Abstract
Studies exploring the rate of fatigue in isolated muscle at 37°C have produced mixed results. In the present study, muscle fibre bundles from the mouse foot were used to study the effect of temperature on the rate of muscle fatigue. Provided iron was excluded from the solutions, time to fatigue at 37°C was increased compared to 22°C (125 ± 8% of 22°C fatigue time). In contrast, when iron was present (∼1 μm), fatigue was accelerated (68 ± 10%). Iron can increase reactive oxygen species (ROS), which are believed to accelerate fatigue. The addition of 25–100 μm H2O2 at 22°C reduced time to fatigue to 80–20% of the control, respectively. Iron was added to cultured primary skeletal muscle cells to determine if iron could increase ROS production. Neither iron entry nor ROS production were detected in non-contracting muscle cells. The addition of 8-hydroxyquinoline, which facilitates iron entry, to iron–ascorbic acid solutions caused a rapid rise in intracellular iron and ROS. Our results indicate that time to fatigue in vitro is increased at 37°C relative to 22°C, but the addition of ROS can accelerate fatigue. An increase in muscle iron can accelerate ROS production, which may be important during or following exercise and in haemochromatosis, disuse atrophy and sarcopenia.
It is generally recognised that temperature affects muscle performance, but the mechanisms are complex and poorly understood. Humans performing endurance exercise at an elevated ambient temperature reach exhaustion earlier than at cooler temperatures, but this appears to be primarily a central phenomenon despite a large increase in skeletal muscle temperature (Nybo, 2008). In contrast, short-term anaerobic performance in humans such as sprinting is enhanced by a small increase (Bergh & Ekblom, 1979; Davies & Young, 1983), and impaired by a small decrease, in muscle temperature (Crowley et al. 1991). In the above exercise regimes there are changes in both core temperature and muscle temperature. Experiments on small groups of muscles are easier to interpret as they avoid the rise of core temperature. Isometric contractions of the forearm (Clarke et al. 1958; Holewijn & Heus, 1992) or quadriceps muscles in humans (Edwards et al. 1972) are sustained for the longest time period before volitional fatigue within a muscle temperature range of ∼25–30°C. Fatigue is accelerated outside of this range.
Volitional fatigue incorporates a central component and this confounding factor can be by-passed by electrical stimulation. de Ruiter et al. (1999, 2000) used intermittent electrical stimulation of the ulnar nerve to stimulate the adductor pollicis muscle in humans and demonstrated that fatigue can be accelerated at 37°C relative to muscle cooled to between ∼22–30°C. The accelerated fatigue at 37°C in these studies was observed in the presence of a cuff, used to occlude the blood vessels supplying the muscle.
Intermittent contractions without vessel occlusion minimise the build-up of metabolites and ionic shifts that are thought to contribute to accelerated fatigue (for review see Allen et al. 2008). Blomstrand et al. (1985) used intermittent electrical stimulation in a rat model with an intact blood supply. No difference in time to fatigue was observed between 28°C and 36°C, suggesting that with adequate perfusion no difference in fatigue time occurs within the physiological range.
Recent in vitro studies have been performed in an attempt to identify the mechanisms of the temperature-dependent modification of skeletal muscle fatigue. Moopanar & Allen (2005) showed that skeletal muscle fatigue in mouse single fibres was accelerated at 37°C relative to room temperature due to a loss in Ca2+ sensitivity of the contractile proteins. The rapid fatigue at 37°C was prevented by the ROS scavenger Tiron, leading the authors to conclude that an increase in ROS at 37°C reduced Ca2+ sensitivity and accelerated fatigue. The accelerated fatigue at 37°C was probably due to the production of disulfide bonds, as dithiothreitol, a reducing agent that converts disulfide bonds to free sulphide groups, reversed the loss in Ca2+ sensitivity and force (Moopanar & Allen, 2006).
An increase in fatigue time in isolated muscle with increasing temperature has also been observed. Roots et al. (2009) isolated small bundles of rat muscle and produced fatigue with either isometric or shortening contractions. Under both isometric and shortening conditions, the muscle fatigued more slowly as the temperature was increased.
Given these conflicting findings, we reinvestigated the effect of temperature on isolated muscle fatigue. Initial findings appeared to demonstrate that the time to fatigue at 37°C was longer than at room temperature, a finding which conflicts with previous work from our laboratory. In the present experiments an aluminium heat exchanger was used to warm the perfusate to 37°C, whereas in the previous study (Moopanar & Allen, 2005) a stainless steel heat exchanger was used.
Iron can increase the production of ROS, such as the highly reactive hydroxyl radical (OH•) (Bacic et al. 2008). Iron can be detected in a solution after a single pass through a stainless steel needle (Buettner, 1990). We hypothesise that the iron from the stainless steel heat exchanger passes into solution, generates ROS and accelerates fatigue at 37°C.
Methods
Ethical approval
All experiments were approved by the Animal Ethics Committee of The University of Sydney.
Muscle bundle dissection
Male mice (Balb-C strain) were used in this study as in previous work (Lännergren & Westerblad, 1987; Moopanar & Allen, 2005, 2006). Mice aged 8–12 weeks were killed by rapid cervical dislocation and the flexor digitorum brevis (FDB) muscle was removed from the hind foot. The FDB is a fast, mixed muscle containing approximately 75% type IIX fibres; the remaining are a mixture of type IIA and type I fibres (Allen et al. 1993). Small bundles of two to five fibres were dissected from the middle of the FDB. Aluminium foil crimps were used to attach one tendon and the other to a force transducer (Akers AE 801). Parallel platinum electrodes were used for stimulation. The pulse width was 0.5 ms, set at 100 Hz and lasted 400 ms. The stimulation was set at 1.5 times the minimum voltage required to activate all fibres.
Muscle fatigue experiments
Muscles dissection took place at room temperature (∼22°C) in a non-bubbled, slightly alkaline (pH ∼8.0) solution (see Lännergren & Westerblad, 1987). During experiments, the following solution was used (mm): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24; glucose, 5.5; pH 7.4 when bubbled with 95% O2 and 5% CO2.
EGTA (100 μm) was used as a broad-spectrum chelator of metal ions. Transition metals such as iron have the potential to produce increased levels of ROS (Bacic et al. 2008) and ROS scavengers have been shown to prevent rapid fatigue at 37°C (Moopanar & Allen, 2005). EGTA was therefore used to determine if chelating transition metals could also prevent the rapid fatigue observed at 37°C. Desferrioxamine (DFO) was used as a more specific and higher affinity iron chelator.
Fatigue protocol
Muscle bundles were stimulated until the force attained was less than 50% of the initial tetanus. This duration is the ‘fatigue time’ (T1/2). To fatigue the muscle, tetani were produced every 4 s for the first 2 min then every 3.5, 3, 2.5 s and so on until fatigue (Westerblad et al. 1990). In all experiments, a fatigue run was first performed at room temperature (22 ± 3°C). After the muscle bundle was fatigued, it was allowed to recover for 40 min. Tetani were elicited at 20 and 40 min to ensure the preparation had recovered. Recovery was defined as ≥ 85% of the initial force (Fi) of the fatigue run. The second fatigue run was performed either at room temperature or at 37°C. T1/2 for the second fatigue run is expressed as a percentage of the first fatigue run at room temperature (T1/2 normalised).
Heating the muscle bundle to 37°C
Muscle bundles were heated to 37°C using two different heat exchangers which work on similar principles. Both heat exchangers were heated by circulating water, maintained at ∼43°C. In the stainless steel heat exchanger, the experimental solution passed through stainless steel tubing, while in the aluminium heat exchanger, the experimental solution passed through a channel of anodised aluminium. A miniature thermocouple (Jenway 8500) was used to set and maintain the temperature of the solution in the muscle bath.
Iron released from stainless steel heat exchanger
An ascorbate assay was used to determine the concentration of iron in solutions. The oxidation of ascorbate by transition metals causes a decrease in absorbance at 265 nm over time (Buettner, 1988). Freshly made ascorbic acid was added to the solution of interest in a quartz cuvette at a final concentration of 125 μm. The percentage fall in absorbance after 15 min was used for analysis.
For measurements of iron levels, the experimental solution was continuously recirculated through the apparatus under conditions identical to fatigue experiments. A control solution was treated identically except it by-passed the heat exchanger. The fall in absorbance measured by the assay was converted to iron concentration by constructing a standard curve for iron.
Muscle fatigue in the presence of H2O2
H2O2 was used in the current study at room temperature to mimic the increase in ROS that may occur during fatigue at elevated temperatures (Reid et al. 1992; Moopanar & Allen, 2005; Edwards et al. 2007). Bundle preparations were incubated in H2O2 immediately prior to the second fatigue run at a concentration range of 25–100 μm, for 5 or 10 min. H2O2 was present for the duration of the fatigue run.
Fatigue in the presence of iron
In experiments designed to mimic iron release from the stainless steel heat exchanger, exogenous iron was added to solutions passing through the aluminium heat exchanger. In an oxygen-rich environment, iron is primarily observed in the ferric (Fe3+) form (Halliwell & Gutteridge, 1998). We therefore added iron as ferric citrate.
Isolation and culturing of skeletal muscle fibres
Mice aged 12–16 weeks were used for culture experiments. Each experiment used a minimum of three mice. The total number of cells studied is indicated by n. The FDB from both feet were dissected in Hepes-buffered physiological saline (HBPS) which contained (mm): NaCl, 138; KCl, 2.7; CaCl2, 1.8; MgCl2, 1.06; N-2-hydroxy-ethylpiperazine-N′-2-ethanesulphonic acid (Hepes), 12.4; glucose, 5.5; pH 7.3 using 1 m NaOH. Following dissection, the FDB muscles were incubated in HBPS supplemented with 10% fetal bovine serum (FBS) and 0.2% collagenase type 1 at 37°C for 90 min. After incubation the muscles were transferred to a Dulbecco's modified essential medium containing 10% FBS and 1% penicillin–streptomycin. Fibres were separated by trituration with Pasteur pipettes and then concentrated by centrifugation at 250g. Fibres were then seeded onto glass-bottomed micro-well dishes which had been coated with 20 μg ml−1 laminin.
Fluorescent indicators
To determine if iron uptake was detectable, the membrane-permeant form of the intracellular iron indicator Phen green SK, diacetate was used (14313; Invitrogen, Carlsbad, CA, USA). A 20 mm stock solution using DMSO was made and then stored in aliquots. The final loading concentration was 10 μm. The ROS indicator CM-H2DCFDA (5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate) (C6827; Invitrogen) was used to detect non-specific ROS production. A 5 mm stock solution was prepared daily in DMSO. The final loading concentration was 5 μm. HPF (3′-(p-hydroxyphenyl) fluorescein) (H36004; Invitrogen) is selective for highly reactive oxygen species (hROS) and was used to determine if hydroxyl radicals were present. HPF is 6.5 times more sensitive to OH• than to ONOO− and is 100-fold more sensitive to OH• than to other ROS (Setsukinai et al. 2003). The final loading concentration was 5 μm. The impermeable ROS indicator OxyBURST Green H2HFF BSA (O-13291 Invitrogen) was used to determine extracellular ROS production. One millilitre of HBPS was added per vial to make a stock solution of 1 mg ml−1. The final concentration was 100 μg ml−1.
Dye loading for ROS indicators
Dishes of cultured, primary skeletal myocytes were removed from the incubator and allowed to cool to room temperature then washed in HBPS 3 times. The intracellular indicators were then loaded into cells at room temperature for 30 min. The extracellular ROS indicator was added to the dish and was present for the duration of the experiment.
Fluorescence imaging
Live cell imaging was performed on a Zeiss LSM 510 Meta inverted confocal microscope. Fluorescence measurements for all the indicators were performed using an excitation wavelength of 488 nm and an emission wavelength >505 nm. Cells were heated to 37°C using a sealed heating unit (Tempcontrol 37-2 digital and Heating Insert; Pecon, Germany) set at 45°C.
Live cell imaging was performed using a 4-dimensional acquisition technique (z stack over time) (Thomsen et al. 2002). After imaging, the z stack for each time point was compressed into a single 2-dimensional image (z projection) and the fluorescence (average intensity of the signal from the fibre) was plotted against time. The final value obtained for each intervention was expressed relative to a predicted value. The predicted value was constructed by a line of best fit (linear for the CM-H2DCFDA and OxyBURST dyes and exponential for the Phen green and HPF dye) generated from the 5 min baseline period.
Experimental solutions used in cell culture experiments
The lipophilic additive 8-hydroxyquinoline (HQ) was used to facilitate passive iron entry into cells. HQ was added to solutions at 4 times the molar concentration of iron (Fe). Ascorbic acid (AA) was added at 1 mm to maintain iron in the ferrous form (Halliwell & Gutteridge, 1998). Initial experiments showed that when Fe + AA + HQ was freshly made up from stock solutions, very little ROS production was detectable (data not shown). In contrast, when stock solutions were mixed on the previous day, ‘day-old’ stock solutions produced a substantial increase in ROS production. This difference may result from iron oxidising some of the ascorbic acid (AA) to dehydroascorbic acid (DHA) (Buettner, 1988). The resulting solution may then contain a small amount of DHA which enters cells at a greater rate than AA (Lane & Lawen, 2008). This complex phenomenon was not explored further, but is noted in Fig. 8B. All results reported are from experiments performed using stock solutions mixed on the previous day.
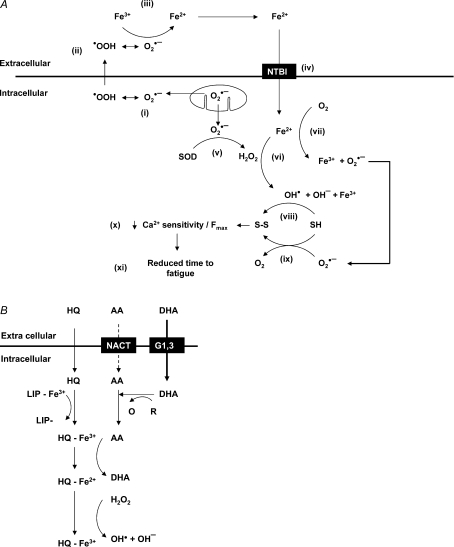
Figure 8. Iron-induced ROS production and the labile iron pool.
A, proposed mechanism of iron-induced ROS production and subsequent fatigue. •OOH, protonated, uncharged, membrane-permeable (Korshunov & Imlay, 2002) form of superoxide; SOD, superoxide dismutase; NTBI, non-transferrin iron uptake channel; OH•, hydroxyl radical; OH−, hydroxyl ion; SH, thiol group; S–S, disulfide bridge. B, the production of OH• via LIP modification. LIP, labile iron pool; HQ, 8-hydroxyquinoline; AA, ascorbic acid; DHA, oxidised ascorbic acid; NACT, sodium–ascorbate co-transporter; G1,3, facilitative glucose transporters 1 and/or 3; R/O, unspecified redox couple.
Statistics
All values are expressed as the mean ±s.e.m. Statistical significance was tested with paired t test or one-way ANOVA (Holm–Sidak method) as appropriate. Results were considered significant at P < 0.05.
Results
Muscle fatigue at 37°C
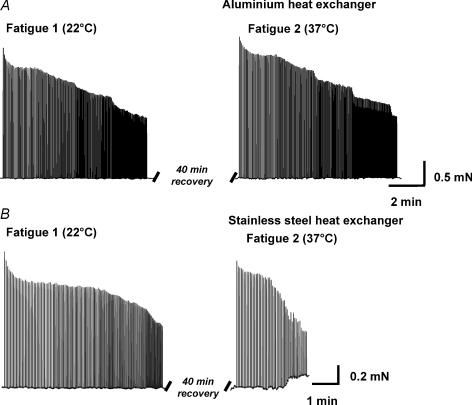
Initial findings in the current study demonstrated that the time to fatigue at 37°C was longer than at room temperature, a finding which conflicts with previous work from our laboratory (Moopanar & Allen, 2005). Investigation revealed that the source of difference was the type of heat exchanger used. Representative fatigue runs using the two heat exchangers are shown in Figs 1 and 2.
Figure 1. Fatigue at 22°C and 37°C.
Representative fatigue runs performed at room temperature then at 37°C using the aluminium heat exchanger (A) or stainless steel heat exchanger (B). Muscle fibre bundles were fatigued using supra-maximal electrical stimulation every few seconds. An increase in baseline force was observed in some bundles using the stainless steel heat exchanger without iron chelators present and may represent one fibre going into contracture. The effect only occurred when force was ∼50%, therefore the rapid fatigue observed in these preparations was not dependent on this process. This effect did not occur under any other condition.
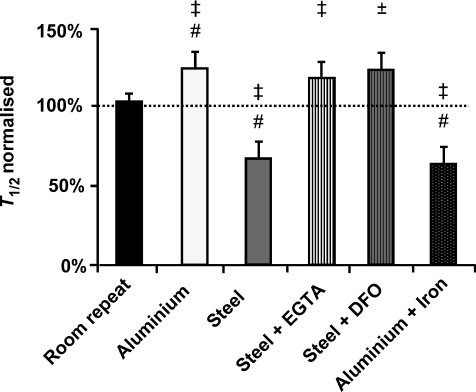
Figure 2. Skeletal muscle fatigue times at room temperature and 37°C.
Data are represented as normalised fatigue time (fatigue time 2 as a percentage of fatigue time 1). Dashed line represents fatigue 1 (internal control). #Significantly different to room repeat (P < 0.05). ‡Significantly different to internal control (P < 0.05); ±Significant trend (P= 0.08).
A group of experiments were performed using two fatigue runs at room temperature to determine if the time to fatigue changes between fatigue runs, independent of a temperature change. This group of experiments is referred to as ‘room repeat’ (Fig. 2). No difference in fatigue time was observed between the first (5.7 ± 0.5 min) and the second fatigue run performed on the same preparation at room temperature (5.6 ± 0.4 min; T1/2 normalised = 104 ± 5%, n= 17)
Fatigue runs performed at 37°C using the aluminium heat exchanger (T1/2 7.6 ± 1.0 min) were significantly longer compared to the room temperature control (T1/2 6.0 ± 0.6 min; T1/2 normalised = 125 ± 8%; Figs 1A and 2; P < 0.05; n= 7) and the room repeat experiments (P < 0.05).
Fatigue runs performed at 37°C using the stainless steel heat exchanger (4.6 ± 0.8 min) were significantly shorter compared to the room temperature control (T1/2 7.0 ± 1.0 min; T1/2 normalised = 68 ± 10%; Figs 1B and 2; P < 0.05; n= 6) and the room repeat experiments (P < 0.05). In some experiments an increase in baseline force was observed (Fig. 1B). This effect was only observed when force had fallen to ∼50%.
Stainless steel heat exchanger using iron chelators
Fatigue runs were performed at 37°C using the stainless steel heat exchanger in the presence of the non-specific metal chelator EGTA (n= 9). EGTA (100 μm) prevented the rapid fatigue observed at 37°C using the stainless steel heat exchanger. The T1/2 in the presence of EGTA at 37°C (7.3 ± 1.0 min) was significantly longer compared to the room temperature control (6.2 ± 0.8 min; T1/2 normalised = 119 ± 12%; Fig. 2; P < 0.05; n= 6).
Fatigue runs were also performed at 37°C using the stainless steel heat exchanger in the presence of the iron specific chelator DFO (100 μm; n= 10). DFO prevented the rapid fatigue observed at 37°C that was observed using the stainless steel heat exchanger. The T1/2 in the presence of DFO at 37°C (5.6 ± 0.5 min) was marginally longer compared to the internal control performed at room longer (T1/2 normalised = 124 ± 12%; paired t test; P= 0.08). Combining the DFO and EGTA results, the time to fatigue was longer at 37°C compared to the room temperature control (T1/2 normalised = 121 ± 8%; paired t test; P < 0.01)
Iron addition
After completing the iron chelation experiments, iron was added to solutions at 37°C to determine if adding iron while using the aluminium heat exchanger would accelerate fatigue. Iron was added as ferric citrate at a concentration of 1 μm. Adding iron significantly reduced fatigue time at 37°C compared to the internal control performed at room temperature (T1/2 normalised = 63 ± 10%; Fig. 3; P < 0.05; n= 10) and the room repeat experiments.
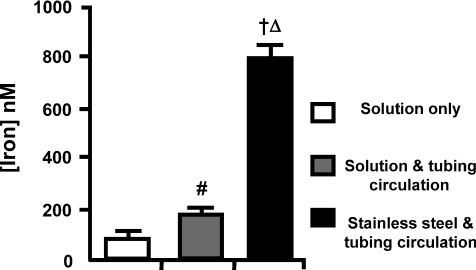
Figure 3. Iron release from the stainless steel heat exchanger.
An experimental solution was circulated through the heat exchanger and the tubing or the tubing only and assessed for iron content using an absorbance based assay. #Significantly different to solution only (P < 0.05), †(P < 0.001). ΔSignificantly different to both groups (P < 0.001).
Iron released from the stainless steel heat exchanger
After circulating the perfusate through the stainless steel heat exchanger (n= 3), the iron concentration was 793 ± 48 nm (see Methods). The solution passed through the tubing alone (n= 3) gave an iron concentration of 173 ± 7 nm, the perfusate alone (n= 8) 76 ± 20 nm. All conditions were significantly different to one another (Fig. 3; P < 0.05).
Muscle fatigue in the presence of H2O2
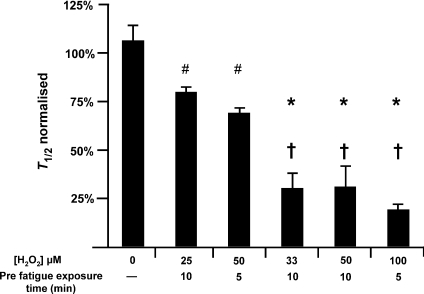
Iron is known to produce ROS so we added H2O2 to determine if ROS can accelerate fatigue. The effect of H2O2 on fatigue was dose and duration dependent. As Fig. 4 shows, fatigue at 22°C was accelerated in the presence of H2O2. Figure 4 also shows a negative correlation between fatigue time and the H2O2 concentration or the concentration–duration product. The lowest concentration (25 μm) reduced T1/2 to 80 ± 1%, while the highest concentration reduced T1/2 to 19 ± 1%. A paired t test revealed a small but significant (P < 0.05) increase in Fi after H2O2 treatment consistent with previous reports (Andrade et al. 1998). These data demonstrate that ROS can accelerate fatigue in the absence of reduced initial force.
Figure 4. Muscle fatigue using H2O2.
H2O2 was added to the experimental solution at a concentration of 25–100 μm, 5–10 min prior to initiating fatigue and remained present during the fatigue run. #Significantly different to no H2O2 exposure (P < 0.05); †(P < 0.001). *Significantly different to 25 μm H2O2 (10 min) and 50 μm H2O2 (5 min) exposure (P < 0.01).
Iron uptake using the iron indicator Phen green
In the current study rapid fatigue was observed at 37°C in the presence of iron. Iron has been shown to enter hepatocytes (Petrat et al. 1999; Rauen et al. 2000) and human erythroleukaemia K-562 cells (Breuer et al. 1995) through a non-transferrin bound iron uptake mechanism (NTBI) in the ferrous form (Fe2+).
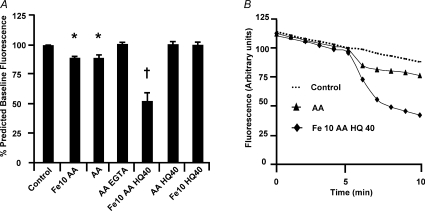
The fluorescent iron indicator Phen green was used to measure iron uptake in muscle cells. All results are expressed relative to their predicted fluorescence value (see Methods). As can be seen in Fig. 5, fluorescence quenching, which represents an increase in intracellular iron, was observed following the addition of Fe (10 μm) and ascorbic acid (AA) (80 ± 5%; P≤ 0.05, n= 6). Increasing Fe to 50 μm did not increase the effect further (data not shown). However, AA alone also produced a quenching of fluorescence (87 ± 1%; P≤ 0.05, n= 13). The addition of EGTA prevented the drop in signal observed with AA (100 ± 1%; P≤ 0.05, n= 4), suggesting that contaminating iron, interacting with AA, might produce fluorescence quenching. 8-Hydroxyquinoline (HQ, 40 μm; used to facilitate iron entry) was added to Fe (10 μm) + AA solutions to facilitate iron entry and produced a greater fluorescence quenching than all other groups (52 ± 6%; P≤ 0.001, n= 9), indicating an increase in intracellular iron. No fluorescence quenching was observed using AA + HQ (40 μm) in the absence of iron, confirming Fe2+ entry is the cause of the above signal. Fluorescence quenching was also absent for Fe (10 μm) + HQ (40 μm), showing that AA is essential, presumably to maintain iron in the Fe2+ form.
Figure 5. Iron loading using the iron indicator Phen green.
A, pooled data showing quenching after 5 min induced by the addition of each experimental solution. Control represents the addition of HBPS. *Significantly different to control (P < 0.05). †Significantly different to all groups (P < 0.001). Numbers indicate the concentration of iron (Fe) or 8-hydroxyquinoline (HQ) used, in μm. [AA] was 1 mm. B, representative records of fluorescence measurements over time.
Based on these findings, the fluorescent iron indicator Phen green is a useful tool for detecting a large increase in the intracellular iron concentration of skeletal muscle, but may not be sensitive enough to detect the intracellular change caused by 1 μm extracellular iron.
Intracellular ROS production using the non-specific indicator CM-H2DCFDA
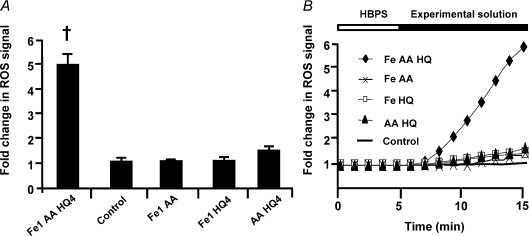
Based on the Phen green results, HQ facilitates iron entry. For ROS experiments, iron was added at 1 μm to simulate the extracellular iron present during fatigue experiments. The addition of Fe (1 μm) + AA + HQ (4 μm) to cultured primary myocytes produced a substantial increase in signal, indicating an increase in ROS production (Fig. 6; P < 0.001; n= 8). In contrast, no difference in fluorescence was found for the following solutions: 50 μm Fe + AA (1.1 ± 0.1, n= 8), Fe (1 μm) + HQ (4 μm) (1.1 ± 0.1, n= 4) or AA + HQ (4 μm) (1.5 ± 0.1, n= 10), demonstrating that all three compounds are necessary for ROS production. These data demonstrate that a sufficiently large increase in intracellular iron causes a large and rapid increase in intracellular ROS production.
Figure 6. Intracellular ROS production using the general ROS indicator CM-H2DCFDA.
A, pooled data demonstrating ROS production 10 min after the addition of each experimental solution. †Significantly different to control (P < 0.001). Numbers represent concentration in μm, [AA] was 1 mm. B, representative records displaying the rate of change of the ROS signal following the addition of different experimental solutions.
Intracellular ROS production using the OH• selective indicator HPF
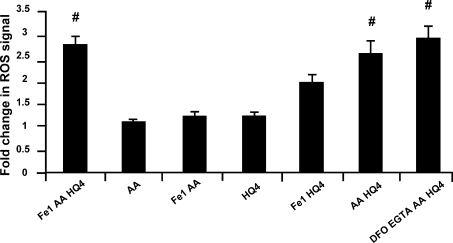
The highly reactive ROS indicator HPF, which is very selective for OH•, was used to determine if OH• is produced in the presence of increased intracellular Fe2+. Fe (1 μm) + AA + HQ produced a significant increase in fluorescence (2.8 ± 0.2, n= 23) relative to AA alone (1.0 ± 0.0, n= 3; Fig. 7; P < 0.05). Adding Fe (10 μm) + AA, in the absence of HQ had no effect (1.2 ± 0.0, n= 4), nor did HQ alone (1.2 ± 0.1, n= 4). However, adding AA + HQ produced a significant increase in fluorescence (2.6 ± 0.3, n= 11), which was not significantly different to Fe (1 μm) + AA + HQ. If the signal increase caused by AA + HQ was due to contaminating iron, then the application of iron chelators to these solutions should prevent the increase in signal. The addition of the iron chelators desferrioxamine (DFO) and EGTA (both 100 μm) to the solutions did not prevent the increase in signal (2.9 ± 0.3, n= 14), suggesting that external iron did not contribute to the signal increase. These data suggest that intracellular OH• can be increased in the presence of AA + HQ, even in the absence of an increase in intracellular iron. A potential mechanism is presented in Fig. 8B and is considered in the Discussion.
Figure 7. Intracellular ROS production using the OH• selective indicator HPF.
#Significantly different to AA (P < 0.05). [AA] was 1 mm. Numbers represent concentration in μm. Both DFO and EGTA were 100 μm.
Extracellular ROS production using OxyBURST Green
The impermeable ROS indicator OxyBURST Green was used to determine if exogenously applied iron could increase ROS production on the cell surface which would not be apparent with intracellular ROS indicators. Initial experiments performed with Fe (1–10 μm) ± AA did not cause an increase in signal (data not shown). Fe + AA was added in the presence of H2O2 (10 μm) to increase the production of OH•. The addition of H2O2 produced a large increase in signal at the fibre edge and within the area occupied by the fibre, assumed to be from the t-tubules (Fe + AA + H2O2; 5.5 ± 0.9, n= 5); however, this increase was not different to H2O2+ DFO (4.0 ± 0.9, n= 6). These data suggest that the addition of Fe + AA to non-contracting skeletal muscle cells does not cause an increase in extracellular ROS or the OxyBURST Green indicator is not able to detect it.
Discussion
The rate of muscle fatigue as a function of temperature is variable (see introduction). The current study provides evidence that the time taken to reach fatigue is greater at 37°C compared to room temperature, provided Fe is absent or chelated. Inorganic phosphate (Pi) and hydrogen ions (H+) both increase during fatigue (Sahlin et al. 1976; Cady et al. 1989; Dahlstedt et al. 2000) and both can independently reduce force at room temperature (Fabiato & Fabiato, 1978; Millar & Homsher, 1990). Given the correlation between reduced force and the accumulation of Pi and H+ during fatigue (Cady et al. 1989), both have been proposed as causal contributors to fatigue (for review see Fitts, 2008). Recent evidence suggests, however, that the effects of both Pi and H+ are reduced at elevated temperatures (Pate et al. 1995; Westerblad et al. 1997; Debold et al. 2004). The reduced effect of Pi and H+ at elevated temperatures may be one reason the time taken to reach fatigue at 37°C was greater in our study.
ROS and muscle fatigue
Another potential mediator of muscle fatigue is reactive oxygen species (ROS). Evidence supporting a role of ROS in muscle fatigue has come from studies adding ROS to unfatigued muscle (Andrade et al. 1998) and from studies utilising ROS scavengers (Reid et al. 1992; McKenna et al. 2006). Our observation that exogenous H2O2 accelerates fatigue confirms these findings.
Fatigue in isolated muscle at elevated temperatures has been shown to be both accelerated (Moopanar & Allen, 2005) and slowed (Roots et al. 2009). Our results support a slowing of fatigue at elevated temperatures, but this contrasts with the majority of studies performed in vivo. Human studies demonstrating a reduced time to fatigue at elevated temperatures have been performed using sustained contractions (Clarke et al. 1958; Edwards et al. 1972; Holewijn & Heus, 1992) or intermittent contractions performed in the presence of a cuff (de Ruiter et al. 1999; de Ruiter & de Haan, 2000, 2001), both of which involve reduced blood flow. Conversely, in a rat model with intermittent tetani and unimpaired blood flow, no difference in time to fatigue occurred between 28°C and 36°C (Blomstrand et al. 1985). We propose that the effect of reduced blood flow on fatigue may be exaggerated at elevated temperatures. ROS production in muscle increases with temperature (Edwards et al. 2007) and contraction (Reid et al. 1992). When fatigue is produced under conditions of reduced blood flow, ROS removal from the muscle will be compromised. The subsequent build-up of extracellular and intracellular ROS could then reduce force via effects on the membrane or contractile proteins, respectively, consistent with our H2O2 data. The preparation used in the current study and by Roots et al. (2009) minimise the accumulation of endo-genously produced ROS which may have reduced the rate of fatigue. We therefore propose that the conflicting results between experiments performed on isolated preparations (current data and Roots et al. 2009) and in humans where muscle blood flow is impaired (Clarke et al. 1958; Edwards et al. 1972; de Ruiter et al. 1999; de Ruiter & de Haan, 2001) may be due to differential ROS accumulation.
Iron and muscle fatigue
The current study demonstrates that iron can accelerate fatigue at 37°C in isolated muscles and highlights the dangers of release of iron from stainless steel. An increase in extracellular iron may accelerate fatigue by entering the cells and accelerating ROS production. In support of this idea, an increase in intracellular iron has been shown to increase ROS in cardiac cells (Oudit et al. 2003, 2004). The scheme we propose is described in Fig. 8A and shows a fibre during fatigue producing O2•− (i) and H2O2 (v). In summary, Fe2+ enters the cell though a non-transferrin bound mechanism iron uptake mechanism (iv), increasing ROS production (vi, vii). The resulting ROS may then oxidise thiol groups (viii, ix), reduce Ca2+ sensitivity and/or Fmax (van der Poel & Stephenson, 2002; Moopanar & Allen, 2006; Murphy et al. 2008) (x) and accelerate fatigue (xi).
Iron uptake and ROS production
Skeletal muscle iron uptake was studied using the fluorescent iron indicator Phen green, which has been used previously in hepatocytes (Petrat et al. 1999; Rauen et al. 2000). Our results suggest that the iron indicator Phen green is a useful tool for detecting a large change in the intracellular iron concentration of skeletal muscle, but may not be sensitive enough to detect the intracellular change caused by 1 μm extracellular iron.
The general ROS indicator demonstrated that an acute increase in intracellular iron causes a large increase in ROS production. An interesting feature of our results was that in contrast to the results obtained using the general ROS indicator, the OH• selective indicator showed a smaller, Fe-independent increase. This suggests that ascorbic acid added in the presence of hydroxyquinoline (used to facilitate iron entry), can increase OH• without interacting with extracellular iron. We propose (Fig. 8B) that hydroxyquinoline moves into the cell and binds Fe3+, making it accessible to redox cycling. Fe3+ is then reduced by ascorbic acid forming Fe2+. Fe2+ then reacts with H2O2 to produce OH•.
ROS identity
As expected, increasing the intracellular concentration of Fe2+ increased general ROS production (Fig. 6). In contrast, the highly reactive ROS indicator, selective for OH•, appeared insensitive to an increase in intracellular iron, responding equally well to solutions containing ascorbic acid and hydroxyquinoline with or without iron (Fig. 7). One possibility is that the increase in intracellular Fe2+ produced O2•− (Fig. 8A; vii) (Halliwell & Gutteridge, 1998), which is detectable with the general ROS indicator, but not the highly reactive ROS indicator. Another possibility is that the highly reactive ROS indicator is saturated by the OH• produced by the ascorbic acid + hydroxyquinoline solutions. If saturation is occurring, an increasing in intracellular Fe2+ may produce more OH• without an increase in fluorescence.
Iron in health and disease
Iron may play a role in healthy muscle during or following exercise. Redox-active iron increases in the blood following eccentric exercise (Childs et al. 2001) and may cause iron to move into cells during or following the exercise bout. The O2•− released within muscle during contraction (Reid et al. 1992) can also release iron from myoglobin, increasing redox-active iron. Increased intracellular redox-active iron may therefore contribute to the increase in ROS production, damage and adaptation associated with regular exercise. Iron-induced ROS production is also believed to occur in skeletal muscle with ageing (Jung et al. 2008) and disuse atrophy (Hofer et al. 2008). A redox-active labile iron pool may therefore underlie sarcopaenia (Jung et al. 2008), a condition characterised by reduced muscle mass and function with age.
Our results demonstrate that iron can accelerate fatigue and that an acute increase in iron can increase ROS; however, we did not measure ROS production during fatigue. Preliminary stimulation experiments, using primary cultured muscle cells suggest that a few contractions in the presence of iron are not enough to stimulate ROS production (data not shown). Iron-induced ROS production may therefore require fatiguing contractions, perhaps by allowing iron entry through store-operated calcium channels which are believed to activate during fatigue (Ducret et al. 2006). Future studies may measure iron-induced ROS production during muscle fatigue in conjunction with store-operated calcium channel blockers as well as other proposed NTBI blockers. If successful, NTBI blockers could be used with a skeletal muscle iron overload protocol similar to our previous study (Reardon & Allen, 2009) to determine if NTBI blockers reduce iron uptake, oxidative stress and the loss in exercise performance associated with iron overload conditions.
People with iron overload disorders display symptoms of weakness and fatigue (Brandhagen et al. 2002; Davidsen et al. 2007) which may be explained by increased ROS generated from an increase in the redox-active labile iron pool. In support of this theory, Reardon & Allen (2009) demonstrated that iron overloaded skeletal muscles are under more oxidative stress and show a greater force loss over time on a strength test compared to controls.
Conclusion
The current study demonstrates that the time to fatigue of an isolated skeletal muscle bundle is longer at 37°C than at room temperarture, but shorter in the presence of extracellular iron or following the direct application of ROS. We show that an acute increase in intracellular iron can cause a substantial increase in ROS production, suggesting that iron accelerates fatigue by entering muscle cells and increasing ROS production. Iron-induced ROS production may be important in the weakness and fatigue observed in iron overload conditions and may contribute to the reduced muscle function associated with disuse atrophy and sarcopaenia.
References
- Allen DG, Duty S, Westerblad H. Metabolic changes in muscle during exercise: their effects on muscle function. Proc Aust Physiol Pharmacol Soc. 1993;24:65–75. [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacic G, Spasojevic I, Secerov B, Mojovic M. Spin-trapping of oxygen free radicals in chemical and biological systems: New traps, radicals and possibilities. Spectrochim Acta A Mol Biomol Spectrosc. 2008;69:1354–1366. doi: 10.1016/j.saa.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Bergh U, Ekblom B. Influence of muscle temperature on maximal muscle strength and power output in human skeletal-muscles. Acta Physiol Scand. 1979;107:33–37. doi: 10.1111/j.1748-1716.1979.tb06439.x. [DOI] [PubMed] [Google Scholar]
- Blomstrand E, Larsson L, Edstrom L. Contractile properties, fatiguability and glycolytic metabolism in fast-twitch and slow-twitch rat skeletal-muscles of various temperatures. Acta Physiol Scand. 1985;125:235–243. doi: 10.1111/j.1748-1716.1985.tb07712.x. [DOI] [PubMed] [Google Scholar]
- Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65:853–860. [PubMed] [Google Scholar]
- Breuer W, Epsztejn S, Millgram P, Cabantchik IZ. Transport of iron and other transition-metals into cells as revealed by a fluorescent-probe. Am J Physiol Cell Physiol. 1995;37:C1354–C1361. doi: 10.1152/ajpcell.1995.268.6.C1354. [DOI] [PubMed] [Google Scholar]
- Buettner GR. In the absence of catalytic metals ascorbate does not autoxidize at Ph-7 – ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988;16:27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Buettner GR. Ascorbate oxidation: UV absorbance of ascorbate and ESR spectroscopy of the ascorbyl radical as assays for iron. Free Radic Res Commun. 1990;10:5–9. doi: 10.3109/10715769009145927. [DOI] [PubMed] [Google Scholar]
- Cady EB, Jones DA, Lynn J, Newham DJ. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol. 1989;418:311–325. doi: 10.1113/jphysiol.1989.sp017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- Clarke RSJ, Hellon RF, Lind AR. The duration of sustained contractions of the human forearm at different muscle temperatures. J Physiol. 1958;143:454–473. doi: 10.1113/jphysiol.1958.sp006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley GC, Garg A, Lohn MS, Van Someren N, Wade AJ. Effects of cooling the legs on performance in a standard Wingate anaerobic power test. Br J Sports Med. 1991;25:200–203. doi: 10.1136/bjsm.25.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB J. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- Davidsen ES, Liseth K, Omvik P, Hervig T, Gerdts E. Reduced exercise capacity in genetic haemochromatosis. Eur J Cardiovasc Prev Rehabil. 2007;14:470–475. doi: 10.1097/HJR.0b013e3280ac151c. [DOI] [PubMed] [Google Scholar]
- Davies CTM, Young K. Effect of temperature on the contractile properties and muscle power of triceps surae in humans. J Appl Physiol. 1983;55:191–195. doi: 10.1152/jappl.1983.55.1.191. [DOI] [PubMed] [Google Scholar]
- Debold EP, Dave H, Fitts RH. Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am J Physiol Cell Physiol. 2004;287:C673–C681. doi: 10.1152/ajpcell.00044.2004. [DOI] [PubMed] [Google Scholar]
- de Ruiter CJ, de Haan A. Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflügers Arch. 2000;440:163–170. doi: 10.1007/s004240000284. [DOI] [PubMed] [Google Scholar]
- de Ruiter CJ, de Haan A. Similar effects of cooling and fatigue on eccentric and concentric force-velocity relationships in human muscle. J Appl Physiol. 2001;90:2109–2116. doi: 10.1152/jappl.2001.90.6.2109. [DOI] [PubMed] [Google Scholar]
- de Ruiter CJ, Jones DA, Sargeant AJ, de Haan A. Temperature effect on the rates of isometric force development and relaxation in the fresh and fatigued human adductor pollicis muscle. Exp Physiol. 1999;84:1137–1150. doi: 10.1017/s0958067099018953. [DOI] [PubMed] [Google Scholar]
- Ducret T, Vandebrouck C, Cao ML, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol. 2006;575:913–924. doi: 10.1113/jphysiol.2006.115154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JN, Macdonald WA, Van Der Poel C, Stephenson DG. O2(•−) production at 37°C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am J Physiol Cell Physiol. 2007;293:C650–C660. doi: 10.1152/ajpcell.00037.2007. [DOI] [PubMed] [Google Scholar]
- Edwards RHT, Harris RC, Hultman E, Kaijser L, Koh D, Nordesjo L-O. Effect of temperature on muscle energy metabolism and endurance during successive isometric contractions, sustained to fatigue, of quadriceps muscle in man. J Physiol. 1972;220:335–352. doi: 10.1113/jphysiol.1972.sp009710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscles. J Physiol. 1978;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd edn. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- Hofer T, Marzetti E, Xu JZ, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holewijn M, Heus R. Effects of temperature on electromyogram and muscle function. Eur J Appl Physiol Occup Physiol. 1992;65:541–545. doi: 10.1007/BF00602362. [DOI] [PubMed] [Google Scholar]
- Jung SH, DeRuisseau LR, Kavazis AN, DeRuisseau KC. Plantaris muscle of aged rats demonstrates iron accumulation and altered expression of iron regulation proteins. Exp Physiol. 2008;93:407–414. doi: 10.1113/expphysiol.2007.039453. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Imlay JA. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Mol Microbiol. 2002;43:95–106. doi: 10.1046/j.1365-2958.2002.02719.x. [DOI] [PubMed] [Google Scholar]
- Lane DJ, Lawen A. Non-transferrin iron reduction and uptake are regulated by transmembrane ascorbate cycling in K562 cells. J Biol Chem. 2008;283:12701–12708. doi: 10.1074/jbc.M800713200. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NC, Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers; a steady-state and transient kinetic study. J Biol Chem. 1990;265:20234–20240. [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37°C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol. 2006;571:191–200. doi: 10.1113/jphysiol.2005.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Dutka TL, Lamb GD. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol. 2008;586:2203–2216. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L. Hyperthermia and fatigue. J Appl Physiol. 2008;104:871–878. doi: 10.1152/japplphysiol.00910.2007. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP, Backx PH. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Trivieri MG, Khaper N, Husain T, Wilson GJ, Liu P, Sole MJ, Backx PH. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation. 2004;109:1877–1885. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrat F, Rauen U, de Groot H. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology. 1999;29:1171–1179. doi: 10.1002/hep.510290435. [DOI] [PubMed] [Google Scholar]
- Rauen U, Petrat F, Li TJ, de Groot H. Hypothermia injury/cold-induced apoptosis – evidence of an increase in chelatable iron causing oxidative injury in spite of low O2–/H2O2 formation. FASEB J. 2000;14:1953–1964. doi: 10.1096/fj.00-0071com. [DOI] [PubMed] [Google Scholar]
- Reardon TF, Allen DG. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp Physiol. 2009;94:720–730. doi: 10.1113/expphysiol.2008.046045. [DOI] [PubMed] [Google Scholar]
- Reid MR, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- Roots H, Ball G, Talbot-Ponsonby J, King M, McBeath K, Ranatunga KW. Muscle fatigue examined at different temperatures in experiments on intact mammalian (rat) muscle fibers. J Appl Physiol. 2009;106:378–384. doi: 10.1152/japplphysiol.90883.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle samples obtained after dynamic exercise. Pflugers Archiv. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel C, Stephenson DG. Reversible changes in Ca2+-activation properties of rat skeletal muscle exposed to elevated physiological temperatures. J Physiol. 2002;544:765–776. doi: 10.1113/jphysiol.2002.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lee JA, Lamb AG, Bolsover SR, Allen DG. Spatial gradients of intracellular calcium in skeletal muscle during fatigue. Pflügers Arch. 1990;415:734–740. doi: 10.1007/BF02584013. [DOI] [PubMed] [Google Scholar]