Abstract
During walking, a change in speed is accomplished by varying the duration of the stance phase, while the swing phase remains relatively invariant. To determine if this asymmetry in the control of locomotor cycles is an inherent property of the spinal central pattern generator (CPG), we recorded episodes of fictive locomotion in decerebrate cats with or without a complete spinal transection (acute or chronic). During fictive locomotion, stance and swing phases typically correspond to extension and flexion phases, respectively. The extension and flexion phases were determined by measuring the duration of extensor and flexor bursts, respectively. In the vast majority of locomotor episodes, cycle period varied more with the extension phase. This was found without phasic sensory feedback, supraspinal structures, pharmacology or sustained stimulation. We conclude that the control of walking speed is governed by an asymmetry within the organization of the spinal CPG, which can be modified by extraneous factors.
A change in walking speed is accomplished by varying the locomotor cycle period, which is composed of stance and swing phases. During treadmill or normal walking, a change in cycle period is associated with a change in the stance phase, while the swing phase remains relatively unchanged (Goslow et al. 1973; Grillner et al. 1979; Halbertsma, 1983). In other words, the cycle period of walking changes as a function of the stance phase. What causes this asymmetry in the walking pattern that biases the control of cycle period in favour of stance? The basic pattern of locomotion is generated within the spinal cord by a central pattern generator (CPG), which interacts dynamically with supraspinal signals and with sensory inputs from the periphery (Rossignol et al. 2006); this means that the asymmetry can arise within any of these locomotor control systems. It was suggested that the asymmetry in the control of cycle period is accomplished within the spinal network (Grillner & Dubuc, 1988; Dubuc et al. 1988), or that bias is induced by descending supraspinal signals (Armstrong, 1988) and/or phasic sensory inputs from peripheral receptors (Yakovenko et al. 2005; Juvin et al. 2007; Musselman & Yang, 2007). Some have stated that more evidence is required before concluding that the organization of rhythm generation within the locomotor CPG is asymmetric (Brownstone & Wilson, 2008; McCrea & Rybak, 2008).
Fictive locomotion is used to investigate the centrally generated pattern of locomotion by the spinal network, as phasic sensory feedback from the moving limbs is absent (Grillner, 1981; Kiehn, 2006). In these preparations, activity is recorded from peripheral nerves, or ventral roots, and a pattern of alternating extension and flexion phases, which roughly corresponds to stance and swing phases of walking, can be observed. Cycle period, defined as the time between two successive extensor or flexor bursts of activity, is composed of a flexion and an extension phase, and can change by varying the duration of either phase, or both concurrently. The flexion and extension phases are determined by measuring the duration of flexor and extensor bursts, respectively. Using phase durations and cycle period we can plot the relationship between the flexion and extension phases as a function of cycle period, which provides a phase–cycle period relationship for flexion and extension, respectively. A cycle period that changes as a function of the extension phase is termed an ‘extensor-dominated’ locomotion, whereas a cycle period that changes as a function of the flexion phase is considered ‘flexor dominated’ (Fleshman et al. 1984; Yakovenko et al. 2005).
Recent studies suggest that the presence of phasic sensory inputs from the periphery favours an extensor-dominated locomotion (Yakovenko et al. 2005; Juvin et al. 2007), as in normal walking. For example, phase–cycle period relationships were investigated during fictive locomotion in neonatal rats induced by neurochemicals or brainstem electrical stimulation (Juvin et al. 2007). It was shown that extension and flexion phases, approximated from ventral root burst durations, varied similarly with cycle period. However, when the hindlimbs were left attached to the cord, movement-related sensory feedback induced an extensor-dominated pattern. In another study, Yakovenko et al. (2005) showed that fictive locomotion evoked by stimulating the mesencephalic locomotor region (MLR) was primarily flexor dominated in decerebrate curarized cats. Both studies concluded that the absence of phasic sensory inputs during fictive locomotion was responsible for the rarity of extensor-dominated locomotion. However, in these preparations the expression of the spinal rhythmic generation may have been obscured or biased by brainstem electrical stimulation or neurochemicals (see Discussion).
In the present study, we first investigated whether fictive locomotion is primarily flexor- or extensor-dominated without phasic sensory feedback by studying episodes of fictive locomotion occurring spontaneously (i.e. without drugs or brainstem electrical stimulation). We further investigated whether the isolated spinal CPG is able to produce extensor-dominated fictive locomotion after removal of all supraspinal inputs in complete spinal cats.
Methods
Ethical information, animal care, and surgical procedures
All procedures were in accordance with the Guide for Care and Use of Experimental Animals (Canada) and approved by the Ethics Committee of the Université de Montréal. All animals were obtained from a designated breeding establishment for scientific research. Before the experiments, animals were housed and fed within designated areas, which were monitored daily by veterinarians and trained personnel. All experimental procedures meet the guidelines set-out in The Journal of Physiology for ethical matters (Drummond, 2009). Fifty one adult cats weighing between 2.5 and 4.5 kg were used. The data presented in the current study are derived from experiments performed within the last 15 years for answering other scientific questions. In other words, there were no additional animals used to compile the current data set. This is part of our effort to maximize the scientific output from every animal experiment.
Prior to surgery, cats were injected with an analgesic (Anafen 2 mg kg−1; subcutaneously) and premedicated (Atravet 0.1 mg kg−1, glycopyrrolate 0.01 mg kg−1, ketamine 0.01 mg kg−1; intramuscularly). Cats were then anaesthetized with a mask using a mixture of oxygen (∼50%), nitrous oxide (∼50%) and halothane (∼2–3%; MTC Pharmaceuticals, Cambridge, Ontario, Canada) or isoflurane (2–4%, Abbott Laboratories, Montréal, Québec, Canada). Once the animal was deeply anaesthetized (10–15 min), a tracheotomy was performed and cats were intubated to provide the anaesthesia. The right common carotid artery was cannulated to monitor blood pressure and the right jugular and cephalic veins were cannulated for fluid administration. The level of anaesthesia was confirmed and adjusted throughout the experiment by monitoring blood pressure, applying pressure to the paw to detect limb withdrawal, and by verifying the size and reactivity of the pupils.
Following a craniotomy, the cortex was removed and all tissue was removed rostral to the colliculi and mammillary bodies (i.e. a pre-collicular/pre-mammillary decerebration). At this point, the animals are considered to have complete lack of sentience. Anaesthesia was discontinued and animals were paralysed with an injection (1 mg kg−1) of Pancuronium Bromide (Sandoz Canada Inc., Quebec, Canada) through the right jugular or cephalic veins. Paralysis is required to remove movement-related sensory feedback to study the centrally generated pattern of locomotion (i.e. ‘fictive’ locomotion). Immediately after this injection, the animals were artificially ventilated for the duration of the experiment. An identical injection of Pancuronium Bromide was administered every 45 min until the end of the experiment. A lethal injection of pentobarbital anaesthetic was administered at the end of the experiment through the right jugular or cephalic veins.
In 16 cats, the spinal cord was completely transected at low thoracic levels under aseptic conditions 1 or 3 months before the acute experiment. The complete spinal transection was performed under aseptic conditions in an operation room with sterilized equipment. Prior to surgery, cats were injected with an analgesic (Anafen 2 mg kg−1; subcutaneously) and premedicated (Atravet 0.1 mg kg−1, glycopyrrolate 0.01 mg kg−1, ketamine 0.01 mg kg−1; intramuscularly). Cats were then anaesthetized with a mixture of oxygen (∼50%), nitrous oxide (∼50%) and isoflurane (2–4%, Abbott Laboratories, Montréal, Québec, Canada) while heart rate and respiration were monitored. A laminectomy was performed at T13, the dura was removed, and after local lidocaine application (xylocaine, 2%), the spinal cord was completely transected with surgical scissors. Hemostatic material (Surgicel) was inserted within the gap, and muscles and skin were sewn back to close the opening. A fentanyl patch (Duragesic, 25 μg; Janssen-Ortho, Markham, Canada) was sewn on the back of the animal. After surgery, an analgesic (Buprenorphine 0.01 mg kg−1) was administered subcutaneously. An oral antibiotic (cephatab or apo-cephalex, 100 mg day−1) was given for 10 days following surgery. The bladder was manually emptied 1–2 times each day up to the acute experiment. The animals were monitored daily by experienced personnel and veterinarians to ensure that they were not suffering and that no infections were present.
Nerve recording and stimulation
To monitor locomotor episodes, the electroneurography (ENG) of selected muscle nerves of the left hindlimb was recorded by dissecting and mounting the following nerves on bipolar silver chloride electrodes: posterior biceps–semitendinosus (PBSt), semimembranosus–anterior biceps (SmAB), lateral gastrocnemius–soleus (LGS), medial gastrocnemius (MG), plantaris (Pl), flexor hallucis longus/flexor digitorum longus (FDHL), tibialis anterior (TA), extensor digitorum longus (EDL), and sciatic nerve (uncut). The superficial peroneal (SP) nerve was also mounted uncut. In some experiments some peripheral nerves such as MG, LGS, Pl and FDHL were left uncut. After a laminectomy exposing spinal segments L5–S1, the animals were transferred to a stereotaxic frame and skin flaps surrounding the spinal cord and the hindlimb nerves were used to construct paraffin oil pools. The cord dorsum potential (CDP) was recorded with a silver chloride ball electrode and stimulation intensity was expressed as multiples of the threshold for the most excitable fibres in the nerve determined by the first negative deflection of the CDP.
Electroneurography was band-pass filtered (10 Hz–10 kHz) and amplified. Episodes of fictive locomotion were digitized on-line with interactive custom-made software (Spinal Cord Research Center, University of Manitoba, Winnipeg, Canada) or off-line from recordings on videotape (15 channels; Vetter 4000A; A.R. Vetter, Rebersburg, PA, USA). Data were analysed using the same custom-made software.
Fictive locomotion
A spontaneous fictive locomotor rhythm is frequently observed (>50%) after a pre-collicular/pre-mammillary decerebration. Manually stimulating the neck region and pinna or a short series of electrical trains of stimuli to the sciatic nerve were sometimes used to initiate episodes of rhythmic activities, which could last for several minutes. In two cats with a spontaneous locomotor rhythm, the mesencephalic locomotor region (MLR) (Shik et al. 1966) was electrically stimulated (100–200 μA at 20 Hz) unilaterally using a monopolar tungsten electrode.
In 16 cats a complete spinal transection was performed at low thoracic levels under aseptic conditions 1 or 3 months before the acute experiment. In these cats, fictive locomotion was generally induced by intravenous clonidine (α2-noradrenergic agonist; 500 μg kg−1; Sigma) injection combined with perineal stimulation (Cote et al. 2003; Cote & Gossard, 2004). However, in a few cases, we evoked locomotor rhythms before clonidine injection with perineal stimulation, and in one cat a fictive locomotor episode was elicited with trains of electrical stimuli to the sciatic nerve before clonidine and without perineal stimulation.
In five cats, the spinal cord was completely transected at low thoracic levels during the acute terminal experiment a few hours after decerebration and fictive locomotion was evoked by intravenous injection of nialamide (50 mg kg−1; Sigma-Aldrich) and l-dihydroxyphenylalanine (l-DOPA; 50–100 mg kg−1; Pfizer, New York, NY, USA) (Grillner & Zangger, 1979).
Data recording and analysis
Overall, 68 episodes of fictive locomotion were analysed in 51 cats (35 of spontaneous locomotion in 28 cats, 1 MLR-evoked locomotion in 1 cat, 6 with l-DOPA/nialamide locomotion in 5 spinal cats, and 26 with clonidine locomotion in 16 spinal cats). Some different locomotor episodes were studied in the same cat. We did not reject any episode from the analysis as long as the onset and offset of extensor and flexor bursts could be clearly established. A locomotor episode was defined as an alternation between flexion and extension of at least 10 cycles. Overall, we had an average of 40 ± 6 cycles per episodes. Burst duration was defined as the time between burst onset and termination and was determined from the raw ENG waveforms. The ENG with the best signal-to-noise ratio was used to calculate phase duration. Flexor and extensor burst durations were used to delineate flexion and extension phases, respectively. The ENG from LGS, SmAB, MG, Pl or FDHL, which have approximately the same onsets and offsets during fictive locomotion, were used as extensor bursts, whereas the ENG from TA or EDL was used for flexor bursts. Cycle period was defined as the time between successive flexor or extensor burst onsets. Regression lines were fitted to scatter plots of flexion and extension phase durations relative to its corresponding cycle period (i.e. the phase–cycle period relationship). For example, flexion phase duration was plotted against cycle period calculated from flexor burst onsets. Coefficients of linear regression (r, the slope of the phase–cycle period relationship) were calculated using Sigmaplot 10.0 (Systat Software Inc., Germany).
Statistical analysis
All statistical tests were done using statistical software (SPSS 15.0, Chicago, IL, USA). A simple linear regression was performed between phase duration (i.e. dependent variable) and cycle period (i.e. independent variable) and an F test was used to determine if the regression was significant. A multiple linear regression was performed to determine significant differences between the slopes of the extension (re) and flexion (rf) phase regression lines. The dependent variable (phase duration) was compared against three independent variables (cycle period, phase (flexion or extension), and cycle period × phase). The regression coefficient for cycle period × phase was used to determine if the slopes of the extension and flexion regression lines are different for a given episode. This analysis was performed because cycle period can co-vary (or remain invariant) with the extension or flexion phase concomitantly. Significance was set at P < 0.05. A correlation matrix was also constructed to perform a two-tailed Spearman test with the following eight variables for spontaneous fictive locomotion: flexor cycle period, extensor cycle period, flexion phase duration, extension phase duration, re, rf, % flexion relative to cycle period, and % extension relative to cycle period. Spearman's rank-order correlation coefficient (ρ) was calculated and significance was set at P < 0.005 to correct for multiple comparisons.
To determine if a locomotor episode was extensor- or flexor-dominated we used three criteria: (1) a slope of the phase–cycle period relationship greater than 0.5 or more negative than −0.5, (2) a significant relationship, and (3) a significant difference between the slopes of extension and flexion regression lines; for example, an extensor- or flexor-dominated episode has a slope greater than 0.5 (or more negative than −0.5), the relationship is significant, and extension and flexion slopes are different from each other. An episode that co-varies has one or both slopes greater than 0.5 (or more negative than −0.5) and both slopes are not significantly different from each other. An episode that does not vary has both slopes smaller than 0.5 or less negative than −0.5, or both relationships are not significant.
Results
Spontaneous fictive locomotion
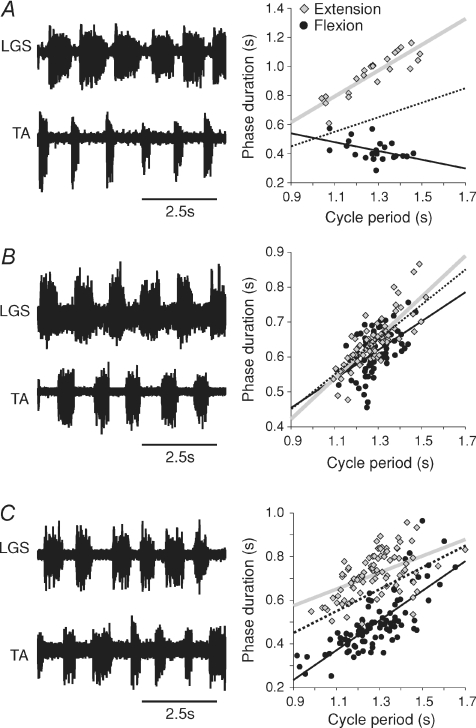
Fictive locomotion was recorded in decerebrate cats to determine if the locomotor CPG controls cycle period by changing the duration of the extension and/or flexion phase. Following a pre-collicular/pre-mammillary decerebration spontaneous fictive locomotion can be generated after anaesthesia is discontinued. We analysed 35 episodes of such spontaneous fictive locomotion in 28 cats (1–2 episodes per cat). Figure 1 shows three episodes with similar average cycle periods in three different cats, providing examples of extensor-dominated (Fig. 1A), covariance (Fig. 1B), and flexor-dominated (Fig. 1C) episodes of spontaneous fictive locomotion. Left panels show the ENG of an extensor and flexor nerve while right panels show the phase–cycle period relationships for extension (grey) and flexion (black). Phases were determined by measuring flexor and extensor burst durations, whereas cycle period was defined as the time between two successive flexor or extensor bursts. The dotted lines in Figs 1, 2, 4 and 5 has a slope of 0.5 and passes through the origin and is used as a reference line to illustrate extensor- or flexor-dominated episodes. All values, unless stated otherwise, are the mean ± standard error of the mean (s.e.m.). Using a multiple linear regression analysis the coefficients of regression (r, or slope) for extension (re) and flexion (rf) phases as a function of cycle period (i.e. the phase–cycle period relationship) are compared and significance is determined at Pef < 0.05 (see Methods). In Fig. 1A, cycle period (1.28 s ± 0.02 s) changed as a function of the extension phase (re= 0.89, P < 0.001; rf=−0.30, P < 0.05; Pef < 0.001). In Fig. 1B, cycle period (1.28 s ± 0.01 s) changed as a function of extension and flexion phases (re= 0.59, P < 0.001; rf= 0.41, P < 0.001; Pef= 0.11) concomitantly because there was no significant difference between the extension and flexion slopes. In Fig. 1C, cycle period (1.28 s ± 0.01 s) changed as a function of the flexion phase (re= 0.38, P < 0.001; rf= 0.68, P < 0.05; Pef < 0.001).
Figure 1. Phase–cycle period relationships during spontaneous fictive locomotion in the decerebrate cat.
Left panels show ENG from extensor (LGS) and flexor (TA) nerves during (A) extensor-dominated, (B) co-varying, and (C) flexor-dominated locomotor episodes in 3 cats. Right panels show scatter plots and regression lines for extension (grey) and flexion (black) phases relative to cycle period. The dotted line has a slope of 0.5 and passes through the origin and is used as a reference line.
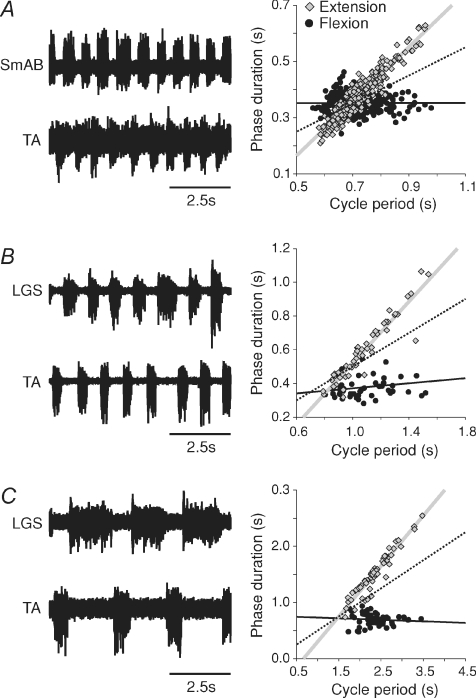
Figure 2. Three episodes of extensor-dominated, spontaneous fictive locomotion, with different cycle periods.
Left panels show ENG from extensor (LGS or SmAB) and flexor (TA) nerves in 3 cats. Right panels show scatter plots and regression lines for extension (grey) and flexion (black) phases relative to cycle period. The dotted line has a slope of 0.5 and passes through the origin and is used as a reference line.
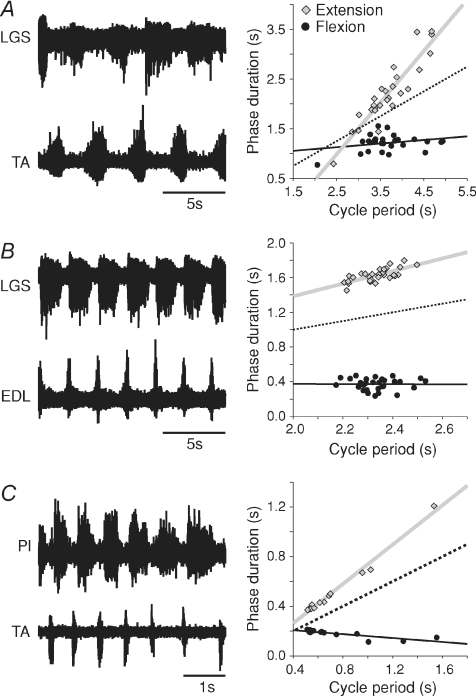
Figure 4. Extensor-dominated locomotor episodes following a complete spinal transection induced by electrical stimuli or neurochemicals.
Left panels show ENG from extensor (Pl or LGS) and flexor (EDL or TA) nerves (A) during an episode of spinal/l-DOPA locomotion in one cat, (B) during an episode of spinal/clonidine locomotion in another cat, and (C) during an episode evoked with electrical trains of stimuli to the sciatic nerve in a third cat. Right panels show scatter plots and regression lines for extension (grey) and flexion (black) phases relative to cycle period. The dotted line has a slope of 0.5 and passes through the origin and is used as a reference line.
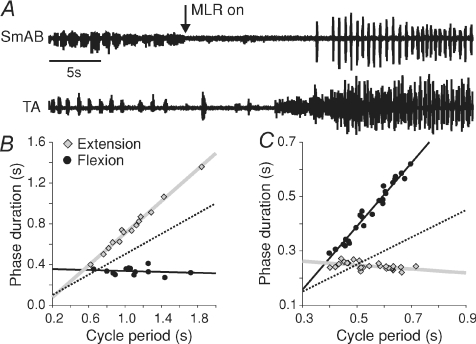
Figure 5. Effects of stimulating MLR during spontaneous fictive locomotion in a decerebrate cat.
A, ENG from extensor (SmAB) and flexor (TA) nerves during spontaneous fictive locomotion followed by MLR stimulation in one cat. Scatter plots and regression lines for extension (grey) and flexion (black) phases relative to cycle period during spontaneous fictive locomotion (B) and MLR-evoked locomotion (C) in the episode illustrated in A. The dotted line has a slope of 0.5 and passes through the origin and is used as a reference line.
Across the group, extensor-dominated locomotion (24/35 episodes; cycle period = 1.22 s ± 0.08 s) accounted for the majority of spontaneous fictive locomotion episodes whereas covariance (6/35; cycle period = 0.91 s ± 0.07 s), invariance (2/35; cycle period = 0.68 s ± 0.04 s) and flexor-dominated (3/35 episodes; cycle period = 0.97 ± 0.11 s) episodes were less frequent. In the 24 extensor-dominated episodes, mean extensor and flexor slopes were, respectively, 0.87 ± 0.03 and 0.07 ± 0.08 (P < 0.01, paired t tests). In the 8 episodes that co-varied or did not vary, mean extensor and flexor slopes were, respectively, 0.46 ± 0.05 and 0.35 ± 0.08 (P= 0.24, paired t tests). In the 3 flexor-dominated episodes, mean extensor and flexor slopes were, respectively, 0.12 ± 0.13 and 0.71 ± 0.08 (P= 0.07, paired t tests). Figure 2 shows three extensor-dominated episodes during fast (Fig. 2A), intermediate (Fig. 2B) and slow (Fig. 2C) spontaneous fictive locomotion to demonstrate that ‘speed’ is not a determining factor in extensor or flexor bias. All 3 episodes were clearly extensor-dominated despite differences in the range of cycle periods.
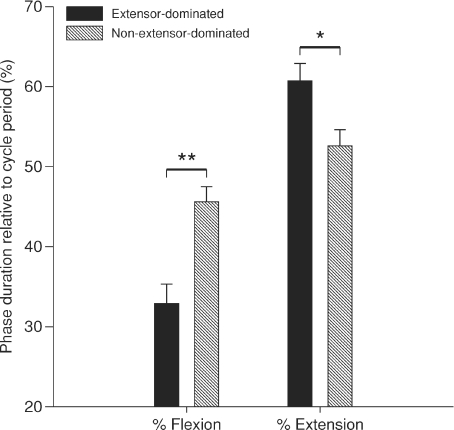
Although, the speed of fictive locomotion does not appear to be a factor in bias, the proportion or per cent time occupied by extension and flexion phases relative to cycle period appears to be involved (Fig. 3). In non-extensor-dominated episodes, flexion occupied a greater percentage (P < 0.01, one-way ANOVA) of the cycle period (45.6 ± 1.9%) compared to extensor-dominated episodes (32.9 ± 2.4%). Conversely, in extensor-dominated episodes, extension occupied a greater percentage (P < 0.05, one-way ANOVA) of the cycle period (60.7 ± 2.2%) compared to non-extensor-dominated episodes (52.6 ± 2.0%). There was a positive (ρ= 0.49; P < 0.005) and negative (ρ=−0.63; p < 0.005) correlation between the per cent time spent by flexion relative to cycle period and the regression coefficients rf and re, respectively. There was also a positive (ρ= 0.58; P < 0.005) and negative (ρ=−0.66; P< 0.005) correlation between per cent time spent by extension relative to cycle period and the regression coefficients re and rf, respectively. Thus, per cent time of extension or flexion relative to cycle period correlates with the slopes of the phase–cycle period relationships. Moreover, per cent time spent by flexion relative to cycle period was negatively correlated with cycle period (ρ=−0.52; P < 0.005). Per cent time spent by extension relative to cycle period did not correlate with cycle period (ρ= 0.16; P= 0.36). Thus, per cent time of flexion relative to cycle period correlated with cycle period.
Figure 3. Phase percentage relative to cycle period during spontaneous fictive locomotion.
Each bar is the mean ± standard error for 24 extensor-dominated and 11 non-extensor-dominated episodes. *P < 0.05, **P < 0.01.
Fictive locomotion after complete spinal transection
To determine if extensor or flexor bias requires signals from supraspinal structures we analysed fictive locomotor rhythms following a complete spinal transection. In cats acutely spinalized, fictive locomotion was induced by intravenous injection of nialamide and l-dihydroxyphenylalanine (l-DOPA) (Grillner & Zangger, 1979). We analysed six episodes of spinal/l-DOPA fictive locomotion in five cats (1–2 episodes per cat). To determine if a bias remains following a long-term spinal section, we studied cats completely spinalized 1 or 3 months before the acute experiment. Fictive locomotion in these chronic spinal cats was induced by intravenous clonidine injection combined with perineal stimulation (Cote et al. 2003; Cote & Gossard, 2004). We analysed 26 episodes of spinal/clonidine fictive locomotion in 16 cats (1–2 episodes per cat).
Figure 4A shows an episode of fictive locomotion in an acute spinal cat approximately 2 h following administration of l-DOPA/nialamide. Cycle period (3.67 ± 0.08 s) changed as a function of extension (re= 1.04, P < 0.001; rf= 0.07, P= 0.17; Pef < 0.001). Figure 4B shows an episode of fictive locomotion in a cat spinalized 1 month before the terminal experiment after clonidine injection and with perineal stimulation. Cycle period (2.33 ± 0.01 s) changed as a function of the extension phase (re= 0.73, P < 0.001; rf=−0.01, P= 0.97; Pef < 0.01). Figure 4C shows an episode of fictive locomotion, which followed six short trains of stimuli to the sciatic nerve (50 pulses, 10 T at 200 Hz), before clonidine injection and without perineal stimulation, in a cat spinalized 3 months before the terminal experiment. Cycle period (0.73 ± 0.06 s) also changed as a function of extension (re= 0.79, P < 0.001; rf=−0.08, P < 0.001; Pef < 0.001). We also recorded another two episodes of extensor-dominated fictive locomotion before clonidine injection with perineal stimulation, in two different cats and both episodes were extensor-dominated (not shown).
For the spinal/clonidine and spinal/l-DOPA groups, extensor-dominated locomotion accounted for 24/26 (cycle period = 1.03 ± 0.08 s) and 6/6 (cycle period = 5.48 ± 0.60 s) episodes, respectively. In the two episodes of spinal/clonidine that were not extensor-dominated, one episode did not vary (re= 0.42, P < 0.01; rf= 0.12, P < 0.05; Pef < 0.01) whereas the other episode co-varied (re= 0.54, P < 0.001; rf= 0.43, P < 0.001; Pef= 0.462). For the spinal/clonidine group, in the 24 extensor-dominated episodes, mean extension and flexion slopes were, respectively, 0.75 ± 0.03 and 0.11 ± 0.02 (P < 0.001, paired t tests) and extension occupied 65.9 ± 2.1% of the cycle period compared to 27.6 ± 1.6% for flexion (P < 0.001, paired t tests). For the spinal/l−DOPA group, the six extensor-dominated episodes, mean extension and flexion slopes were, respectively, 0.79 ± 0.08 and 0.09 ± 0.02 (P < 0.001, paired t tests) and extension occupied 67.4 ± 4.1% of the cycle period compared to 29.9 ± 1.3% for flexion (P < 0.001, paired t tests). Thus, fictive locomotion in cats after a complete spinal transection is primarily extensor-dominated and the extension phase occupies a larger proportion of the cycle period.
Modulation by MLR
Stimulating the mesencephalic locomotor region (MLR) in decerebrate cats can evoke fictive locomotor-like activity (Shik et al. 1966; Jordan et al. 2008). During MLR-evoked fictive locomotion, it was shown that cycle period varied predominantly with the flexion phase (Yakovenko et al. 2005). We wanted to determine what effects adding electrical stimulation of the brainstem would have on episodes of spontaneous fictive locomotion. Figure 5A shows an episode of spontaneous fictive locomotion followed by an episode of MLR-evoked locomotion. The spontaneous fictive locomotor episode (cycle period = 1.08 ± 0.06 s) was extensor-dominated (Fig. 5B; re= 0.79, P < 0.001; rf=−0.02, P= 0.55; Pef < 0.001). Once MLR stimulation began, the spontaneous rhythm stopped and was followed approximately 15 s later by a faster and more vigorous rhythm (cycle period = 0.55 ± 0.01 s) that was flexor-dominated (Fig. 5C; re=−0.07, P < 0.05; rf= 1.17, P < 0.001; Pef < 0.001). In seven trials in two cats, MLR stimulation did not appear to modify the ongoing spontaneous rhythm but replaced it with another rhythm. In such instances, the spontaneous rhythm became disorganized, stopped, and was followed by an episode of a more vigorous and faster rhythm. During the spontaneous locomotor episode shown in Fig. 5, extension and flexion, respectively, occupied 71.1% and 30.6% of cycle period, whereas during MLR-evoked locomotion this changed to 44.4% and 81.4%. Thus, under identical experimental conditions, the control of cycle period can be switched from extensor- to flexor-dominated, and the per cent time occupied by extension and flexion relative to cycle period can also change from a greater extension proportion during spontaneous fictive locomotion to a greater flexion percentage during MLR-evoked locomotion.
Discussion
In the present study, we showed that there is a clear asymmetry in the control of cycle periods by the spinal rhythm generator. Cycle period during fictive locomotion, evoked spontaneously in pre-mammillary/pre-collicular decerebrate cats, or in complete spinal cats with clonidine or nialamide/l-DOPA administration, was predominantly altered by modifying the extension phase. Therefore, without phasic sensory inputs or supraspinal stimulation, fictive locomotion is primarily extensor-dominated in decerebrate cats. A change in the stance phase, which is similar to the extension phase of fictive locomotion, also regulates changes in cycle period during normal or treadmill locomotion (Goslow et al. 1973; Grillner et al. 1979; Halbertsma, 1983). We conclude that the asymmetry in the control of cycle periods observed during normal locomotion resides within the spinal rhythm generator.
Asymmetry of spinal rhythm generators
During normal locomotion, cycle period varies as a function of the stance phase, or extension (Goslow et al. 1973; Grillner et al. 1979; Halbertsma, 1983), which would suggest that the spinal rhythm generators are organized asymmetrically with a clear extensor bias (Grillner & Dubuc, 1988; Dubuc et al. 1988). However, some have proposed that rhythm generators are organized symmetrically and that peripheral sensory inputs favours an extensor bias (Yakovenko et al. 2005; Juvin et al. 2007; Musselman & Yang, 2007). In the present study, we provide evidence that phasic sensory inputs are not required to produce predominantly extensor-dominated locomotion, because during fictive locomotion, the animal is curarized and phasic sensory inputs associated with movements are absent. Kiehn & Kjaerulff (1996) also showed in one neonatal rat that extensor-dominance, during a dopamine-induced fictive rhythm, remained after dorsal rhizotomy.
It is conceptually useful to consider the core of the rhythm generator within the CPG (the ‘clock function’) as a simple set of coupled oscillators, one for flexion and one for extension, with reciprocal connections (Koshland & Smith, 1989; Orsal et al. 1990; Stein & Smith, 1997; Kiehn, 2006; McCrea & Rybak, 2008). Dominance of a particular phase in determining cycle period is explained by an increased excitability in either oscillator. From analysis of episodes of MLR-evoked fictive locomotion, Yakovenko et al. (2005) concluded that cycle period is controlled symmetrically and that the rhythm can operate in flexor- or extensor-dominated modes. Our data also indicate that episodes of spontaneous fictive locomotion could be either flexor- or extensor-dominated. It is thus clear that, because of the inherent variability in excitability of the decerebrate cat, the rhythm generator can operate in either mode. We further showed that per cent time spent by extension or flexion as a function of cycle period correlated with the slope of the phase–cycle period relationship during spontaneous fictive locomotion (Fig. 3). Specifically, in episodes that co-varied or were flexor-dominated, flexor bursts occupied a greater proportion of the step cycle, and conversely extensor bursts occupied a smaller percentage. In MLR-evoked fictive locomotion, flexion occupies a greater percentage of the locomotor cycle (Yakovenko et al. 2005), whereas during spontaneous fictive locomotion extension occupies a greater percentage of cycle period. Presumably, it is easier for various inputs to influence the phase that occupies a greater percentage of the cycle. Functionally, there might be situations that require greater excitability within the flexor rhythm generator and thus a different type of locomotor control. For instance, consider walking through mud or deep snow and having to forcefully pick up the foot with each step, both of which would require greater control of flexors.
However, symmetry in the control of cycle period would predict a similar proportion of extensor- and flexor-dominated episodes and/or greater occurrence of co-varying episodes. In this study we investigated the presence of co-variance and found it could only explain a small proportion of our episodes (6/35 of episodes in spontaneous locomotion, 1/26 and 0/6 episodes in the locomotor pattern of chronic and acute spinal cats, respectively). The strong flexor bias found in Yakovenko et al. (2005) and the strong extensor bias found in our study combined with the rarity of co-variance does not support symmetric operation of rhythm generation. Also, per cent time of flexion relative to cycle period correlated significantly with cycle period but not per cent time of extension. Thus, even if extension occupies a small proportion of the step cycle, its variation still determines cycle period. On the other hand, only when the flexion phase occupies a larger proportion of the locomotor cycle does it contribute to cycle period variations. This suggests that flexor-dominance is achieved only when the oscillator for flexion receives an increase in excitability.
Our results also revealed that extensor-dominance does not require signals from supraspinal structures because, after a complete spinal transection, extensor-dominated fictive locomotion was observed in the great majority of episodes induced by clonidine or nialamide/l-DOPA. Moreover, extensor-dominance does not require particular pharmacology either because it was observed without drug injection. For example, in two 3 month chronic spinal cats, locomotor episodes induced by perineal stimulation alone were extensor-dominated without any drug injection. Furthermore, as shown in Fig. 4C, trains to the sciatic nerve generated an episode of extensor-dominated fictive locomotion, without perineal stimulation and without drug injection in another 3 month chronic spinal cat. In such conditions, all possible biases were removed (supraspinal, pharmacology or sustained stimulation) allowing the spinal cord networks to express the basic locomotor rhythm in the adult animal. Thus, irrespective of the methods used to evoke rhythmic activity or time after spinalization (acute and 1 or 3 month chronic spinal cats) the vast majority of fictive locomotor episodes are extensor-dominated. The overall evidence indicates that varying extension more than flexion to alter cycle period is an inherent property of the spinal rhythm generator.
In a recent study, excitatory and inhibitory synaptic inputs to flexor and extensor motoneurons were investigated in the isolated neonatal mice spinal cord during locomotor-like activity induced by NMDA, serotonin and/or dopamine (Endo & Kiehn, 2008). It was shown that excitation and inhibition varied out of phase, with increased excitation during the active phase and increased inhibition during the inactive phase in flexor and extensor motoneurons, which is consistent with a ‘push–pull’ organization of synaptic drive (Grillner, 1981; Orsal et al. 1986; Jordan, 1991; Kiehn, 2006; McCrea & Rybak, 2008). Overall, however, the variation in inhibition was larger than excitation, and moreover, variation in inhibition was larger in extensor than flexor motoneurons, which suggests some asymmetry in the synaptic drives from the CPG.
Breathing is another example of a rhythmic activity with an intrinsic asymmetry in the regulation of cycle period (Del Negro et al. 2009). A change in ventilation (i.e. cycle period) is accompanied by changes in the interval of time between inspiratory bursts (i.e. expiration + silent period), whereas inspiratory phase duration remains invariant (Del Negro et al. 2009). Therefore, an asymmetry in the control of cycle period appears to be a fundamental property of rhythm generation.
Preparation-dependent differences and methodological issues
Differences in phase–cycle period relationships observed in different preparations are probably due to multiple factors. The first one is certainly the level of the decerebration. Note that our decerebration involves a pre-mammillary section, sparing the subthalamic locomotor region (SLR), which is important for initiating goal-directed locomotion (Orlovsky, 1969; Whelan, 1996). The resulting reticulospinal commands, coupled with the intrinsic excitability of spinal locomotor networks, are probably responsible for the spontaneous occurrence of rhythmic activity. An additional lesion caudal to the mammillary body immediately stops the spontaneous rhythm (Gossard JP and Frigon A, unpublished results). For MLR-evoked fictive locomotion, the lesion is usually post-mammillary (Shik et al. 1966), which excludes the SLR. Consequently, the spinal cord networks are silent and require the reticulospinal drive evoked by the brainstem stimulation to produce rhythm.
Our results indicate that the descending synaptic drive evoked by brainstem electrical stimulation may alter the excitability within the spinal rhythm generator to operate in a flexor-dominated mode, as often seen in decerebrate cats (Yakovenko et al. 2005), and decerebrate rats (Iles & Nicolopoulos-Stournaras, 1996). In our Fig. 5, the ability of the MLR to switch fictive locomotion to a flexor-dominated pattern was clearly illustrated. Also, Fleshman et al. (1984) showed that varying MLR stimulation parameters could switch MLR-evoked locomotion from extensor-dominated to flexor-dominated. Although no analysis was done to confirm extensor- or flexor-dominance in that study, which was not the goal, data showed that low-frequency MLR stimulation generated fictive locomotion that was relatively extensor-dominated, with long extensor bursts, whereas with increasing stimulation frequency, locomotion became flexor-dominated, with longer flexor bursts (Fleshman et al. 1984).
One reason for the difference between spontaneous and MLR-evoked fictive locomotion is that they may represent distinct locomotor behaviours. Although they share much of the same locomotor circuitry they may be activated by quite different descending drives. For example, in Xenopus tadpoles, different types of excitatory neurons within the hindbrain are activated during swimming and struggling, with different discharge properties, suggesting that separate excitatory drives mediate the two locomotor behaviours (Li et al. 2007; Frigon, 2009).
Different drugs used to initiate fictive locomotion could also induce a bias in the control of cycle period. In neonatal rats, fictive rhythmic activities induced by different neurochemicals can differ from one another (Cowley & Schmidt, 1994; Kiehn & Kjaerulff, 1996). For instance, the timing and magnitude of flexor and extensor bursts, differed whether induced by dopamine or serotonin (Kiehn & Kjaerulff, 1996). Fictive rhythmic activities induced by dopamine were slow and irregular, whereas rhythms induced by serotonin were fast and regular (Kiehn & Kjaerulff, 1996). These results suggest that different neurochemicals may produce different functional configurations of the spinal network and hence different rhythmic patterns. The application of neuromodulators can mimic the descending drives from several supraspinal structures, which could induce an operational bias, some with an extensor bias, others with a flexor bias.
Lastly, the asymmetry in the control of cycle period might be acquired during development. In Juvin et al. (2007), fictive locomotor-like activity was induced in the isolated spinal cord of neonatal rats (0–12 days after birth) by bath application of a mixture of N-methyl-d,l-aspartate (NMDA), serotonin and dopamine, or by electrically stimulating the brainstem. They showed that both flexor and extensor motor bursts correlated positively with cycle period, as would be predicted by a set of coupled symmetric oscillators. Whether this symmetric operation is due to the particular choice of chemicals or immaturity or both is unknown. They further showed that movement-related sensory feedback from the hindlimbs could switch the locomotor pattern into extensor-dominance. It would be interesting to know if, after a life-long training effect of walking and associated sensory feedback, the spinal CPG is reconfigured in the adult with an inherent bias for extensor-dominance.
Concluding remarks
Our results showed that the predominance of extensor-dominated locomotor patterns, as seen in normal walking, was observed without phasic sensory feedback or supraspinal structures or pharmacology or sustained stimulation. It is thus concluded that there is an inherent asymmetry in the organization of the spinal rhythm generator in the control of phase durations and cycle period. It is also clear that a number of systems can modulate the operation of the rhythm generator to produce different modes of phase–cycle period changes. Future studies should continue elucidating the multiple inputs that influence rhythm generation because, from a clinical perspective, this could have important implications in ameliorating locomotor function, particularly speed, after spinal cord injury.
Acknowledgments
We thank France Lebel for technical assistance and Miguel Chagnon for help with the statistical analysis. We also thank Patrick Noué, Jennifer Sirois, Marie-Pascale Côté, Ariane Ménard, Isabelle Poulin and Hugues Leblond for participating in some of the experiments. The present work was supported by the Canadian Institutes of Health Research (J.-P.G.) and by the Christopher and Dana Reeve Foundation (A.F.). All studies were conducted in the Department of Physiology at the Université de Montréal.
Glossary
Abbreviations
- CDP
cord dorsum potential
- CPG
central pattern generator
- EDL
extensor digitorum longus
- ENG
electroneurography
- FDHL
flexor hallucis longus/flexor digitorum longus
- l-DOPA
l-dihydroxyphenylalanine
- LGS
lateral gastrocnemius–soleus
- MG
medial gastrocnemius
- MLR
mesencephalic locomotor region
- NMDA
N-methyl-d,l-aspartate
- PBSt
posterior biceps–semitendinosus
- Pl
plantaris
- SLR
subthalamic locomotor region
- SmAB
semimembranosus–anterior biceps
- SP
superficial peroneal
- TA
tibialis anterior
Author contributions
A.F. and J.-P.G. were involved in project design, data analysis/interpretation and writing of the manuscript.
References
- Armstrong DM. The supraspinal control of mammalian locomotion. J Physiol. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev. 2008;57:64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci. 2004;24:11317–11327. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett. 1994;171:147–150. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Kam K, Hayes JA, Feldman JL. Asymmetric control of inspiratory and expiratory phases by excitability in the respiratory network of neonatal mice in vitro. J Physiol. 2009;587:1217–1231. doi: 10.1113/jphysiol.2008.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc R, Cabelguen J-M, Rossignol S. Rhythmic fluctuations of dorsal root potentials and antidromic discharges of single primary afferents during fictive locomotion in the cat. J Neurophysiol. 1988;60:2014–2036. doi: 10.1152/jn.1988.60.6.2014. [DOI] [PubMed] [Google Scholar]
- Endo T, Kiehn O. Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J Neurophysiol. 2008;100:3043–3054. doi: 10.1152/jn.90729.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman JW, Lev-Tov A, Burke RE. Peripheral and central control of flexor digitorium longus and flexor hallucis longus motoneurons: the synaptic basis of functional diversity. Exp Brain Res. 1984;54:133–149. doi: 10.1007/BF00235825. [DOI] [PubMed] [Google Scholar]
- Frigon A. Reconfiguration of the spinal interneuronal network during locomotion in vertebrates. J Neurophysiol. 2009;101:2201–2203. doi: 10.1152/jn.00003.2009. [DOI] [PubMed] [Google Scholar]
- Goslow GEj, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973;141:1–42. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control. Bethesda, Maryland: American Physiological Society; 1981. pp. 1179–1236. [Google Scholar]
- Grillner S, Dubuc R. Control of locomotion in vertebrates: spinal and supraspinal mechanisms. In: Waxman SG, editor. Functional Recovery in Neurological Disease. New York: Raven Press; 1988. pp. 425–453. [PubMed] [Google Scholar]
- Grillner S, Halbertsma J, Nillsson J, Thorstensson A. The adaptation to speed in human locomotion. Brain Res. 1979;165:177–182. doi: 10.1016/0006-8993(79)90059-3. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Halbertsma JM. The stride cycle of the cat: the modelling of locomotion by computerized analysis of automatic recordings. Acta Physiol Scand Suppl. 1983;521:1–75. [PubMed] [Google Scholar]
- Iles JF, Nicolopoulos-Stournaras S. Fictive locomotion in the adult decerebrate rat. Exp Brain Res. 1996;109:393–398. doi: 10.1007/BF00229623. [DOI] [PubMed] [Google Scholar]
- Jordan LM. Brainstem and spinal cord mechanisms for the initiation of locomotion. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Tokyo: Japan Scientific Societies Press; 1991. pp. 3–20. [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Juvin L, Simmers J, Morin D. Locomotor rhythmogenesis in the isolated rat spinal cord: a phase-coupled set of symmetrical flexion extension oscillators. J Physiol. 2007;583:115–128. doi: 10.1113/jphysiol.2007.133413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- Koshland GF, Smith JL. Mutable and immutable features of paw-shake responses after hindlimb deafferentation in the cat. J Neurophysiol. 1989;62:162–173. doi: 10.1152/jn.1989.62.1.162. [DOI] [PubMed] [Google Scholar]
- Li WC, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: specific neuron recruitment and context-dependent synaptic plasticity. J Neurosci. 2007;27:12267–12276. doi: 10.1523/JNEUROSCI.3694-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman KE, Yang JF. Loading the limb during rhythmic leg movements lengthens the duration of both flexion and extension in human infants. J Neurophysiol. 2007;97:1247–1257. doi: 10.1152/jn.00891.2006. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Spontaneous and induced locomotion of the thalamic cat. Biophysics. 1969;14:1154–1162. [Google Scholar]
- Orsal D, Cabelguen J-M, Perret C. Interlimb coordination during fictive locomotion in the thalamic cat. Exp Brain Res. 1990;82:536–546. doi: 10.1007/BF00228795. [DOI] [PubMed] [Google Scholar]
- Orsal D, Perret C, Cabelguen J-M. Evidence of rhythmic inhibitory synaptic influences in hindlimb motoneurons during fictive locomotion in the thalamic cat. Exp Brain Res. 1986;64:217–224. doi: 10.1007/BF00238216. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mid-brain (in Russian) Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Stein PSG, Smith JL. Neural and biochemical control strategies for different forms of vertebrate hindlimb locomotor tasks. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks and Motor Behaviour. Cambridge: MIT Press; 1997. pp. 61–73. [Google Scholar]
- Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol. 1996;49:481–515. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, McCrea DA, Stecina K, Prochazka A. Control of locomotor cycle durations. J Neurophysiol. 2005;94:1057–1065. doi: 10.1152/jn.00991.2004. [DOI] [PubMed] [Google Scholar]