Abstract
We have investigated the role of the kinesin I isoform Kif5b in the trafficking of a cardiac voltage-gated potassium channel, Kv1.5. In Kv1.5-expressing HEK293 cells and H9c2 cardiomyoblasts, current densities were increased from control levels of 389 ± 50.0 and 317 ± 50.3 pA pF−1, respectively, to 614 ± 74.3 and 580 ± 90.9 pA pF−1 in cells overexpressing the Kif5b motor. Overexpression of the Kif5b motor increased Kv1.5 expression additively with several manipulations that reduce channel internalization, suggesting that it is involved in the delivery of the channel to the cell surface. In contrast, expression of a Kif5b dominant negative (Kif5bDN) construct increased Kv1.5 expression non-additively with these manipulations. Thus, the dominant negative acts by indirectly inhibiting endocytosis. The increase in Kv1.5 currents induced by wild-type Kif5b was dependent on Golgi function; a 6 h treatment with Brefeldin A reduced Kv1.5 currents to control levels in Kif5b-overexpressing cells but had little effect on the increase associated with Kif5bDN expression. Finally, expression of the Kif5bDN prior to induction of Kv1.5 in a tetracycline inducible system blocked surface expression of the channel in both HEK293 cells and H9c2 cardiomyoblasts. Thus, Kif5b is essential to anterograde trafficking of a cardiac voltage-gated potassium channel.
Voltage-gated K+ channels (Kv channels) are required for repolarization and the termination of electrical excitation in all cardiomyocytes, and their function depends upon the presence of active channels at the plasmalemma. Surface expression has long been known to be regulated by changes in gene expression, phosphorylation and interactions with accessory subunits (reviewed in Steele et al. 2007), and several groups have investigated the roles of motifs within K+ channels that affect trafficking of the channels to the cell surface (Li et al. 2000; Manganas et al. 2001; Zhu et al. 2001, 2003). More recently, we have shown the dynein motor to be important in the maintenance of normal expression levels of several Kv channels (Choi et al. 2005; Loewen et al. 2009). Also, several Rab GTPases (Seebohm et al. 2007; McEwen et al. 2007; Zadeh et al. 2008) and dynamin (Nesti et al. 2004; Choi et al. 2005) have all been implicated in Kv channel trafficking. Beyond the roles of the motifs mentioned above, however, little has been learned about the forward trafficking of newly synthesized channels.
Surface expression requires movement from the endoplasmic reticulum (ER) through the Golgi apparatus to the plasma membrane, and several studies have investigated channel determinants that affect this trafficking process (Li et al. 2000; Manganas et al. 2001; Zhu et al. 2001, 2003). In neurons, a kinesin isoform, Kif17, has been implicated in the trafficking of Kv4.2 (Chu et al. 2006) and there is evidence that Kif5b is involved in axonal but not dendritic trafficking of some Kv1 channels (Rivera et al. 2007). The involvement of kinesins in the trafficking of cardiac ion channels has not, however, been established to date. Here we have used electrophysiology, molecular biology and imaging techniques to show that the major isoform of kinesin I (Kif5b) is required for the normal trafficking of Kv1.5 to the cell surface in both heterologous cells and in a cardiomyoblast cell line. Overexpression of a Kif5b construct caused a significant increase in Kv1.5 surface expression as did expression of a dominant negative Kif5b construct, the latter probably because of negative interactions with the dynein motor. The delivery of newly synthesized Kv1.5, on the other hand, was almost completely blocked by the dominant negative. Thus, Kif5b is required for the normal delivery of Kv1.5 to the plasma membrane.
Methods
Cell preparation and transfection
HEK293 cells and H9c2 cardiac myoblasts were cultured and transfected as described previously (Fedida et al. 2003). Transfections were by a liposome-mediated method. One day before transfection, cells were plated on a coverslip in 35 mm dishes at 40–50% confluence. After 1 day's growth, transfections were performed using 1.5 μg of each relevant plasmid and Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions.
Plasmid constructs
Human p50 in pEGFP was a gift of Richard Vallee (Columbia University, NY, USA). p50 was amplified by PCR and inserted in the C-terminus of mCherry in pcDNA3 as described previously (Zadeh et al. 2008). Human Kif5b was a gift of Ronald Vale (University of San Francisco, CA, USA). Kif5b–EGFP was similarly constructed by inserting PCR-amplified Kif5b at the C-terminus of EGFP in pcDNA3. A dominant negative version of Kif5b–EGFP (Kif5bDN–EGFP) was constructed by removing the motor domain, 366 amino acids from the N-terminus of Kif5b and inserting PCR-amplified Kif5bDN at the C-terminus of EGFP in pcDNA3. pcDNA3–T7–Kv1.5–HA(haemagglutinin Antigen)(S1–S2) was described previously (Zadeh et al. 2008). In generating a tetracycline-inducible Kv1.5, EGFP fused to the N-terminus of Kv1.5 was inserted into pcDNA4-TO. Plasmid DNA was prepared for transfection using the Qiagen Plasmid Midi Kit (Qiagen Inc., Valencia, CA, USA).
Electrophysiological experiments and solutions
Solutions and methodology for the recording of ionic currents were as previously reported from our laboratory (Fedida et al. 2003). The standard bath solution contained (in mm): NaCl, 135; KCl, 5; MgCl2, 1; sodium acetate, 2.8; Hepes, 10; CaCl2, 1; adjusted to pH 7.4 using NaOH. The standard pipette filling solution contained (in mm): KCl, 130; EGTA, 5; MgCl2, 1; Hepes, 10; Na2ATP, 4; GTP, 0.1; adjusted to pH 7.2 with HCl. All chemicals were from Sigma (Mississauga, ON, Canada). Whole-cell current recording and data analysis were done using an Axopatch 200B amplifier and pClamp 9 software (Axon Instruments, Foster City, CA, USA). Patch electrodes were fabricated using thin-walled borosilicate glass (World Precision Instruments, Sarasota, FL, USA) and polished by heating. Pipette resistances were between 1 and 3 MΩ. Compensation for capacitance and series resistance was performed manually in all whole-cell recordings. All recordings were performed at room temperature (RT, 20–23°C).
Imaging
Cells were prepared for imaging according to previously published methods (Zadeh et al. 2008). Briefly, the cells were rinsed and fixed with 4% paraformaldehyde for 12 min at room temperature (RT). After three 5 min washes with 1× phosphate-buffered saline (PBS; 137 mm NaCl, 2.7 mm KCl, 4.3 mm Na2HPO4, 1.4 mm KH2PO4), cells were incubated with monoclonal mouse-anti-HA antibody (Clone 12CA5, Roche) diluted (1: 1000) in PBS supplemented with 0.2% BSA to label the externally tagged Kv1.5 for 1 h at RT. The cells were washed three times for 5 min in PBS on a rotator before incubation with secondary antibody, Alexa 594-conjugated goat anti-mouse IgG antibody (1: 1000; Molecular Probes) for 1 h on the rotator at RT. Cells were then washed three times with PBS prior to mounting with 10 μl of a 90% glycerol, 2.5% w/v DABCO (1,4-diazabicyclo2.2.2octane)–PBS solution. In experiments testing the effects of pre-expression of Kif5bDN on Kv1.5 surface expression, H9c2 myoblasts and HEK293 cells were transfected with Kif5bDN–EGFP then, 24 h later, with T7–Kv1.5–HA. Twenty-four hours after the second transfection, the cells were fixed and stained with rabbit-α-HA (Zymed Laboratories) without permeabilization to label the surface Kv1.5. The cells were then permeabilized and stained with mouse-α-T7 (Novagen) to label total Kv1.5. α-Mouse-Alexa 647 and α-rabbit-Alexa 594 were used to detect the total or surface Kv1.5, respectively. Images were collected on an Olympus Fluoview 1000 laser scanning confocal microscope equipped with a 60× (NA 1.4) oil immersion objective. EGFP was excited using the 488 nm line of an Ar laser set at 5% transmission and emission collected using the variable bandpass filter set at 500–530 nm. Alexa 594 was excited using a 543 nm He–Ne laser set at 25% transmission and emission collected using the variable bandpass filter set at 555–625 nm. Alexa 647 was excited with a 633 nm He–Ne laser set at 5% transmission and emission collected using the variable longpass filter set at >650 nm. A 60×, 1.35 NA oil-immersion objective was used for imaging. An optical zoom of 2× was often used. Images were acquired at 512 × 512 pixel resolution. Identical acquisition settings were used for producing the images. The images were analysed using ImageJ software (NIH).
Tetracycline induction experiments
pcDNA4/TO–Kv1.5–EGFP was co-transfected with tetracycline repressor, pcDNA6/TR, at a ratio of 1: 6 into HEK293 cells or H9c2 myoblasts in control cells. Cells used to test the effects of Kif5bDN and p50 were co-transfected also with Kif5bDN–Cherry or p50–Cherry, respectively. Twenty-four hours later, tetracycline was added at a final concentration of 2 μg ml−1 to one culture to induce Kv1.5 expression on the evening prior to electrophysiological analysis. Electrophysiology was performed on the next day. Western analysis for Kv1.5–EGFP expression was performed using 15 μg of protein loaded, and probed with anti-GFP.
Data statistics
Results are expressed as mean ±s.e.m. Statistical analyses were conducted using Student's t test (paired and unpaired) or by one-way ANOVA, as appropriate.
Results
Kv1.5 functional expression is significantly increased by overexpression of kinesin I (Kif5b)
There are many kinesin isoforms. Some are expressed only in specific cell types; others are present in all cell types (reviewed in Hirokawa & Noda 2008). As the kinesin I isoform Kif5b is expressed across essentially all cell types, we chose to investigate whether this form of kinesin was involved in the trafficking of Kv1.5. Unlike Kif5a and Kif5c, the expression of which appear to be restricted to neurons, Kif5b is ubiquitously expressed (Hirokawa & Noda 2008). To carry out this work, we constructed an N-terminally EGFP-tagged wild-type Kif5b clone (Kif5bWT) in pcDNA3 and a similarly tagged dominant negative Kif5b construct (Kif5bDN) in which the N-terminal 366 amino acid residues, comprising the kinesin motor domain, were deleted. H9c2 cardiomyoblast cells and HEK293 cells each stably expressing a Kv1.5 construct (T7–Kv1.5–HA), tagged N-terminally with T7 and externally (in the S1–S2 linker) with an HA tag, were transfected with these Kif5b–EGFP and Kif5bDN–EGFP as a first test of whether Kif5b is important to Kv1.5 trafficking.
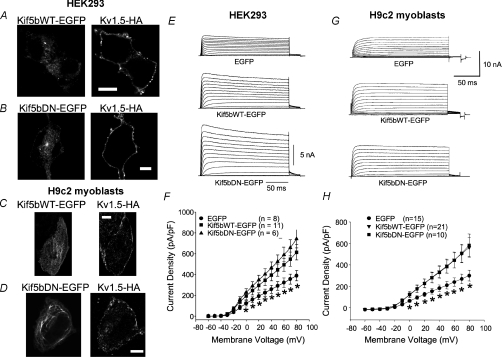
Figure 1A and B, and C and D show, respectively, representative images of HEK293 cells and H9c2 cardiomyoblasts stably expressing T7–Kv1.5–HA, transfected with the Kif5bWT and Kif5bDN constructs. Both the Kif5bWT (Fig. 1A and C, left panels) and DN constructs (Fig. 1B and D, left panels) were expressed at high levels in both cell types. Surface Kv1.5 distribution, detected with anti-HA was quite uniform in HEK cells, whether transfected with the Kif5b wild-type or dominant negative (Fig. 1A and B, right panels). The distribution of the channel at the surface of H9c2 myoblasts was also robust, but more punctate than in the HEK cells (Fig. 1C and D, right panels). No obvious difference in Kv1.5 surface staining could be detected between the cells expressing the wild-type and dominant negative Kif5b isoforms.
Figure 1. Transfections with Kif5bWT and Kif5bDN similarly affect Kv1.5 functional expression.
A–D, representative images of cells stably expressing Kv1.5 transfected with the Kif5bWT–EGFP and Kif5bDN–EGFP constructs. A, HEK293 cell stably expressing Kv1.5–HA transfected with Kif5bWT–EGFP showing Kif5bWT expression (left) and Kv1.5–HA surface expression (right). B, HEK293 cells stably expressing Kv1.5–HA transfected with Kif5bDN–EGFP showing Kif5bDN expression (left) and surface expression of Kv1.5–HA (right). C, H9c2 myoblasts stably expressing Kv1.5–HA transfected with Kif5bWT–EGFP showing Kif5bWT expression (left) and Kv1.5–HA surface expression (right). D, H9c2 myoblasts stably expressing Kv1.5–HA transfected with Kif5bDN–EGFP showing Kif5bDN expression (left) and Kv1.5–HA surface expression (right). Scale bar, 10 μm. E–H, effects of Kif5bWT and Kif5bDN on Kv1.5 current density in myoblasts and HEK293 cells. Cells were depolarized from −60 to +80 mV in 10 mV, 200 ms steps and repolarized to −80 mV between pulses. Data are represented as mean ±s.e.m. Current densities from control cells (EGFP), Kif5bWT–EGFP and Kif5bDN–EGFP transfected cells are plotted against voltage. E and G, sample traces of control, Kif5bWT and Kif5bDN-transfected HEK293 cells and H9c2 myoblasts, respectively. Both cell lines stably express Kv1.5. F and H, current density versus voltage plot for data recorded 36–48 h post-transfection for HEK293 cells and H9c2 myoblasts, respectively. *P < 0.05, comparing Kif5bWT–EGFP and Kif5bDN–EGFP to EGFP.
Electrophysiological analysis indicated that both the wild-type and dominant negative Kif5b proteins increased Kv1.5 functional expression relative to control, EGFP-transfected cells. Overexpression of Kif5bWT significantly increased Kv1.5 current densities in both the HEK293 cells (Fig. 1E and F) and in the H9c2 myoblasts (Fig. 1G and H). Current density was increased from 389 ± 50.0 pA pF−1 in control, EGFP-tranfected HEK293 cells to 614 ± 74.3 pA pF−1 in the Kif5bWT overexpressing cells at +80 mV and from 317 ± 50.3 to 580 ± 90.9 pA pF−1, respectively, in the H9c2 myoblasts (Fig. 1F and H). Kif5bDN similarly increased current densities in these cell lines. In the myoblasts, the current density in Kif5bDN-transfected cells was 600 ± 105 pA pF−1 (Fig. 1H) and in the HEK293 cells, the density was 742 ± 86.8 pA pF−1 (Fig. 1F). Activation and inactivation kinetics were unchanged by any of the treatments (data not shown). Thus, overexpression of both wild-type and dominant negative Kif5b increases Kv1.5 surface expression in these cells that stably express Kv1.5.
Kif5bWT and Kif5bDN increase in Kv1.5 current densities by different mechanisms
One explanation for the fact that both wild-type and dominant negative forms of Kif5b increase Kv1.5 surface expression is that overexpression of the wild-type kinesin is promoting the anterograde trafficking of newly synthesized channel to the cell surface whereas the dominant negative is interfering generally with the microtubule-dependent trafficking system. It is well established that blocking retrograde microtubule-dependent trafficking by p50 overexpression, which blocks dynein function, causes an increase in surface expression of several ion channels (Dhani et al. 2003; Loewen et al. 2009), including Kv1.5 (Choi et al. 2005), very probably by interfering indirectly with channel endocytosis (Choi et al. 2005). It is also established that block of retrograde trafficking can cause a reduction in anterograde trafficking and vice versa (Hamm-Alvarez et al. 1993; Valetti et al. 1999; Martin et al. 1999). Thus, it is expected that a dominant negative Kif5b would cause an increase in Kv1.5 functional expression.
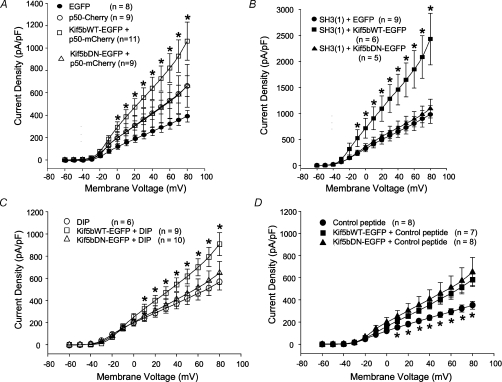
As a first test of whether a negative effect on retrograde transport indeed underlies the increase in currents seen when the Kif5bDN is expressed, we co-transfected the Kv1.5-expressing HEK293 line with the Kif5b dominant negative, plus p50. p50 expression alone increased Kv1.5 currents by approximately 50% above control levels (Fig. 2A), as we have reported previously (Choi et al. 2005). Co-expression of the Kif5bDN with p50 had no further effect. Current densities in p50-overexpressing cells were 661 ± 91.9 pA pF−1 at +80 mV and 662 ± 192 pA pF−1 in cells co-expressing also Kif5bDN. In striking contrast, the effects of co-overexpression of p50 and Kif5bWT on Kv1.5 currents were additive. p50 and Kif5bWT co-overexpression increased Kv1.5 current density to 1062 ± 170 pA pF−1 at +80 mV, significantly higher than the 661 pA pF−1 in cells overexpressing p50 alone.
Figure 2. Differential interactions of Kif5bWT and Kif5bDN with direct inhibitions of retrograde trafficking.
HEK293 cells stably expressing Kv1.5 were depolarized from −60 to +80 mV in 10 mV, 200 ms steps and repolarized to −80 mV between pulses. Data are represented as mean ±s.e.m. A, current densities, 36–48 h post-transfection, measured from cells transfected with EGFP (control), p50–Cherry, Kif5bWT–EGFP + p50–Cherry and Kif5bDN–EGFP + p50–Cherry plotted against voltage. *P < 0.05, comparing Kif5bDN–EGFP + p50–mCherry to Kif5b–EGFP +p50 and p50–Cherry alone. B, current densities, 36–48 h post-transfection, from control Kv1.5ΔSH3(1) + EGFP expressing cells, Kv1.5ΔSH3(1) + Kif5bWT–EGFP and Kv1.5ΔSH3(1) + Kif5bDN–EGFP-transfected cells plotted against voltage. *P < 0.05 comparing Kv1.5ΔSH3(1) + Kif5bWT–EGFP to Kv1.5ΔSH3(1) + Kif5bDN–EGFP and to Kv1.5ΔSH3(1) + EGFP. C and D, current densities from control cells (EGFP), Kif5bWT–EGFP transfected cells + DIP (C) or control peptide (D) and Kif5bDN–EGFP transfected cells + DIP (C) or control peptide (D) plotted against voltage. C, current density versus voltage plot for data from DIP-treated cells recorded 36–48 h post-transfection. D, current density versus voltage plot for data from control peptide-treated cells recorded 36–48 h post-transfection. *P < 0.05, comparing, in C, Kif5bWT–EGFP + DIP to Kif5bDN–EGFP + DIP and to DIP alone and, in D, Kif5bWT–EGFP + control peptide and Kif5bDN–EGFP + control peptide to control peptide alone.
To confirm that Kif5bDN acts through a mechanism similar to that of p50, and that the mechanism of action of Kif5bWT is different, we tested for additivity of the wild-type and dominant negative with two additional manipulations known to be non-additive with p50 overexpression, an SH3-binding domain deletion in the N-terminus of Kv1.5 and a direct block of endocytosis using dynamin inhibitory peptide. In previously published work, we showed that the p50-dependent increase in Kv1.5 expression depends on the presence of a specific SH3-binding domain in the channel (Choi et al. 2005). Removal of this domain (generating Kv1.5ΔSH3(1)), increases Kv1.5 functional expression to a level that cannot be further increased by p50 overexpression. We tested whether the effect of the Kif5bDN is similarly attenuated in cells expressing Kv1.5ΔSH3(1). As shown in Fig. 2B, this was indeed the case. Co-expression of Kif5bDN failed to increase the functional expression levels of this mutant channel. Current densities at +80 mV were 981 ± 174 pA pF−1 in EGFP-transfected HEK293-Kv1.5ΔSH3(1) cells and 1094 ± 179 pA pF−1 in the cells transfected with Kif5bDN. Again indicating that Kif5bWT acts by a different mechanism, overexpression of this motor protein increased Kv1.5ΔSH3(1)-dependent currents nearly 2.5-fold. Current densities were enormous in these cells, reaching 2433 ± 495 pA pF−1.
The results of the experiments with dynamin inhibitory peptide (DIP), which blocks endocytosis by interfering with nascent vesicle scission from the plasma membrane, were similar. Kif5bWT overexpression increased Kv1.5 current density over and above that produced by a 16 h treatment with 50 μm DIP whereas the Kif5bDN did not. DIP alone increased Kv1.5 current densities from control levels of 352 ± 35.5 pA pF−1 at +80 mV in scrambled peptide-treated cells to 567 ± 71.9 pA pF−1 in the DIP-treated cells (compare Fig. 2C and D). When added to Kif5bWT-overexpressing cells, DIP further increased current densities to a similar extent but there was no additivity of DIP and Kif5bDN expression. Current densities in Kif5bWT-overexpressing cells were 908 ± 104 pA pF−1 at +80 mV in the DIP-treated Kif5bWT-expressing cells (Fig. 2C), some 50% higher than the 583 ± 51.5 pA pF−1 measured in Kif5bWT-transfected cells treated with the control peptide (Fig. 2D). In the Kif5bDN-expressing cells, current densities in the Kif5bDN-expressing cells ± DIP were not significantly different. Densities at +80 mV were 648 ± 103 pA pF−1 in the DIP-treated cells and 651 ± 132 pA pF−1 in the Kif5bDN-expressing cells treated with the scrambled control peptide. Thus, Kif5bDN very probably acts by generally impeding microtubule-dependent trafficking and, like p50, indirectly interfering with channel endocytosis. Kif5bWT, on the other hand, clearly operates by a different mechanism.
Enhancement of Kv1.5 current density by Kif5bWT requires intact Golgi function
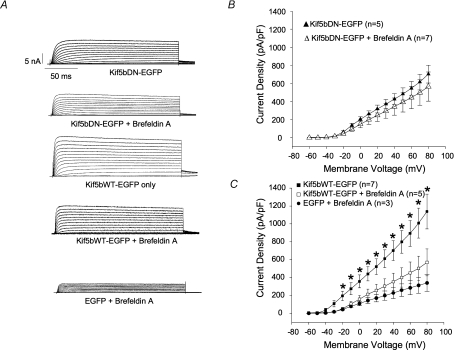
To further test whether wild-type Kif5b is indeed promoting delivery of new channels to the plasma membrane, experiments with Brefeldin A were conducted. Brefeldin A blocks ER to Golgi transport (Klausner et al. 1992) and thus should deprive kinesin of cargo to deliver to the plasma membrane. Immunocytochemistry confirmed that 5 μg ml−1 of the drug caused dissolution of the Golgi apparatus in these cells (data not shown). Whereas treatment with the drug for 6 h only marginally attenuated the increase in currents associated with Kif5bDN expression (Fig. 3A and B), it completely blocked the increase in Kv1.5 expression associated with Kif5bWT expression (Fig. 3A and C). Current densities at +80 mV were 341 ± 94.0 pA pF−1 in EGFP-transfected cells treated with Brefeldin A only (shown in Fig. 3C) and 703 ± 98.8 and 560 ± 161 pA pF−1 in cells transfected with KifbDN untreated and treated with Brefeldin A, respectively. Current densities in cells overexpressing Kif5bWT were reduced from 1139 ± 194 pA pF−1 in untreated cells to 570 ± 152 pA pF−1 when treated with Brefeldin A.
Figure 3. Brefeldin A treatment prevents increase in Kv1.5 currents associated with Kif5bWT overexpression.
HEK293 cells stably expressing Kv1.5 were depolarized from −60 to +80 mV in 10 mV, 200 ms steps and repolarized to −80 mV between pulses. Data are represented as mean ±s.e.m. A, sample traces of Brefeldin A-treated EGFP-, Kif5bWT- and Kif5bDN-transfected cells, and untreated Kif5bDN- and Kif5bWT-transfected cells. B, current density versus voltage plot for data recorded from Kif5bDN-transfected cells ± Brefeldin A treatment 36–48 h post-transfection. C, current density versus voltage plot for data recorded from Kif5bWT-transfected cells ± Brefeldin A treatment and EGFP-transfected Brefeldin A-treated cells 36–48 h post-transfection. *P < 0.05, comparing Kif5bWT–EGFP to Kif5bWT–EGFP + Brefeldin A.
Together, the experimental results described above strongly indicate that overexpression of Kif5bWT promotes the forward trafficking of new Kv1.5 channel to the plasma membrane whereas the dominant negative probably acts by reducing microtubule-dependent trafficking in both directions and, thereby, indirectly interfering with endocytosis of plasma membrane resident channels.
Kif5b function is specifically required for Kv1.5 delivery to the plasma membrane
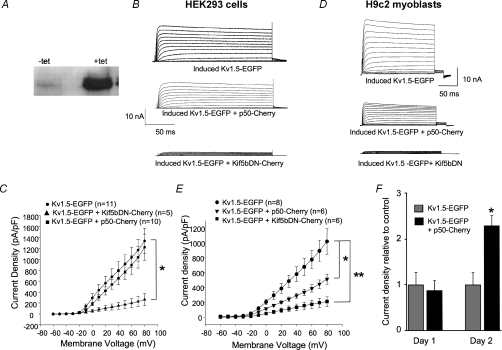
That Kif5bWT overexpression increases delivery of Kv1.5 to the cell surface does not prove that the kinesin isoform is a requirement for delivery to occur at all. In order to test whether Kif5bWT is required for this delivery, we employed a system in which Kv1.5 expression could be delayed relative to that of transfected motor. HEK293 cells and H9c2 myoblasts were co-transfected with tetracycline-inducible EGFP-tagged Kv1.5 (Kv1.5–EGFP) and the tetracycline repressor (see Methods). As shown in Fig. 4A, Kv1.5 expression, as assayed by Western blot, is very low under control conditions but levels of the protein increased 24 h after induction with 2 μg ml−1 tetracycline. For electrophysiology, these cells were simultaneously co-transfected with the inducible Kv1.5–EGFP system ± either p50 tagged with mCherry fluorescent protein (p50–Cherry) or similarly tagged Kif5bDN (Kif5bDN–Cherry). On the next day, Kv1.5 expression was induced with tetracycline and, 12 to 24 h later, patch clamping was performed. As shown in the upper panels of Fig. 4B and D, tetracycline treatment increased Kv1.5 current densities to very high levels in control cells in both HEK293 cells and H9c2 cardiomyoblasts. In all cases, induction of Kv1.5–EGFP expression was clearly visible under the light microscope as bright green fluorescence, and p50 or Kif5bDN was visible as red fluorescence.
Figure 4. Kif5bDN expression prevents functional expression of newly synthesized Kv1.5.
HEK293 cells and H9c2 myoblasts were transfected with Kv1.5–EGFP in pcDNA4-TO and pcDNA6-TR and either Kif5bDN–Cherry or p50–Cherry. Kv1.5–EGFP expression was induced 24 h later and electrophysiological experiments were performed the following day. A, Western blot showing induction of Kv1.5 by 2 μg ml−1 tetracycline in HEK293 cells 24 h after transfection with the pcDNA4 and pcDNA6 constructs. B and D, sample traces of control, p50 and Kif5bDN-transfected HEK293 cells and H9c2 myoblasts co-transfected with the tetracycline-inducible Kv1.5 system, respectively, 12–24 h post-tetracycline-induction of Kv1.5 expression. C and E, current density versus voltage plot for data recorded 12–24 h post-induction from HEK293 and H9c2 cells, respectively. F, current densities in HEK293 cells co-expressing p50 and tetracycline-inducible Kv1.5–EGFP relative to control cells expressing tetracycline-inducible Kv1.5–EGFP only at 12–24 h (Day 1) and 36–48 h (Day 2) post-tetracycline induction of Kv1.5–EGFP expression. Values are normalized to control values for each day ±s.e.m. Day 1: control, n= 11; p50, n= 10; Day 2: control, n= 10; p50, n= 10. *P < 0.05, **P < 0.01.
As expected, induction of Kv1.5–EGFP expression in p50-transfected HEK cells and H9c2 myoblasts resulted in moderately lower, but still very high, Kv1.5 current densities than were obtained in control cells transfected with the inducible Kv1.5 system alone (Fig. 4C and E). This is consistent with previous reports that block of dynein function also causes a reduction in kinesin function (Hamm-Alvarez et al. 1993; Valetti et al. 1999; Martin et al. 1999). Tetracycline-induced current densities in the control cells were 1530 ± 458 and 1020 ± 172 pA pF−1 in the HEK293 cells and H9c2 myoblasts, respectively. Induced current densities in p50-expressing HEK293 cells were reduced to 1222 ± 237 pA pF−1 and, in H9c2 myoblasts to 512 ± 68.9 pA pF−1. By 2 days post-induction, current densities in p50-co-expressing cells substantially exceeded those of the control cells (Fig. 4F). This is consistent with p50 overexpression strongly interfering with Kv1.5 internalization and more moderately impeding forward trafficking of the channel. In striking contrast to the p50 results, the Kif5bDN nearly eliminated surface expression of the induced Kv1.5–EGFP. In HEK293 cells, densities were 262 ± 111 pA pF−1 and in the H9c2 myoblasts, current densities were 210 ± 67.4 pA pF−1 in these Kif5bDN-expressing cells.
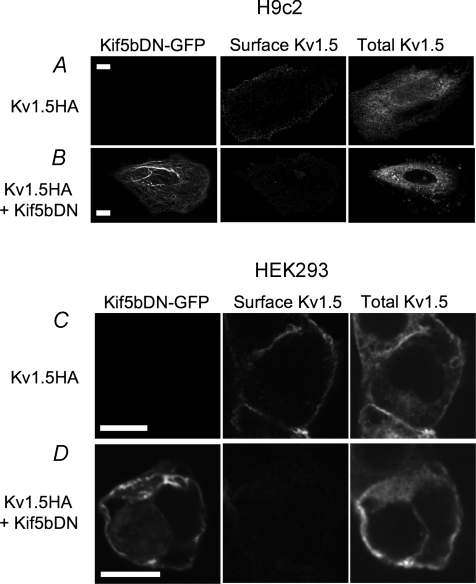
Separate immunocytochemical experiments, in which H9c2 myoblasts and HEK293 cells were transfected with Kif5bDN–GFP 24 h prior to transfection with Kv1.5–HA yielded similar results. All cells expressed high levels of internal Kv1.5 (Fig. 5A–D, right panels). H9c2 myoblasts and HEK293 cells untransfected with Kif5bDN showed robust surface expression of Kv1.5–HA (Fig. 5A and C, middle panels) whereas Kif5bDN-transfected cells expressed almost no surface Kv1.5 (Fig. 5B and D, middle panels). This was despite the fact that internal expression of the channel was robust (Fig. 5B and D, right panels). Kif5b function is clearly essential to the normal trafficking of Kv1.5 to the cell surface.
Figure 5. Kif5bDN blocks surface expression of newly synthesized Kv1.5.
H9c2 myoblasts and HEK293 cells were transfected with Kif5bDN–EGFP then, 24 h later, with T7–Kv1.5–HA. A and C, examples of control H9c2 (A) and HEK293 (C) cells transfected with T7–Kv1.5–HA but untransfected with Kif5bDN. Kif5bDN–EGFP is absent (left panel) and T7–Kv1.5–HA surface expression is robust (right panel). B and D, examples of H9c2 myoblasts (B) and HEK293 cells (D) transfected first with Kif5bDN–EGFP then, 24 h later, with T7–Kv1.5–HA. Scale bar, 10 μm.
Discussion
Kif5b is essential for forward trafficking of Kv1.5
Using both immunocytochemistry and quantitative electrophysiological techniques, we have shown that the ubiquitous kinesin I isoform Kif5b is essential for the normal trafficking of a cardiac potassium channel to the plasma membrane in both HEK293 cells and H9c2 cardiac myoblasts. This is the first identification of a role for a kinesin isoform in the trafficking of any such cardiac ion channel. Block of Kif5b function by expression of a dominant negative isoform prevents the delivery of newly synthesized channels to the plasma membrane, as indicated by current density measurements and immunocytochemistry. Overexpression of Kif5b dramatically increased Kv1.5 functional expression. Interestingly, expression of the dominant negative increased Kv1.5 current density in cells pre-expressing the channel. This is almost certainly due to an indirect reduction in the endocytosis of the channel, as it is well established that interference with kinesin function interferes also with dynein function (Hamm-Alvarez et al. 1993; Valetti et al. 1999). Further, we have previously established that interference with dynein function by p50 overexpression increases Kv1.5 surface expression by both imaging and current density measurements. This effect requires the presence of a specific SH3-binding domain in the channel and is not additive with block of endocytosis by a dynamin inhibitory peptide (Choi et al. 2005), all properties shared by the Kif5bDN. Our demonstration that the effect of the Kif5bDN to increase channel surface density is also non-additive with that of p50 overexpression firmly establishes that the Kif5bDN acts in the same way as does the p50, i.e. indirectly inhibiting endocytosis of the channel. That the Kif5b wild-type is additive with the effects of p50, DIP and the SH3-binding domain deletion in increasing Kv1.5 functional expression firmly establishes that this kinesin isoform carries the channel to the cell surface.
Kv1.5–kinesin interaction
The motifs in Kv1.5 required for interaction with Kif5b have yet to be identified. Attempts to co-immunoprecipitate Kif5b with Kv1.5 proved unsuccessful (data not shown), indicating that the channel probably does not interact directly with the motor. This is not surprising. The ion channels GluR2 and NR2B interact with kinesins via PDZ-containing proteins that interact with those channels’ C-terminal PDZ-binding motifs (Setou et al. 2000, 2002). GluR2, for example, is linked to Kif5b via the GRIP PDZ protein (Setou et al. 2002). Like GluR2 and NR2B, Kv1.5 contains a PDZ-binding motif at its extreme C-terminal end that could be involved in indirect kinesin binding. Deletion of this motif, however, does not appreciably reduce surface expression of this channel (Eldstrom et al. 2003; Mathur et al. 2006). Another possibility is that a non-canonical PDZ-binding domain present in the Kv1.5 N-terminus (Eldstrom et al. 2002) is involved in linking the channel to the kinesin. Alternatively, pore (Zhu et al. 2001) and/or C-terminal motifs (Li et al. 2000) in the channel may be responsible for linkage to the kinesin motor. Clearly, further work is required to resolve this issue.
Microtubule transport as regulator of functional expression
The involvement of both the dynein (Choi et al. 2005) and kinesin motors in the trafficking of Kv1.5 to and from the cell surface implies that modulation of microtubule-dependent trafficking is probably an important mechanism by which cardiomyocytes regulate the functional expression of its resident ion channels. Several additional potassium channels are known to be affected by modulation of dynein motor function (Loewen et al. 2009) and this further supports the hypothesis that the microtubule transport system is fundamental to the regulation of channel surface localization. It has been reported that the abundance of microtubules is increased in the cardiomyocytes of patients with congestive heart failure (Aquila et al. 2004). It will be of great interest to determine if changes in trafficking along these microtubules are associated with the channel remodelling inherent in this condition and other chronic cardiac diseases.
Acknowledgments
This study was supported by funding to D.F. from the Canadian Institutes for Health Research and the Heart and Stroke Foundation of British Columbia and the Yukon.
Author contributions
All of the authors of this manuscript contributed to the conception and design of this study as well as to the analysis and interpretation of the data. D.F.S. and D.F. wrote the manuscript with important intellectual input from all of the other authors and all authors have approved the final version of this manuscript.
References
- Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J Heart Lung Transplant. 2004;23:954–963. doi: 10.1016/j.healun.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281:365–373. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- Dhani SU, Mohammad-Panah R, Ahmed N, Ackerley C, Ramjeesingh M, Bear CE. Evidence for a functional interaction between the ClC-2 chloride channel and the retrograde motor dynein complex. J Biol Chem. 2003;278:16262–16270. doi: 10.1074/jbc.M209828200. [DOI] [PubMed] [Google Scholar]
- Eldstrom J, Choi WS, Steele DF, Fedida D. SAP97 increases Kv1.5 currents through an indirect N-terminal mechanism. FEBS Lett. 2003;547:205–211. doi: 10.1016/s0014-5793(03)00668-9. [DOI] [PubMed] [Google Scholar]
- Eldstrom J, Doerksen KW, Steele DF, Fedida D. N-terminal PDZ-binding domain in Kv1 potassium channels. FEBS Lett. 2002;531:529–537. doi: 10.1016/s0014-5793(02)03572-x. [DOI] [PubMed] [Google Scholar]
- Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, Van Wagoner DR. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ Res. 2003;93:744–751. doi: 10.1161/01.RES.0000096362.60730.AE. [DOI] [PubMed] [Google Scholar]
- Hamm-Alvarez SF, Kim PY, Sheetz MP. Regulation of vesicle transport in CV-1 cells and extracts. J Cell Sci. 1993;106:955–966. doi: 10.1242/jcs.106.3.955. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88:1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Takimoto K, Levitan ES. Surface expression of Kv1 channels is governed by a C-terminal motif. J Biol Chem. 2000;275:11597–11602. doi: 10.1074/jbc.275.16.11597. [DOI] [PubMed] [Google Scholar]
- Loewen ME, Wang ZR, Eldstrom J, Zadeh AD, Khurana A, Steele DF, Fedida D. Shared requirement for dynein function and intact microtubule cytoskeleton for normal surface expression of cardiac potassium channels. Am J Physiol Heart Circ Physiol. 2009;296:H71–H83. doi: 10.1152/ajpheart.00260.2008. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of KV1.5 in atrial myocytes. J Biol Chem. 2007;282:29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Wang Q, Scannevin RH, Antonucci DE, Rhodes KJ, Trimmer JS. Identification of a trafficking determinant localized to the Kv1 potassium channel pore. Proc Natl Acad Sci U S A. 2001;98:14055–14059. doi: 10.1073/pnas.241403898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R, Choi WS, Eldstrom J, Wang Z, Kim J, Steele DF, Fedida D. A specific N-terminal residue in Kv1.5 is required for upregulation of the channel by SAP97. Biochem Biophys Res Commun. 2006;342:1–8. doi: 10.1016/j.bbrc.2006.01.110. [DOI] [PubMed] [Google Scholar]
- Nesti E, Everill B, Morielli AD. Endocytosis as a mechanism for tyrosine kinase dependent suppression of a voltage gated potassium channel. Mol Biol Cell. 2004;15:4073–4088. doi: 10.1091/mbc.E03-11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Chu PJ, Lewis TL, Arnold DB. The role of Kif5B in axonal localization of Kv1 K+ channels. Eur J Neurosci. 2007;25:136–146. doi: 10.1111/j.1460-9568.2006.05277.x. [DOI] [PubMed] [Google Scholar]
- Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, et al. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res. 2007;100:686–692. doi: 10.1161/01.RES.0000260250.83824.8f. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Steele DF, Eldstrom J, Fedida D. Mechanisms of cardiac potassium channel trafficking. J Physiol. 2007;582:17–26. doi: 10.1113/jphysiol.2007.130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C, Wetzel DM, Schrader M, Hasbani MJ, Gill SR, Kreis TE, Schroer TA. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol Biol Cell. 1999;10:4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh AD, Xu HJ, Loewen ME, Noble GP, Steele DF, Fedida D. Internalized Kv1.5 traffics via Rab-dependent pathways. J Physiol. 2008;586:4793–4813. doi: 10.1113/jphysiol.2008.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Watanabe I, Gomez B, Thornhill WB. Determinants involved in Kv1 potassium channel folding in the endoplasmic reticulum, glycosylation in the Golgi, and cell surface expression. J Biol Chem. 2001;276:39419–39427. doi: 10.1074/jbc.M107399200. [DOI] [PubMed] [Google Scholar]
- Zhu J, Watanabe I, Gomez B, Thornhill WB. Trafficking of Kv1.4 potassium channels: interdependence of a pore region determinant and a cytoplasmic C-terminal VXXSL determinant in regulating cell-surface trafficking. Biochem J. 2003;375:761–768. doi: 10.1042/BJ20030885. [DOI] [PMC free article] [PubMed] [Google Scholar]