Abstract
Paired associative stimulation (PAS) is an effective non-invasive method to induce human motor plasticity by the repetitive pairing of peripheral nerve stimulation and transcranial magnetic stimulation (TMS) at the primary motor cortex (M1) with a specific time interval. Although the repetitive pairing of two types of afferent stimulation might be a biological basis of neural plasticity and memory, other types of paired stimulation of the human brain have rarely been studied. We hypothesized that the repetitive pairing of TMS and interhemispheric cortico-cortical projection or paired bihemispheric stimulation (PBS), in which the right and left M1 were serially stimulated with a time interval of 15 ms, would produce an associative long-term potentiation (LTP)-like effect. In this study, 23 right-handed healthy volunteers were subjected to a 0.1 Hz repetition of 180 pairings of bihemispheric TMS, and physiological and behavioural measures of the motor system were compared before, immediately after, 20 min after and 40 min after PBS intervention. The amplitude of the motor evoked potential (MEP) induced by the left M1 stimulation and its input–output function increased for up to ∼20 min post-PBS. Fine finger movements were also facilitated by PBS. Spinal excitability measured by the H-reflex was insensitive to PBS, suggesting a cortical mechanism. The associative LTP-like effect induced by PBS was timing dependent, occurring only when the interstimulus interval was 5–25 ms. These findings demonstrate that using PBS in PAS can induce motor cortical plasticity, and this approach might be applicable to the rehabilitation of patients with motor disorders.
The human primary motor cortex (M1) has a great reorganization ability in adults following repetitions of simple movement and motor skill training (Karni et al. 1995; Pascual-Leone et al. 1995; Classen et al. 1998), environmental change (Muellbacher et al. 2000; Nilsson & Pekny, 2007) and injury to the sensorimotor system (Brasil-Neto et al. 1993; Chen et al. 2002; Nudo, 2003; Rossini et al. 2007). This maintained plasticity helps us to adjust to various external and internal conditions. Moreover, the plasticity in the motor cortex might be a physiological basis of motor learning and memory (Rioult-Pedotti et al. 2000; Ziemann et al. 2004). One intensively studied method to produce human M1 plasticity experimentally is paired associative stimulation (PAS), that is, the low-frequency repetitive pairing of electrical peripheral nerve stimulation and transcranial magnetic stimulation (TMS) over the M1 with a specific time interval (Stefan et al. 2000, 2002; Wolters et al. 2003; Ueki et al. 2006). Previous studies showed that the M1 plasticity induced by PAS is similar to associative long-term potentiation (LTP) or long-term depression (LTD). This LTP-like effect is likely to be Hebbian plasticity (Hebb, 1949), because it is lost or even reversed when TMS is applied immediately prior to the cortical arrival time of peripheral nerve stimulation.
However, it is not known whether other types of afferent stimulation of the M1 can produce associative plasticity when they are time-locked to the TMS over the M1. This scenario is considered likely to occur in humans if associative plasticity is a general principle of brain development and learning, as suggested by Hebb (1949).
One possible origin of afferent projection to the M1 is the contralateral M1. The human motor control of complex bimanual coordination is based on the intense functional connections within the bilateral M1. Lesion studies in patients have shown the functional relevance of transcallosal fibres for bimanual coordination (Serrien et al. 2001; Kennerley et al. 2002; Seitz et al. 2004). Although the transcallosal connections between M1 areas are more prominent in the proximal muscles of primates (Jenny, 1979; Pappas & Strick, 1981; Gould et al. 1986), several studies have suggested the existence of short interhemispheric conduction pathways for the hand M1 area, possibly through transcallosal fibres (Shibasaki et al. 1978; Wilkins et al. 1984; Brown et al. 1991; Hanajima et al. 2001). When paired TMS was applied to the right and left M1, the conditioning effect of the contralateral M1 stimulation began as early as 6 ms and reached a maximum at ∼10 ms (Ferbert et al. 1992; Gerloff et al. 1998).
Thus, we hypothesized that the repetitive pairing of TMS and interhemispheric cortico-cortical projection or paired bihemispheric stimulation (PBS) would produce an associative LTP-like effect. For the PBS, we utilized left M1 stimulation preceded by right M1 stimulation with a time interval of 15 ms, which can combine the interhemispheric projection from the right to left M1 and the TMS at the left M1. As the order of the paired inputs is important for effectively producing associative plasticity (Bi & Poo, 1998; Wolters et al. 2003, 2005), we decided to apply a delay of 15 ms, so that the inputs from the contralateral M1 would arrive at the targeted M1 area before the TMS. A previous animal study indicated that a time delay between two spikes of <20 ms could produce associative plasticity (Bi & Poo, 1998). We therefore also tested whether the effects of PBS were timing dependent by changing the interstimulus interval (ISI) at intervals of 10 ms from −25 ms to 25 ms.
In addition to the motor evoked potential (MEP) amplitudes, we also systematically examined the spinal motor neuronal excitability, the input–output (I–O) function of the M1 and various motor behaviours following PBS. We found PBS to be a promising method to induce associative plasticity in the human motor system (Koganemaru et al. 2008). A similar method to induce plastic change in order to increase cortico-spinal excitability was reported recently (Rizzo et al. 2009).
Methods
Subjects
The study was approved by the Committee of Medical Ethics of the Graduate School of Medicine, Kyoto University, Japan, and written informed consent was obtained from all participants. The experiments were performed with 23 healthy volunteers (22 men and 1 woman) aged 22–34 years (mean, 27.7 ± 4.6 years). None of the participants had a history of neurological illness or was taking medication. All of the volunteers were right handed, according to the Oldfield handedness inventory (Oldfield, 1971).
Recording procedures
The electromyographic activity was recorded from the right and left abductor pollicis brevis (APB) muscles, and the right and left flexor carpi radialis (FCR) muscles, using a pair of silver electrodes. The EMGs were amplified and filtered (bandpass, 5–2000 Hz), and digitized at a sampling rate of 10 kHz using the Neuroscan system (Neuroscan Co., Herndon, VA, USA).
Each subject was seated comfortably in an armchair. Focal TMS was performed using a flat figure-of-eight-shaped magnetic coil (outer diameter of each wing, 9 cm) connected to a Magstim 200 magnetic stimulator (Magstim, Whitland, Dyfed, UK). The coil was placed tangentially to the scalp with the handle pointing backwards and 45 deg lateral to the midline. For each subject, the optimal scalp positions to induce the motor response for both the right and the left APB were determined.
The resting motor threshold (rMT) was defined as the minimal stimulator output eliciting MEPs of >50 μV in 5 out of 10 consecutive pulses (Rossini et al. 1994). Complete muscle relaxation was continuously monitored by visual feedback of the surface EMGs. For each subject, the peak-to-peak amplitudes of the MEPs were measured in each single trial and averaged to evaluate the cortico-spinal excitability.
PBS
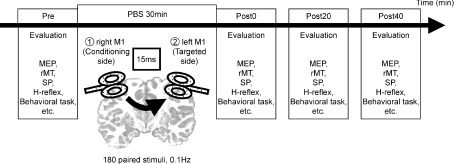
The PBS intervention consisted of paired TMS over each hemisphere; the first TMS over the right M1 (the conditioned side) was followed by the second TMS over the left M1 (the targeted side) with an ISI of 15 ms. In total, 180 repetitions of the paired TMS were delivered every 10 s (0.1 Hz) over a period of 30 min. The stimulus intensity was 120% of the rMT for each M1 area. If the PBS intervention was repeated, the experiments were separated by at least 1 day (Fig. 1).
Figure 1. Design of the main experiments.
The PBS consisted of 0.1 Hz repetitions of 180 paired stimulations of the TMS at the right M1, followed by the TMS at the left M1, with a delay of 15 ms. We examined motor functional parameters (for example, the amplitudes of MEP, rMT, SP and behavioural tasks in each experiment) before, immediately after, 20 min after and 40 min after the end of PBS (the pre, post-0, post-20 and post-40 conditions, respectively).
Experimental protocol
We performed several experiments to examine the effects of PBS. The order of the side and the intensities of the TMS were randomized in all experiments. The measurements took ∼10 min. The evaluations were done before, immediately after, 20 min after and 40 min after the PBS in the following experiments (designated as the ‘pre’, ‘post-0’, ‘post-20’ and ‘post-40’ conditions, respectively; Fig. 1) unless otherwise stated.
Experiment 1: Effects of PBS on motor excitability
The recruitment of the cortico-spinal projection (I–O function) from the left hand M1 was measured in 10 subjects, to investigate the motor cortical excitability. The intensities of the single TMS stimuli were individually adapted according to the rMT to evaluate the I–O function.
Eight MEPs were recorded from the right APB muscle at intensities of 50, 70, 80, 90, 100, 110, 120, 130 and 150% of the rMT, respectively.
Experiment 2: Effects of PBS on rMT and silent period (SP)
In seven subjects, the effects of PBS on the rMT and the duration of the cortical SP at the left M1, which was targeted by the PBS, were further studied. To assess the cortico-spinal excitability, the amplitudes of the MEPs were measured with the fixed intensity of the TMS machine adjusted to produce an MEP of ∼1 mV from both the right and the left APB muscle (stimulus intensity, SI 1 mV) before the PBS.
To investigate the cortical inhibitory system, the SP of the left M1 with a stimulation intensity of 120% of the rMT (before the PBS) was assessed using surface electromyographic recordings of the APB isometrically contracted at 15% of the maximum force. The duration of the SP was defined as the time from the onset of the magnetic stimulation until the return of voluntary electromyographic activity. The force was measured using a force transducer (range, 0–50 lbs; diameter of contact surface area, 2 cm) and was fed back into an oscilloscope. The individual 15% force level was marked directly on the oscilloscope screen in front of the subject.
Experiment 3: Effects of PBS on H-reflex
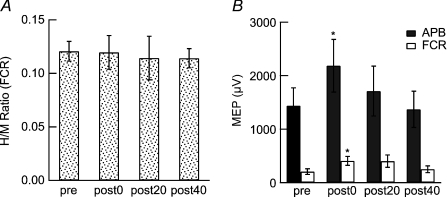
The H-reflex in the right FCR muscle was measured to test the effects of the PBS at the spinal level in six subjects. To produce an H-reflex in the FCR muscle, the right median nerve was stimulated at the elbow with an electric pulse of 1 ms duration during wrist flexion at 20% of the maximum force. The stimulus intensity was gradually increased from a level below the H-wave threshold to a level at which a stable maximum M-wave was elicited. Both H-waves and M-waves were recorded. The spinal excitability was assessed by the ratio of the maximum H-reflex amplitude (Hmax) to the maximum M-wave amplitude (Mmax).
The FCR muscle was selected because reliable H-reflexes cannot be produced in the APB muscle. To confirm that the change in the FCR motor excitability was comparable to that in the APB following the targeted PBS, we also evaluated the MEP of the right FCR in this experiment.
Experiment 4: Effects of PBS on motor behaviour
The effects of the PBS on motor behaviour were examined using the choice reaction time (cRT) task, nine-hole peg test (9HPT; Mathiowetz et al. 1985), pinch force and grip power, as well as by measuring the MEPs of the right APB with an SI of 1 mV in 10 subjects. All of the behaviour tasks were assessed for both hands.
In the cRT task, the subjects had to select one of two button-press responses with the right or left thumb, according to the direction of an arrow presented on a monitor set 1 m in front of them. When an arrow was presented, the subjects had to respond as quickly and accurately as possible. The probability of appearance of each of the right and left arrows was set at 50%. Following one or two practice trials, data from 40 trials were collected for each subject at each session. The mean reaction times (RTs) of each hand for a subject were calculated. Incorrect responses and RTs of >500 ms were excluded.
The 9HPT is a standardized quantitative test requiring the accurate rotation and translation with stabilization of the arm and hand. In this test the subjects were presented with a wooden block containing nine empty holes and a small, shallow container holding nine pegs. Upon receiving the start command, the subjects picked up the nine pegs one by one with one hand as quickly as possible, put them into the nine holes and then removed them again one by one as quickly as possible, returning them to the shallow container. The total time taken to complete the task was recorded.
The maximal pinch force using the thumb and index finger of each hand was measured using the same force transducer as that used in Experiment 2. The maximal grip power (kilograms) of each hand was measured. The order of the tasks was randomized both within and across subjects.
In addition, we repeated the experiment with the PBS targeted to the right M1 in seven different subjects, in order to confirm that the behavioural differences were not simply due to the difference in dexterity between the dominant and non-dominant sides in motor learning.
Experiment 5: Site specificity of PBS
PBS of the left M1 (target) and the right parietal area (conditioning site) was performed to test the site specificity of the conditioning stimulus with an ISI of 15 ms in six subjects who had participated in Experiment 1. The conditioning TMS of the right parietal area was applied at position P4 on the electroencephalogram according to the International 10–20 System, which was approximately 6 cm posterior to the right hand M1.
The cortico-spinal excitability was assessed by the mean amplitudes of 20 MEPs recorded from the right APB with SI 1 mV measured before the PBS.
Experiment 6: Differences of the effects of side
To examine the differences of the effects of side, the serial order of the right and left M1 stimulation was reversed during the PBS in six subjects. For the right M1 (the targeted side), 20 MEPs recorded from the left APB with SI 1 mV were also measured. These were compared to the MEPs with SI 1 mV from the right APB before and after normal ordered PBS for the left M1 (the targeted side), which was performed on a different day in the same six subjects.
Experiment 7: Influence of interstimulus interval (ISI) on PBS
PBS was performed with different ISIs (−25, −15, −5, 5, 15 and 25 ms) to test the effect of the timing of the paired stimulation in six subjects. The ISI was defined as the time interval between the TMS of the conditioned side and the targeted side. A minus value for the ISI indicated that the serial order of stimulation during the PBS was reversed; for example, the experimental condition using TMS of the right M1 followed by TMS of the left M1 with a delay of 15 ms was regarded as having an ISI of 15 ms for the MEP of the right APB and −15 ms for that of the left APB.
The mean amplitudes of 20 MEPs for the right and left APB with SI 1 mV were measured. The order of the experiments using different ISIs was randomized across subjects. The effects of the PBS on the left and right M1 were measured separately. To clarify the effects of different ISIs on the PBS, the ratio for MEPs in the post-0 condition compared with the baseline (post-0/pre) condition was measured for each ISI.
Experiment 8: Influence of conditioning intensity
To test whether the conditioning stimulus had the optimum intensity value, PBS was applied with an intensity of 90% of the rMT on the conditioning side in six subjects. The mean amplitudes of 20 MEPs for the right APB with SI 1 mV were measured.
Statistical analysis
The paired t test was used to compare the rMT and the test intensities for SI 1 mV between both M1 areas. To assess the effects of PBS, data on the MEP amplitudes for the right and left M1, the intensity for the rMT, the duration of the SP, and the behavioural performance measured in the right and left hand were subjected to repeated-measures ANOVA with Time (pre, post-0, post-20 and post-40) as a within-subject factor. In addition, for Experiment 1, two-way repeated-measures ANOVA was performed using Intensity (50, 70, 80, 90, 100, 110, 120, 130 and 150%) and Time. For Experiment 7, two-way repeated-measures ANOVA was performed using Side (right and left M1) and ISI.
If necessary, the Greenhouse–Geisser correction was used to adjust for the sphericity, changing the degrees of freedom using a correction coefficient epsilon. The Bonferroni correction for multiple comparisons was used for the post hoc t test. Effects were considered significant at P < 0.05. All data are given as the mean ±s.e.m.
Results
None of the subjects experienced any side effects from TMS during the experiments.
The rMTs for the right and left APB muscles were 49.5 ± 6.0 and 50.7 ± 7.5% of the maximum stimulator output, respectively (n= 23). There was no significant main effect of Side. The test intensities for SI 1 mV for the right and left APB were 62.1 ± 9.6% (Experiments 2–8) and 64.8 ± 9.1% (Experiments 2, 4, 6 and 7), respectively.
Experiment 1: Effects of PBS on motor excitability
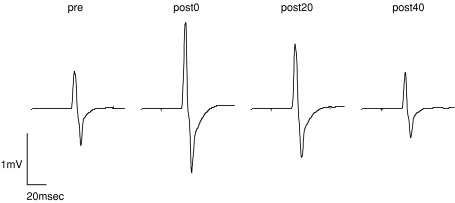
Figure 2 illustrates the waveforms of the mean MEPs from one representative subject with an intensity of 120% of the rMT before, immediately after, 20 min after and 40 min after the intervention. The MEPs recorded from the right APB were enhanced immediately after and 20 min after the intervention.
Figure 2. Mean MEP waveforms before and after PBS in one subject.
MEPs were recorded from the right APB with a stimulus intensity of 120% of the rMT and were averaged (8 trials for each). MEPs were facilitated immediately after and 20 min after PBS (post-0 and post-20 conditions) compared with the baseline (pre condition), and returned to the baseline 40 min after PBS (post-40 condition).
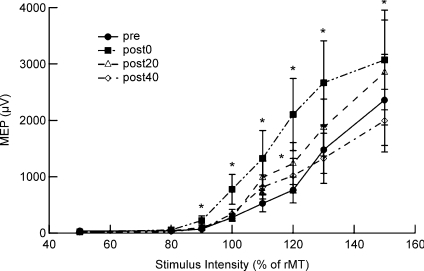
Repeated-measures ANOVA showed significant main effects of Time (F= 16.63, P < 0.001), and the Time × Strength interaction (F= 2.0, P= 0.029). The MEP amplitudes as a function of the TMS strength were significantly different in the post-0 and post-20 conditions compared with the pre condition (pre vs. post-0, F= 27.0, P < 0.001; pre vs. post-20, F= 9.3, P= 0.003; Fig. 3). The post hoc t test revealed significant increases of the MEP amplitude compared with the pre condition at intensities of 90, 100, 110, 120, 130 and 150% of that of the post-0 condition, and 120% of that of the post-20 condition (P= 0.026, 0.05, 0.024, 0.001, 0.01, 0.047 and 0.032, respectively).
Figure 3. MEP amplitudes as a function of TMS intensity before and after PBS.
The mean MEP amplitudes were calculated for the right APB with TMS at intensities of 50, 70, 80, 90, 100, 110, 120, 130 and 150% of the rMT (8 trials for each; 10 subjects). The post hoc t test showed an increase of the MEP amplitudes compared with the pre condition at intensities of 90, 100, 110, 120, 130 and 150% of the post-0 condition, and 120% of the post-20 condition (t test, *P < 0.05).
Experiment 2: Effects of PBS on rMT and SP
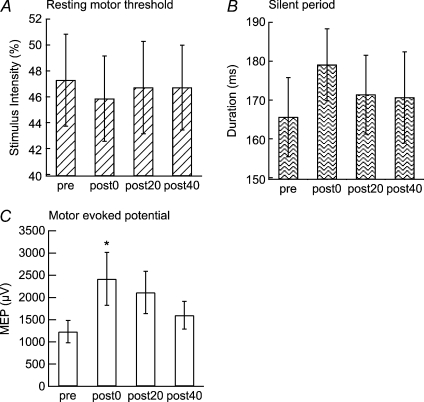
The rMTs and SPs for the left M1 had a tendency to decrease or increase, respectively, but neither was significantly modulated by the PBS (rMT, F= 1.36, P= 0.29; SP, F= 1.79, P= 0.19; Fig. 4A and B). The MEPs for the right APB muscle were significantly increased by the PBS (F= 4.96, P= 0.01; post hoc t test, pre vs. post-0, P= 0.042; Fig. 4C). The MEPs for the left APB muscle were not significantly modulated by the PBS (F= 0.78, P= 0.5).
Figure 4. Effects of PBS on rMT and SP.
PBS did not have any significant effects on the rMT (A) or SP (B) of the right APB, but significantly enlarged its MEP amplitudes (C) (t test, *P < 0.05).
Experiment 3: Effects of PBS on H-reflex
The mean H-latency was 15.78 ± 0.79 ms. There was no significant change in the Hmax/Mmax ratio (Fig. 5A). The amplitudes of the MEPs of the right FCR muscles, as well as the right APB, were significantly enhanced immediately after the PBS (FCR, F= 4.3, P= 0.022; pre vs. post-0, P= 0.001; APB, F= 3.4, P= 0.046; post hoc t test, pre vs. post-0, P= 0.005; Fig. 5B).
Figure 5. Effects of PBS on H-reflex and FCR muscles.
A, the H-reflex in the right FCR muscles was measured by stimulating the right median nerve at the elbow during wrist flexion at 20% of the maximum force. The Hmax/Mmax ratio was not significantly changed after PBS. B, the MEP amplitudes for the right FCR, as well as the APB, were significantly enhanced immediately after the PBS (t test, *P < 0.05).
Experiment 4: Effects of PBS on motor behaviour
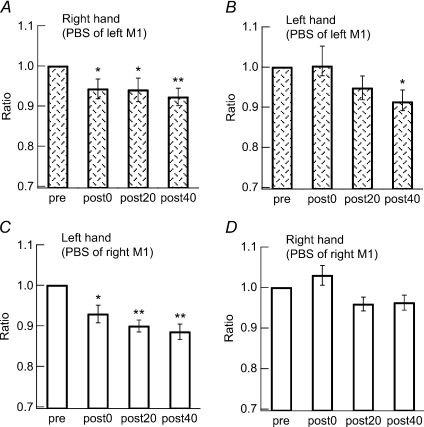
For the PBS targeting the left M1, a significant decrease in the completion time in the 9HPT was found in the right hand in the post-0, post-20 and post-40 conditions (F= 10.0, P= 0.0004; post hoc t test, pre vs. post-0, P= 0.005, pre vs. post-20, P= 0.003, and pre vs. post-40, P < 0.0003; Fig. 6A) and in the post-40 condition in the left hand (F= 5.3, P= 0.005; post hoc t test, pre vs. post-40, P= 0.0045; Fig. 6B).
Figure 6. Effects of PBS on time taken to complete 9HPT.
The times required to complete the 9HPT by the right hand (A) and the left hand (B) were measured. A significant enhancement was found in the right hand immediately after PBS (post-0 condition), which continued for over 40 min (post-40 condition), whereas in the left hand it was seen only at 40 min post-PBS (post-40 condition) (t test, *P < 0.05).
For the pre, post-0, post-20 and post-40 conditions, respectively, the cRT (right hand, 353.2 ± 8.6, 338.9 ± 8.2, 342.7 ± 6.0 and 338.3 ± 10.0 ms; left hand, 349.8 ± 9.0, 343.7 ± 8.6, 340.7 ± 4.1 and 340.1 ± 7.6 ms), pinch force (right hand, 12.9 ± 0.6, 14.2 ± 0.8, 13.7 ± 0.7 and 13.9 ± 1.0 lbs; left hand, 12.4 ± 0.8, 11.9 ± 0.8, 12.6 ± 0.6 and 12.9 ± 0.6 lbs) and grip power (right hand, 36.0 ± 1.6, 37.0 ± 1.5, 37.1 ± 1.2 and 36.4 ± 1.5 kg; left hand, 34.4 ± 1.5, 34.6 ± 1.5, 35.7 ± 1.4 and 34.8 ± 1.5 kg) were not significantly modified by the PBS.
For PBS targeting the right M1, a significant decrease of the completion time in the 9HPT was found in the left hand in the post-0, post-20 and post-40 conditions (F= 14.9, P < 0.0001; post hoc t test, pre vs. post-0, P= 0.01, pre vs. post-20, P < 0.001, and pre vs. post-40, P < 0.001; Fig. 6C), whereas no significant change was observed in the right hand (F= 5.0, P= 0.03; post hoc t test, pre vs. post-0, P > 0.05, pre vs. post-20, P > 0.05, and pre vs. post-40, P > 0.05; Fig. 6D).
For the pre, post-0, post-20 and post-40 conditions, respectively, the cRT of the left hand was also significantly modified by PBS targeting the right M1 (393.9 ± 17.4, 370.0 ± 18.7, 370.1 ± 14.7 and 377.3 ± 20.0 ms; F= 4.2, P= 0.02; post hoc t test, pre vs. post-0, P= 0.02, and pre vs. post-20, P= 0.02), while that of the right hand was not (380.4 ± 14.4, 376.7 ± 16.1, 369.7 ± 16.4, 364.7 ± 18.2 ms; F= 1.3, P > 0.05).
For the pre, post-0, post-20 and post-40 conditions, respectively, the pinch force (left hand, 11.9 ± 1.1, 11.7 ± 0.8, 12.8 ± 1.6 and 12.3 ± 1.3 pounds; right hand, 13.7 ± 1.3, 12.4 ± 0.8, 11.9 ± 1.0 and 12.8 ± 1.1 pounds) and grip power (left hand, 40.5 ± 3.2, 38.6 ± 2.6, 38.9 ± 2.7 and 35.4 ± 2.0 kg; right hand, 42.6 ± 2.8, 41.7 ± 2.7, 41.5 ± 3.4 and 41.2 ± 1.3 kg) were not significantly modified.
Experiment 5: Site specificity of PBS
When the conditioning TMS of the PBS was applied to the right parietal area, the MEPs for the right APB were not significantly modulated (1042.2 ± 310.6, 653.1 ± 245.2, 792.4 ± 203.6 and 974.0 ± 263.9 μV for the pre, post-0, post-20 and post-40 conditions, F= 1.3, P= 0.3).
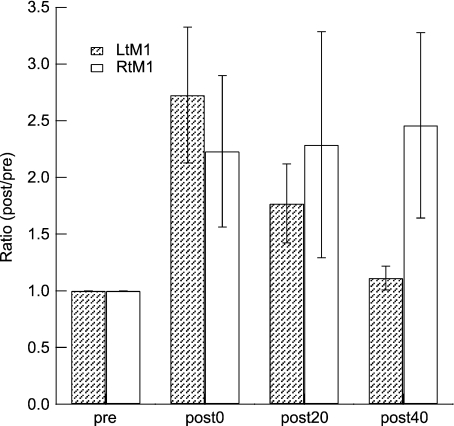
Experiment 6: Differences of side
The MEP change in the right APB induced by PBS targeted to the left M1 was compared with that in the left APB induced by PBS targeted to the right M1. Time had a significant effect (F= 4.57, P= 0.023), but the Time × Side interaction did not (F= 2.76, P= 0.08; Fig. 7).
Figure 7. Effects of PBS targeting the left and right M1.
The effects of PBS targeting the left M1 measured by the MEP amplitudes of the right APB, and the right M1 measured by the MEP amplitudes of the left APB, are shown. There was no significant interaction between Side (right and left) and Time (pre, post-0, post-20 and post-40). For presentation purposes, the ratio of MEPs compared to the pre condition is shown here.
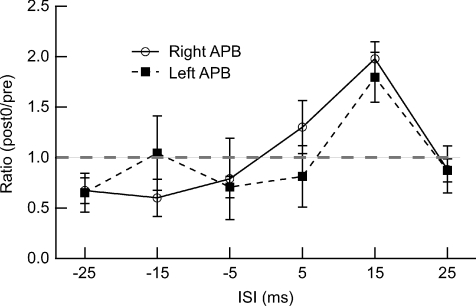
Experiment 7: Influence of ISI on PBS
PBS targeting the left or right M1 with an ISI of 15 ms produced a significant increase of MEP amplitudes only in the right APB (F= 4.0, P= 0.029; post hoc t test, pre vs. post 0, P= 0.009) and the left APB (F= 3.9, P= 0.031; post hoc t test, pre vs. post-0, P= 0.021). PBS with different ISIs (−25, −15, −5, 5 and 25 ms) did not induce any significant change in the MEP amplitudes.
Two-way repeated-measures ANOVA of the MEP ratio (post-0/pre) demonstrated a significant effect of the ISI (F= 5.6, P= 0.002; Fig. 8). The interaction of Side × ISI was not significant (F= 1.1, P= 0.4).
Figure 8. Influence of ISI on effects of PBS.
The ratio of the MEP amplitudes of the right or left APB muscles (post-0/pre) is presented as a function of the ISI measured from the time of conditioning stimulation to that of target M1 stimulation. The largest MEP ratio was observed at an ISI of 15 ms.
Experiment 8: Influence of conditioning intensity
When the conditioning stimulus intensity was fixed at 90% of the rMT and the target intensity was 120% of the rMT during the PBS, the MEPs for the right APB were not significantly modulated (1526.2 ± 38.1, 1459.9 ± 496.2, 1416.3 ± 666.7 and 1473.3 ± 358.6 μV for the pre, post-0, post-20 and post-40 conditions, respectively).
Discussion
The present results showed that paired low-frequency TMS over bihemispheric motor areas with a specific time interval (PBS) produced transiently sustained increments of motor cortical excitability and enhanced motor performances on the targeted side. The effects of the PBS were temporary and reversible; they developed immediately, continued for a period (20–40 min) and then returned to the baseline. PBS seemed to show timing specificity between two different inputs. Thus, it is likely that PBS combining TMS and interhemispheric projection induces associative LTP-like changes in the human motor system.
Extending a previous study by Rizzo et al. (2009), we found that the bihemispheric stimulation of homologous areas (that is, M1–M1 but not the parietal area–M1) was necessary to produce plasticity. The behavioural consequences of PBS were found to include an improvement in the performance of the 9HPT as well as the cRT task. The H-reflex experiment demonstrated that PBS did not modify the spinal excitability as measured by the Hmax/Mmax ratio. Therefore, it is likely that the increase in motor excitability after PBS was produced at the supraspinal level. In addition, we found that the effects of PBS were timing dependent. The increase of excitability was dependent on the specific time interval of the activation of the target M1 through two afferent pathways.
Regarding the behavioural consequences, PBS produced significant changes in the complex fine motor control of digits measured by the 9HPT for the target hand, irrespective of the side of the intervention. The improvement was observed immediately after PBS and continued over 40 min in the target hand. It was not clear why there was enhanced motor performance without a significant increase of the MEP amplitude in the target hand at 40 min post-PBS. One hypothesis is that the PBS-induced plasticity might have enhanced the learning rate or the consolidation of motor learning induced by repeating the 9HPT during the experiment. If this was the case, the improved motor performance might have lasted longer than the change in motor excitability measured by the MEP amplitudes. Repetitive TMS was reported to interfere with the consolidation of motor learning (Muellbacher et al. 2000). It was likely that motor learning contributed to the 9HPT performance change, especially during the later part of the experiment, because similar enhancement occurred in the left (non-target) hand at 40 min post-PBS targeting the left M1.
Alternatively, the neural circuits responsible for the 9HPT performance might only partially overlap with the cortico-spinal neurons associated with the MEP generation. If so, the MEP amplitudes and motor performance of the 9HPT might reflect the different aspects of human motor function, and could behave differently following PBS.
For the cRT task, we found that PBS targeting the right M1 produced a shortening of the RT, which was consistent with the study by Rizzo et al. (2009). The change of the RT in the right hand for PBS targeting the left M1 showed a similar tendency, but failed to reach the level of significance. As dexterity should be superior in the side of the dominant hand, it is possible that the behavioural effects of PBS might be more clearly demonstrated for the left hand.
In primate studies, lesions in the primary sensorimotor or motor area have produced specific failures of fine finger movements such as the precision grip (Passingham et al. 1983; Liu & Rouiller, 1999; Murata et al. 2008), suggesting that the M1 hand area is essential for exerting skilled fine movements. From this viewpoint, it is reasonable to assume that the 9HPT is a more sensitive measure of M1 function than the cRT, power grip or pinch force used in the present study.
In human studies, an enhancement in motor performance has been also reported along with the increased cortico-spinal excitability produced by TMS in healthy adults (Pascual-Leone et al. 1998; Butefisch et al. 2004; Kim et al. 2004). These findings are also consistent with the observed effects of PBS in our motor behaviour tests. Previous studies in stroke patients showed that high-frequency repetitive TMS could enhance the cortico-spinal excitability of the affected hemisphere and improve the activities of daily life in the acute stage (Khedr et al. 2005), as well as the accuracy and speed of the affected hand movement in the chronic stage (Kim et al. 2006). The effects of PBS on cortico-spinal excitability and motor behaviour, and especially on hand dexterity, suggest that it might be useful in stroke rehabilitation.
Although neuronal plasticity has been recognized to occur at multiple levels of the central nervous system, the PBS-induced changes in the present experiment were likely to have occurred at the supraspinal level, because the Hmax/Mmax ratio did not change significantly, suggesting that the excitability at the spinal level was insensitive to PBS. Testing the spinal excitability is particularly important for understanding the physiological mechanism of PBS, because ipsilateral projection to the spinal motor neuron has been reported in anatomical and physiological studies (Shahani & Young, 1971; Roby-Brami & Bussel, 1987; Delwaide & Pepin, 1991; Nathan et al. 1996). It is possible that paired bihemispheric cortical stimulation can produce paired afferent inputs to the spinal motor circuits, which might cause plasticity only at the spinal motoneurons that receive descending inputs but are not involved in the generation of H-reflex.
A recent study by Meunier et al. (2007) showed that conventional PAS produced the changes in spinal excitability measured by H-reflex recruitment curves. Thus, it is also possible that MEPs and H-reflexes might not necessarily reflect the same motor neuronal pools. However, Nielsen et al. (1993) and Petersen et al. (2003) reported on the modulation of the H-reflex induced by TMS, suggesting that the TMS recruits the same motor neuronal pool that receives the afferent input from the H-reflex. As we could not find any change in the H-reflex following PBS, it was likely that the contribution of the spinal plasticity might have been smaller for our PBS protocol than for the conventional PAS protocol combining peripheral nerve stimulation and TMS.
Di Lazzaro et al. (2009) reported on the enhancement of the amplitudes of the descending volleys evoked by TMS using cervical epidural recording following the conventional PAS protocol, which provided direct evidence of the plasticity at the cortical level. To understand fully the cortical and spinal contribution to the PBS-induced plasticity, further studies in these comparatively rare patients or animal models will be necessary.
Moreover, the MEP amplitudes as a function of the stimulus intensity (I–O function), which are thought to represent the motor cortical excitability (Ridding & Rothwell, 1997), were affected by PBS for up to 20 min post-PBS. The I–O function reflects not only the size of the population of firing neurons activated by the suprathreshold stimuli, but also the excitability of the neurons produced by the subthreshold stimuli. A change of the I–O function means a change of the excitability of the motor representation, with increasing or decreasing numbers of firing neurons seen at varying stimulus intensities (Ridding & Rothwell, 1997). The change of I–O function in the present study also supported the hypothesis that PBS produced motor cortical plasticity.
The rMT, which reflected the neuronal membrane excitability level in the motor cortex (Mavroudakis et al. 1994; Ziemann et al. 1996; Hallett, 2000), showed a tendency to decrease. By contrast, the SP, the later part of which represented the cortical inhibitory system (Inghilleri et al. 1993; Chen et al. 1999), showed a tendency to increase. However, both failed to reach the level of significance.
PBS seems to share various features with ‘associative LTP’, which has been previously reported in animal studies. Low-frequency presynaptic stimulation coupled with concurrent postsynaptic depolarization was reported to induce LTP in hippocampal slices from rats (Kelso & Brown, 1986; Robinson, 1986; Sastry et al. 1986). In cat studies, pairing the stimulation of various cortical afferents including the callosal system with depolarization (Baranyi & Szente, 1987) or stimulation-induced firing of the postsynaptic cells in the motor cortex (Baranyi & Feher, 1981) produced associative LTP, which required the coupled stimulation of different afferent pathways.
In our current PBS protocol, the plastic changes were induced only when conditioning TMS was applied 15 ms prior to TMS of the target M1, suggesting timing-dependent plasticity according to the Hebbian rule (Hebb, 1949). Rizzo et al. (2009) found LTP-like effects using a similar protocol with an ISI of 8 ms. We found an absence of LTP-like effects at ISIs of 5 and 25 ms, and so the critical time window for LTP might be within this range. Although conventional PAS studies using TMS and peripheral nerve stimulation showed bidirectional plasticity depending on the ISI of two stimuli (Wolters et al. 2003, 2005), we could not find an associative LTD-like effect in the present experimental setting. A previous study also failed to produce an associative LTD-like effect using an ISI of 1 ms (Rizzo et al. 2009). However, there was a non-significant tendency towards LTD-like effects when the conditioning stimulus was applied 5–25 ms after the test stimulus. It was possible that the associative LTD-like effect might have been difficult to observe due to the narrower time window within which the ISI could induce depressive effects.
Although the time interval between the presynaptic input and the postsynaptic firing is critical for the modification of synaptic strength, the stimulus parameters that can effectively produce the associative plasticity might vary depending on the types of synapse studied. Some previous animal experiments reported on the importance of the specific time interval (Dan & Poo, 2004, 2006; Caporale & Dan, 2008). LTP in excitatory postsynaptic potentials (EPSPs) was produced when postsynaptic action potentials (APs) were preceded by 10 ms, while LTD in the EPSPs was produced when the APs were delayed by 10 ms, and critical time window was –100 ms to about ∼100 ms for the AP–EPSP interaction in cortical synapses of rats (Markram et al. 1997). In the cerebellum-like structure of fish, EPSPs were enhanced after postsynaptic spikes preceded EPSPs onset by 8∼90 ms, while EPSPs were depressed after the postsynaptic spikes followed EPSPs onset within 60 ms, as though according to an anti-hebbian rule (Bell et al. 1997). LTP was induced when single presynaptic APs preceded the postsynaptic APs by 15 ms, while LTD was induced when single presynaptic APs were synchronously paired or asynchronously delayed with postsynaptic APs by 25∼200 ms in CA3 pyramidal cells of rats hippocampus (Debanne et al. 1998). LTD was produced when the presynaptic excitatory input arrived between 10 ms before and 25 ms after the postsynaptic firing in layer 4 of the somatosensory cortex of rats (Egger et al. 1999). If the inhibitory presynaptic input arrived 410–510 ms after the onset of the postsynaptic firing, LTP occurred in presynaptic inhibition, whereas LTD occurred if the presynaptic input arrived up to 250 ms after the onset of the postsynaptic firing in the neocortical pyramidal cells of rats (Holmgren & Zilberter, 2001). In cell cultures of hippocampal glutamategic neurons, repetitive postsynaptic spiking within a time window of 20 ms after presynaptic activation resulted in long-term potentiation (LTP), whereas postsynaptic spiking within a window of 20 ms before the repetitive presynaptic activation led to long-term depression (LTD) (Bi & Poo, 1998).
The induction of LTP in the mature M1, especially in the horizontal pathways, might require the additional modulation of the vertical (thalamocortical and corticocortical) inputs (Hess et al. 1996; Hess & Donoghue, 1996). As for the associative LTP-like effects observed in the present study and by Rizzo et al. (2009), TMS applied to the target M1 is known predominantly to activate the cortico-spinal neurons in the M1 transsynaptically (Di Lazzaro et al. 2004). Altering the target M1 activity induced by the direct and indirect cortico-cortical projections connecting both M1 areas (Gerloff et al. 1998) might work as an associative factor to produce LTP-like modulation of the synaptic efficacy in the target M1.
Another possible mechanism for the LTP-like effect of PBS is persistent non-synaptic neuronal change in intrinsic excitability due to the altered membrane conductance, which has been reported in brain tissue from several species during the enhancement of learning and memory (Sah & Bekkers, 1996; Moyer et al. 2000; Saar et al. 2001, 2002; Stackman et al. 2002; Barkai, 2005). Further studies will be necessary to determine whether the PBS-induced plasticity is the same phenomenon as the associative plasticity at the cellular level. Nonetheless, the present results, as well as those of Rizzo et al. (2009), confirmed that the motor threshold of the target M1 was not significantly affected by PBS. The MEP threshold is a general measure of cortico-spinal excitability, and is closely linked to the neuronal and interneuronal membrane excitability, which can be altered by sodium channel blockers (Ziemann et al. 1996).
Rizzo et al. (2009) reported on the asymmetry of the cortico-spinal excitability change using a similar intervention protocol. PBS targeting the right, but not the left, M1 produced LTP-like effects. However, in the present study, there was no significant difference in the effect of PBS. Moreover, the LTP-like effect as a function of the ISI was also similar for the right and left M1. One possible reason for this divergence might have been the difference in the PBS protocol. The frequency of paired stimulation was higher (0.1 vs. 0.05 Hz) and the ISI was longer (15 vs. 8 ms) in our current study. As various inhibitory and excitatory neural circuits are involved in the generation of interhemispheric interaction (Daskalakis et al. 2002; Chen et al. 2003; Lee et al. 2007), PBS using different ISIs might preferentially activate different neuronal populations within and between the M1 areas. In this regard, the neural basis of the LTP-like effects observed in the present study and by Rizzo et al. (2009) might not be exactly the same. Further research evaluating the afferent inhibition or intracortical inhibition/facilitation of short or long latencies will be useful to clarify this point. However, it is reasonable to assume that the asymmetric plasticity of two hemispheres might reflect the quantitative, but not qualitative, differences, and that a sufficiently powerful protocol could produce LTP-like effects in the right and left M1.
As we were able to induce LTP-like effects immediately after the intervention in the left and right M1, it is likely that PBS with an ISI of 15 ms is better at producing plasticity than that with an ISI of 8 ms. The ISI should be a relevant parameter that determines the plasticity, because the effectiveness of associative LTP is often dependent on the spike timing (Hess et al. 1996; Hess & Donoghue, 1996; Bell et al. 1997; Bi & Poo, 1998; Holmgren & Zilberter, 2001). In studies of interhemispheric inhibition using TMS (Ferbert et al. 1992), a double shock with an ISI of 6–20 ms had an inhibitory effect mainly in the M1, possibly through the transcallosal pathway. In addition, the conduction time estimated from the interhemispheric inhibition of the MEP was similar to those investigated by the human transcallosal electroencephalographic response (Cracco et al. 1989) or in patients with cortical myoclonus (Shibasaki et al. 1978; Wilkins et al. 1984; Brown et al. 1991; Hanajima et al. 2001).
In a previous study, Rizzo et al. (2009) showed significant attenuation of the interhemispheric inhibition (IHI) with ISIs of 10 and 40 ms induced by PBS with an ISI of 8 ms, suggesting that the facilitation of MEP amplitudes was due to the reduction of the IHI. Thus, modulation of the IHI is one possible mechanism underlying the PBS-induced plasticity observed in the present study. However, it is also possible that the excitability of a specific subset of intracortical interneurons in the targeted M1 was altered. The interhemispheric projection and various intracortical interneurons are known to interact with each other (Daskalakis et al. 2002; Chen et al. 2003; Lee et al. 2007).
The IHI with an ISI of ∼10 ms was probably due to excitatory inputs from transcallosal fibres to the contralateral cortical inhibitory interneurons, as the transcallosal and descending cortico-spinal tract seem to have different origins in some animal studies (Chang, 1953; Jacobson & Marcus, 1970; Jacobson & Trojanowski, 1974; Catsman-Berrevoets et al. 1980). Moreover, although the cortico-spinal neurons are known to originate from layer V of the M1, the transcallosal neurons of the M1 are localized in layers II–VI (Jacobson & Trojanowski, 1974; Catsman-Berrevoets et al. 1980). So far, direct corticocortical interhemispheric projections to large pyramidal cells in layer V have not been demonstrated in animal studies (Chang, 1953; Jacobson & Marcus 1970).
It is not clear why the associative LTP-like effects in humans, such as PAS or PBS, are induced by the afferent stimulation, which by itself has inhibitory effects on the M1. Median nerve stimulation used in PAS is known to produce afferent inhibition of the MEP at an ISI of ∼20 ms (Inghilleri et al. 1990; Tokimura et al. 2000). In the case of PBS, contralateral M1 stimulation at an ISI of ∼10 ms can cause interhemispheric inhibition (Ferbert et al. 1992; Ni et al. 2008). It is possible that the afferent stimulation might affect the interneuron network in the M1 through multiple synaptic pathways, which might partly involve the excitatory system as well as the inhibitory one. Indeed, it should be noted that the exact timing of the paired stimulation of the conventional PAS (Stefan et al. 2000) or PBS in the present study was longer than the ISI at which the inhibition of the MEP was maximal. Afferent inhibition of the MEP amplitude was reduced when the ISI between the median nerve stimulation and TMS was 25 ms (Kotb et al. 2005). Recently, Ni et al. (2008) reported that the IHI showed inhibitory peaks at ISIs of 10 and 40 ms, with a trough or non-significant inhibition at ∼16 ms.
In non-human primates, the transcallosal connections between motor representations of hand area are known to be sparse (Jenny 1979; Pappas & Strick, 1981; Gould et al. 1986). However, the stimulation of the corpus callosum in the monkey activated the precentral neurons related to finger and wrist movement orthodromically (Matsunami & Hamada, 1984), suggesting that the connections function in the hand M1. Behaviourally, the human corpus callosum might be important for achieving bimanual coupled movements. Patients with a callosotomy or with an acquired callosal lesion exhibited specific impairments in synchronous bilateral hand movement (Serrien et al. 2001; Kennerley et al. 2002; Seitz et al. 2004). However, their unilateral hand movement, or bilateral but different movement in each hand, was preserved as normal. Moreover, Rizzo et al. (2009) demonstrated that the effects of PBS were mainly produced through the transcallosal tract, because a patient with callosal agenesis showed no significant changes of cortico-spinal excitability after the intervention. Therefore, it is likely that the effects of PBS are mediated through the transcallosal tract.
A previous PAS study using median nerve stimulation and TMS reported topographical specificity of the LTP-like effects within the M1, which might have been due to the tight connection of the homologous somatosensory and motor areas (Stefan et al. 2000). However, we found that the MEP amplitudes of the FCR were enhanced when the positioning of the coils was optimized for the APB representation area of the hand M1, suggesting that the effects of PBS were not strictly muscle specific. As cortico-spinal neurons have projections to several nearby muscles (Humphrey et al. 1991), the topographic organization of the motor map might be less strict than that of the somatosensory map.
With regard to the inter-regional site specificity of the LTP-like effects, when the conditioned stimuli were applied over the contralateral parietal area that was not directly connected with the targeted M1, no significant LTP-like effects were produced in the present study. This finding strongly suggests that the stimulation of homotopic areas in both hemispheres is necessary for the induction of changes by PBS. However, if PAS is a general way in which to generate associative LTP-like effects in the human brain, it is still possible that the paired stimulation of the parietal area and the M1 at an appropriate ISI might modulate the cortico-spinal excitability.
A threshold intensity of PBS appeared to be required for conditioning TMS in order to induce the LTP-like effects. An intensity of 90% of the rMT is thought to represent a subthreshold level for producing interhemispheric effects in paired TMS (Ferbert et al. 1992). It was understandable that PBS using the subthreshold conditioning TMS, which had no modulation effects on the other M1 excitability measured by TMS, failed to produce LTP-like effects. It might be necessary to activate the cortical circuits sufficiently strongly to transmit signals to the targeted area through interhemispheric pathways.
In conclusion, we showed that human LTP-like plasticity at the M1 area could be induced by PBS, suggesting that repeated pairings of stimuli applied to the brain could be an effective general tool for producing plasticity. The present PBS protocol with an ISI of 15 ms might have been more powerful than that used in a previous study by Rizzo et al. (2009), because we successfully induced plasticity in both the left and the right M1 to a similar extent. In behavioural and physiological studies in both animals and humans (Rioult-Pedotti et al. 2000), the LTP/LTD is likely to be a physiological mechanism underlying learning and memory. Thus, the associative LTP-like changes induced by PBS prove the importance of associative plasticity in human motor control, and have the potential to influence our motor behaviour by modulating the function of the M1. In particular, this methodology might be clinically applied for the rehabilitation of hemiparetic patients (Hummel & Cohen, 2006) or the treatment of movement disorders (Quartarone et al. 2003, 2008).
Acknowledgments
This study was partly supported by Grant-in-Aid for Scientific Research on Priority Areas (Integrative Brain Research) (20019023) to T.M., by the Strategic Research Program for Brain Sciences (SRPBS) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to T.M., by Grant-in-Aid for Scientific Research (C) 18500239 from the Japan Society for the Promotion of Science to T.M., and by Research Grant (2007) from the Neurocreative Lab to T.M.
Glossary
Abbreviations
- APs
action potentials
- APB
abductor pollicis brevis
- cRT
choice reaction time
- EPSPs
excitatory postsynaptic potentials
- FCR
flexor carpi radialis
- Hmax
maximum H-reflex amplitude
- 9HPT
nine-hole peg test
- I–O
input–output
- IHI
interhemispheric inhibition
- ISI
interstimulus interval
- LTD
long-term depression
- LTP
long-term potentiation
- M1
primary motor cortex
- MEP
motor evoked potential
- Mmax
maximum M-wave amplitude
- PAS
paired associative stimulation
- PBS
paired bihemispheric stimulation
- rMT
resting motor threshold
- RT
reaction time
- SI
stimulus intensity
- SI 1 mV
stimulus intensity required to produce a motor evoked potential of ∼1 V
- SP
silent period
- TMS
transcranial magnetic stimulation
Author contributions
S.K. contributed to the conception and design of the study, performed the analysis and interpretation of the data, drafted the article and approved the final version. T.M., M.N., Y.U., H.F. and K.D. contributed to the analysis and interpretation of the data, critically reviewed the intellectual content and approved the final version.
References
- Baranyi A, Feher O. Synaptic facilitation requires paired activation of convergent pathways in the neocortex. Nature. 1981;290:413–415. doi: 10.1038/290413a0. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Szente MB. Long-lasting potentiation of synaptic transmission requires postsynaptic modifications in the neocortex. Brain Res. 1987;423:378–384. doi: 10.1016/0006-8993(87)90867-5. [DOI] [PubMed] [Google Scholar]
- Barkai E. Dynamics of learning-induced cellular modifications in the cortex. Biol Cybern. 2005;92:360–366. doi: 10.1007/s00422-005-0564-0. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Brown P, Day BL, Rothwell JC, Thompson PD, Marsden CD. Intrahemispheric and interhemispheric spread of cerebral cortical myoclonic activity and its relevance to epilepsy. Brain. 1991;114:2333–2351. doi: 10.1093/brain/114.5.2333. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Catsman-Berrevoets CE, Lemon RN, Verburgh CA, Bentivoglio M, Kuypers HG. Absence of callosal collaterals derived from rat corticospinal neurons. A study using fluorescent retrograde tracing and electrophysiological techniques. Exp Brain Res. 1980;39:433–440. doi: 10.1007/BF00239308. [DOI] [PubMed] [Google Scholar]
- Chang HT. Cortical response to activity of callosal neurons. J Neurophysiol. 1953;16:117–131. doi: 10.1152/jn.1953.16.2.117. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:417–424. doi: 10.1016/0168-5597(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–48. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol. 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Pepin JL. The influence of contralateral primary afferents on Ia inhibitory interneurones in humans. J Physiol. 1991;439:161–179. doi: 10.1113/jphysiol.1991.sp018662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F, Tonali PA. Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex. 2009 doi: 10.1093/cercor/bhn255. Epub ahead of print DOI 10.1093/cercor/bhn255. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Egger V, Feldmeyer D, Sakmann B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat Neurosci. 1999;2:1098–1105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Okabe S, Yuasa K, Shiio Y, Iwata NK, Kanazawa I. Interhemispheric interaction between the hand motor areas in patients with cortical myoclonus. Clin Neurophysiol. 2001;112:623–626. doi: 10.1016/s1388-2457(01)00477-1. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behaviour: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Wars) 1996;56:397–405. doi: 10.55782/ane-1996-1143. [DOI] [PubMed] [Google Scholar]
- Holmgren CD, Zilberter Y. Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J Neurosci. 2001;21:8270–8277. doi: 10.1523/JNEUROSCI.21-20-08270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Humphrey DR, Freund HJ, Freie Universität Berlin, Berlin (Germany: West) Senat & Stifterverband für die Deutsche Wissenschaft . Motor Control: Concepts and Issues: Report of the Dahlem Workshop on Motor Control: Concepts and Issues, Berlin, 1989, December 3–8. Chichester, New York: Wiley; 1991. [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Priori A, Manfredi M. Motor potentials evoked by paired cortical stimuli. Electroencephalogr Clin Neurophysiol. 1990;77:382–389. doi: 10.1016/0168-5597(90)90060-q. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Marcus EM. The laminar distribution of fibres of the corpus callosum: a comparative study in the rat, cat, rhesus monkey and chimpanzee. Brain Res. 1970;24:517–520. doi: 10.1016/0006-8993(70)90189-7. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Trojanowski JQ. The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res. 1974;74:149–155. doi: 10.1016/0006-8993(74)90118-8. [DOI] [PubMed] [Google Scholar]
- Jenny AB. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol. 1979;188:137–145. doi: 10.1002/cne.901880111. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Brown TH. Differential conditioning of associative synaptic enhancement in hippocampal brain slices. Science. 1986;232:85–87. doi: 10.1126/science.3952501. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB. Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci. 2002;5:376–381. doi: 10.1038/nn822. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett. 2004;367:181–185. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, Yoo WK, Hallett M. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Koganemaru S, Mima T, Ueki Y, Nakatuska M, Fukuyama H, Domen K. Induction of plasticity in the human motor cortex by modified paired associative stimulation through transcallosal tract. Brain Stimulat. 2008;1:283. [Google Scholar]
- Kotb MA, Mima T, Ueki Y, Begum T, Khafagi AT, Fukuyama H, Nagamine T. Effect of spatial attention on human sensorimotor integration studied by transcranial magnetic stimulation. Clin Neurophysiol. 2005;116:1195–1200. doi: 10.1016/j.clinph.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R. The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol. 2007;580:1021–1032. doi: 10.1113/jphysiol.2006.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- Matsunami K, Hamada I. Effects of stimulation of corpus callosum on precentral neuron activity in the awake monkey. J Neurophysiol. 1984;52:676–691. doi: 10.1152/jn.1984.52.4.676. [DOI] [PubMed] [Google Scholar]
- Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of diphenylhydantoin on motor potentials evoked with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:428–433. doi: 10.1016/0168-5597(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Meunier S, Russmann H, Simonetta-Moreau M, Hallett M. Changes in spinal excitability after PAS. J Neurophysiol. 2007;97:3131–3135. doi: 10.1152/jn.01086.2006. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20:5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behaviour. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Murata Y, Higo N, Oishi T, Yamashita A, Matsuda K, Hayashi M, Yamane S. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J Neurophysiol. 2008;99:773–786. doi: 10.1152/jn.01001.2007. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996;119:1809–1833. doi: 10.1093/brain/119.6.1809. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex. 2008;19:1654–1665. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol. 1993;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Pekny M. Enriched environment and astrocytes in central nervous system regeneration. J Rehabil Med. 2007;39:345–352. doi: 10.2340/16501977-0084. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003;41:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pappas CL, Strick PL. Anatomical demonstration of multiple representation in the forelimb region of the cat motor cortex. J Comp Neurol. 1981;200:491–500. doi: 10.1002/cne.902000404. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Perry VH, Wilkinson F. The long-term effects of removal of sensorimotor cortex in infant and adult rhesus monkeys. Brain. 1983;106:675–705. doi: 10.1093/brain/106.3.675. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Pyndt HS, Nielsen JB. Investigating human motor control by transcranial magnetic stimulation. Exp Brain Res. 2003;152:1–16. doi: 10.1007/s00221-003-1537-y. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant’angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HS, Morgante F, Mastroeni C, Girlanda P, Quartarone A. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb Cortex. 2009;19:907–915. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- Robinson GB. Enhanced long-term potentiation induced in rat dentate gyrus by coactivation of septal and entorhinal inputs: temporal constraints. Brain Res. 1986;379:56–62. doi: 10.1016/0006-8993(86)90254-4. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Long-latency spinal reflex in man after flexor reflex afferent stimulation. Brain. 1987;110:707–725. doi: 10.1093/brain/110.3.707. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Altamura C, Ferreri F, Melgari JM, Tecchio F, Tombini M, Pasqualetti P, Vernieri F. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eura Medicophys. 2007;43:241–254. [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Long-lasting cholinergic modulation underlies rule learning in rats. J Neurosci. 2001;21:1385–1392. doi: 10.1523/JNEUROSCI.21-04-01385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Learning-induced enhancement of postsynaptic potentials in pyramidal neurons. J Neurophysiol. 2002;87:2358–2363. doi: 10.1152/jn.2002.87.5.2358. [DOI] [PubMed] [Google Scholar]
- Sah P, Bekkers JM. Apical dendritic location of slow after hyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci. 1996;16:4537–4542. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry BR, Goh JW, Auyeung A. Associative induction of posttetanic and long-term potentiation in CA1 neurons of rat hippocampus. Science. 1986;232:988–990. doi: 10.1126/science.3010459. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Kleiser R, Butefisch CM, Jorgens S, Neuhaus O, Hartung HP, Wittsack HJ, Sturm V, Hermann MM. Bimanual recoupling by visual cueing in callosal disconnection. Neurocase. 2004;10:316–325. doi: 10.1080/13554790490505373. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Wiesendanger M. Role of the corpus callosum in bimanual coordination: a comparison of patients with congenital and acquired callosal damage. Eur J Neurosci. 2001;14:1897–1905. doi: 10.1046/j.0953-816x.2001.01798.x. [DOI] [PubMed] [Google Scholar]
- Shahani BT, Young RR. Human flexor reflexes. J Neurol Neurosurg Psychiatry. 1971;34:616–627. doi: 10.1136/jnnp.34.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, Yamashita Y, Kuroiwa Y. Electroencephalographic studies myoclonus. Brain. 1978;101:447–460. doi: 10.1093/brain/101.3.447. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, Begum T, Reza F, Nagamine T, Fukuyama H. Altered plasticity of the human motor cortex in Parkinson's disease. Ann Neurol. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- Wilkins DE, Hallett M, Berardelli A, Walshe T, Alvarez N. Physiologic analysis of the myoclonus of Alzheimer's disease. Neurology. 1984;34:898–903. doi: 10.1212/wnl.34.7.898. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]