Abstract
Endometrial cancer is the most common gynecological cancer. Estrogen-dependent endometrioid carcinoma is the most common type of endometrial cancer, and alterations in the expression of PTEN and K-ras have been associated with this disease. To study the roles of Pten and K-ras in endometrial cancer, we generated Pten ablation and oncogenic K-ras mutation in progesterone receptor positive cells (PR cre/+ Pten f/f K-ras G12D). Double mutant mice dramatically accelerated the development of endometrial cancer compared to a single mutation of either gene. Histological analysis showed that all of the 1-month old double mutant female mice developed endometrial cancer with myometrial invasion. The expression of PR was downregulated in double mutant mice compared to a single mutation of either gene which resulted in decreased suppression of estrogen signaling. Therefore, these results suggest a synergistic effect of dysregulation of the Pten and K-ras signaling pathways during endometrial tumorigenesis.
1. Introduction
Endometrial cancer is the most common type of gynecological cancer in the United States with approximately 40 100 cases diagnosed and about 7470 deaths from the disease each year [1]. Therefore, there is great interest in identifying novel ways to treat and prevent this disease. Estrogen-dependent endometrioid carcinoma is the most common type of endometrial cancer. An increased incidence of endometrial cancer has been found in association with prolonged, unopposed estrogen (E2) exposure [2, 3] and alterations in the expression of the tumor suppressor gene, PTEN, as well as the oncogenes, β-catenin and Ras [4, 5]. Currently, progesterone (P4) therapy is used as a means to prevent the development of endometrial cancer associated with unopposed E2 as a means to block E2 actions [6].
The uterus consists of heterogeneous cell types that undergo dynamic changes in response to the ovarian steroid hormones E2 and P4 to support embryo development and implantation. E2 stimulates the proliferation of epithelial cells in the mouse uterus [7, 8]. In contrast, P4 is inhibitory to this E2-mediated proliferation of the luminal and glandular epithelial cells. However, P4, alone or in conjunction with E2, leads to uterine stromal cell proliferation [8–10]. The ability of the ovarian steroid hormones to regulate uterine cell proliferation depends upon the ability of hormonal stimulation to regulate growth factor communication networks between the uterine stroma and epithelium. For instance, P4 attenuates E2-stimulated uterine epithelial cell proliferation by regulating uterine stromal cell gene expression [11]. An imbalance caused by increased E2 action and/or decreased P4 action can result in abnormal endometrial proliferation and endometrial adenocarcinoma. Therefore, elucidating the molecular mechanisms by which the steroid hormones control uterine physiology is important to understanding the pathology of these diseases.
Two common mutations that occur in endometrial cancer are in the tumor suppressor Pten and the oncogene K-ras [4, 5]. K-ras encodes a guanine nucleotide binding protein of 21 kDa that has a central role in the regulation of cell growth and differentiation by transducing signals from activated transmembrane receptors. It has been shown to be mutated in 10%–30% of endometrial cancers [4]. These mutations have been found in all grades of endometrioid carcinoma and have been reported in complex atypical hyperplasia, suggesting a relatively early role for K-ras mutations in endometrial tumorigenesis [12]. PTEN (phosphatase and tensin homologue deleted from chromosome 10) is one of the most frequently mutated tumor suppressor genes in human cancers [13, 14]. PTEN is completely lost or mutated in >50% of primary endometrioid endometrial cancer [15] and in at least 20% of endometrial hyperplasias, the precancerous lesions of the endometrium [15, 16]. Thus, loss of PTEN is a very early event in the multistep process leading to endometrioid endometrial cancer [16, 17]. Phosphoinositide 3-kinases (PI3K) regulates a number of cellular functions through the activation of Akt [18]. PTEN acts as a negative regulator of PI3K signaling [19]. Previously, loss of Pten (either as a heterozygote or by uterine specific ablation) has been shown to induce endometrial cancer in mice highlighting its important role in cancer development [20, 21]. This mutation and subsequent Akt activation resulted in the activation of ERα-dependent pathways that play an important role in the tumorigenesis of endometrial cancer [21]. Interestingly, the PTEN/PI3K/AKT signaling pathway can also be activated by E2 suggesting a complex interaction between these two signaling pathways [22].
In this study, we utilized conditional Pten ablation and oncogenic K-ras mutation in the uteri of mice to demonstrate a synergistic effect of dysregulation of the Pten and K-ras signaling pathways during endometrial tumorigenesis. Pten ablation and oncogenic K-ras mutation dramatically accelerated the development of endometrial cancer compared to single mutation of either gene. Thus, these results demonstrate the importance of Pten and K-ras regulation in the tumorigenesis of endometrial cancer.
2. Materials and Methods
2.1. Animals
mice were maintained in the designated animal care facility at Baylor College of Medicine according to the institutional guidelines for the care and use of laboratory animals. PR Cre/+ mice were previously generated [23]. The Pten f/f were acquired from Dr. Hong Wu (University of California, Los Angeles, Los Angeles, CA) [24]. The lox-stop-Lox Kras G12D mice were acquired from Dr. Tyler Jacks (MIT, Cambridge, MA) [25]. Mice of various genotypes were sacrificed at 2 and 4 weeks of age. At the time of dissection, uterine tissues were placed in the appropriate fixative or flash frozen and stored at −80°C. For the genotyping of the oncogenic K-ras mutation, total RNA was isolated from uterus according to, Qiagen minieasy kit protocol. cDNA was generated from RNA samples by reverse transcription, followed by PCR amplification using primers K-ras1S, 5′-GCCATTTCGGACCCGGAGCGA and K-ras1A, 5′-CCTACCAGGACCATAGGCACATC. RNA expression of wild-type K-ras and mutant K-ras G12D was determined by digestion of 5 μL of the reverse transcription-PCR products with HindIII for 1 hour at 37°C. The restriction products were resolved in a 2% agarose gel. The mutant K-ras G12D allele contains a HindIII restriction site engineered in exon 1, which is absent in the wild-type allele. Therefore, digestion of the 488-bp products generates 300-bp and 148-bp restriction fragments in the mutant but not in the wild-type PCR product.
2.2. Western Blot Analysis
Samples containing 15 μg of proteins were applied to SDS-PAGE. The separated proteins were transferred onto a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Membranes were blocked overnight with 0.5% casein (wt/vol) in PBS with 0.1% Tween 20 (vol/vol) (Sigma-Aldrich, St. Louis, MO) and probed with anti-Akt (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-phospho-Akt (Cell Signaling Technology, Inc., Danvers, MA), anti-PR (DAKO Corp., Capinteria, CA), or anti-ERα (DAKO Corp., Capinteria, CA) antibodies. Immunoreactivity was visualized by incubation with a horseradish peroxidase-linked secondary antibody and treatment with ECL reagents. To control for loading, the membrane was stripped and probed with anti-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and developed again.
2.3. Immunohistochemistry and TUNEL Assay
Uterine sections from paraffin-embedded tissue were cut at 5 μm and mounted on silane-coated slides, deparafinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal serum in PBS (pH 7.5) and then incubated with anti-PR antibody (DAKO Corp., Capinteria, CA) or anti-ERα (DAKO Corp., Capinteria, CA) in 10% normal serum in PBS (pH 7.5). On the following day, sections were washed in PBS and incubated with a secondary antibody (5 μL/mL; Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Immunoreacitivity was detected using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). The TUNEL assay was performed according to manufacturer's instructions using the Roche In Situ Cell Death Detection Kit, Fluorescein (Roche, Boulder, CO).
2.4. RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA was extracted from uterine tissues using the Qiagen RNeasy total RNA isolation kit (Valencia, CA). Quantitative real-time RT-PCR analysis was conducted on isolated RNA. Expression levels of Ltf, Clca3, and C3 were measured by real-time RT-PCR TaqMan analysis (Applied Biosystems, Foster City, CA). cDNA was made from 1 μg of total RNA using random hexamers and M-MLV Reverse Transcriptase (Invitrogen Corp., Carlsbad, CA). RT-PCR was performed using RT-PCR Universal Master Mix reagent (Applied Biosystems, Foster City, CA). All real-time RT-PCR results were normalized against 18S RNA using ABI rRNA control reagents. Statistical analyses were performed using one-way ANOVA followed by Tukey's post hoc multiple range test with the Instat package from GraphPad (San Diego, CA, USA).
3. Results
3.1. Generation of Pten Ablation and Oncogenic K-Ras Mutation in the Murine Uterus
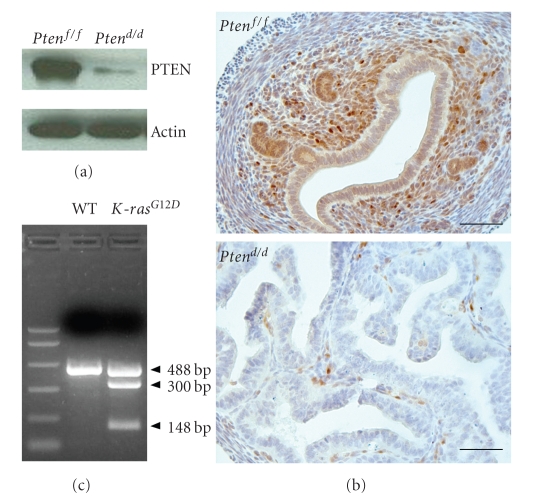
Homozygous Pten −/− mouse embryos die around E8.5 and heterozygous Pten +/− mice develop numerous pathologies and have decreased longevity [21, 26] making our ability to investigate the role of Pten in the mouse uterus severely limited. Likewise, constitutive activation of a K-ras mutation results in embryonic lethality [27]. In order to achieve ablation of Pten and activation of K-ras in the uterus, mice with floxed Pten (Pten f/f) [24] and mice with loxP-Stop-loxP-Kra s G12D/+ (LSL-K-ras G12D/+) [25] were bred to the P R Cre mouse [23]. Using this mouse with Cre recombinase inserted into the progesterone receptor (PR) locus, floxed genes are edited in PR expressing cells including all compartments of the mouse uterus. This model was previously used to ablate Pten in the uterus resulting in endometrial adenocarcinoma [20]. Therefore, in order to effectively investigate the effects of the Pten and K-ras signaling pathways in endometrial cancer, mice with uterine Pten ablation (PRcre/+Pten f/f; Pten d/d) and oncogenic K-ras mutation (K-ras G12D) were generated and mated to generate double mutant mice (PRcre/+Ptenf/fK-ras G12D; Ptend/dK-ras G12D) [23–25]. Ablation of Pten in the Ptend/d mice was assayed by Western blot and immunohistochemical analysis (Figures 1(a) and 1(b)). PTEN protein was expressed in the endometrium of Ptenf/f mice. However, the level of PTEN protein was significantly decreased in the uteri of Ptend/d mice demonstrating efficient ablation of Pten. To confirm the oncogenic K-ras mutation in the uterus, the PCR products encompassing the K-ras mutation were digested with HindIII (see Section 2). The mutant K-ras G12D allele contains a HindIII restriction site engineered in exon 1, which is absent in the wild-type allele. Therefore, digestion of the 488-bp products generates 300-bp and 148-bp restriction fragments in the mutant but not in the wild-type PCR product. Analysis of the HindIII digestion revealed the presence of the 300-bp and 148-bp fragments in the K-ras G12D but not in the wild type uteri (Figure 1(c)). These results suggest that PR-cre efficiently generated uterine Pten ablation and oncogenic K-ras mutation.
Figure 1.
Analysis of conditionally ablated Pten and oncogenic K-ras mutation in the murine uterus. (a) Western blot analysis of PTEN in whole uterine extracts from Ptenf/f and Ptend/d mice. (b) Immunohistochemical analysis for PTEN in Ptenf/f and Ptend/d mice uteri. Four-week-old Ptenf/f and Ptend/d mice were used for Western blot analysis and immunohistochemical analysis. These experiments demonstrate that Pten is conditionally ablated in the uteri of these mice. Scale bar: 50 μm. (c) PR-cre mediated recombination of K-ras G12D in uterus. The mutant K-ras G12D allele contains a HindIII restriction site engineered in exon 1, which is absent in the wild-type allele. Therefore, digestion of the 488-bp products generates 300-bp and 148-bp restriction fragments in the mutant but not in the wild-type PCR product.
3.2. Development of Vaginal Papillomas in Mice with the Oncogenic K-ras Mutation in PR-Expressing Cells
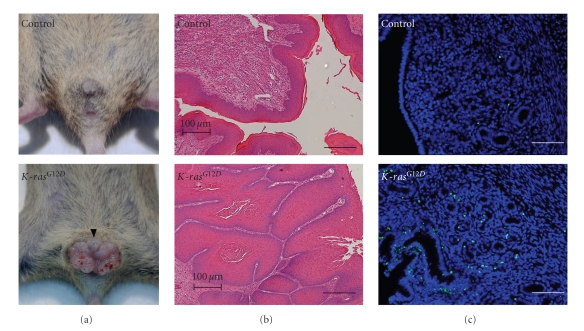
Introduction of the oncogenic K-ras mutation in all PR-positive cells resulted in the development of vaginal papillomas. Lesions were present at the vaginal opening of K-ras G12D mice as early as 2 months of age but were never observed in control mice (Figure 2(a)). Examination of the histology of these lesions revealed an abnormal vaginal architecture in the K-ras G12D mice compared to controls (Figure 2(b)). The vaginal epithelium exhibited increased keratinization accompanied by disorganization of the vaginal lumen. In addition, there was a decrease in the vaginal stroma. Interestingly, these lesions remained benign, never developing into a cancerous lesion. K-ras G12D mice did not show any pathological phenotype in the uterus. Proliferation was not affected in the K-ras mutant mice; however, there was increased apoptosis in the K-ras G12D uteri compared to controls (Figure 2(c)).
Figure 2.
Development of vaginal papiloma and increased apoptosis in K-ras G12D mice. (a) The development of vaginal papiloma. Control and K-ras G12D mice at 2 months of age. (b) H&E staining of vagina of control and K-ras G12D mice. The histology of these lesions revealed an abnormal vaginal architecture in the K-ras G12D mice compared to controls. (c) TUNEL assay in the uterus of control and K-ras G12D mice at 2 months of age. The number of apoptotic cells was significantly increased in epithelial cells of K-ras G12D uteri compared to controls. Scale bar: 100 μm.
3.3. Development of Endometrial Cancer in Mice with Pten Ablation and the Oncogenic K-ras Mutation in PR-Expressing Cells
To investigate the impact of Pten and K-ras signaling on endometrial cancer development and progression, control, Ptend/d, K-ras G12D, and Ptend/dK-ras G12D mice were sacrificed, excised uteri were weighed, and morphology was examined at gross and histological levels. Ptend/dK-ras G12D mice showed a significant increase in uterine weight at 2 weeks of age compared to control, Ptend/d, and K-ras G12D mice (Figures 3(a) and 3(b)). Ptend/d and Ptend/dK-ras G12D mice showed a significant increase in uterine wet weight compared to control mice at 4 weeks of age. The uterine weight of Ptend/d K-ras G12D mice was significantly increased compared to other mice including Ptend/d mice at 4 weeks of age (Figures 3(a) and 3(b)). The uterine weight of K-ras G12D mice did not change compared to control mice at either timepoint.
Figure 3.
Development of endometrial cancer in mice with Pten ablation and oncogenic K-ras mutation. (a) Gross anatomy of control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D uteri at 2 and 4 weeks of age. Scale bar: 1 cm (b) The ratio of uterine weight to body weight in control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D mice at 2 and 4 weeks of age. Uterine weight was determined for females 2 and 4 weeks old. Increased uterine weight was observed for Ptend/dK-ras G12D mice after 2 weeks of age. *, P < .05; ***, P < .001, one-way ANOVA followed by Tukey's post hoc multiple range test.
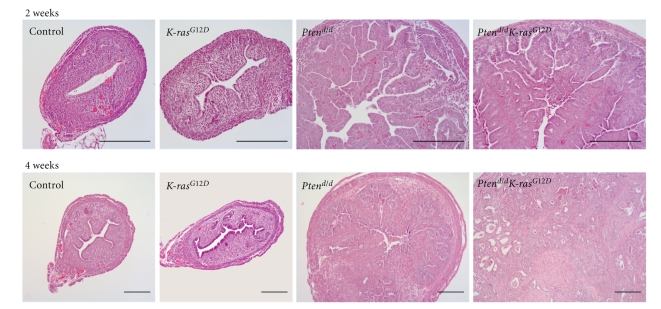
Histological analysis of the uteri showed an increase in the number of endometrial glands and in the gland/stroma ratio in the uteri of 2-week-old Ptend/d and Ptend/dK-ras G12D mice (Figure 4); however, the myometrium was not enlarged. These histological changes demonstrate that the uteri of 2 week old Ptend/d and Ptend/dK-ras G12D mice display endometrial hyperplasia, a predisposing factor to endometrial adenocarcinoma in humans. Thus, even though the uterine weight of the Ptend/dK-ras G12D mice was increased compared to the Ptend/d mice, the uteri of Ptend/d and Ptend/dK-ras G12D mice exhibit a similar hyperplastic phenotype at 2 weeks of age.
Figure 4.
Histology of uteri from mice with Pten ablation and oncogenic K-ras mutation. H&E staining of control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D mice at 2 and 4 weeks of age. Endometrial cancer was induced in the uteri of Ptend/dK-ras G12D mice, but not in other mice at 4 weeks of ages. Scale bar: 200 μm.
Interestingly, all of the Ptend/dK-ras G12D mice developed invasive endometrioid-type endometrial adenocarcinoma by 4 weeks of age. The neoplastic endometrial glands in the Ptend/dK-ras G12D mice invaded through the uterine muscle wall and invaded adjacent structures such as the colon, pancreas, and skeletal muscle (Figure 3(a)). The Ptend/d mice displayed endometrial hyperplasia at 4 weeks of age (Figure 4). Although the Ptend/d mice have been previously shown to develop endometrial hyperplasia and endometrial cancer [20], they did not develop invasive endometrial cancer within 4 weeks of age. Thus, histological analysis showed that Pten ablation in conjunction with the oncogenic K-ras mutation dramatically accelerated the development of endometrial cancer compared to single ablation of either gene (Figure 4). These results suggest that Pten ablation in addition to the oncogenic K-ras mutation dramatically accelerated the development of endometrial cancer compared to single mutation of either gene.
3.4. Down-regulation of P4 Signaling in Mice with Pten Ablation and the Oncogenic K-ras Mutation
Ablation of Pten resulted in increased activation of AKT as expected (Figure 5(a)). To determine if the hyperplastic phenotype observed was due to altered ovarian steroid hormone signaling, we examined the expression of ERα and PR in control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D uteri at 2 weeks of age using Western blot analysis. The expression of ERα was decreased in Ptend/d and Ptend/dK-ras G12D uteri compared to control and K-ras G12D uteri (Figure 5(a)). Interestingly, the level of PR, both the PR-A and PR-B isoforms, was decreased only in the Ptend/d K-ras G12D uteri compared to control, K-ras G12D, and Ptend/d uteri at 2 weeks of age (Figure 5(a)).
Figure 5.
The decrease of PR in the 2-week-old Ptend/dK-ras G12D mice. (a) Western blot analysis of PTEN, AKT, phosphor-AKT, ERα, and PR in 2 week old control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D mice. (b)-(c) Immunohistochemical analysis of ERα (b) and PR (c) in uteri of control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D mice. Immunohistochemical analysis of PR shows that it is decreased in the Ptend/dK-ras G12D uteri compared to other mice. Scale bar: 100 μm.
To analyze the spatial expression of ERα and PR, we performed immunohistochemical analysis in control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D uteri at 2 weeks of age. The expression of ERα was decreased in the endometrial stroma of Ptend/d K-ras G12D compared to Ptend/d mice. However, the level of ERα was increased in the epithelium of Ptend/dK-ras G12D compared to Ptend/d mice. These immunohistochemical results suggest that the decreased level of ERα in the whole uterus was due primarily to decreased ERα expression in the endometrial stroma (Figure 5(b)). The spatial expression of PR was altered in the Ptend/d uteri compared to control and K-ras G12D mice. Instead of uniform expression throughout the endometrial epithelium, PR was localized to the distal regions of the epithelium in the Ptend/d uteri (Figure 5(c)). However, this strong PR expression was almost lost in the Ptend/dK-ras G12D mice.
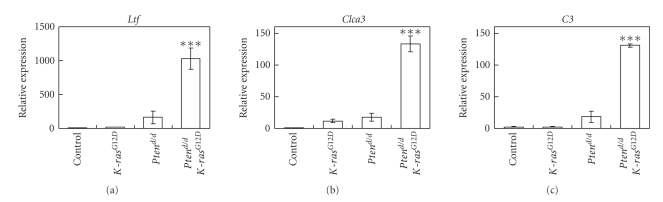
We next investigated the molecular impact of Pten ablation and the oncogenic K-ras mutation on the expression of ER target genes (Ltf, Clca3, and C3) in control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D uteri at 2 weeks of age. Ltf and C3 were increased in the Ptend/d mice as compared to control and K-ras G12D mice (Figure 6). Ltf, Clca3, and C3 were significantly increased in the Ptend/dK-ras G12D mice as compared to Ptend/d mice. Since P4 attenuates E2 regulation of proliferation and gene expression by regulating the expression of a yet to be identified paracrine signal from the stromal cells to the epithelial cells, the regulation of the expression of ERα and PR in the endometrial stroma and epithelium by Pten ablation and the oncogenic K-ras mutation is critical for the expression of ER target genes and the tumorigenesis of endometrial cancer.
Figure 6.
The increase of ERα signaling in the 2-week-old Ptend/dK-ras G12D mice. Real-time RT-PCR analysis of Ltf, Clca3, and C3 was performed in uteri of control, K-ras G12D, Ptend/d, and Ptend/dK-ras G12D mice at 2 weeks of age. The results represent the mean ± SEM of three independent RNA sets. ***, P < .001, one-way ANOVA followed by Tukey's post hoc multiple range test.
4. Discussion
Endometrial cancer is the most common gynecological cancer and has been shown to be associated with mutations in the tumor suppressor Pten and the oncogene K-ras among others [4]. Previous mouse model studies in the skin, ovary, and lung suggest that these mutations exert cooperative or antagonistic effects on tumorigenesis depending upon their interactions with tissue-specific factors [28–30]. Dinulescu et al. generated Pten loss and oncogenic K-ras mutations in ovarian surface epithelium using adenoviral vector delivery of Cre recombinase [28]. These mice developed endometriosis and endometrioid ovarian adenocarcinoma. K-ras mutations occur in a very small percentage of human cases of ovarian cancer but the mutation is important for the development of ovarian cancer [31]. However, whether Pten loss and oncogenic K-ras mutations interact to promote or inhibit the development of endometrial cancer has not yet been defined. In order to study the role of Pten and K-ras in the development of endometrial cancer, we generated mice in which Pten was ablated and K-ras was activated in the reproductive tract using the PRCre mouse model [23–25].
PTEN is completely lost or mutated in >50% of primary endometrioid endometrial cancer [15] and in at least 20% of endometrial hyperplasias, the precancerous lesions of the endometrium [15, 16]. Thus, loss of PTEN is a very early event in the multistep process leading to endometrioid endometrial cancer. Pten +/− and mice with Pten conditionally ablated in the uterus (Ptend/d) develop endometrioid endometrial adenocarcinoma [20, 32]. This mutation and subsequent Akt activation results in the activation of ERα-dependent pathways that play an important role in the tumorigenesis of endometrial cancer [21]. Introduction of the oncogenic K-ras mutation into the Ptend/d mice accelerated the tumorigenesis of endometrial cancer as compared to Pten ablation. The neoplastic endometrial glands in the double mutant mice invaded through the uterine muscle wall and invaded adjacent structures such as the colon, pancreas, and skeletal muscle.
The K-ras mutation alone was not sufficient enough to exert a pathological phenotype in the uterus even though mutations in K-ras have been consistently identified in 10%–30% of endometrial cancers [4]. Interestingly, K-ras G12D mice exhibited abnormal vaginal architecture due to increased keratinization in the vaginal epithelium resulting in vaginal papilloma. The development of vaginal papillomas confounds its impact on the tumorigenesis of endometrial cancer. Thus, we have only examined these mice up to 3 months of age without evidence of any hyperplasia or pathological phenotype in the uterus. To determine why endometrial cancer failed to develop in the K-ras G12D uteri, we examined proliferation and apoptosis in these mice. Proliferation was normal; however, apoptosis was significantly increased in the epithelial cells of K-ras G12D uteri compared to control mice as shown by the TUNEL assay (Figure 2(c)) and immunohistochemistry of cleaved caspase 3 (data not shown). These results suggest that activation in K-ras is not sufficient for the development of hyperplasia or endometrial cancer due to increased apoptosis of the endometrial epithelial cells.
Mutations in the β-catenin gene have been found in approximately 15%–20% of endometrioid carcinomas, with nuclear accumulation of the protein found in 38% of cases [33]. Subsets of endometrial carcinomas, especially those with nuclear translocation of β-catenin [34], are associated with squamous morule differentiation. Some of the tumors in Ptend/d mice exhibited squamous differentiation, which can also be observed in human endometrial cancers while we did not observed squamous differentiation in the tumors from Ptend/dK-ras G12D mice (data not shown). Nuclear translocation of β-catenin was not observed in the Ptend/d mice and Ptend/dK-ras G12D mice (data not shown). It indicates that the histopathology of the endometrial cancer lesions in the mice is very similar to human endometrial cancer but does not totally resemble human endometrial cancer which is mainly composed of glandular components with squamous differentiation.
ER and PR are usually found in high concentration in endometrial hyperplasia and endometrioid carcinomas of low grade and stage. However, the level of ER and PR diminishes with increases in stage and grade [35, 36]. The level of total ERα was decreased in Ptend/d and Ptend/dK-ras G12D uteri compared to control and K-ras G12D uteri using Western analysis (Figure 5(a)). The amount of epithelial cells in Ptend/d and Ptend/dK-ras G12D uteri is much higher than control and K-ras G12D mice because of the hyperplasia phenotype. The amount of epithelial cells was different in the 4 different groups of the Western analysis because we have not purified epithelial cells and normalized to epithelial marker proteins. Therefore, we performed immunohistochemical analysis to determine these possible compartmental differences. The results demonstrated that the expression of ERα was decreased in the endometrial stroma but was increased in the endometrial epithelium of Ptend/dK-ras G12D compared to Ptend/d mice. These results support that epithelial ERα is important for the tumorigenesis of endometrial cancer [4].
E2 induces cell proliferation in the luminal and glandular epithelium. In the uterine luminal epithelium, E2 inhibits GSK3β action by the stimulation of a protein kinase B-(AKT)-mediated inhibitory phosphorylation of Ser9. AKT is in turn regulated through activation of phosphoinositide 3-kinase [37]. P4 inhibits this pathway by blocking AKT phosphorylation and, thus, the inactivation of GSK3β with the resultant loss of nuclear cyclin D1 [37]. PR status is considered an independent prognostic factor for endometrial cancer patients [38, 39]. PR exists as two isoforms, PR-A and PR-B, and reduced expression of either one or both of the PR isoforms has been observed in a great majority of endometrial cancers, compared with hyperplastic or normal endometrium [40]. Loss of PR in human endometrioid endometrial carcinoma results in more aggressive biological characteristics which play important roles in the prognosis and recurrence of the disease [40–42]. We observed reduced expression of both PR isoforms in Ptend/dK-ras G12D uteri, but not in Ptend/d or other control uteri. Thus, this loss of PR in the double mutant mice may be the reason for the accelerated tumorigenesis of the Ptend/dK-ras G12D mice as compared to other mice including the Ptend/d mice.
Our results demonstrate that the synergistic effect of conditional Pten loss and oncogenic K-ras mutation on endometrial cancer development occurs via decreased expression of PR. This study has established an endometrial cancer mouse model which replicates common characteristics of the human disease. Using this mouse model, further studies can be undertaken to investigate the genetic and molecular events involved in the transition from normal to hyperplastic/neoplastic endometrium. In summary, these results greatly contribute to the understanding of the molecular mechanism of tumorigenesis and to the development of therapeutic approaches for endometrial cancer.
Acknowledgments
The authors thank Jinghua Li and Jie Yang for technical assistance. They thank Dr. Hong Wu for floxed Pten mice and Dr. Tyler Jacks for K-ras G12D mice. The anti-PR antibody was kindly provided by Dr. Dean Edwards. This work was supported by the NIH Grant R01HD042311 and U54HD0077495 (to F. J. D.), SPORE in Uterine Cancer NIH P50CA098258 (to R. R. B.), NIH Grant R01HD057873 (to J. W. J.), and NIH Grant R01-CA77530 and the Susan G. Komen Award BCTR0503763 (to J. P. L).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: Cancer Journal for Clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jick SS, Walker AM, Jick H. Estrogens, progesterone, and endometrial cancer. Epidemiology. 1993;4(1):20–24. doi: 10.1097/00001648-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. New England Journal of Medicine. 1975;293(23):1167–1170. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]
- 4.Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annual Review of Pathology. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- 5.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. Journal of Clinical Oncology. 2006;24(29):4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 6.Jick SS. Combined estrogen and progesterone use and endometrial cancer. Epidemiology. 1993;4(4):p. 384. doi: 10.1097/00001648-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. Journal of Endocrinology. 1973;56(1):133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 8.Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125(3):1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 9.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst's state of activity determines the ‘window’ of implantation in the receptive mouse uterus. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(21):10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. Journal of Endocrinology. 1973;57(3):549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 11.Kurita T, Lee K-J, Cooke PS, Lydon JP, Cunha GR. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biology of Reproduction. 2000;62(4):831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- 12.Esteller M, Garcia A, Martinez-Palones JM, Xercavins J, Reventos J. The clinicopathological significance of K-RAS point mutation and gene amplification in endometrial cancer. European Journal of Cancer. 1997;33(10):1572–1577. doi: 10.1016/s0959-8049(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 13.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genetics. 1997;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 14.Li D-M, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor β . Cancer Research. 1997;57(11):2124–2129. [PubMed] [Google Scholar]
- 15.Sun H, Enomoto T, Fujita M, et al. Mutational analysis of the PTEN Gene in endometrial carcinoma and hyperplasia. American Journal of Clinical Pathology. 2001;115(1):32–38. doi: 10.1309/7JX6-B9U9-3P0R-EQNY. [DOI] [PubMed] [Google Scholar]
- 16.Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Research. 1998;58(15):3254–3258. [PubMed] [Google Scholar]
- 17.Maxwell GL, Risinger JI, Gumbs C, et al. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Research. 1998;58(12):2500–2503. [PubMed] [Google Scholar]
- 18.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nature Reviews Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 19.Jiang B-H, Liu L-Z. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochimica et Biophysica Acta. 2008;1784(1):150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Daikoku T, Hirota Y, Tranguch S, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Research. 2008;68(14):5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilgelm A, Lian Z, Wang H, et al. Akt-mediated phosphorylation and activation of estrogen receptor α is required for endometrial neoplastic transformation in Pten +/− mice. Cancer Research. 2006;66(7):3375–3380. doi: 10.1158/0008-5472.CAN-05-4019. [DOI] [PubMed] [Google Scholar]
- 22.Chambliss KL, Yuhanna IS, Anderson RGW, Mendelsohn ME, Shaul PW. Erβ has nongenomic action in caveolae. Molecular Endocrinology. 2002;16(5):938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 23.Soyal SM, Mukherjee A, Lee KYS, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 24.Lesche R, Groszer M, Gao J, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32(2):148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 25.Johnson L, Mercer K, Greenbaum D, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410(6832):1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 26.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nature Genetics. 1998;19(4):348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 27.Tuveson DA, Shaw AT, Willis NA, et al. Endogenous oncogenic K-rasG12D stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5(4):375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 28.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nature Medicine. 2005;11(1):63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 29.Iwanaga K, Yang Y, Raso MG, et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Research. 2008;68(4):1119–1127. doi: 10.1158/0008-5472.CAN-07-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao J-H, To MD, Perez-Losada J, Wu D, Del Rosario R, Balmain A. Mutually exclusive mutations of the Pten and ras pathways in skin tumor progression. Genes and Development. 2004;18(15):1800–1805. doi: 10.1101/gad.1213804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurman RJ, Shih I-M. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. International Journal of Gynecological Pathology. 2008;27(2):151–160. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lian Z, De Luca P, Di Cristofano A. Gene expression analysis reveals a signature of estrogen receptor activation upon loss of Pten in a mouse model of endometrial cancer. Journal of Cellular Physiology. 2006;208(2):255–266. doi: 10.1002/jcp.20681. [DOI] [PubMed] [Google Scholar]
- 33.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S. β-catenin mutation in carcinoma of the uterine endometrium. Cancer Research. 1998;58(16):3526–3528. [PubMed] [Google Scholar]
- 34.Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Upregulation of TCF4 expression as a transcriptional target of β-catenin/p300 complexes during trans-differentiation of endometrial carcinoma cells. Laboratory Investigation. 2005;85(6):768–779. doi: 10.1038/labinvest.3700273. [DOI] [PubMed] [Google Scholar]
- 35.Emons G, Fleckenstein G, Hinney B, Huschmand A, Heyl W. Hormonal interactions in endometrial cancer. Endocrine-Related Cancer. 2000;7(4):227–242. doi: 10.1677/erc.0.0070227. [DOI] [PubMed] [Google Scholar]
- 36.Kleine W, Maier T, Geyer H, Pfleiderer A. Estrogen and progesterone receptors in endometrial cancer and their prognostic relevance. Gynecologic Oncology. 1990;38(1):59–65. doi: 10.1016/0090-8258(90)90012-a. [DOI] [PubMed] [Google Scholar]
- 37.Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase → AKT → GSK-3β → cyclin D1 → pRB pathway to block uterine epithelial cell proliferation. Molecular Endocrinology. 2005;19(8):1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- 38.Mohsin SK, Weiss H, Havighurst T, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Modern Pathology. 2004;17(12):1545–1554. doi: 10.1038/modpathol.3800229. [DOI] [PubMed] [Google Scholar]
- 39.Rose PG. Endometrial carcinoma. New England Journal of Medicine. 1996;335(9):640–649. doi: 10.1056/NEJM199608293350907. [DOI] [PubMed] [Google Scholar]
- 40.Arnett-Mansfield RL, deFazio A, Wain GV, et al. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Research. 2001;61(11):4576–4582. [PubMed] [Google Scholar]
- 41.Fukuda K, Mori M, Uchiyama M, Iwai K, Iwasaka T, Sugimori H. Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecologic Oncology. 1998;69(3):220–225. doi: 10.1006/gyno.1998.5023. [DOI] [PubMed] [Google Scholar]
- 42.Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma—new developments in potential endocrine therapy for endometrial cancer. Endocrine Journal. 2007;54(5):667–679. doi: 10.1507/endocrj.kr-114. [DOI] [PubMed] [Google Scholar]