurs (Dunbar, 1988). Although the 2%–4% difference may seem small in absolute terms, it is large given the horse’s TBM of roughly 500 kg, a value that is 10, 11 and 250 times greater than that of humans, chimpanzees and lemurs, respectively. Furthermore, the equine neck is larger in mass than the head (6% vs 4% TBM, respectively) (Clayton, 2004). Mass measurements for the head and neck of primates and other mammals are rarely reported, however, the lemur neck is much smaller than its head (1% vs 5% TBM, respectively) (Dunbar, 1988). Thus, the inertial properties of the massive and elongated axial segments of horses, compared with the relatively small segments of humans and other primates, most certainly contribute to the reduced rotational displacements and velocities of the equine trunk, neck and head.
Inertial influences on the equine head are revealed through a comparison of pitch displacements (Table 2) and velocities (Table 3) in horses with those in humans (Pozzo et al., 1990) and monkeys (Dunbar et al., 2004). Equine head pitch displacements and velocities are, with few exceptions, smaller than those of humans or monkeys across all three gaits. By contrast, equine head vertical displacements (Table 2) and velocities (Table 3) are, with few exceptions, greater than those of humans and monkeys. During walks, these large vertical head displacements can be attributed almost entirely to the equine head pitching in unison with the elongated neck at the neck–trunk axis, resulting in a large arc length. During canters, neck pitch is certainly a contributing factor but so is the vertical excursion of the trunk in the oscillation between the support and the airborne phase, an excursion that is more than double that seen for walks and trots. During trots, the smaller head vertical displacements are attributed primarily to the small range in vertical movements of the entire body and the small, compensatory rotations of the head and neck, which characterize this symmetrical running gait.
The impact of differences in mass and inertia between horses and monkeys (Dunbar, 2004; Dunbar et al., 2004) is most clearly revealed (through comparisons of trunk angular motion during canters and slow gallops, respectively (Tables 2 and 3). Trunk pitch displacements and velocities are much smaller in horses than in monkeys, reflecting differences in strategy: horses flex and extend the limb joints to clear the hooves from the ground, effectively shortening limb length whereas monkeys rotate the trunk in the pitch plane to lift the extended limbs for clearance during the airborne phase.
Inertia and its role in equine head stabilization
Does inertia itself play a significant role in segmental stabilization? That is, if an external object (e.g. neck) applies a force to the head, will the head, in the absence of muscle activation, react by rotating in the opposite direction (~180 deg. out-of-phase) thereby maintaining its spatial orientation? This question could be asked of the neck or trunk but it is most relevant to the head and whether a stabilized vestibular apparatus provides a reference frame for spatial orientation and an inertial guidance platform for navigation (Pozzo et al., 1990). A method, the details of which can be found elsewhere (Dunbar et al., 2004; Jones and Spells, 1963; Peterson and Goldberg, 1981), has been developed that predicts the frequency range within which inertia becomes a significant factor for stabilizing the head of animals of any given size. The method employs a model that is based on the general dimensional similarity between species (Jones and Spells, 1963) and uses the estimated stabilizing frequency of 5 Hz for a domestic feline head (Peterson and Goldberg, 1981) as a reference point. Based upon this model, the frequency range within which inertia should become a significant stabilizing factor for the equine head is 1–3 Hz. This estimated range encompasses the actual 1–2.5 Hz mean peak frequency range revealed in this study for both angular and vertical head displacements during all three gaits. Thus, inertia, in itself, can play a significant role in stabilizing the equine head. By contrast, the frequencies of pitch plane and vertical displacements of hanuman langur and bonnet macaque heads during quadrupedal walks and gallops over natural terrain (1–2 Hz) fall outside the theoretical range (hanuman langurs, 3–4 Hz; bonnet macaques, 4–5 Hz) in which inertia would play a significant stabilizing role (Dunbar et al., 2004). It should be noted, however, that a treadmill study (Xiang et al., 2008) reports that one cynomolgus macaque subject had a head pitch frequency during walks of 3.6 Hz, which approaches the lower limit at which inertia would become a significant stabilizing factor in bonnet macaques, a monkey species of similar body size. For the human head, inertia also is probably not a significant factor during bipedal walks but it is likely to have an impact during bipedal runs (Pozzo et al., 1990). Thus, with the latter exception, head stabilization in monkeys and humans during gait depends on coordinated muscle activity.
Whereas inertia can potentially play a dominant stabilizing role for the equine head by producing compensatory pitch rotations, its actual expression is variable or absent. During canters and trots, head pitch and vertical displacement are often largely out of phase, particularly in horses 1 and 2, as one would expect with inertial dominance (Figs 8 and 9). This pattern is inconsistent, however, commonly shifting closer to an in-phase relationship, as seen especially in horse 3. Furthermore, walks contrast with both trots and canters in that head pitch and vertical displacement are largely in phase indicating that inertial stabilization is not being expressed in this gait (Figs 8 and 9). Thus, Hypothesis 5, that pitch rotations of the equine head will not be consistently 180 deg. out-of-phase with head vertical translations, is supported. As reported for monkeys in the wild (Dunbar et al., 2004), inertial influences are frequently overridden by active mechanisms during natural overground equine locomotion.
In regard to coordination of head pitch and vertical displacements, horse 3 differs notably from the other two horses, perhaps due to age-related changes. Horse 3 was 24-years-old, twice the age of horse 1 (10 years) and horse 2 (9 years). Reduced viscoelasticity of the nuchal ligament and other soft tissues of the neck, and developing osteoarthritis in the cervical joints are two characteristics of the equine aging process that can reduce the degree of head–neck and neck–trunk flexibility. Even though the overall locomotor pattern of horse 3 is not distinctive from the two younger horses, these factors, if present, could underlie the difference in phasing of head pitch to vertical displacement compared with horses 1 and 2. Horse 3 also differs from the other two horses in being the only pure Thoroughbred. Despite heritage differences, the three horses were similar physically and behaviorally. Thus, whether or not the decreased flexibility observed in Horse 3 can be attributed in part to breeding is unknown.
Equine segmental stabilization and gaze
In addition to the inertial stabilization issue discussed above, tine finding that equine head pitch is primarily in-phase with vertical displacement during walks and inconsistently out-of-phase during trots and canters, also indicates that compensatory head movements are not essential for gaze stabilization during natural overground quadrupedal locomotion. The lack of such an ‘eye–head synergy’ (Pozzo et al., 1990) contradicts the findings of several studies on bipedal humans attempting to maintain gaze on a fixed target. Pozzo and colleagues found that when human subjects look at a target while performing a bipedal locomotor task, they pitch the head counter-clockwise during vertical head drops and clockwise during vertical head rises, thereby maintaining a fixed gaze (Pozzo et al., 1990). Several other laboratory studies have reported these consistent compensatory head rotations (Cromwell et al., 2001; Cromwell et al., 2004; Moore et al., 1999; Paige, 1989), which appear to parallel compensatory eye-in-orbit (VOR) rotations (Grossman et al., 1988; Liao et al., 2008; Moore et al., 1999; Paige, 1989; Schwarz and Miles, 1991). In line with the above studies on humans, an investigation of two Japanese macaques and one gibbon (Hylobates lar) walking bipedally overground in a laboratory revealed that head rotations are largely compensatory for vertical head translations in these species (Hirasaki and Kumakura, 2004), The authors note, however, that one of the macaques was not trained to walk bipedally, and that the degree to which its head rotations were compensatory was variable. By contrast, both horses and quadrupedal monkeys (Dunbar et al., 2004) demonstrate non-compensatory (in-phase) head pitch-plane rotations (equine walk, monkey gallop) or no consistent phase relationships with vertical displacement (equine trot and canter, monkey walk). A study of cynomolgus macaques walking on a treadmill supports our findings (Xiang et al., 2004). Note, however, that largely compensatory head pitch rotations are reported for two Japanese macaques walking quadrupedally overground in the laboratory (Hirasaki and Kumakura, 2004). Based on the published figures by Pozzo and colleagues, even bipedal humans show a variable phase relationship during walks (Pozzo et al., 1990). The discrepancies are probably attributed to the differences between the natural and volitional locomotor conditions under which the overground equine and monkey data were collected, the unnatural and highly controlled conditions of the laboratory and study protocols used in the human studies, and whether or not the subjects looked at a specific target. The horses (arena) and monkeys (natural terrain) were unrestrained, moving overground and not required to maintain gaze on any particular target. Thus, fixing gaze on a specific target under natural conditions may be transitory (even within a single cycle) because free-ranging animals visually inspect their surroundings frequently for food or predators and other dangers. The distance to a visual target is also a potential factor, with the need for compensatory head rotations diminishing with increasing visual target distance (Dunbar et al., 2004). But, with the exception of the closest visual targets, this possibility also seems unlikely. We revealed that equine head rotations are not compensatory but rather in-phase with vertical displacements during walks when the head is most closely oriented towards the ground. Furthermore, a human treadmill locomotor study reported that, although head pitch rotations are compensatory, viewing distance has little impact on the magnitude of either head movement or phase relationships between head pitch rotation and vertical translation (Moore et al., 1999).
Human head pitch may also compensate for the significantly greater ground reaction forces generated during bipedal locomotion. Quadrupedal horses (Lanovaz et al., 1998) and monkeys (Schmitt, 2003) and bipedal humans (Cappozzo, 1982) each incorporate damping mechanisms to attenuate ground impact forces. Nevertheless, peak ground reaction forces relative to TBM for the forelimbs and hind limbs, respectively, during quadrupedal walks are only 68% and 49% in horses (Ueda et al., 1981) and only approximately 60% and 65% in macaque monkeys (Kimura et al., 1979). By contrast, the forces are doubled (130%) in human limbs during bipedal walks (Schmitt, 2003). Thus, the impact forces may be sufficiently attenuated during quadrupedal locomotion to avoid generating and transmitting vibrations from the hooves or hands and feet to the head that would result in head pitch rotations, such as those that are reported to be typical of human bipedal locomotion (Moore et al., 1999; Pozzo et al., 1990).
Based upon the results of the current equine study and those discussed above, we propose that head pitch rotation is not a typical compensatory component of gaze stabilization during natural quadrupedal locomotion. It is more likely that gaze is stabilized primarily through eye-in-orbit rotations alone. Alternatively, gaze fixation may be a transient activity relative to the length of the gait cycle or, it may not be commonly used or essential for successful locomotion.
Unlike humans and monkeys (Dunbar et al., 2004) horses avoid lateral (yaw) head rotations during overground walks as well as during trots and canters, a behavior that may relate to their differing visual system, the latter of which is characteristic of many plains-living or open habitat-living species. The equine eye is more laterally placed in the head than in primates with a field of view of 160–170 deg. (Harman et al., 1999) to 190–195 deg. (Rochon-Duvigneaud, 1943) horizontally and 178 deg. (Roberts, 1992) vertically, and binocular overlap between 55 deg. and 65 deg. (Duke-Elder, 1958; Hughes, 1977) and even up to 80 deg. (Harman et al., 1999). The only blind spots are immediately in front of the forehead and directly behind the rump (Timney and Macuda, 2001). Equine retinas concentrate the ganglionic cells for visual acuity in a horizontally oriented band called a ‘visual streak’, providing a sharp field of view that is very broad horizontally but very restricted vertically (Harman et al., 1999; Hughes, 1977), thus obviating the need for lateral head turns. Harmon and colleagues (Harmon et al., 1999), however, challenge the assumption that horses can see both forwards and backwards simultaneously (Simpson, 1961), proposing instead that horses must choose between a forward field of view when the head is raised, as during locomotion, and a lateral view when the head is lowered, as during quiet stance when grazing. If correct, then large monocular fields of view with laterally placed eyes fails to explain the absence of head yaw rotations during walks. Even if the high acuity field of view is restricted during gait, the peripheral field still provides a large lateral field of view for motion detection, to alert the horse to the approach of other animals. A behavioral explanation for lack of yaw rotations is that such head turns may trigger unwanted neck tonic reflexes, which are known to extend one forelimb while flexing the other forelimb (Roberts, 1978), thereby negatively impacting stability and steering. This possibility is not very likely, however, because humans, monkeys and many other animals that possess these neck reflexes commonly rotate the head during walks. Furthermore, tonic vestibular (labyrinthine) reflexes triggered by the same head turns are opposite to neck reflexes (Lindsay et al., 1976; Roberts, 1978), leading Roberts (Roberts, 1978) to propose that cervical and vestibular tonic reflexes should be considered a single reflexive system that underlies (he ability to turn the head without destabilizing the body. Another possibility, which we feel is most likely, is that horses actively avoid lateral head rotations for mechanical reasons. Pitch-plane rotations of the long and heavy head and neck during the gait cycle are known to create large torques at the cervicothoracic junction, and cause an increased shift of the center of mass (CM) anteroposteriorly between the forelimbs and hind limbs (Clayton, 2004; Vorstenbosch et al., 1997). Similarly, lateral head and neck turns would suddenly shift the CM mediolaterally, most probably generating a lateral sway that could adversely affect steering, stability, or both.
Equine head stabilization and the vestibular apparatus
Knowing that their horizontal semicircular canals are held in the pitch plane near earth horizontal, horses join humans (Pozzo et al., 1990), monkeys (Dunbar et al., 2004) and many other species of mammals and birds (Graf et al., 1995a) in adopting a head position that maintains the vestibular apparatus in a relatively fixed orientation relative to gravity vertical or to the associated gravito-inertial acceleration vector (Imai et al., 2001). Thus, horses lend further support to the hypothesis that the stabilized head allows the vestibular apparatus to provide information about whole-body spatial orientation and to function as an inertial guidance system for navigation (Pozzo et al., 1990). Horses, however, hold an advantage in fulfilling these functions. Compared with humans and monkeys, the large ranges of pitch rotation permitted between the head and the neck, and the neck and the trunk, enable the equine head to adopt an angular spatial orientation that is much more independent of neck and trunk orientation. In addition, the greater inertial properties of the equine head, along with viscoelastic properties of the nuchal ligament and other neck tissues, provide a much greater passive, energy efficient mechanism for maintaining head orientation.
The vestibular apparatus, like the eyes, experiences curvilinear displacements during head rotations because these sensory organs are located a distance from the cranial rotational axes. Unlike the eyes, however, which can rotate independently within the orbits, the skull-fixed vestibular apparatus must faithfully follow head rotations. Thus, placing the vestibular apparatus close to the cranial rotational axes dramatically reduces the magnitude of curvilinear displacement, which is experienced during rotations.
Owing to morphological differences in the skull, the position of the equine vestibular apparatus relative to the axes (pitch and yaw) of cranial rotation on the neck differs from that in humans and monkeys. During natural posture and locomotion, the vestibular apparatus is located anteriorly (rostrally) in horses whereas it is located superiorly (dorsally) in humans and other primates. Given the same degree of head-on-neck pitch, the vertical vector component of curvilinear displacement is larger than the horizontal component in horses but the horizontal component is larger than the vertical component in primates (Fig. 10). Pitch rotation within the normal range for horse locomotion (±5 deg.) would produce a very small horizontal displacement in the posterior direction in the horse but a significant motion in the primate, in both the anterior (+pitch) and posterior (−pitch) directions. Thus, for comparable head pitch rotations, the pattern of saccular and utricular stimulation differs for horses and primates. This morphological difference may be an advantage in the horse in that linear accelerations due to forward progression will be minimally confounded by the linear components of pitch rotations, unlike primates in who this confound is a significant issue.
Fig. 10.
Schematic drawing comparing vector components of equine and primate (human and monkey) vestibular acceleration in response to clockwise pitch rotation. Rectangle in the 3 o’clock position represents equine vestibular apparatus whereas rectangle in the 12 o’clock position represents primate vestibular apparatus. Circle with small arrowheads depicts direction of rotation about the pitch axis. Resultant vectors (Rh, Rp) and their horizontal (Hh, Hp) and vertical (Vh, Vp) components are depicted for horses and primates, respectively.
This leads to a fundamental problem of how the nervous system can distinguish among and interpret the complex sensory signals generated by the vestibular apparatus. During natural behaviors such as locomotion, the otolith signal encodes not only the linear acceleration of the head in space, but also that due to the off-axis rotations, as discussed above, and the gravitational acceleration induced by head tilts. Following Einstein’s equivalence principle of gravity and inertial acceleration (Einstein, 1908), the otolith organs do not distinguish between inertial acceleration due to translation (linear head movements) and that due to gravitational acceleration (head tilt) (Angelaki et al., 1999; Angelaki et al., 2004; Hess, 2007; Merfeld et al., 1999). How, then, does the nervous system successfully interpret vestibular signals that arise from the multiple segmental and whole body movements that characterize locomotion?
Experimental studies over the last decade have largely resolved this problem. Firstly, studies of VOR, which are based on eye movement measurements in humans (Merfeld et al., 1999) and single unit recordings in rhesus monkeys (Angelaki et al., 1999; Angelaki et al., 2004), reveal that the nervous system resolves the ambiguity of head tilt vs linear translation through a computation that combines otolith and semicircular canal signals. This computed signal enables the nervous system to determine head-to-gravity orientation regardless of how the head rotates or translates in space (Macpherson et al., 2007). Secondly, a psychophysical study exposed seated human subjects (no vision, diminished proprioceptive and hearing information) to constant velocity yaw rotations with and without galvanically induced illusory rotations while the subject held the head in various tilt positions in the pitch plane, in order to examine perception of the route traveled (Day and Fitzpatrick, 2005). They concluded that the nervous system combines the otolith gravitational signal with the net canal signal to accurately compute the distance traveled in the yaw plane. Thus, using the above mechanisms, the nervous system is able to parse out the various components of the vestibular signal to determine segmental and whole-body orientation and to successfully navigate through space. Furthermore, the problem of differing patterns of saccular and utricular stimulation in horses and primates is most likely moot because the meaning of the different resultant signals to the nervous system of each species most certainly will be comparable for the same segmental or whole body movements.
Equine neck
The long neck, which enables the horse to graze while standing, is also of central importance to equine locomotion and head stabilization. Powerful ligaments and muscles stabilize and move the head and neck. The well-developed and structurally complex nuchal ligament, which is poorly developed or absent in primates and carnivores, extends from the elongated spinous processes of the cranial thoracic vertebrae across the cervical vertebrae to the occipital region of the skull. Gellman and Bertram (Gellman and Bertram, 2002a; Gellman and Bertram, 2002b) found that the equine nuchal ligament, which consists of a cord-like dorsal (funicular) part and a sheet-like ventral (lamellar) part, does more than simply support head and neck position. The cranial portion of the lamellar part and caudal portion of the funicular part, in particular, store and release elastic strain energy generated by head and neck oscillations during locomotion (Gellman and Bertram, 2002a), thereby helping to reduce neck pitch excursions to the ranges that we present in the current study (Gellman and Bertram, 2002b). Indeed, the nuchal ligament provides the majority (55%) of energy required to move the head and neck during walks and approximately one third of that required during trots (33%) and canters (31%) (Gellman and Bertram, 2002b). Rotating the head in-phase with the neck may reduce the energy required to maintain head position against gravity by use of the energy stored in the passive elements of the nuchal ligament. For example, out-of-phase or counter-rotation of the head as the neck pitches down would shorten the cranial component of the nuchal ligament and reduce the energy stored due to elastic stretch, thus requiring a greater contribution from the neck muscles to control head position as the neck begins its upward trajectory.
Gellman and colleagues also found that the epaxial cervical musculature associated with the equine nuchal ligament has identifiable structural and histochemical properties to meet the multiple functional demands of head and neck stabilization and movement for head positioning, posture and locomotion (Gellman et al., 2002). Specifically, the uncompartmentalized splenius muscle, with its long-fibers and mix of slow-twitch oxidative (SO) and fast-twitch glycolytic (FG) fiber types, is considered to turn the head, maintain static postural support and prevent unwanted flexion and descent of the head and neck during locomotion. The semispinalis capitis muscle, by contrast, with its central tendon, division into multiple neuromuscular compartments and opposing gradients of SO fibers in the cranial portion of the dorsal and ventral regions (i.e. high percentage dorsally, low percentage ventrally), is proposed to have separate functions for each region. The dorsal region is considered to provide passive head and neck support by adjusting the spring-like qualities of the central tendon whereas the ventral region raises the lowered head through active muscular effort. Structural compartmentalization, however, does not necessarily translate into a capacity to function independently or differentially (Dunbar and Macpherson, 1993; Gans and Gaunt, 1992). Thus, this capacity in equine semispinalis capitis compartments, regions or both needs to be determined experimentally.
The distribution and degree of joint mobility within the equine neck and overall vertebral column also have a profound impact on head, neck and trunk movements. Horses and other large ungulates built for high running endurance and low energy expenditure have evolved relatively rigid vertebral columns, largely by shortening the lumbar region and elongating the vertebral spines to limit movement (Slijper, 1946; Wake, 1979). Other than at the lumbosacral joint (Hildebrand, 1959; Townsend et al., 1983), vertebral flexion and extension is limited primarily to the cervical region in horses; the majority of the cervical joints (between the 3rd cervical and 1st thoracic vertebrae) allow around 25–35 deg. of flexion and extension but the largest range (87 deg.) occurs at the atlanto–occipital joint at the base of the skull (Clayton and Townsend, 1989). The equine atlanto–occipital joint’s range of pitch-plane rotation, which contributes 32% of the total flexion and extension in the cervical spine (Clayton and Townsend, 1989), is similar to the feline rotational range (92deg.) (Graf et al., 1995b). This large range contrasts with the smaller ranges in rhesus (32deg.) and squirrel monkeys (19deg.) (Graf et al., 1995b) and in humans, the latter reported to be from around 11 deg. (Dvorak, 1988; Graf et al., 1995b) to 25 deg. (White and Panjabi, 1990). Thus, unlike in humans and monkeys, head angular position in horses is not heavily dependent upon cervical spine orientation. Rather, the equine head achieves and maintains a variety of head orientations quite independently from the neck to successfully complete a variety of behavioral tasks (e.g. stance and gaze maintenance, locomotion, grazing).
The equine neck plays a significant role not only in locomotion but also in respiration. The neck is probably intimately linked to the central pattern generators (CPGs) for locomotion, based upon its specific spatial and temporal coordination with the head and trunk but in addition, the timing of neck pitch is known to be a critical component of locomotor-respiratory coupling (LRC) during running (Bramble, 1989). A limited number of coupling ratios (i.e. number of locomotor cycles to number of ventilatory cycles) occur during trots (Attenburrow, 1982) but the ratio is limited to 1:1 during canters and faster gallops (Bramble and Carrier, 1983). Inhalation occurs when the neck is ventroflexing clockwise whereas exhalation occurs when the neck is dorsiflexing counter-clockwise (Bramble, 1989). The neck is considered part of a cranio–cervical unit that, along with thoracic and lumbo–pelvic units, comprises a LRC biomechanical model. The model explains how axial movements can be coordinated with limb movements to enable the pelvic viscera to act, via inertia, as a piston on the diaphragm to time inhalation and exhalation (Bramble, 1989). In support of this model, subsequent experimental work (Attenburrow and Goss, 1994) demonstrates that the primary factor coupling equine breathing to locomotion is the degree of abdominal muscular contraction to alter intra-abdominal pressure. Other experiments with trotting dogs, however, indicate that the vertical and horizontal trunk movements produce elliptical, rather than linear, visceral movements, resulting in a more complicated respiratory-locomotor coupling than predicted by the piston-like LRC model (Bramble and Jenkins, 1993). Nevertheless, the equine neck is an important component in the coordination of respiration and locomotion.
In conclusion, our results do not demonstrate that the equine neck, in itself, can provide the reference frame for the body relative to space because it is never the only stabilized segment. Nevertheless, the neck of horses is remarkable in that its morphological features enable it to minimize its own rotations and those of the head and trunk to the degree that all three segments remain stabilized (≤20deg.) simultaneously during locomotion. Therefore, these equine movement patterns further support the hypothesis that the nervous system requires at least one stabilized axial segment to determine and maintain whole-body spatial orientation (Dunbar et al., 2004).
Acknowledgments
The authors wish to thank the following individuals and institutions: Ms Anderson, Mrs Coit and Ms Hadden (Trainer), who own the horses, and Mr B. Milligan, who manages the Rancho Riding Club in Rancho Santa Fe, California, kindly provided us with the equine subjects, location and facilities for video collection; skull and cervical spine measurements were made possible at the University of Puerto Rico by Dr Jean E. Tumquist (horse) and the Department of Anatomy and Neurobiology (human) in the School of Medicine, and by the Laboratory of Primate Morphology and Genetics (rhesus monkey) in the Caribbean Primate Research Center; Dr Stéphane Vieilledent (Universíté de Bretagne Occidentale, Brest, France) contributed to the head frequency analyses; and two anonymous reviewers provided helpful comments on the manuscript. Funded by the Research Centers in Minority Institutions Program (NIH RR-03051), the Caribbean Primate Research Center (NIH P40 RR003640), and the University of Puerto Rico School of Medicine.
LIST OF ABBREVIATIONS
- a.d.
angular dispersion
- ANOVA
analysis of variance
- CM
center of mass
- CPG
central pattern generator
- FG
fast-twitch glycolytic
- Hh
horse horizontal vector component
- Hp
primate horizontal vector component
- LF
left forelimb
- LH
left hind limb
- LRC
locomotor-respiratory coupling
- NIH
National Institutes of Health (USA)
- RF
right forelimb
- RH
right hind limb
- Rh
horse resultant vector
- Rp
primate resultant vector
- s.d
standard deviation
- s.e.m
standard error of the mean
- SO
slow-twitch oxidative
- TBM
total body mass
- VCR
vestibulo-collic reflex
- Vh
horse vertical vector component
- VOR
vestibulo-ocular reflex
- Vp
primate vertical vector component
- α
head angle relative to the trunk
- β
trunk angle relative to space
- δ
neck angle relative to space
- ε
neck angle relative to the trunk
- μ
head angle relative to the neck
- θ
head angle relative to space
References
- Alexander RM. Locomotion in Animals. Glasgow: Blackle; 1982. [Google Scholar]
- Angelakl DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJM. Computation of inertial motion: neural strategies to resolve ambiguous ololith information. J Neurosci. 1999;19:316–327. doi: 10.1523/JNEUROSCI.19-01-00316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakl DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564. doi: 10.1038/nature02754. [DOI] [PubMed] [Google Scholar]
- Ankel-Simons F. Primate Anatomy: An Introduction. 2. San Diego: Academic Press; 2000. [Google Scholar]
- Attenburrow DP. Time relationships between the respiratory cycle and the limb cycle in the horse. Equine Vet J. 1982;14:69–72. doi: 10.1111/j.2042-3306.1982.tb02340.x. [DOI] [PubMed] [Google Scholar]
- Attenburrow DP, Goss VA. The mechanical coupling of lung ventilation to locomotion in the horse. Med Eng Phys. 1994;16:188–192. doi: 10.1016/1350-4533(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Bramble DM. Axial-appendicular dynamics and the integration of breathing and gait in mammals. Am Zool. 1989;29:171–186. [Google Scholar]
- Bramble DM, Carrier DR. Running and breathing in mammals. Science. 1983;219:251–256. doi: 10.1126/science.6849136. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Jenkins FA., Jr Mammalian locomotor-respiratoty Integration: implications for diaphragmatic and pulmonary design. Science. 1993;262:235–240. doi: 10.1126/science.8211141. [DOI] [PubMed] [Google Scholar]
- Cappozzo A. Low frequency self-generated vibration during ambulation in normal men. J Biomech. 1982;8:599–609. doi: 10.1016/0021-9290(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Clayton HM. The Dynamic Horse. Mason, Ml: Sport Horse; 2004. [Google Scholar]
- Clayton HM, Townsend HGG. Kinematics of the cervical spine of the adult horse. Equine Vet J. 1989;21:189–192. doi: 10.1111/j.2042-3306.1989.tb02139.x. [DOI] [PubMed] [Google Scholar]
- Crompton RH, Li Y, Alexander RM, Wang W, Gunther MM. Segmental inertial properties of primates: new techniques for laboratory and field studies of locomotion. Am J Phys Anthropol. 1996;99:547–570. doi: 10.1002/(SICI)1096-8644(199604)99:4<547::AID-AJPA3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cromwell R, Wellmon R. Sagittal plane head stabilization during level walking and ambulation on stairs. Physiother Res Int. 2001;6:179–192. doi: 10.1002/pri.226. [DOI] [PubMed] [Google Scholar]
- Cromwell RL, Aadland-Monahan TK, Nelson AT, Stem-Sylvestre SM, Seder B. Sagittal plane analysis of head, neck, and trunk kinematics and electromyographic activity during locomotion. J Orthop Sports Phys Ther. 2001;31:255–262. doi: 10.2519/jospt.2001.31.5.255. [DOI] [PubMed] [Google Scholar]
- Cromwell RL, Pldcoe PE, Griffin LA, Sotillo T, Ganninger D, Feagin M. Adaptations in horizontal head stabilization in response to altered vision and gaze during natural walking. J Vestib Res. 2004;14:367–373. [PubMed] [Google Scholar]
- Das P, McCollum G. Invariant structure in locomotion. Neuroscience. 1988;25:1023–1034. doi: 10.1016/0306-4522(88)90055-3. [DOI] [PubMed] [Google Scholar]
- Day BL, Fltzpatrick RC. Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol (Lond) 2005;567:591–597. doi: 10.1113/jphysiol.2005.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBeer GR. How animals hold their heads. Proc Linn Soc Lond. 1947;159:125–139. [Google Scholar]
- Dempster WT. Wright Air Development Center (WADC) Technical Report 55–159. Ohio: Wright-Patterson Air Force Base; 1955. Space requirements of the seated operator. [Google Scholar]
- Duke-Elder SS. System of Ophthalmology. Vol. 1, The Eye in Evolution. London: Henry Kimpton; 1958. [Google Scholar]
- Dunbar DC. Aerial maneuvers of leaping lemurs: the physics of whole-body rotations while airborne. Am J Primatol. 1988;16:291–303. doi: 10.1002/ajp.1350160402. [DOI] [PubMed] [Google Scholar]
- Dunbar DC. The influence of segmental movements and design on whole-body rotations during the airborne phase of primate leaps. Z Morphol Anthropol. 1994;80:109–124. [Google Scholar]
- Dunbar DC. Stabilization and mobility of the head and trunk in vervel monkeys (Cereopithecus aethiops) during treadmill walks and gallops. J Exp Biol. 2004;207:4427–4438. doi: 10.1242/jeb.01282. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Macpherson JM. Activity of neuromuscular compartments in lateral gastrocnemius evoked by postural corrections during stance. J Neurophysiol. 1993;70:2337–2349. doi: 10.1152/jn.1993.70.6.2337. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Badam GL, Hallgrimsson B, Vlellledent S. Stabilization and mobility of the head and trunk in wild monkeys during terrestrial and flat-surface walks and gallops. J Exp Biol. 2004;207:1027–1042. doi: 10.1242/jeb.00863. [DOI] [PubMed] [Google Scholar]
- Dvorak J. Funktionelle Anatomie der oberen Halswirbelsäule unter besonderer Berückersichtigung des Bandapparates. In: Wolff HD, editor. Die Sonderstellung des Koplgelenkbereichs. Berlin: Springer; 1988. pp. 19–46. [Google Scholar]
- Edwards EH. The New Encyclopedia of the Horse. New York: Dorling Kindersley; 2001. [Google Scholar]
- Einstein A. Über das Relativitätsprinzip und die aus demselben gezogenen Folgerungen. Jahrb Radioakt. 1908;4:411–462. [Google Scholar]
- Frohlich C. Do springboard divers violate angular momentum conservation? Am J Phys. 1979;47:583–592. [Google Scholar]
- Frohlich C. The physics of somersaulting and twisting. Sci Am. 1980;242:154–164. doi: 10.1038/scientificamerican0380-154. [DOI] [PubMed] [Google Scholar]
- Fuchs AF. Eye-head coordination. In: Towe AL, Luschei ES, editors. Handbook of Behavioral Neurobiology, Vol. 5: Motor Coordination. New York: Plenum Press; 1981. pp. 303–366. [Google Scholar]
- Gans C, Gaunt AS. Muscle architecture and control demands. Brain Behav Evol. 1992;40:70–81. doi: 10.1159/000113904. [DOI] [PubMed] [Google Scholar]
- Gellman KS, Bertram JEA. The equine nuchal ligament 1, structural and material properties. Vet Comp Orthop Traumatol. 2002a;15:1–6. [Google Scholar]
- Gellman KS, Bertram JEA. The equine nuchal ligament 2, passive dynamic energy exchange in locomotion. Vet Comp Orthop Traumatol. 2002b;15:7–14. [Google Scholar]
- Gellman KS, Bertram JEA, Hermanson JW. Morphology, histoehemistry, and function of epaxial cervical musculature in the horse (Equus caballus) J Morphol. 2002;251:182–194. doi: 10.1002/jmor.1082. [DOI] [PubMed] [Google Scholar]
- Goody PC. Horse Anatomy: A Pictorial Approach to Equine Structure. London: J.A. Allen; 1983. [Google Scholar]
- Graf W, de Waele C, Vidal PP. Functional anatomy of the head-neck system of quadrupedal and bipedal mammals. J Anat. 1995a;186:55–74. [PMC free article] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal PP, Wang DH, Evinger C. The orientation of the cervical vertebral column in unrestrained awake animals. II: movement strategies. Brain Behav Evol. 1995b;45:209–231. doi: 10.1159/000113551. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbation during locomolion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Harmon AM, Moore S, Hoskins R, Keller P. Horse vision and an explanation for the visual behaviour originally explained by the “ramp retina”. Equine Vet J. 1999;31:384–390. doi: 10.1111/j.2042-3306.1999.tb03837.x. [DOI] [PubMed] [Google Scholar]
- Hess BJM. Sensorimotor transformations in spatial orientation relative to gravity. In: Mast FW, Jäncke L, editors. Spatial Processing in Navigation, Imagery and Perception. New York: Springer; 2007. pp. 281–300. [Google Scholar]
- Hildebrand M. Motions of the running cheetah and horse. J Mammal. 1959;40:481–495. [Google Scholar]
- Hildebrand M. Analysis of symmetrical gaits of tetrapods. Folia Biotheoret. 1966;13:9–22. [Google Scholar]
- Hildebrand M. Analysis of Vertebrate Structure. New York: John Wiley; 1974. [Google Scholar]
- Hildebrand M. Analysis of symmetrical gaits. J Mammal. 1977;58:131–156. [Google Scholar]
- Hill AV. The dimensions of animals and their muscular dynamics. Sci Prog. 1950;38:209–230. [Google Scholar]
- Hirasaki E, Kumakura H. Head movements during locomotion in a gibbon and Japanese macaques. NeuroReport. 2004;15:643–647. doi: 10.1097/00001756-200403220-00014. [DOI] [PubMed] [Google Scholar]
- Howell AB. Speed in Animals. Chicago: University of Chicago Press; 1944. [Google Scholar]
- Hughes A. The topography of vision in mammals of contrasting lifestyle: comparative optics and retinal organization. In: Crescitelli F, editor. Handbook of Sensory Physiology: the Visual System in Vertebrates. VII/5. Berlin: Springer Verlag; 1977. pp. 615–756. [Google Scholar]
- Imai T, Moore ST, Raphan T, Cohen B. Interaction of the body, head, and eyes during walking and turning. Exp Brain Res. 2001;138:1–18. doi: 10.1007/s002210000533. [DOI] [PubMed] [Google Scholar]
- Jakobs T, Miller JAA, Schultz AB. Trunk position sense in the frontal plane. Exp Neurol. 1985;90:129–138. doi: 10.1016/0014-4886(85)90046-9. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wehrly T. Measures and models for angular correlation and angular-linear correlation. J R Stat Soc Ser B Methodol. 1977;39:222–229. [Google Scholar]
- Jones GM, Spells KE. A theoretical and comparative study of functional dependence of the semicircular canal upon physical dimensions. Proc R Soc Lond, B, Biol Sci. 1963;157:403–419. doi: 10.1098/rspb.1963.0019. [DOI] [PubMed] [Google Scholar]
- Kimura T, Okada M, Ishida H. Kinesiological characteristics of primate walking: its significance in human walking. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, Behavior, and Morphology: Dynamic Interactions in Primates. New York: Gustav Fischer; 1979. pp. 297–311. [Google Scholar]
- Kleine JF, Guan Y, Kiplani EL, Gionti L, Hoshi M, Büttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol. 2004;91:2090–2100. doi: 10.1152/jn.00849.2003. [DOI] [PubMed] [Google Scholar]
- Lanovaz JL, Clayton HM, Watson LG. In vitro attenuation of impact shock in equine digits. Equine Vet J Suppl. 1998;26:96–102. doi: 10.1111/j.2042-3306.1998.tb05127.x. [DOI] [PubMed] [Google Scholar]
- Liao K, Walker MF, Joshi A, Reschke M, Leigh RJ. Vestibuloocular responses to vertical translation in normal human subjects. Exp Brain Res. 2008;185:553–582. doi: 10.1007/s00221-007-1181-z. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Raichien DA, Pontzer H, Bramble DM, Cutright-Smith E. The human gluteus maximus and its role in running. J Exp Biol. 2006;209:2143–2155. doi: 10.1242/jeb.02255. [DOI] [PubMed] [Google Scholar]
- Lindsay KW, Roberts TDM, Rosenberg JR. Asymmetrical tonic labyrinth reflexes and their interaction with neck reflexes in the decerebrate cat. J Physiol (Lond) 1976;261:583–601. doi: 10.1113/jphysiol.1976.sp011575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson JM, Everaert DG, Stapley PJ, Ting LH. Bilateral vestibular loss in cats leads to active destabilization of balance during pitch and roll rotations of the support surface. J Neurophysiol. 2007;97:4357–4367. doi: 10.1152/jn.01338.2006. [DOI] [PubMed] [Google Scholar]
- Mardia KV. Linear-circular correlation coefficients and rhythmometry. Biometrika. 1976;63:403–405. [Google Scholar]
- Merfeld DM, Zupan L, Peterka RJ. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- Mergner T, Nardl GL, Becker W, Keeke L. The role of canal-neck interaction for the perception of horizontal trunk and head rotation. Exp Brain Res. 1983;49:198–208. doi: 10.1007/BF00238580. [DOI] [PubMed] [Google Scholar]
- Mergner T, Siebold C, Schwelgart G, Becker W. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res. 1991;85:389–404. doi: 10.1007/BF00229416. [DOI] [PubMed] [Google Scholar]
- Mergner T, Rottler G, Kimmig H, Becker W. Role of vestibular and neck inputs for the perception of object motion in space. Exp Brain Res. 1992;89:655–668. doi: 10.1007/BF00229890. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. The information processing structure of the subjective vertical: a cybernetic bridge between its psychophysics and its neurobiology. In: Marko H, Hauske G, Struppler A, editors. Processing Structures For Perception and Action. Weinheim: V. C. H. Verlagsgesellschaft; 1988. pp. 217–263. [Google Scholar]
- Mittelstaedt H. Evidence of somatic graviception from new and classical investigations. Acta Otolaryngol Suppl. 1995;520:186–187. doi: 10.3109/00016489509125224. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Somatic graviception. Biol Psychol. 1996;42:53–74. doi: 10.1016/0301-0511(95)05146-5. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Interaction of eye-, head-, and trunk-bound information in spatial perception and control. J Vestib Res. 1997;7:283–302. [PubMed] [Google Scholar]
- Mittelstaedt H. Origin and processing of postural information. Neurosci Biobehav Rev. 1998;22:473–478. doi: 10.1016/s0149-7634(97)00032-8. [DOI] [PubMed] [Google Scholar]
- Moore ST, Hirasaki E, Cohen B, Raphan T. Effect of viewing distance on the generation of vertical eye movements during locomotion. Exp Brain Res. 1999;129:374–361. doi: 10.1007/s002210050903. [DOI] [PubMed] [Google Scholar]
- Paige GD. The influence of larget distance on eye movement responses during vertical linear motion. Exp Brain Res. 1989;77:585–593. doi: 10.1007/BF00249611. [DOI] [PubMed] [Google Scholar]
- Patla AE. Understanding the control of human locomotion: a prologue. In: Patla AE, editor. Adaptability of Human Gait. Amsterdam: North-Holland; 1991. pp. 3–17. [Google Scholar]
- Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture. 1997;5:54–69. [Google Scholar]
- Peterka RJ. Sensorimotor integration in human posture control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Boyle RD. Vestibulocollic reflexes. In: Highstein SM, Fay RR, Popper AN, editors. The Vestibular System. New York: Springer; 2004. pp. 343–374. [Google Scholar]
- Peterson BW, Goldberg J. Role of vestibular and neck reflexes in controlling eye and head position. In: Roucoux A, Crommelinck M, editors. Physiological and Pathological Aspects of Eye Movements. The Hague: Dr W. Junk; 1931. pp. 352–364. [Google Scholar]
- Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans. I: Normal subjects Exp Brain Res. 1990;82:97–106. doi: 10.1007/BF00230842. [DOI] [PubMed] [Google Scholar]
- Roberts SM. Equine vision and optics. Vet Clin North Am Equine Pract. 1992;8:451–457. doi: 10.1016/s0749-0739(17)30435-2. [DOI] [PubMed] [Google Scholar]
- Roberts TDM. Neurophysiology of Postural Mechanisms. 2. London: Butterworths; 1978. [Google Scholar]
- Rochon-Duvigneaud A. Les Yeaux et la Vision des Vértebrés. Paris: Mason; 1943. [PubMed] [Google Scholar]
- Russell NA, Horil A, Smith PF, Darlington CL, Bilkey DK. Long-term effects of permanent vestibular lesions on hippocampal spatial firing. J Neurosci. 2003;23:6490–6498. doi: 10.1523/JNEUROSCI.23-16-06490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt D. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J Exp Biol. 2003;206:1437–1448. doi: 10.1242/jeb.00279. [DOI] [PubMed] [Google Scholar]
- Schubert M, Bohner C, Berger WV, Sprundel M, Duysens JEJ. The role of vision in maintaining heading direction: effects of changing gaze and optic flow on human gait. Exp Brain Res. 2003;150:163–173. doi: 10.1007/s00221-003-1390-z. [DOI] [PubMed] [Google Scholar]
- Schwarz U, Miles FA. Ocular responses to translation and their dependence on viewing distance. I: Motion of the observer. J Neurophysiol. 1991;66:851–864. doi: 10.1152/jn.1991.66.3.851. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott MH. Motor Control. 2. Philadelphia: Lipplncott Williams and Wilkins; 2001. [Google Scholar]
- Simpson GG. Horses. New York: Doubleday Anchor; 1961. [Google Scholar]
- Slijper EJ. Comparative biologic-anatomical investigations on the vertebral column and spinal musculature of mammals. Verh Kon Nederl Akad Wetensch Amsterdam II. 1946;42:1–128. [Google Scholar]
- Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input . Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, McCloskey DI. Proprioceptive sensation in rotation of the trunk. Exp Brain Res. 1990;81:413–416. doi: 10.1007/BF00228134. [DOI] [PubMed] [Google Scholar]
- Timney B, Macuda T. Vision and hearing in horses. J Am Vet Med Assoc. 2001;218:1567–1574. doi: 10.2460/javma.2001.218.1567. [DOI] [PubMed] [Google Scholar]
- Townsend HG, Leach DH, Fretz PB. Kinematics of the equine thoracolumbar spine. Equine Vet J. 1933;15:117–122. doi: 10.1111/j.2042-3306.1983.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Niki Y, Yoshida K, Masumitsu H. Force plate study of equine biomechanics. Floor reaction force of normal walking and trotting horses. Bull Equine Res Inst. 1981;18:28–41. [Google Scholar]
- Vaitl D, Mittelstaedt H, Balsch F. Shifts in blood volume alter the perception of posture. Int J Psychophysiol. 1997;27:99–105. doi: 10.1016/s0167-8760(97)00053-6. [DOI] [PubMed] [Google Scholar]
- Vaitl D, Mittelstaedt H, Saborowski R, Stark R, Baisch F. Shifts in blood volume alter the perception of posture: further evidence for somatic graviception. Int J Psychophysiol. 2002;44:1–11. doi: 10.1016/s0167-8760(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Vorstenbosch MATM, Buchner HHF, Savelberg HHCM, Schamhardt HC, Barneveld A. Modeling study of compensatory head movements in lame horses. Am J Vet Res. 1997;58:713–718. [PubMed] [Google Scholar]
- Wake DB. The endoskeleton: the comparative anatomy of the vertebral column and ribs. In: Wake MH, editor. Hyman’s Comparative Vertebrate Anatomy. 3. Chicago: Univerisity of Chicago Press; 1979. pp. 192–237. [Google Scholar]
- White AA, Panjabi MM. Clinical Biomechanics of the Spine. 2. Philadelphia: Lippincolt; 1990. [Google Scholar]
- Wilson VJ, Peterson BW. Vestibulospinal and reticulospinal systems. In: Brooks VB, editor. Handbook of Physiology. Sec. 1, Vol. II: Motor Control, Part 1. Bethesda, MD: American Physiological Society; 1981. pp. 667–702. [Google Scholar]
- Xiang Y, Yakushin SB, Kunin M, Raphan T, Cohen B. Head stabilization by Vestibulocollic reflexes during quadrupedal locomotion in monkey. J Neurophysiol. 2008;100:763–780. doi: 10.1152/jn.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


