Abstract
Major cardiovascular changes occur at birth, including increased pulmonary blood flow (PBF) and closure of the ductus arteriosus (DA), which acts as a low resistance shunt between the fetal pulmonary and systemic circulations. Although the pressure gradient between these circulations reverses after birth, little is known about DA blood flow changes and whether reverse DA flow contributes to PBF after birth. Our aim was to describe the changes in PBF and DA flow before, during and after the onset of pulmonary ventilation at birth. Flow probes were implanted on the left pulmonary artery (LPA) and DA in preterm fetal sheep (n= 8) ∼3 days before they were delivered and ventilated. Blood flow was measured in the LPA and DA, before and after umbilical cord occlusion (UCO) and for 2 h after ventilation onset. Following UCO, DA flow decreased from 534 ± 57 ml min−1 to 237 ± 29 ml min−1 which reflected a similar reduction in right ventricular output. Within 5 min of ventilation onset, PBF increased from 11 ± 6 ml min−1 to 230 ± 13 ml min−1 whereas DA flow decreased to −172 ± 54 ml min−1; negative values indicate reverse DA flow (left-to-right shunting). Reverse flow through the DA contributed up to 50% of total PBF at 30 min and a decrease in this contribution accounted for 71 ± 13% of the time-related decrease in PBF after birth. DA blood flow is very dynamic after birth and depends upon the pressure gradient between the pulmonary and systemic circulations. Following ventilation, reverse DA flow provided a significant contribution to total PBF after birth.
The transition to air-breathing at birth requires substantial changes in cardiovascular physiology, particularly a large increase in pulmonary blood flow (PBF) and closure of vascular shunts that allow blood to by-pass the lungs (Rudolph, 1979, 1985; Reid & Thornburg, 1990; Teitel et al. 1990; Friedman & Fahey, 1993; Heymann, 1999). In the fetus, almost 90% of right ventricular output (RVO) by-passes the lungs and enters the systemic circulation via the ductus arteriosus (DA), which connects the main pulmonary artery with the descending thoracic aorta (Fig. 1). Flow through the DA is determined by the pressure gradient across it and, as pressure in the fetal pulmonary circulation is ∼5 mmHg above systemic arterial pressure (Rudolph, 1979; Reid & Thornburg, 1990; Hooper, 1998), blood flows from the pulmonary circulation through the DA and into the systemic circulation, referred to as right-to-left shunting. In the fetus, pulmonary arterial pressure is high and PBF is low because pulmonary vascular resistance (PVR) is high (Rudolph, 1979; Reid & Thornburg, 1990). The high PVR, in combination with the presence of the DA, confers unique characteristics to the blood flow waveform in the left and right pulmonary arteries downstream from the junction with the DA (Rudolph, 1979; Reid & Thornburg, 1990; Polglase et al. 2004, 2005). In particular, forward flow (towards the lungs) only occurs briefly during systole, whereas throughout most of diastole, flow is retrograde with blood flowing away from the lungs and exiting the pulmonary circulation via the DA (Rudolph, 1979; Reid & Thornburg, 1990; Polglase et al. 2004, 2005).
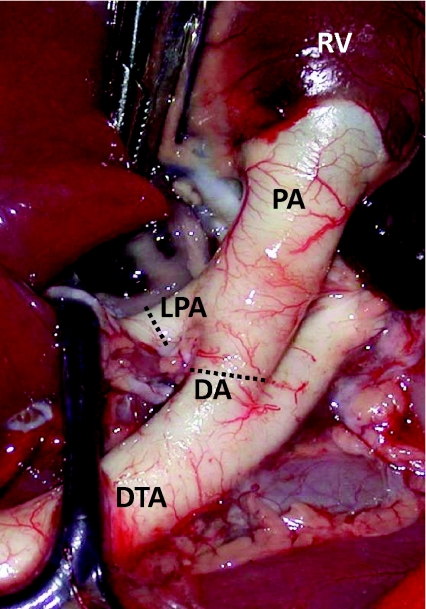
Figure 1. An image from a fetal sheep demonstrating the anatomy of the ductus arteriosus (DA) in relation to the main pulmonary artery (PA), the left pulmonary artery (LPA), the right ventricle (RV) and descending thoracic aorta (DTA).
Flow probes were placed around the LPA and the DA, at the positions indicated by the dotted lines, for the simultaneous measurement of blood flow in the LPA and DA, respectively.
The successful transition to pulmonary gas exchange at birth is largely dependent on airway liquid clearance and a large decrease in PVR so that the entire output of the right ventricle can pass through the lungs (Friedman & Fahey, 1993; Hooper & Harding, 2005). As a result, the DA must close to prevent blood by-passing the lungs and to separate the pulmonary and systemic circulations, allowing mean pulmonary arterial pressure to decrease towards adult levels (∼15 mmHg) (West, 2003). However, any reduction in pulmonary arterial pressure while the DA remains patent must result in blood flow from the systemic into the pulmonary circulation through the DA; this is referred to as left-to-right shunting. Patency of the DA is relatively common in infants born prematurely or with pulmonary hypoplasia and manifests as either right-to-left (particularly in infants with pulmonary hypertension) or left-to-right shunting (Kluckow & Evans, 2000; Clyman, 2006; Hermes-DeSantis & Clyman, 2006). However, relatively little is known of the factors controlling the shunting of blood through the DA after birth or the effect that a patent DA and dynamic changes in the direction of shunting has on pulmonary and cardiovascular function in the newborn.
Although it is well established that PBF increases after birth due to a large decrease in PVR (Rudolph, 1979; Heymann, 1999), the relative contributions of the right-ventricle and left-to-right shunting through the DA (blood derived from the left ventricle) to the increase in PBF after birth are unknown. Furthermore, the dynamics of the changes in the direction of blood flow through the DA after birth are also unknown. We have previously demonstrated that, following the large increase in PBF immediately after birth, PBF gradually decreases by ∼50% within 2 h of ventilation onset in preterm lambs (Polglase et al. 2005, 2008, 2009; Crossley et al. 2007). In this study, we hypothesized that a rapid reversal of the pressure gradient across the DA reverses flow through the DA (causing left-to-right shunting) following ventilation onset and that left-to-right flow through the DA significantly contributes to the increase in PBF after birth. We also hypothesised that the gradual decrease in PBF following ventilation onset is largely due to reduced left-to-right flow through the DA caused by its partial closure. To test these hypotheses, we have simultaneously measured blood flow in the left pulmonary artery (LPA) and the DA before, during and after delivery and with the onset of mechanical ventilation in preterm lambs.
Methods
Experimental protocol
All experimental procedures on animals were approved by the Monash University Animal Ethics Committee, according to the guidelines of the National Health and Medical Research Council of Australia Code of Practice for the Care and Use of Animals for Scientific Purposes. Aseptic surgery was conducted on eight pregnant ewes (Border–Leicester × Merino) at ∼125 ± 2 days of gestation (term is ∼147 days) as described previously (Polglase et al. 2004). Anaesthesia was induced via an intravenous bolus of 5% sodium thiopentone (Pentothal; 1 g in 20 ml) and, following intubation, anaesthesia was maintained with inhalation of 1.5–3% halothane in O2. Antibiotics (1 g of ampicillin, i.v.) were also administered to the ewe prior to any incisions and the ewes were mechanically ventilated throughout surgery. Post-operative analgesia was maintained for 3 days using transdermal fentanyl patches (75 ug h−1; Janssen-Cilag, North Ryde, NSW, Australia). Catheters were inserted into a fetal carotid artery and jugular vein, the amniotic sac and left pulmonary artery; the tip of the carotid artery catheter was located just distal of the junction with the aortic arch. Ultrasonic flow probes (4 mm; Transonic Systems; Ithaca, NY, USA) were placed around the left pulmonary artery and the DA. Surgery was performed prenatally, rather than following caesarean delivery, to avoid extensive thoracic surgery after birth, which could have influenced the respiratory and cardiovascular measurements made before and following ventilation onset.
Before experimentation, fetal well-being was monitored daily by measuring fetal arterial 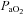 ,
, 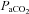 , pH and per cent oxygen saturation of haemoglobin
, pH and per cent oxygen saturation of haemoglobin 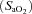 (ABL30, Radiometer, Denmark). Instantaneous blood flows in the LPA and the DA were recorded digitally using a data acquisition system (Powerlab; ADInstruments, Castle Hill, Australia). Arterial pressures were measured using pressure transducers (PD10; DTX Plus Transducer; Becton Dickinson, Singapore) and also recorded digitally.
(ABL30, Radiometer, Denmark). Instantaneous blood flows in the LPA and the DA were recorded digitally using a data acquisition system (Powerlab; ADInstruments, Castle Hill, Australia). Arterial pressures were measured using pressure transducers (PD10; DTX Plus Transducer; Becton Dickinson, Singapore) and also recorded digitally.
At 128 ± 2 days of gestation, ewes and fetuses were anaesthetised by an intravenous bolus of 5% sodium thiopentone (20 ml Pentothal) and was maintained following intubation with 1.5–3% halothane in O2 throughout delivery. The fetal head and neck were exposed via hysterotomy and the trachea was intubated with a 3.5 mm cuffed tube and lung liquid drained passively before the umbilical cord was clamped and cut. The lambs were then delivered, dried, weighed, placed under a radiant heater and ventilated with a Babylog 8000+ ventilator (Dräger, Lübeck, Germany) using volume guarantee mode with a set expired tidal volume (VT) of 5 ml kg−1, at 60 inflations min−1, a variable fraction of inspired oxygen 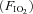 and a positive end expiratory pressure (PEEP) of 4 cmH2O as previously described (Probyn et al. 2004; Polglase et al. 2005). The inspiratory and expiratory times and
and a positive end expiratory pressure (PEEP) of 4 cmH2O as previously described (Probyn et al. 2004; Polglase et al. 2005). The inspiratory and expiratory times and 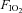 were altered to maintain arterial
were altered to maintain arterial 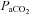 between 40 and 55 mmHg and
between 40 and 55 mmHg and 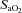 between 90 and 95%. Lambs received 5% dextrose infusion (i.v.) and were sedated (pentobarbitone, i.v.) to prevent spontaneous breathing. All lambs were ventilated for 120 min after birth; mean delivery weight was 3.3 ± 0.2 kg and the gender distribution was three males and five females. Following delivery of the lamb, the ewe was killed using an overdose of sodium pentobarbitone (130 mg kg−1, i.v.), while it was still under general anaesthesia.
between 90 and 95%. Lambs received 5% dextrose infusion (i.v.) and were sedated (pentobarbitone, i.v.) to prevent spontaneous breathing. All lambs were ventilated for 120 min after birth; mean delivery weight was 3.3 ± 0.2 kg and the gender distribution was three males and five females. Following delivery of the lamb, the ewe was killed using an overdose of sodium pentobarbitone (130 mg kg−1, i.v.), while it was still under general anaesthesia.
Analytical methods
Mean PBF and DA flow, heart rate (HR), blood pressures in both the left pulmonary and carotid arteries and pulse height of the blood flow waveform in both PA and DA were averaged over a 10 s period before and after the umbilical cord occlusion (UCO) and at 30 s, 1 min, 2 min, 5 min, 10 min, 30 min, 1 h and 2 h after the onset of ventilation. Periods of recording with obvious artefacts (e.g. caused by blood sampling or body movement) were avoided during these times. Total PBF was calculated by assuming that flow in the left pulmonary artery equalled 40% of total PBF, which is based on the weight difference between the right and left lungs (Moessinger et al. 1990; Keramidaris et al. 1996). RVO was then calculated by summing DA flow and total PBF, whereas the percentage contribution of DA flow to total PBF after birth was calculated by expressing DA flow as a percentage of total PBF when mean DA flow was left to right (i.e. at 3 min after ventilation onset, see below).
Statistical analysis
One-way repeated measures ANOVAs were used to analyse the changes in mean PBF and DA flow, percentage of PBF derived from the DA, heart rate and DA and PBF waveform pulse heights with time after birth. Two-way repeated measures ANOVAs were used to analyse the time-related changes in pressure and differences between the carotid and pulmonary arterial pressures. Where significant effects were found, a least significant difference (LSD) post hoc test was used to detect differences between groups and time points. Data in the text are presented as the mean ±s.e.m. The level of statistical significance was P < 0.05 for all statistical analyses.
Results
Before ventilation onset
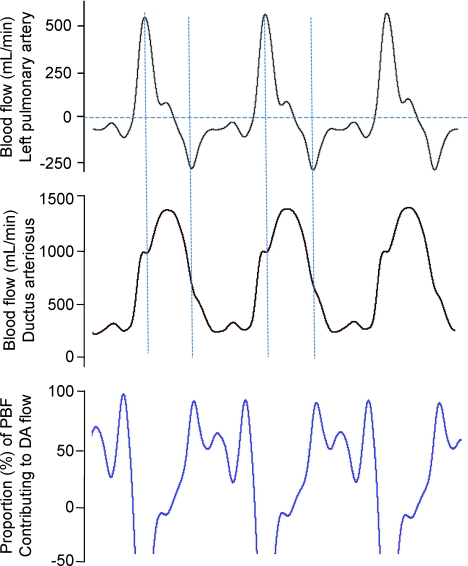
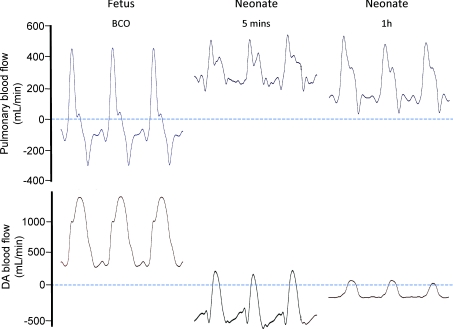
During fetal life (before UCO) blood flow in the DA was significantly greater (534 ± 57 ml min−1) than PBF (11 ± 6 ml min−1), with forward flow occurring throughout the cardiac cycle (Figs 2 and 3). In contrast, PBF was directed towards the lungs only briefly during systole, whereas throughout diastole, blood flowed away from the lungs (retrograde, assigned negative value), thereby contributing to flow in the DA (Fig. 3). Assuming that all retrograde flow in the PA exits the pulmonary circulation via the DA during diastole, up to 100% of flow in the DA during diastole was derived from the pulmonary circulation, although this contribution to DA flow was very variable (Fig. 3). The finding of continuous forward flow (right to left) through the DA is consistent with the findings of higher arterial pressures in the LPA compared to the carotid artery (Fig. 4; Table 1).
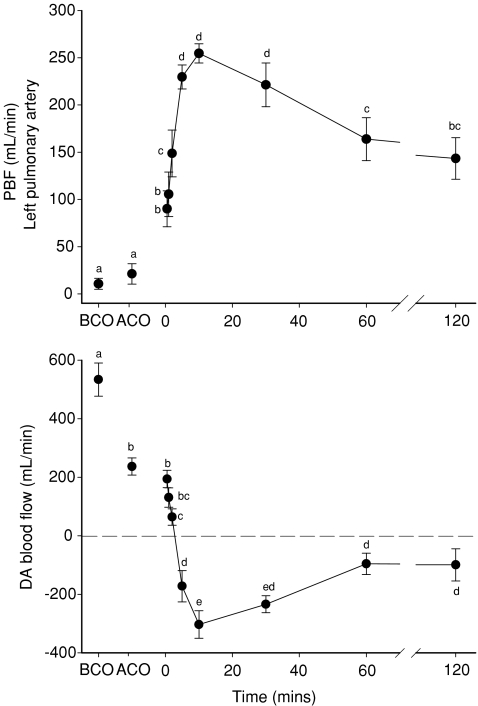
Figure 2. Mean blood flow, averaged over a 10 s period, measured in the left pulmonary artery (top panel) and in the ductus arteriosus (DA; bottom panel) before umbilical cord occlusion (BCO), after cord occlusion (ACO) and at selected intervals after ventilation onset (designated as time 0).
For each panel, values that do not share a common letter are significantly different (P < 0.05) from each other. For DA blood flow, positive values reflect blood flow from the pulmonary artery into the aorta (right-to-left shunting), whereas negative values reflect blood flow from the aorta into the pulmonary circulation (left-to-right shunting).
Figure 3.
Instantaneous measures of blood flow in the left pulmonary artery (top panel) and ductus arteriosus (middle panel) acquired over 3 consecutive cardiac cycles from a fetal sheep. The bottom panel shows the percentage of total pulmonary blood flow (PBF) contributing to right-to-left (from pulmonary into systemic circulation) flow of blood through the ductus arteriosus. Negative PBF values (top panel) indicate retrograde flow of blood away from the lungs.
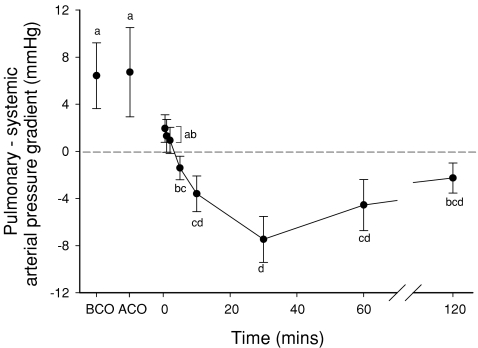
Figure 4. The pressure gradient between the pulmonary and systemic circulations averaged over a 10 s period and measured before umbilical cord occlusion (BCO), after cord occlusion (ACO) and selected intervals after the onset of ventilation (indicated as time 0).
Values that do not share a common letter are significantly different (P < 0.05) from each other.
Table 1.
Mean heart rate (HR, beats min−1), right ventricular output (RVO, ml min−1), pulmonary arterial pressure (PAP, mmHg), systemic arterial pressure (SAP, mmHg), amplitude of the pulmonary blood flow (PBF) waveform (PBF pulse, ml min−1) and amplitude of the ductus arteriosus (DA) blood flow waveform (DA pulse) measured before umbilical cord occlusion (BCO), after umbilical cord occlusion (ACO) and at 0.5, 1, 2, 5, 10, 30, 60 and 120 min after ventilation onset
| Time | HR (beats min−1) | RVO (ml min−1) | PAP (mmHg) | SAP (mmHg) | PBF pulse (ml min−1) | DA pulse (ml min−1) |
|---|---|---|---|---|---|---|
| BCO | 167 ± 9 | 623 ± 55a | 55.6 ± 4.2a | 49.4 ± 4.9 | 707 ± 62a | 794 ± 68a |
| ACO | 166 ± 18 | 315 ± 14b | 56.3 ± 2.9a | 49.6 ± 3.7 | 443 ± 58b | 791 ± 40a |
| 0.5 | 188 ± 17 | 406 ± 65b | 55.4 ± 4.0a | 53.5 ± 4.0 | 547 ± 73b | 1043 ± 106b |
| 1 | 191 ± 15 | 398 ± 66b | 56.2 ± 4.7a | 54.9 ± 4.7 | 494 ± 72b | 1069 ± 124b |
| 2 | 194 ± 10 | 434 ± 54b | 53.9 ± 5.0a | 52.9 ± 5.1 | 470 ± 74b | 1078 ± 103b |
| 5 | 193 ± 13 | 405 ± 50b | 53.8 ± 4.8a | 55.2 ± 5.3 | 426 ± 47b | 1061 ± 69b |
| 10 | 181 ± 10 | 287 ± 45b | 49.6 ± 5.7ab | 53.2 ± 5.5 | 428 ± 36b | 911 ± 109ab |
| 30 | 166 ± 11 | 317 ± 59b | 37.4 ± 3.5b | 44.9 ± 3.2 | 473 ± 34b | 379 ± 103c |
| 60 | 152 ± 14 | 336 ± 64b | 42.7 ± 4.1b | 47.3 ± 2.4 | 468 ± 43b | 368 ± 90c |
| 120 | 160 ± 8 | 263 ± 40b | 42.7 ± 3.2ab | 45.0 ± 2.6 | 477 ± 55b | 457 ± 92c |
Values that do not share a common letter within the same column are significantly different (P < 0.05) from each other.
Immediately following cord occlusion, mean DA blood flow was significantly reduced from 534 ± 57 ml min−1 to 237 ± 29 ml min−1 (Fig. 2), whereas PBF tended to increase (from 11 ± 6 ml min−1 to 21 ± 11 ml min−1), but this was not significant. In contrast, the amplitude of the blood flow waveform in the left pulmonary artery was significantly reduced whereas the pulse height of the DA blood flow wave form was unaltered (Table 1). The reduction in DA blood flow caused by UCO was also associated with a large reduction in RVO (Table 1). However, the pressure gradient between the pulmonary and carotid arteries was not altered by UCO (Fig. 4; Table 1).
Immediately following ventilation onset
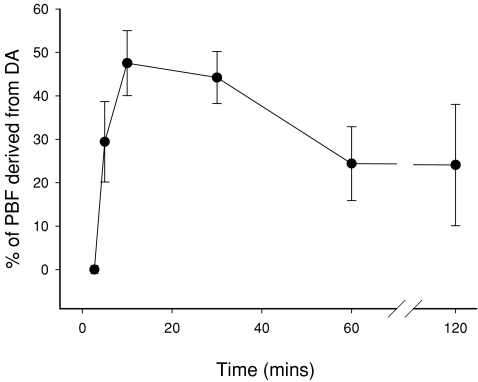
Immediately after ventilation began, PBF rapidly increased from 21 ± 11 ml min−1 to 90 ± 19 ml min−1 within 30 s and increased further to 255 ± 10 ml min−1 within 10 min (Fig. 2). This increase in PBF coincided with a major vertical shift and change in shape of the PBF waveform, resulting in forward flow towards the lungs throughout the cardiac cycle (Fig. 5). In contrast, following a small delay (30 s to 1 min), mean DA flow was markedly reduced from 237 ± 29 ml min−1 (right-to-left direction) to −303 ± 47 ml min−1 after 10 min of ventilation (Fig. 2); a negative value reflects left-to-right shunting (from systemic towards the pulmonary circulation) through the DA. These blood flow changes caused a major vertical shift in the DA blood flow waveform, resulting in left-to-right shunting throughout the cardiac cycle, except for a brief period during systole (Fig. 5). As a result, left-to-right shunting through the DA provided a significant contribution to the increase in PBF after birth. Left-to-right shunting began to significantly contribute to PBF after 3.0 ± 0.5 min of ventilation and rapidly increased to 47.5 ± 8.6% of total PBF within 10 min (Fig. 6).
Figure 5. Examples of the blood flow wave forms recorded in the left pulmonary artery (top panel) and in the ductus arteriosus (DA) in the fetus (before umbilical cord occlusion; BCO) and in the neonate at 5 min and 1 h after the onset of ventilation.
Negative values of pulmonary blood flow indicate retrograde flow away from the lungs, whereas negative blood flow values through the DA indicate flow that is directed from the systemic (aorta) into the pulmonary circulation.
Figure 6. The percentage of total pulmonary blood flow (PBF) that is derived from left-to-right flow (from the systemic into the pulmonary circulation) through the ductus arteriosus (DA) before umbilical cord occlusion (BCO), after umbilical cord occlusion and at selected intervals after the onset of pulmonary ventilation (designated as time 0).
Values that do not share a common letter are significantly different (P < 0.05) from each other.
Immediately following ventilation onset, the pressure gradient between the pulmonary and carotid arteries was reduced, as pressures in both vessels were similar (Fig. 4; Table 1). However, after 10 min of ventilation, mean pulmonary arterial pressure tended to be lower than mean carotid arterial pressure and by 30 min mean pulmonary arterial pressure was significantly lower than mean carotid arterial pressure (Table 1; Fig. 4).
Time-related changes following ventilation onset
The ventilation-induced increase in mean PBF was maximal at 10 min (255 ± 10 ml min−1), tended to be reduced at 30 min (221 ± 23 ml min−1) and was significantly reduced (P < 0.001 compared to the 10 min value) at 60 min (164 ± 23 ml min−1) and 120 min (144 ± 22 ml min−1; Fig. 2). Similarly, following the ventilation-induced reversal in direction of DA blood flow (from right-to-left to left-to-right flow), the amount of left-to-right shunting through the DA was significantly reduced from a maximum of –303 ± 47 ml min−1 at 10 min to −96 ± 37 ml min−1 at 60 min and −99 ± 55 ml min−1 at 120 min (Fig. 2). As a result, the contribution of DA flow to PBF flow was reduced from 47.5 ± 8.6% of total PBF at 10 min to 24.4 ± 8.5% at 60 min and 24.1 ± 14.0% at 120 min (Fig. 6). Thus, left-to-right shunting of blood through the DA remained a significant contributor to PBF throughout the ventilation period (Fig. 2).
Although the pulse amplitude of the DA flow waveform was initially increased after ventilation began, it was significantly reduced at 30 min (Table 1) whereas the PBF pulse height was not significantly altered following UCO. Similarly, although the fetal heart rate tended to increase when ventilation began and then decrease with ventilation time, there were no significant changes in heart rate throughout the experimental period (Table 1).
Following the onset of ventilation, mean pulmonary arterial pressures gradually decreased to a minimum at 30 min and remained low at 60 and 120 min (Table 1), whereas carotid mean arterial pressures were not significantly altered throughout the experimental period. As a result, the pressure gradient between the pulmonary and systemic circulations was reversed, compared with before ventilation (Fig. 4), decreasing from a mean of 6.7 ± 3.8 mmHg (pulmonary > systemic mean arterial pressure) before ventilation to –7.5 ± 2.0 mmHg (systemic > pulmonary mean arterial pressure) after 30 min of ventilation (Fig. 4). The pressure gradient between the pulmonary and systemic circulations gradually reduced with time (Fig. 4); although systemic arterial pressures remained significantly above pulmonary arterial pressures after 60 min (−4.6 ± 2.2 mmHg), they were not after 120 min (−2.3 ± 1.3 mmHg; P= 0.09) of ventilation.
Blood gas changes
The onset of ventilation significantly altered arterial pH (decreased), 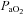 (increased) and
(increased) and 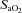 (increased) levels, compared with fetal values, but none of these blood gas parameters were significantly altered throughout the 2 h ventilation period (Table 2).
(increased) levels, compared with fetal values, but none of these blood gas parameters were significantly altered throughout the 2 h ventilation period (Table 2).
Table 2.
Systemic arterial (pre-ductal) blood gas values measured before umbilical cord occlusion (fetal) and at 5, 20, 60 and 120 min after ventilation onset
| Time | pH |
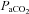 (mmHg) (mmHg) |
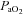 (mmHg) (mmHg) |
Saturation (%) |
|---|---|---|---|---|
| Fetal | 7.36 ± 0.01* | 44.1 ± 2.8 | 21.1 ± 1.1* | 59.1 ± 2.3* |
| 5 min | 7.21 ± 0.03 | 52.6 ± 5.8 | 76.5 ± 16.7 | 93.3 ± 3.8 |
| 20 min | 7.24 ± 0.02 | 45.7 ± 5.8 | 63.0 ± 15.9 | 94.0 ± 3.6 |
| 60 min | 7.25 ± 0.03 | 51.2 ± 6.2 | 41.4 ± 5.8 | 88.5 ± 4.2 |
| 120 min | 7.24 ± 0.02 | 52.3 ± 5.4 | 43.5 ± 5.1 | 88.7 ± 4.2 |
Values indicated by
are significantly different to all other time points within the same column (P < 0.001).
Discussion
The findings of this study demonstrate that the amount and direction of blood flow through the DA (between the pulmonary and systemic circulations) is very dynamic immediately after birth and follows the pressure gradient between the two circulations. During fetal life, mean pulmonary arterial pressures were 6.4 ± 2.3 mmHg above systemic arterial pressures and so flow through the DA was exclusively right to left (from pulmonary into the systemic circulation) throughout the cardiac cycle (Fig. 3). However, after birth, the decrease in PVR associated with the onset of ventilation rapidly (within 3 min) altered the direction of blood flow through the DA, causing the majority of flow to be left-to-right in direction. Right-to-left flow through the DA only occurred briefly during systole and the reversal in mean flow was closely associated with a reversal in the pressure gradient between the pulmonary and systemic circulations. As a result, the contribution of left-to-right shunting of blood through the DA, which originates from the left ventricle, provided almost 50% of flow to total PBF by 10 min after birth. Although the relative contribution of left-to-right shunting to PBF gradually declined with time, it remained a significant contributor to total PBF up to 2 h after birth. However, the time-related reduction in the contribution of left-to-right shunting through the DA to PBF accounted for 71.3 ± 13.2% (at 2 h) of the reduction in PBF that is commonly observed during ventilation after preterm birth (Polglase et al. 2005, 2008, 2009; Crossley et al. 2007).
Before ventilation onset
During fetal life, the retrograde PBF (away from the lungs) that occurs throughout most of diastole (Fig. 2) has been well described (Reid & Thornburg, 1990; Heymann, 1999; Polglase et al. 2004) and the blood is thought to exit the pulmonary circulation and enter the systemic circulation via the DA (Rudolph, 1979). However, simultaneous measures of PBF and DA blood flow have not been reported previously, in either the fetus or newborn. Thus, the relative contributions of retrograde PBF to DA flow prenatally and the contribution of left-to-right DA flow to PBF postnatally, was unknown. Our study shows that, in the fetus, blood flows through the DA from right to left throughout the cardiac cycle, which is a consequence of the sustained ∼5 mmHg pressure gradient between the pulmonary and systemic circulations (Fig. 4). Furthermore, our study shows that retrograde flow exiting the pulmonary circulation during diastole significantly contributes (up to 100%; Fig. 3) to flow through the DA and accounts for most of the continued flow during diastole. Retrograde PBF during diastole is thought to result from a reflected pressure wave bouncing off the vasoconstricted pulmonary vascular bed (Grant et al. 1999) and is likely to be responsible for sustaining the pressure gradient across the DA that facilitates flow from the pulmonary into the systemic circulation during diastole (Fig. 3).
As the DA is a short, large-diameter (Fig. 1), low-resistance shunt between the pulmonary and systemic circulations, it is not surprising that flow in the PA and DA are intimately linked. Indeed, the interaction between flows in the PA and DA is clearly evident in the blood flow waveforms depicted in Fig. 3. Peak PBF during systole corresponds to a clear reduction (notch) in the rate of rise in DA flow, whereas maximum retrograde PBF (most negative flow) during diastole corresponds to a slowing in the rate of decrease in DA flow. This provides further evidence to demonstrate that retrograde PBF contributes to flow through the DA and assists in sustaining high DA flows during diastole; at this time in the cardiac cycle blood flow is zero in the main pulmonary arterial trunk (Rudolph, 1979).
Following UCO, we observed a rapid (within 30 s) and marked reduction in DA flow (50% reduction; Fig. 2) which resulted from a vertical downward shift in the blood flow waveform as pulse amplitude was not affected (Table 1). This reduction in DA flow was closely associated with a 50% reduction in RVO which most probably resulted from a reduction in venous return to the right side of the heart due to the sudden loss of blood returning from the placental circulation. It is surprising that this did not result in a bradycardia, although this may have occurred if the lambs had been delivered vaginally and were not ventilated as soon as possible after birth. In contrast, PBF was not reduced following UCO, indicating that the reduction in RVO only affected DA flow. An increase in downstream resistance within the systemic circulation, due to a loss of the highly compliant placental vascular bed, may have also contributed to the reduction in DA flow following UCO. Increased resistance within the systemic circulation would not be expected to increase systemic arterial pressure under these circumstances, due to the substantial reduction (∼50%) in RVO; RVO contributes to ∼60% of combined ventricular output in the fetus (Rudolph, 1979).
After ventilation onset
The initiation of ventilation caused a large rapid increase in PBF (from 21 ± 11 ml min−1 to 90 ± 19 ml min−1 in 30 s and to 255 ± 10 ml min−1 at 10 min), which was due to a large reduction in PVR, as previously described (Rudolph, 1979, 1985; Reid & Thornburg, 1990; Teitel et al. 1990; Friedman & Fahey, 1993; Heymann, 1999). The increase in PBF was associated with a major change in the shape of the PBF waveform, resulting in forward (positive) flow towards the lungs throughout the cardiac cycle (Polglase et al. 2005). This increase in PBF is thought to result from aeration of the distal gas exchange structures caused by ventilation onset and an associated increase in oxygenation (Iwamoto et al. 1993; Teitel et al. 1990; Hooper & Harding, 2005).
Although we could not detect significant pressure changes in both pulmonary and systemic circulations after 5 min of ventilation, the pressure gradient between the two circulations was lost within 30 s (Fig. 4). Then, after 5 min of ventilation, arterial pressures tended to be lower in the pulmonary circulation than in the systemic circulation, which was significant at 10 min. The reversal of the arterial pressure gradient between the two circulations was closely associated with a reversal in the direction of blood flow through the DA. As a result, within minutes of ventilation onset, blood flow through the DA was left to right (from systemic into the pulmonary circulation) throughout most of the cardiac cycle, with right-to-left flow occurring only briefly during systole.
The reversal in direction of blood flow through DA (from right-to-left to left-to-right direction) occurred rapidly after ventilation began, with mean flow becoming left to right at 3.0 ± 0.5 min. As a result, blood flow from the systemic circulation (i.e. originating from the left ventricle), via the DA, began to contribute to PBF. We calculate that this contribution rapidly increased to almost 50% of total PBF within 10 min of ventilation onset (Fig. 6), indicating that both left and right ventricles contribute to the very large increase in PBF after birth. Although it is relatively clear that both ventricles contribute to PBF immediately after birth, contribution from the left ventricle is counterproductive, forming a short-circuit loop from left ventricle to left atrium through the lungs. Although this may enhance oxygenation of arterial blood, it reduces the efficiency of blood flow distribution to distal structures (e.g. cerebral circulation) and exposes the lung to high vascular pressures at a time when it is clearing large volumes of airway liquid (Hooper & Harding, 1995; Olver et al. 2004) and has a high interstitial tissue pressure (Miserocchi et al. 1994).
We found that PBF gradually decreased with time following ventilation, which is consistent with our previous findings (Polglase et al. 2005, 2008, 2009; Crossley et al. 2007). We hypothesised that this reduction in PBF was due to a reduction in the contribution of left-to-right shunting of blood through the DA. Consistent with our hypothesis, we found that left-to-right shunting was significantly reduced at 2 h after ventilation began (Fig. 2), although it remained as a significant contributor to PBF (∼25% of total) at this time (Fig. 6). Nevertheless, we calculate that this reduction in left-to-right shunting accounts for most (71.3 ± 13.2%) of the reduction in PBF at 2 h after ventilation onset. The remaining 30% of this reduction may have been due to gradually worsening lung disease, caused by mechanical ventilation, or to a gradual reduction in cardiac output (both ventricles), as indicated by the tendency for arterial blood pressures, heart rate and right ventricular output to decrease with time (Table 1).
Although the pulse height of the DA blood flow waveform increased immediately after ventilation began, it was markedly reduced by 30, 60 and 120 min. We consider that this reduction in blood flow pulse height reflects a gradual constriction and partial closure of the DA after birth, which is consistent with the finding that blood flow (left to right) was gradually declining at this time. The factors regulating closure of the DA after birth have been investigated in detail and involve: (1) an increase in 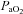 , (2) a decrease in blood pressure within the DA lumen, (3) a decrease in circulating prostaglandins, particularly PGE2 and (4) a decrease in PGE2 receptors in the DA wall (Kajino et al. 2001; Clyman, 2006; Hermes-DeSantis & Clyman, 2006). However, the potential contribution made by the reversal of blood flow through the DA has not been widely considered as a major underlying factor. Indeed, as the junction of the DA and descending thoracic aorta form a relatively acute angle (Fig. 1), left-to-right flow through the DA (from the aorta into the pulmonary artery) must cause substantial turbulence at this junction as well as at the PA end. It is possible that the resulting turbulence and increased shear-stress releases vasoconstrictive factors from the endothelium that may contribute to constriction of the DA after birth. Thus, a larger decrease in PVR may lead to greater left-to-right shunting, which will have a more potent vasoconstrictor effect on the DA; this may partially explain higher rates of patent DA in preterm infants.
, (2) a decrease in blood pressure within the DA lumen, (3) a decrease in circulating prostaglandins, particularly PGE2 and (4) a decrease in PGE2 receptors in the DA wall (Kajino et al. 2001; Clyman, 2006; Hermes-DeSantis & Clyman, 2006). However, the potential contribution made by the reversal of blood flow through the DA has not been widely considered as a major underlying factor. Indeed, as the junction of the DA and descending thoracic aorta form a relatively acute angle (Fig. 1), left-to-right flow through the DA (from the aorta into the pulmonary artery) must cause substantial turbulence at this junction as well as at the PA end. It is possible that the resulting turbulence and increased shear-stress releases vasoconstrictive factors from the endothelium that may contribute to constriction of the DA after birth. Thus, a larger decrease in PVR may lead to greater left-to-right shunting, which will have a more potent vasoconstrictor effect on the DA; this may partially explain higher rates of patent DA in preterm infants.
In conclusion, we have shown, for the first time, that the direction of blood flow through the DA is very dynamic after birth and probably reflects the pressure gradient between the pulmonary and systemic circulations. With a decrease and then reversal of the pressure gradient between the pulmonary and systemic circulations, we found that the primary direction of blood flow through the DA reverses. As a result, immediately (within 3 min) after ventilation begins, left-to-right flow through the DA provides a large (∼50%) contribution to PBF and the gradual reduction in this contribution, as the DA begins to close, results in a gradual and significant reduction in PBF. The close inter-relationship between PBF and DA flow indicates that the factors influencing both the systemic and pulmonary circulations need to be considered together when attempting to understand problems associated with either circulations in the immediate newborn period.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Mr A. Satragno, Ms K. Rodgers, Ms V. Zahra and Mrs A. Thiel. This study was supported by the National Health and Medical Research Council (NHMRC) of Australia and S.B.H. and G.R.P. are recipients of NHMRC and Australian Heart Research Foundation research fellowships.
Glossary
Abbreviations
- DA
ductus arteriosus
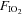
fraction of inspired oxygen
- HR
heart rate
- LPA
left pulmonary artery
- PBF
pulmonary blood flow
- PEEP
positive end expiratory pressure
- PGE2
prostaglandin E2
- PVR
pulmonary vascular resistance
- RVO
right ventricular output
- UCO
umbilical cord occlusion.
Author contributions
All authors have significantly contributed to (1) the conception and design the experiments, (2) analysis and interpretation of data, (3) drafting and critically revising the manuscript and have (4) approved the final version to be published.
References
- Clyman RI. Mechanisms regulating the ductus arteriosus. Biol Neonate. 2006;89:330–335. doi: 10.1159/000092870. [DOI] [PubMed] [Google Scholar]
- Crossley KJ, Morley CJ, Allison BJ, Polglase GR, Dargaville PA, Harding R, Hooper SB. Blood gases and pulmonary blood flow during resuscitation of very preterm lambs treated with antenatal betamethasone and/or Curosurf: effect of positive end-expiratory pressure. Pediatr Res. 2007;62:37–42. doi: 10.1203/PDR.0b013e31806790ed. [DOI] [PubMed] [Google Scholar]
- Friedman AH, Fahey JT. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol. 1993;17:106–121. [PubMed] [Google Scholar]
- Grant DA, Hollander E, Skuza EM, Fauchere JC. Interactions between the right ventricle and pulmonary vasculature in the fetus. J Appl Physiol. 1999;87:1637–1643. doi: 10.1152/jappl.1999.87.5.1637. [DOI] [PubMed] [Google Scholar]
- Hermes-DeSantis ER, Clyman RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006;26:S14–S18. doi: 10.1038/sj.jp.7211465. [DOI] [PubMed] [Google Scholar]
- Heymann MA. Control of the pulmonary circulation in the fetus and during the transitional period to air breathing. Obstet Gynecol. 1999;84:127–132. doi: 10.1016/s0301-2115(98)00321-2. [DOI] [PubMed] [Google Scholar]
- Hooper SB. Role of luminal volume changes in the increase in pulmonary blood flow at birth in sheep. Exp Physiol. 1998;83:833–842. doi: 10.1113/expphysiol.1998.sp004163. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Harding R. Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung. Clin Exp Pharmacol Physiol. 1995;22:235–247. doi: 10.1111/j.1440-1681.1995.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Harding R. Role of aeration in the physiological adaptation of the lung to air-breathing at birth. Curr Respir Med Rev. 2005;1:185–195. [Google Scholar]
- Iwamoto HS, Teitel DF, Rudolph AM. Effects of lung distension and spontaneous fetal breathing on hemodynamics in sheep. Pediatr Res. 1993;33:639–644. doi: 10.1203/00006450-199306000-00021. [DOI] [PubMed] [Google Scholar]
- Kajino H, Chen YQ, Seidner SR, Waleh N, Mauray F, Roman C, Chemtob S, Koch CJ, Clyman RI. Factors that increase the contractile tone of the ductus arteriosus also regulate its anatomic remodelling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R291–R301. doi: 10.1152/ajpregu.2001.281.1.R291. [DOI] [PubMed] [Google Scholar]
- Keramidaris E, Hooper SB, Harding R. Effect of gestational age on the increase in fetal lung growth following tracheal obstruction. Exp Lung Res. 1996;22:283–298. doi: 10.3109/01902149609031776. [DOI] [PubMed] [Google Scholar]
- Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr. 2000;137:68–72. doi: 10.1067/mpd.2000.106569. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Poskurica BH, Del Fabbro M. Pulmonary interstitial pressure in anaesthetized paralyzed newborn rabbits. J Appl Physiol. 1994;77:2260–2268. doi: 10.1152/jappl.1994.77.5.2260. [DOI] [PubMed] [Google Scholar]
- Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. 1990;86:1270–1277. doi: 10.1172/JCI114834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Walters DV, Wilson M. Developmental regulation of lung liquid transport. Annu Rev Physiol. 2004;66:77–101. doi: 10.1146/annurev.physiol.66.071702.145229. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Hooper SB, Gill AW, Allison BJ, McLean CJ, Nitsos I, Pillow JJ, Kluckow M. Cardiovascular and pulmonary consequences of airway recruitment in preterm lambs. J Appl Physiol. 2009;106:1347–1355. doi: 10.1152/japplphysiol.91445.2008. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Morley CJ, Crossley KJ, Dargaville P, Harding R, Morgan DL, Hooper SB. Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J Appl Physiol. 2005;99:1453–1461. doi: 10.1152/japplphysiol.00055.2005. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Moss TJ, Nitsos I, Allison BJ, Pillow JJ, Hooper SB. Differential effect of recruitment maneuvres on pulmonary blood flow and oxygenation during HFOV in preterm lambs. J Appl Physiol. 2008;105:603–610. doi: 10.1152/japplphysiol.00041.2008. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Wallace MJ, Grant DA, Hooper SB. Influence of fetal breathing movements on pulmonary hemodynamics in fetal sheep. Pediatr Res. 2004;56:932–938. doi: 10.1203/01.PDR.0000145254.66447.C0. [DOI] [PubMed] [Google Scholar]
- Probyn ME, Hooper SB, Dargaville PA, McCallion N, Crossley K, Harding R, Morley CJ. Positive end expiratory pressure during resuscitation of premature lambs rapidly improves blood gases without adversely affecting arterial pressure. Pediatr Res. 2004;56:198–204. doi: 10.1203/01.PDR.0000132752.94155.13. [DOI] [PubMed] [Google Scholar]
- Reid DL, Thornburg KL. Pulmonary pressure-flow relationships in the fetal lamb during in utero ventilation. J Appl Physiol. 1990;69:1630–1636. doi: 10.1152/jappl.1990.69.5.1630. [DOI] [PubMed] [Google Scholar]
- Rudolph AM. Fetal and neonatal pulmonary circulation. Annu Rev Physiol. 1979;41:383–395. doi: 10.1146/annurev.ph.41.030179.002123. [DOI] [PubMed] [Google Scholar]
- Rudolph AM. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res. 1985;57:811–821. doi: 10.1161/01.res.57.6.811. [DOI] [PubMed] [Google Scholar]
- Teitel DF, Iwamoto HS, Rudolph AM. Changes in the pulmonary circulation during birth-related events. Pediatr Res. 1990;27:372–378. doi: 10.1203/00006450-199004000-00010. [DOI] [PubMed] [Google Scholar]
- West JB. Thoughts on the pulmonary blood-gas barrier. Am J Physiol Lung Cell Mol Physiol. 2003;285:L501–L513. doi: 10.1152/ajplung.00117.2003. [DOI] [PubMed] [Google Scholar]